Published online Jun 7, 2019. doi: 10.3748/wjg.v25.i21.2675
Peer-review started: March 21, 2019
First decision: April 4, 2019
Revised: April 24, 2019
Accepted: May 8, 2019
Article in press: May 8, 2019
Published online: June 7, 2019
Processing time: 78 Days and 1.9 Hours
Several studies have been conducted to explore the association between the use of proton pump inhibitors (PPIs) and hepatic encephalopathy (HE) risk in patients with liver cirrhosis. However, their results are controversial.
To perform a systematic review and meta-analysis to evaluate the HE risk among PPI users.
A systematic search on PubMed, Web of Science, EMBase, and ScienceDirect databases was conducted up to December 31, 2018 for eligible studies involving PPI use and HE risk. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the fixed or random effects model. Publication bias was evaluated using Begg’s test, Egger’s test, and trim-and-fill method.
Seven studies with 4574 patients were included in the present meta-analysis. The meta-analysis results indicated a significant association between the PPI use and HE risk (OR = 1.50; 95%CI: 1.25-1.75) with low heterogeneity (I2 = 14.2%, P = 0.321). Although publication bias existed when Egger’s tests were used (P = 0.005), the trim-and-fill method verified the stability of the pooled result. Sensitivity analyses suggested that the results of this meta-analysis were robust.
The current evidence indicates that PPI use increases HE risk in patients with liver cirrhosis. Further studies with a large data set and well-designed models are needed to validate our findings.
Core tip: Several studies have been conducted to explore the association between the use of proton pump inhibitors (PPIs) and hepatic encephalopathy (HE) risk in patients with liver cirrhosis. However, their results are controversial. The current evidence indicates that PPI use increases HE risk in patients with liver cirrhosis. Physicians should ban PPI use in patients with liver cirrhosis when they are used without specific indications.
- Citation: Ma YJ, Cao ZX, Li Y, Feng SY. Proton pump inhibitor use increases hepatic encephalopathy risk: A systematic review and meta-analysis. World J Gastroenterol 2019; 25(21): 2675-2682
- URL: https://www.wjgnet.com/1007-9327/full/v25/i21/2675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i21.2675
Proton pump inhibitors (PPIs) are the first choice of treatment for esophagitis and peptic ulcer disease, as well as the prevention of nonsteroidal anti-inflammatory drug associated ulcers, Zollinger-Ellison syndrome, and functional dyspepsia[1]. In acid-related diseases, the benefits of PPI use outweigh their potential harm. Unfortunately, the negative effects of PPI use are generally underestimated due to marketing strategy and neglected reporting bias in published trials. Thus, not all PPIs are used following evidence-based guidelines in the clinical setting, and PPIs are overprescribed in both inpatient and outpatient settings[2-4].
Accumulating data illustrate the potential risks associated with long-term PPI therapy, including pneumonia, spontaneous bacterial peritonitis, gastric cancer, vitamin B12 deficiency, Clostridium difficile-associated diarrhea, myocardial infarction, hypomagnesemia, chronic kidney disease, and hip fracture[5-10]. Regarding concerns over liver adverse effects, a previous meta-analysis showed that PPIs increase the risk of hepatic encephalopathy (HE) in patients with hepatic failure[11]. However, the results are restricted because of the inclusion of a relatively small number of studies. New primary studies[12-15] have also been recently published, and their results are controversial.
Therefore, in this meta-analysis, we aimed to update, compile, and critically review the existing evidence on the HE risk in patients with liver cirrhosis and PPI use and provide a quantitative estimate of the relationship between PPI use and HE risk.
This systematic review was performed in accordance with the standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[16]. Ethical approval was not sought for this study because all the data came from published studies, and no individual-level data were used. The systematic review registration number is CRD42019120845.
A systematic search on PubMed, Web of Science, Embase, and ScienceDirect databases was conducted up to December 31, 2018 for eligible studies involving PPI use and HE risk. The following keywords were used to search for the related literature: “proton pump inhibitors” and “hepatic encephalopathy.” The reference lists of the identified articles were also manually searched to identify additional relevant studies.
Two reviewers independently screened the titles and abstracts of the retrieved studies. Studies were considered eligible if they met the inclusion criteria, as follows: Studies assessed the association between PPI and HE risk, those with full text access, and those which included sufficient data to calculate odds ratios (ORs) and 95% confidential intervals (CI) for extraction. Among duplicate studies, the most recent study was included in this meta-analysis. The exclusion criteria were as follows: Insufficient data for extraction, articles that are available only in abstracts, case reports, conference papers, editor comments, reviews, meta-analysis, and inclusion of duplicate data in other studies.
Data were extracted independently by two reviewers using the data extraction tables. The results were compared, and disagreements were discussed. The following data were extracted: First author’s name, publication year, region, number of patients, age, sex, PPI use duration, HE level, and outcomes.
To evaluate the methodological quality of the included studies, we used the Newcastle-Ottawa scale (NOS)[17]. The range of NOS scores was from 0 to 9, and a score ≥ 6 was defined as “high quality”[18,19].
Statistical analyses were performed using STATA version 12.0 (Stata Corporation, College Station, TX, United States), and two-sided P < 0.05 was considered statistically significant. Pooled ORs with 95%CIs were utilized to evaluate the relationship between PPI use and HE risk. Statistical heterogeneity was assessed based on P- and I2-values by using the standard Chi-squared test. Low, moderate, or high hete-rogeneity among studies was defined as I2 < 25%, 50%-75%, and > 75%, respectively. When I2 > 50%, P < 0.1 was considered significantly heterogeneous, and the random effects model was used for meta-analysis; otherwise, the fixed effects model was used. We performed a sensitivity analysis by excluding one study at a time to assess the effect of individual studies on the summary estimates. Publication bias was evaluated using Begg’s test, Egger’s test, and trim-and-fill method.
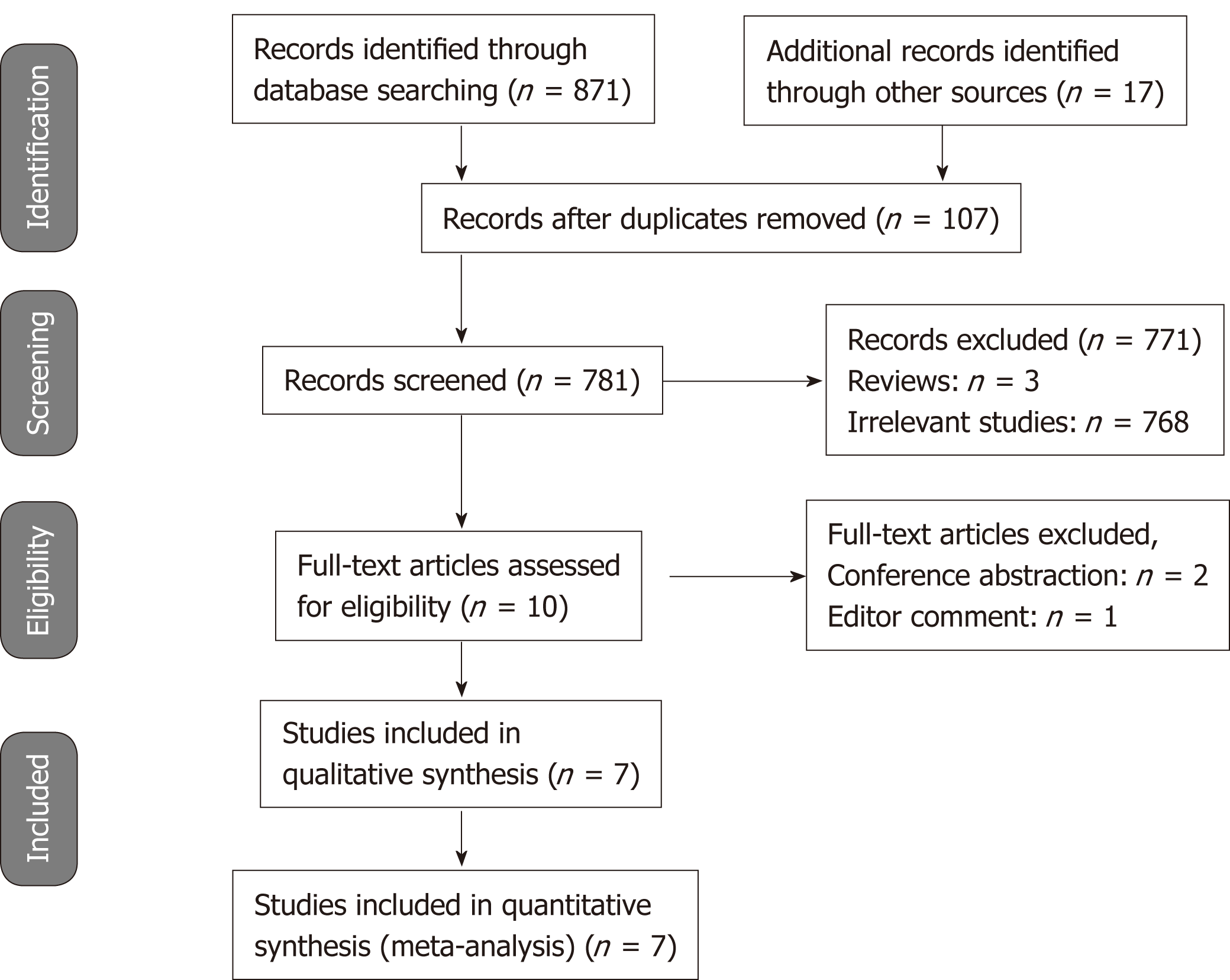
The details of study identification, screening, and selection are presented in Figure 1. The initial database search yielded 888 records, of which 107 duplicates were excluded. Then, 771 records, including 768 irrelevant studies and 3 reviews, were removed through the primary screening of titles and abstracts. After assessing ten full-text studies, two conference abstractions and one editor comment were excluded. Finally, seven articles[12-15,20-22] involving 4574 patients were included in this meta-analysis.
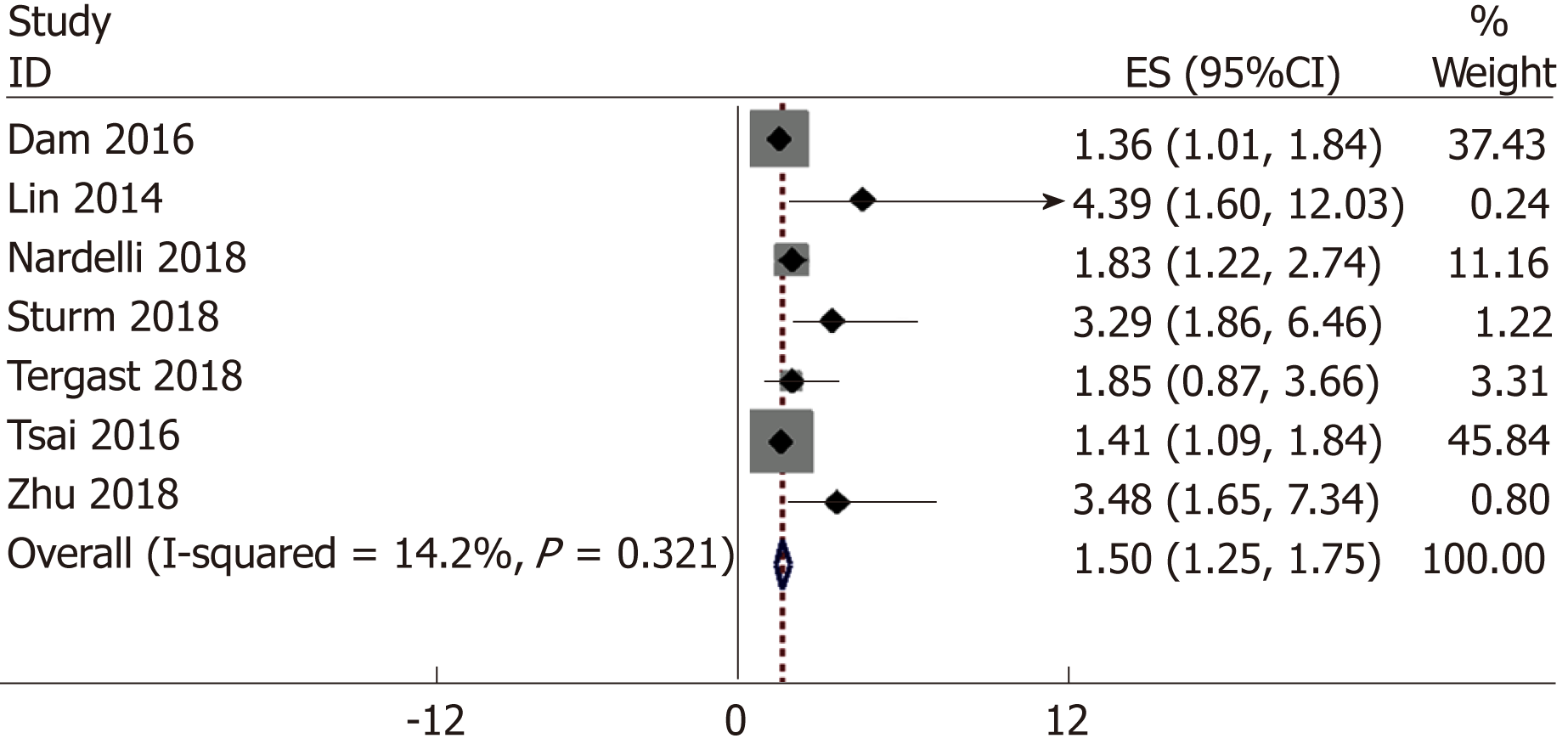
The characteristics of the included studies are summarized in Table 1. The seven included studies[12-15,20-22] were published within the last 5 years, altogether involving 4574 patients. Among the seven articles[12-15,20-22], three were based on Asian populations[15,21,22], and four involved Europeans[12-14,20]. Out of the seven included studies[12-15,20-22], six were retrospective[13-15,20-22], and one was prospective[12]. The NOS scores of the eligible studies[12-15,20-22] ranged from 7 to 9, with a mean of 7.9, thereby indicating that the included studies were of high quality (Table 2).
| Ref. | Region | Design | n | Male (%) | Mean minimal PPI use duration | Number of episodes | HE level1 | Age (yr) | Risk estimate (95%CI) |
| Dam et al[20], 2016 | Denmark | Retrospective case-control study | 865 | 86.7 | At least 1 wk prior to HE episode | Follow-up ended at the onset of the first HE episode | 2-4 | 57.4 | Current PPI use vs current nonuse, HR: 1.36 (95%CI: 1.01-1.84) |
| Lin et al[21], 2014 | China | Retrospective case-control study | 165 | 78.2 | More than 5 d prior to HE episode | Follow-up ended at the onset of the first HE episode | 2-4 | 44.0 | PPI use vs nonuse, OR: 4.392 (95%CI: 1.604-12.031) |
| Nardelli et al[12], 2018 | Rome | Prospective observational study | 310 | 71.3 | PPI use at least 4 wk prior to the admission | Follow-up ended at the onset of the first HE episode | 2-4 | 62.0 | PPI use at least 4 weeks prior to admission vs. PPI nonuse at least 4 wk prior to admission OR: 3.96 (95%CI: 2.27-6.92) |
| Sturm et al[13], 2018 | Germany | Retrospective observational study | 397 | 68.1 | NR | Follow-up ended at the onset of the first HE episode | 1-4 | 59.3 | PPI use vs nonuse, OR: 2.29 (95%CI: 1.86-6.46) |
| Tergast et al[14], 2018 | Germany | Retrospective longitudinal cohort study | 249 | 67.9 | PPI intake within 7 d prior to enrollment | NR | 3-4 | 56.8 | PPI dosage > 40 mg/d vs PPI dosage > 10-40 mg/d, HR: 1.85 (95%CI: 0.87-3.66) |
| Tsai et al[22], 2016 | Taiwan | Retrospective case-control study | 2332 | 74.2 | PPI intake at least 30 d prior to enrollment | Follow-up ended at the onset of the first HE episode | NR | 53.1 | (cDDD > 365 vs cDDD ≤ 30) OR: 3.01 (95%CI: 1.78-5.10); 120 < cDDD < 365 vs cDDD ≤ 30, OR: 1.51 (95%CI: 1.11-2.06) 30 < cDDD < 120 vs cDDD ≤ 30, OR: 1.41 (95%CI: 1.09-1.84) |
| Zhu et al[15], 2018 | China | Retrospective case-control study | 256 | 63.3 | PPI use during hospitalization | HE episode during hospitalization | 2-4 | 58.3 | PPI use during hospitalization vs nonuse during hospitalization OR: 3.481 (95%CI: 1.651-7.340) |
Figure 2 shows the pooled results from the fix-effects model combining ORs for HE risk. Our meta-analysis result indicated a significant association between PPI use and HE risk (OR = 1.50; 95%CI: 1.25-1.75; Figure 2) with low heterogeneity (I2 = 14.2%, P = 0.321).
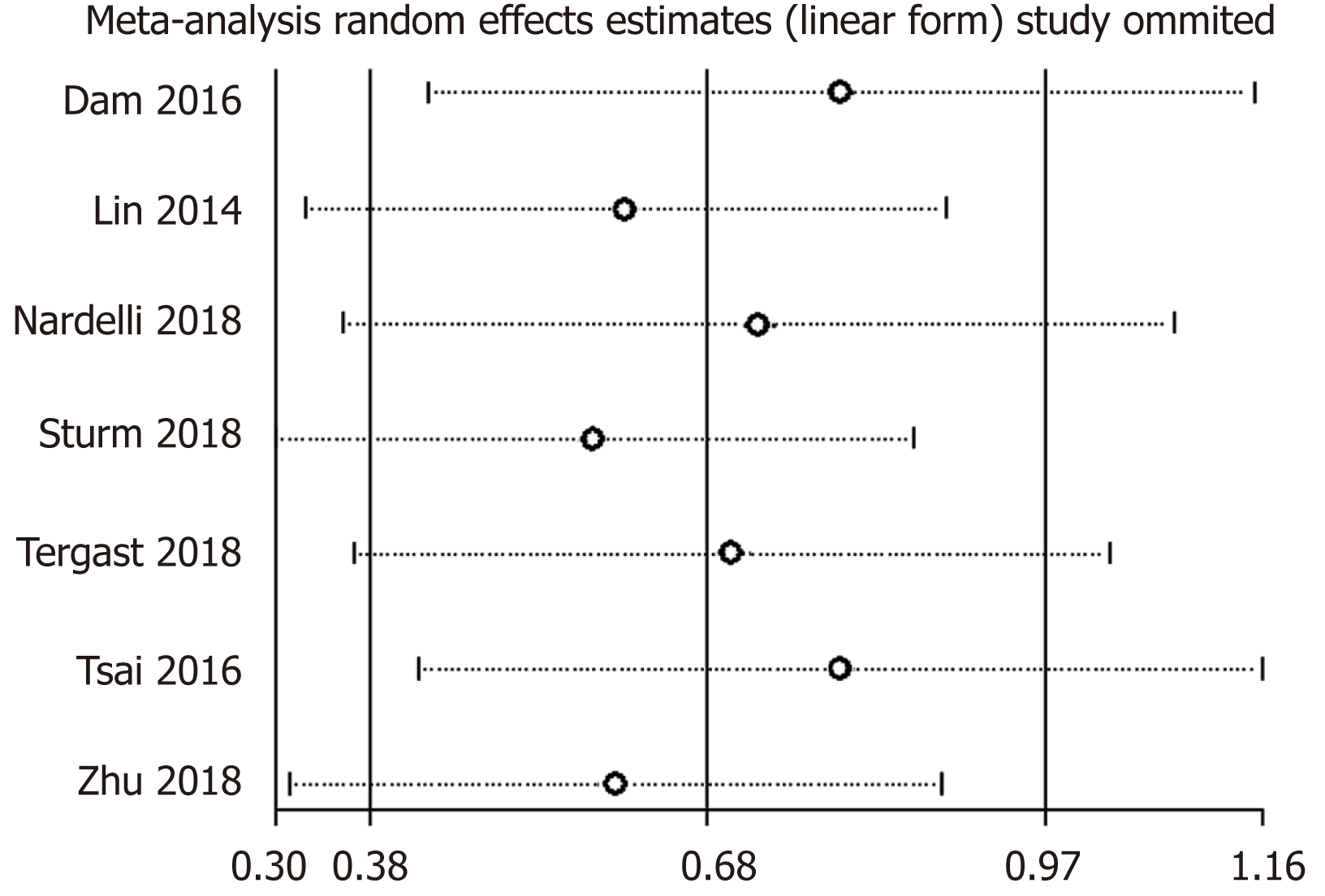
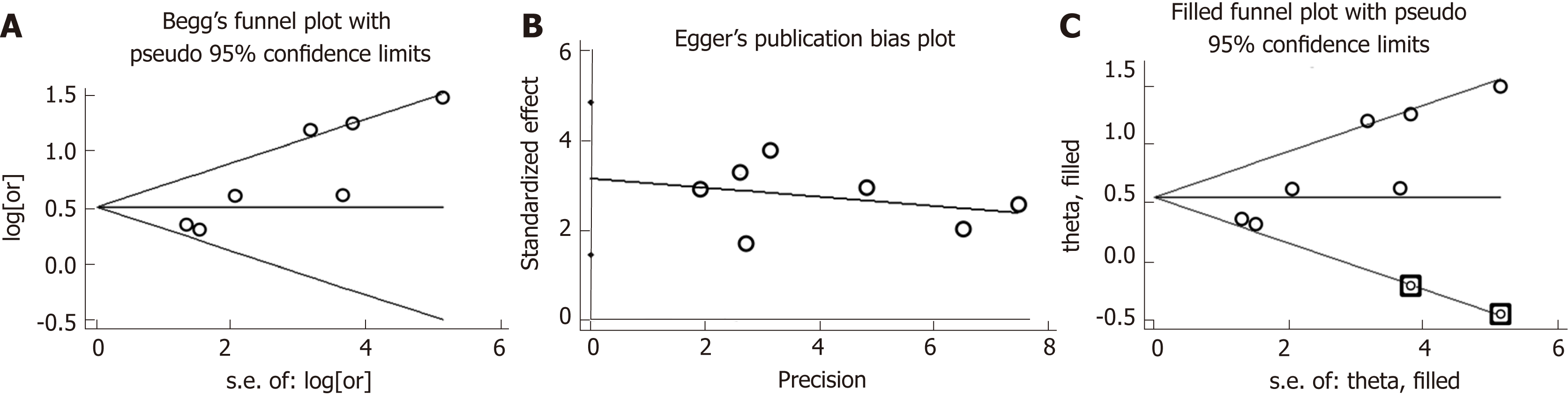
Sensitivity analyses showed that pooled OR for PPI use and HE risk association and the corresponding 95%CIs were unaltered substantially by removing one study, thereby suggesting that the results of this meta-analysis were robust (Figure 3). Although publication bias existed as indicated by the results of the Egger’s tests (Egger’s tests, P = 0.005; Begg’s tests, P = 0.133), the trim-and-fill method verified the stability of the pooled result, and the pooled OR was unaffected (1.58, 95%CI: 1.36-1.85; Figure 4).
In this study, we performed a meta-analysis to observe HE alterations in patients with liver cirrhosis and explore the relationship between PPI use and HE risk. The findings from this study indicated an increase of 50% risk of HE among PPI users, which was consistent with the results obtained in a previous study[11]. Our meta-analysis expanded the results of previous studies and increased the statistical power to evaluate the effects.
The mechanisms of PPIs on the occurrence of HE in cirrhosis are unclear. However, as proposed, PPI use can contribute to the gut dysbiosis that generally exists in patients with cirrhosis, whereas altered gut microbiota can induce or exacerbate HE occurrence[23,24]. Intestinal bacteria in the colon produce ammonia from glutamine and nitrogenous source catabolism. An increased ammonia level in the brain results in HE occurrence by primarily influencing the brain energy metabolism and central nervous system[25,26]. PPI use also inhibits neutrophil-endothelial cell interactions and reduces natural killer cell activities and neutrophils[27], thereby possibly promoting HE occurrence due to the failure of local and systemic immune defense[28].
Low heterogeneity was detected among the included studies and meta-regression analysis was not required. However, we were also concerned about the effect of pub-lication bias because positive results are likely to be published. Although publication bias existed when Egger’s tests were used, the trim-and-fill method verified the stability of the pooled result.
Considering that PPI use is associated with an increased risk of HE occurrence in patients with liver cirrhosis, physicians should ban PPI use in these patients and those with portal hypertension when PPIs are used without specific indications[29]. Adhering to evidence-based guidelines is the only way to ensure effective and safe PPI use[30]. Regulatory authorities should also assume supervision and management responsibilities to avoid inappropriate PPI use[31].
This study has several potential limitations. First, given the heterogeneity of the studies included, some of the results should be regarded with caution. Second, we included only trials published in English. Third, the number of included studies was relatively small. Although we conducted a comprehensive literature search, only seven studies were included.
In conclusion, the results of our meta-analysis suggests that PPI use is independently associated with HE risk. Therefore, randomized multicentric studies with a large sample size should be conducted to provide further insight into the potential impact of PPIs on HE.
Proton pump inhibitors (PPIs) are the first choice of treatment for esophagitis and peptic ulcer disease, as well as the prevention of nonsteroidal anti-inflammatory drug associated ulcers, Zollinger-Ellison syndrome, and functional dyspepsia. In acid-related diseases, the benefits of PPI use outweigh their potential harm. Unfortunately, the negative effects of PPI use are generally underestimated due to marketing strategy and neglected reporting bias in published trials. Thus, not all PPIs are used following evidence-based guidelines in the clinical setting, and PPIs are overprescribed in both inpatient and outpatient settings.
Regarding concerns over liver adverse effects, a previous meta-analysis showed that PPIs increase the risk of hepatic encephalopathy (HE) in patients with hepatic failure. However, the results are restricted because of the inclusion of a relatively small number of studies. New primary studies have also been recently published, and their results are controversial.
In this meta-analysis, we aimed to update, compile, and critically review the existing evidence on the risk of HE in patients with liver cirrhosis and PPI use and provide a quantitative estimate of the relationship between PPI use and HE risk.
A systematic search on PubMed, Web of Science, EMBase, and ScienceDirect databases was conducted up to December 31, 2018 for eligible studies involving PPI use and HE risk. The odd ratios (ORs) and 95% confidence intervals (CIs) were calculated using the fixed- or random-effects model. Publication bias was evaluated using the Begg’s, Egger’s tests, and trim-and-fill method.
The findings from this study indicated an increase of 50% risk of HE among PPI users, which is consistent with the results obtained in a previous study.
Our meta-analysis expanded the results of previous studies and increased the statistical power to evaluate the effects.
Randomized multicentric studies with a large sample size should be conducted to provide further insight into the potential impact of PPIs on HE.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Braillon A, Hillman LC, Lombardo L, Savarino V S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64:935-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 2. | Ahrens D, Chenot JF, Behrens G, Grimmsmann T, Kochen MM. Appropriateness of treatment recommendations for PPI in hospital discharge letters. Eur J Clin Pharmacol. 2010;66:1265-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Patterson Burdsall D, Flores HC, Krueger J, Garretson S, Gorbien MJ, Iacch A, Dobbs V, Homa T. Use of proton pump inhibitors with lack of diagnostic indications in 22 Midwestern US skilled nursing facilities. J Am Med Dir Assoc. 2013;14:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Maes ML, Fixen DR, Linnebur SA. Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf. 2017;8:273-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected]. Am J Gastroenterol. 2009;104 Suppl 2:S27-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, Grams ME. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern Med. 2016;176:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 500] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 8. | Kockerling D, Nathwani R, Forlano R, Manousou P, Mullish BH, Dhar A. Current and future pharmacological therapies for managing cirrhosis and its complications. World J Gastroenterol. 2019;25:888-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 9. | Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, Chiriac SA, Ciobica A, Boiculese L. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol. 2017;23:6500-6515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (5)] |
| 10. | Lv X, Zhang J, Jiang M, Liu Y, Ren W, Fang Z. Clostridium difficile-associated diarrhea following the therapy with antibiotic and proton pump inhibitors in a 77-year-old man with several comorbidities: A case report. Medicine (Baltimore). 2019;98:e15004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Bian J, Wang A, Lin J, Wu L, Huang H, Wang S, Yang X, Lu X, Xu Y, Zhao H. Association between proton pump inhibitors and hepatic encephalopathy: A meta-analysis. Medicine (Baltimore). 2017;96:e6723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Nardelli S, Gioia S, Ridola L, Farcomeni A, Merli M, Riggio O. Proton Pump Inhibitors Are Associated With Minimal and Overt Hepatic Encephalopathy and Increased Mortality in Patients With Cirrhosis. Hepatology. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Sturm L, Bettinger D, Giesler M, Boettler T, Schmidt A, Buettner N, Thimme R, Schultheiss M. Treatment with proton pump inhibitors increases the risk for development of hepatic encephalopathy after implantation of transjugular intrahepatic portosystemic shunt (TIPS). United European Gastroenterol J. 2018;6:1380-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Tergast TL, Wranke A, Laser H, Gerbel S, Manns MP, Cornberg M, Maasoumy B. Dose-dependent impact of proton pump inhibitors on the clinical course of spontaneous bacterial peritonitis. Liver Int. 2018;38:1602-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Zhu J, Qi X, Yu H, Yoshida EM, Mendez-Sanchez N, Zhang X, Wang R, Deng H, Li J, Han D, Guo X. Association of proton pump inhibitors with the risk of hepatic encephalopathy during hospitalization for liver cirrhosis. United European Gastroenterol J. 2018;6:1179-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47174] [Article Influence: 2948.4] [Reference Citation Analysis (0)] |
| 17. | Oremus M, Oremus C, Hall GB, McKinnon MC; ECT & Cognition Systematic Review Team. Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa Scales. