Published online Jun 14, 2019. doi: 10.3748/wjg.v25.i22.2734
Peer-review started: February 6, 2019
First decision: February 26, 2019
Revised: April 29, 2019
Accepted: May 8, 2019
Article in press: May 8, 2019
Published online: June 14, 2019
Processing time: 129 Days and 5.4 Hours
Technological advances and the widespread use of medical imaging have led to an increase in the identification of pancreatic cysts in patients who undergo cross-sectional imaging. Current methods for the diagnosis and risk-stratification of pancreatic cysts are suboptimal, resulting in both unnecessary surgical resection and overlooked cases of neoplasia. Accurate diagnosis is crucial for guiding how a pancreatic cyst is managed, whether with surveillance for low-risk lesions or surgical resection for high-risk lesions. This review aims to summarize the current literature on confocal endomicroscopy and cyst fluid molecular analysis for the evaluation of pancreatic cysts. These recent technologies are promising adjuncts to existing approaches with the potential to improve diagnostic accuracy and ultimately patient outcomes.
Core tip: Current methods for the diagnosis and risk-stratification of pancreatic cysts are suboptimal, resulting in both unnecessary surgical resection and overlooked cases of neoplasia. Novel technologies such as confocal endomicroscopy and cyst fluid molecular analysis are promising adjuncts to the existing standard of care for the management of pancreatic cysts with the potential to improve diagnostic accuracy and ultimately patient outcomes.
- Citation: Durkin C, Krishna SG. Advanced diagnostics for pancreatic cysts: Confocal endomicroscopy and molecular analysis. World J Gastroenterol 2019; 25(22): 2734-2742
- URL: https://www.wjgnet.com/1007-9327/full/v25/i22/2734.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i22.2734
Diagnosing and managing pancreatic cysts is a common problem within gastro-enterology. The number of pancreatic cysts identified has grown over the last several decades, largely due to improved detection during imaging studies. Studies show that the prevalence of pancreatic cysts may be even higher than previously thought with about 2.4%-19.6% of all patients who undergo abdominal magnetic resonance imaging (MRI) and 2.6% who undergo computed tomography (CT) having detectable pancreatic cysts[1-4].
Pancreatic cysts can be classified as either mucinous or non-mucinous lesions, each with distinct characteristics and potential for malignancy (Table 1). Mucinous cysts include intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasms (MCN), while non-mucinous cysts include serous cystadenoma (SCA), pseudocysts, cystic neuroendocrine tumors (cystic-NETs), and solid pseudopapillary neoplasm (SPN).
| (A) Mucinous Cysts |
| 1 Intraductal Papillary Mucinous Neoplasm (IPMN) |
| Branch Duct IPMN |
| Mixed Duct IPMN |
| 2 Mucinous Cystic Neoplasm (MCN) |
| (B) Non-mucinous Cysts |
| Serous Cystadenoma (SCA) |
| Solid Pseudopapillary Tumor (SPT) |
| Cystic Neuroendocrine Tumor (Cystic-NET) |
| Squamous-Lined Cysts |
| Epidermoid Cysts |
| Lymphoepithelial Cysts |
| Pseudocysts |
| (C) Other malignant Cysts |
| Ductal Adenocarcinoma with Cystic Degeneration |
| Acinar Cell Cystadenocarcinoma |
| Cystic Degeneration of Metastatic Lesions to the Pancreas |
In general, mucinous pancreatic cysts have the potential for malignant trans-formation, while non-mucinous SCAs are typically benign neoplasms. Therefore, it is crucial to accurately diagnose and differentiate pancreatic lesions, as it will impact future prognosis and treatment plans. The evaluation of pancreatic cysts is a multimodality approach, utilizing a combination of clinical history, demographics, radiographic and endoscopic ultrasound (EUS) features, cytology, and cyst fluid analysis [i.e., carcinoembryonic antigen (CEA) and amylase][5]. Despite these multidisciplinary techniques, distinguishing pancreatic cyst type prior to surgical intervention remains difficult and there is a need for improved diagnostic strategies.
Multiple guidelines have been developed to aid the diagnosis and treatment of pancreatic cysts, including the International Consensus Guidelines (Sendai 2006, Fukuoka 2012, and Fukuoka 2017), the American Gastroenterological Association (AGA) 2015 guidelines, and clinical guidelines from the American College of Gastroenterology[6-10]. Current guidelines recommend surgical resection of all large cysts (> 4 cm), MCNs (malignancy risk: 17.5%), main duct (MD)-IPMNs (malignancy risk: 61%) and branch duct (BD)-IPMNs with high-risk features (obstructive jaundice, dilated main pancreatic duct > 1 cm, solid enhancing intracystic nodule; malignancy risk: 25%)[7]. A summary of these guidelines can be found in Table 2.
| Guideline | Recommendations |
| Sendai 2006 [8] | Recommended surgical resection if any of the following lesions were suspected: |
| MCNs | |
| Main duct IPMNs | |
| Mixed duct IPMNs | |
| Also recommended surgical resection also based on: | |
| Clinical symptoms | |
| Dilated pancreatic duct (≥6mm) | |
| Intracystic mural nodules | |
| Positive cytology [8] | |
| Fukuoka 2012 [6] | Recommended surgical resection for high-risk criteria: |
| Dilated pancreatic duct (≥10mm) | |
| Presence of an enhancing solid component | |
| Obstructive jaundice [6] | |
| American Gastroenterological Association (AGA) 2015 [9] | Recommended EUS-FNA if 2 out of 3 of the following high-risk features were present: |
| Size ≥ 3 cm | |
| Dilated main pancreatic duct | |
| Solid component | |
| Recommended surgical resection if a cyst had both of the following: | |
| Solid component | |
| Dilated pancreatic duct and/or concerning features on EUS-FNA [9] | |
| Fukuoka 2017 [7] | Enhancing mural nodule is a high risk feature if measuring ≥ 5 mm |
| Added surveillance guidelines for BD-IPMN, noting presence of lymphadenopathy, increased serum CA19-9 and cyst growth rate >5 mm in diameter over 2 years as “worrisome features” [7] |
In a systemic review of the clinical utility of Sendai and Fukuoka Guidelines, both had lower positive predictive value (PPV) (Sendai: 11%-52%; Fukuoka 27%-100%) for worrisome and/or high-risk criteria to predict malignancy in surgical resected IPMNs. While further enhancements are needed to the current consensus guidelines to improve the management of patients with mucinous cysts, additional imaging modalities, such as endoscopic ultrasound needle-based confocal endomicroscopy (EUS-nCLE), and novel pancreatic molecular biomarkers are promising new technologies that may prove crucial in how pancreatic cysts are differentiated and treated. This review aims to summarize the current literature on confocal endomicroscopy and cyst fluid molecular analysis for the evaluation of pancreatic cysts.
Confocal laser endomicroscopy is a novel technology that allows for real-time microscopic imaging of intracystic epithelium in vivo. An endoscopic and mini probe-based modality, CLE probes can be introduced into pancreatic cysts through a 19-gauge FNA needle to allow for high magnification and resolution imaging of cyst epithelium during EUS. Intravenous fluorescein is used during EUS-nCLE to further enhance blood vessels and other structures within the pancreatic cysts. The indication for EUS-nCLE is the evaluation of pancreatic cysts where fine needle aspiration is being considered, typically for lesions measuring ≥ 2 cm in size. EUS-nCLE is contraindicated in patients with allergic reactions to fluorescein.
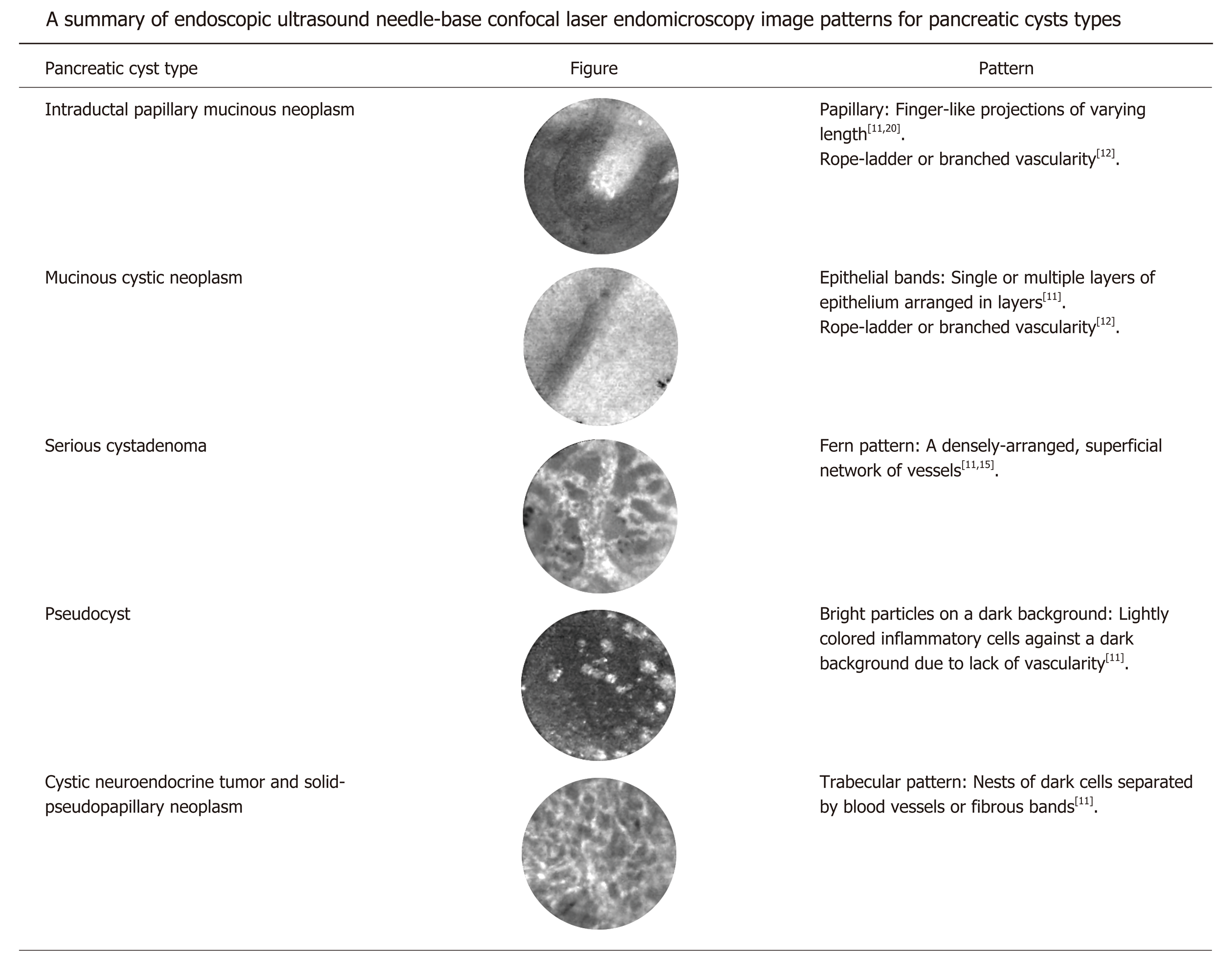
Pancreatic cysts have characteristic patterns on EUS-nCLE imaging that aid in their identification and classification. Figure 1 summarizes pancreatic cysts types and their associated imaging patterns on EUS-nCLE. These images are broadly classified into epithelial and vascular patterns[11,12]. IPMNs are characterized by their finger-like papillae, while MCNs can be identified by their singular or layered epithelial bands[11,13,14]. SCAs have fern-like patterns with a superficial dense network of vessels[14,15]. Pseudocysts appear as bright particles against a dark background, corresponding to inflammatory cells without the presence of a true cysts wall or vascularity[11]. Cystic-NETs and SPNs have a trabecular pattern with high cellularity, appearing as nests or cords of cells separated by fibrous bands[16]. Rarer pancreatic cyst types, for example, lymphoepithelial cysts, have also been described in case reports[17]. Both in vivo and ex vivo nCLE images have been validated and compared with histopathology for IPMNs, MCNs, SCAs, and Cystic-NETs[16,18].
Several innovative studies have established the safety, feasibility, and ability of nCLE to differentiate pancreatic cyst types. These include the INSPECT, DETECT, CONTACT-1 and -2, and INDEX trials. The INSPECT study (2013) established the safety and demonstrated feasibility of nCLE in differentiating mucinous pancreatic cysts. This study also identified IPMNs as having papillary structures on nCLE[13]. The DETECT study (2015) evaluated the technical feasibility, safety, and diagnostic capabilities of cystoscopy and nCLE for diagnosing pancreatic cysts, showing that this combination of novel imaging modalities has a strong concordance with the definitive diagnosis of pancreatic cystic neoplasms[19]. The CONTACT-1 study (2015) evaluated solitary pancreatic cysts in 31 patients using EUS-nCLE. They found that SCAs had a characteristic pattern of superficial vascular networks on nCLE, which correlated microscopically to dense networks of subepithelial capillaries[15]. The CONTACT-2 study (2016) described nCLE patterns for MCNs, pseudocysts, and cystic-NETs that correlated with histology. These cysts appeared as epithelial bands, bright particles on a dark background, and black nests of cells separated by white fibrous bands under nCLE, respectively[11]. The INDEX study (2016) validated previously described nCLE findings for pancreatic cysts, comparing in vivo and ex vivo CLE to surgical histopathology, and showed substantial interobserver agreement and intraobserver reliability for differentiating mucinous pancreatic cyst types in blinded nCLE observers[12,16,20].
In the most recent update of the multicenter CONTACT-2 study (2018), among 78 subjects with reference diagnoses, the sensitivity, specificity, PPV, negative predictive value (NPV) for EUS-nCLE to diagnose premalignant pancreatic cysts (MCNs, BD-IPMNs, cystic-NETs, SPNs, and cystic lymphoma) from benign lesions were 96%, 95%, 98%, and 91%, respectively. To differentiate mucinous from non-mucinous lesions, the sensitivity, specificity, PPV, and NPV were 95%, 100%, 100% and 94% respectively[21].
Procedural expertise for optimal image acquisition during EUS-nCLE can be obtained by directly observing an expert EUS-nCLE in dedicated workshops and subsequently performing at least 10 cases. Since there are no formal studies to address high-quality image acquisition, the limited case requirement is only an opinion among experts.
In addition to recent imaging technologies, DNA-based molecular analysis of cyst fluid has also become a useful tool for diagnosing pancreatic cysts. Epithelial cells that line pancreatic cysts shed DNA into cyst fluid through cell lysis or exfoliation. This DNA can be analyzed for genetic alterations associated with a particular diagnosis and prognosis[22,23]. Multiple studies have identified molecular markers associated with each of the major types of pancreatic cysts and genetic profiles that predict progression into adenocarcinoma[22,24,25]. Several aspects of molecular analysis are important for diagnosis and prognostication: DNA quantity and quality, genetic mutations, and tumor suppressor genes or loss of heterozygosity (LOH).
The amount of DNA contained in pancreatic cysts fluid can be determined through spectrophotometry. When a sample is exposed to ultraviolet light in a spectr-ophotometer, a photo-detector measures the quantity of nucleic acid in the sample and calculates its DNA concentration using the optical density ratio at certain wavelengths (260 nm:280 nm). In a prospective, multicenter study of 113 patients, elevated concentrations of DNA within pancreatic cysts were associated with a diagnosis of malignancy[26]. Furthermore, while traditional techniques used to analyze pancreatic cyst fluid, such as cytopathology and carcinoembryonic antigen (CEA), cannot be optimally performed due to the inadequate volume of cells or fluid, pancreatic cysts typically contain enough DNA to evaluate for mutations[26,27].
LOH is when there is a loss of one of the copies of a gene and its surrounding chromosomal region. When there is LOH of a tumor suppressor gene, it can result in loss of tumor suppressor activity and subsequent development of unregulated growth. This can be detected using microsatellite markers linked to tumor suppressor genes and correlates to malignancy[28].
Next-generation sequencing (NGS) has been crucial in the recent identification of molecular markers for specific pancreatic cyst types. NGS encompasses a number of high-throughput technologies that allow for millions or billions of DNA strands to be sequenced in parallel. It has enabled the rapid sequencing of entire genomes and exomes, as well as more targeted sequencing studies. Prior to NGS, Sanger sequencing was the most widely used method of determining genetic sequences. It was based on selective incorporation of chain-terminating dideoxynucleotides (dATP, dTTP, dGTP, dCTP) by DNA polymerase during in vivo DNA replication. Table 3 contains a summary of the genetic mutations associated with each major pancreatic cyst type.
| Pancreatic cyst type | Molecular biomarkers |
| Intraductal papillary mucinous neoplasm | KRAS, GNAS, RNF43 positive[22,24,29,34,37] |
| Advanced neoplasia: TP53, SMAD4, PIK3CA, PTEN, CDKN2A, AKT1, p16, p53 positive[30-34,38,39] | |
| Mucinous cystic neoplasm | KRAS, RNF3 positive[22,24,29,34,37] |
| GNAS negative[24,28,29] | |
| Advanced neoplasia: TP53, SMAD4, PIK3CA, PTEN, CDKN2A, AKT1 positive[30-34] | |
| Serious cystadenoma | VHL positive[22,24,28] |
| Solid papillary neoplasm | CTNNB1 positive[22,24] |
| Pseudocyst | Negative for DNA |
| Cystic neuroendocrine tumor | Not well described |
Of note, KRAS mutations are common in both IPMNs and MCNs, while GNAS mutations are typically found in IPMNs but not MCNs[22,24,28,29]. Both IPMNs and MCNs are also frequently found to have RNF43 mutations[22,24]. Malignant and high-grade IPMNs have been found to have TP53, SMAD4, PIK3CA, PTEN, CDKN2A, and AKT1 mutations[30-34]. Additionally, VHL mutations are highly specific for SCAs[22,24,28] and Β-catenin gene (CTNNB1) mutations are associated with solid-pseudopapillary neoplasms[22,24].
A recent prospective study of 626 pancreatic cyst fluid specimens showed that KRAS/GNAS mutations were associated with an 89% sensitivity and 100% specificity for mucinous pancreatic cysts and that NGS had improved sensitivity over Sanger sequencing. It also showed that the combination of KRAS/GNAS mutations and alterations in TP53/PIK3CA/PTEN had an 89% sensitivity and 100% specificity for advanced neoplasia, which was better than the presence of ductal dilation, a mural nodule, and malignant cytopathology in identifying high-grade cysts[34].
Table 4 compares the key benefits and drawbacks of EUS-nCLE and molecular analysis of cyst fluid. NGS is not without limitations. Certain pancreatic cyst types are currently poorly identified using molecular analysis, suggesting the need for further exploration of molecular biomarkers. Although MCNs are readily associated with several genetic changes (e.g., KRAS, RNF3), these mutations have low sensitivity (33% for KRAS and 8%-35% for RNF3 in MCNs), which could result in under-detection[28,33-35]. The Sanger sequencing technique is not able to detect the entire loss of the VHL gene but can detect deletions and insertions within exons or complete loss of an exon. Hence Sanger sequencing has low sensitivity for the detection of VHL mutation which is otherwise commonly observed in SCAs[34,36]. Additionally, molecular markers in cystic-NETs are poorly characterized in current literature.
| Molecular analysis of PCL fluid | EUS-nCLE of PCLs |
| (DNA analysis) | |
| High sensitivity and specificity for the diagnosis of mucinous PCLs | High sensitivity and specificity for the diagnosis of mucinous PCLs |
| Markers can detect advanced neoplasia in IPMNs; need validation in multicenter studies | Need further studies to address role of EUS-nCLE in the identification of advanced neoplasia in PCLs |
| Lower sensitivity for the detection of KRAS mutations in MCNs | Detection of flat epithelium in MCNs can be difficult for early adapters of EUS-nCLE |
| Need large multicenter prospective studies with confirmed histopathology to replicate single center results | Need large multicenter prospective studies with confirmed histopathology to replicate single center results |
| Lack of established markers for cystic-NET and squamous lined cysts | EUS-nCLE reveals specific image patterns for different PCL types. Unable to differentiate between cystic-NET and SPN |
| During EUS-FNA, 5%-10% of PCLs may not yield DNA for molecular analysis | There is a 2%-5% risk of technical and procedural issues with failure of image acquisition during EUS-nCLE |
| Low sensitivity for the detection of VHL mutations in SCAs | EUS-nCLE identifies characteristic ‘fern-pattern’ of vascularity for diagnosing SCAs |
This review summarizes recent technological advances in the evaluation of pancreatic cysts: confocal endomicroscopy and molecular biomarkers. Both EUS-nCLE and cyst fluid analysis have demonstrated their ability to help diagnose and differentiate pancreatic cysts with high accuracy. Current methods for diagnosing pancreatic cysts, such as imaging (MRI/CT), endoscopy (EUS), cytology, CEA, and amylase, often yield suboptimal or indeterminate results. Given the limitations of existing diagnostic strategies, these minimally invasive technologies, therefore, have the potential to increase diagnostic accuracy, improve risk stratification, and serve as useful adjuncts to current management protocols. As emerging technologies, both confocal endomicroscopy and DNA analysis currently are utilized predominantly in academic settings and are not yet widely used in clinical practice. Thus, additional studies and clinician training will be needed to incorporate them into routine use. Because the number of large (> 2 cm) pancreatic cysts found on abdominal imaging continues to grow each year, diagnostics like confocal endomicroscopy and molecular analysis are more important than ever for accurately diagnosing pancreatic lesions and guiding the next steps in their management.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiu CC, Ghiorzo P, Mastoraki A S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
| 1. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 436] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 2. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 3. | Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 277] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 5. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 6. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 7. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 8. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1442] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 9. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-822; quize 12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 10. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 11. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Poizat F, Giovannini M. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Krishna SG, Brugge WR, Dewitt JM, Kongkam P, Napoleon B, Robles-Medranda C, Tan D, El-Dika S, McCarthy S, Walker J, Dillhoff ME, Manilchuk A, Schmidt C, Swanson B, Shah ZK, Hart PA, Conwell DL. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: an international external interobserver and intraobserver study (with videos). Gastrointest Endosc. 2017;86:644-654.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK, Saunders MD, Aslanian HR, Wroblewski K, Waxman I. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Modi RM, Swanson B, Muscarella P 2nd, Conwell DL, Krishna SG. Novel techniques for diagnosis of serous cystadenoma: fern pattern of vascularity confirmed by in vivo and ex vivo confocal laser endomicroscopy. Gastrointest Endosc. 2017;85:258-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Filoche B, Giovannini M. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Krishna SG, Modi RM, Kamboj AK, Swanson BJ, Hart PA, Dillhoff ME, Manilchuk A, Schmidt CR, Conwell DL. In vivo and ex vivo confocal endomicroscopy of pancreatic cystic lesions: A prospective study. World J Gastroenterol. 2017;23:3338-3348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Modi RM, Kamboj AK, Swanson B, Conwell DL, Krishna SG. Epidermoid cyst within an intrapancreatic accessory spleen: endosonography and confocal endomicroscopy of an unusual pancreatic cystic lesion. Endoscopy. 2016;48:E332-E333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Krishna SG, Swanson B, Conwell DL, Muscarella P. In vivo and ex vivo needle-based confocal endomicroscopy of intraductal papillary mucinous neoplasm of the pancreas. Gastrointest Endosc. 2015;82:571-572. |
| 19. | Nakai Y, Iwashita T, Park DH, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Krishna SG, Swanson B, Hart PA, El-Dika S, Walker JP, McCarthy ST, Malli A, Shah ZK, Conwell DL. Validation of diagnostic characteristics of needle based confocal laser endomicroscopy in differentiation of pancreatic cystic lesions. Endosc Int Open. 2016;4:E1124-E1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Napoleon B, Pujol B, Palazzo M, Caillol F, Palazzo L, Aubert A, Maire F, Buscail L, Lemaistre A-I, Giovannini M. Needlebased confocal laser endomicroscopy of pancreatic cystic lesions: a prospective multicenter validation study in patients with definite diagnosis. Gastroenterology. 2017;152:S132-S133. [DOI] [Full Text] |
| 22. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-Del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 328] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 23. | Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA. 2011;108:21188-21193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 25. | Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, Zamboni G, Maitra A, Salvia R, Hruban RH, Bassi C, Capelli P, Lawlor RT, Goggins M, Scarpa A. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 26. | Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 27. | Khalid A, McGrath KM, Zahid M, Wilson M, Brody D, Swalsky P, Moser AJ, Lee KK, Slivka A, Whitcomb DC, Finkelstein S. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clin Gastroenterol Hepatol. 2005;3:967-973. [PubMed] |
| 28. | Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 29. | Singhi AD, Nikiforova MN, Fasanella KE, McGrath KM, Pai RK, Ohori NP, Bartholow TL, Brand RE, Chennat JS, Lu X, Papachristou GI, Slivka A, Zeh HJ, Zureikat AH, Lee KK, Tsung A, Mantha GS, Khalid A. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res. 2014;20:4381-4389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Kanda M, Sadakari Y, Borges M, Topazian M, Farrell J, Syngal S, Lee J, Kamel I, Lennon AM, Knight S, Fujiwara S, Hruban RH, Canto MI, Goggins M. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol. 2013;11:719-730.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Schönleben F, Qiu W, Ciau NT, Ho DJ, Li X, Allendorf JD, Remotti HE, Su GH. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res. 2006;12:3851-3855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Garcia-Carracedo D, Chen ZM, Qiu W, Huang AS, Tang SM, Hruban RH, Su GH. PIK3CA mutations in mucinous cystic neoplasms of the pancreas. Pancreas. 2014;43:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Yu J, Sadakari Y, Shindo K, Suenaga M, Brant A, Almario JAN, Borges M, Barkley T, Fesharakizadeh S, Ford M, Hruban RH, Shin EJ, Lennon AM, Canto MI, Goggins M. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. 2017;66:1677-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 34. | Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 35. | Mukewar S, de Pretis N, Aryal-Khanal A, Ahmed N, Sah R, Enders F, Larson JJ, Levy MJ, Takahashi N, Topazian M, Pearson R, Vege SS, Chari ST. Fukuoka criteria accurately predict risk for adverse outcomes during follow-up of pancreatic cysts presumed to be intraductal papillary mucinous neoplasms. Gut. 2017;66:1811-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 36. | Tsiatis AC, Norris-Kirby A, Rich RG, Hafez MJ, Gocke CD, Eshleman JR, Murphy KM. Comparison of Sanger sequencing, pyrosequencing, and melting curve analysis for the detection of KRAS mutations: diagnostic and clinical implications. J Mol Diagn. 2010;12:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 373] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 37. | Nikiforova MN, Khalid A, Fasanella KE, McGrath KM, Brand RE, Chennat JS, Slivka A, Zeh HJ, Zureikat AH, Krasinskas AM, Ohori NP, Schoedel KE, Navina S, Mantha GS, Pai RK, Singhi AD. Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: a clinical experience of 618 pancreatic cysts. Mod Pathol. 2013;26:1478-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Sasaki S, Yamamoto H, Kaneto H, Ozeki I, Adachi Y, Takagi H, Matsumoto T, Itoh H, Nagakawa T, Miyakawa H, Muraoka S, Fujinaga A, Suga T, Satoh M, Itoh F, Endo T, Imai K. Differential roles of alterations of p53, p16, and SMAD4 expression in the progression of intraductal papillary-mucinous tumors of the pancreas. Oncol Rep. 2003;10:21-25. [PubMed] |
| 39. | Garcia-Carracedo D, Turk AT, Fine SA, Akhavan N, Tweel BC, Parsons R, Chabot JA, Allendorf JD, Genkinger JM, Remotti HE, Su GH. Loss of PTEN expression is associated with poor prognosis in patients with intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2013;19:6830-6841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |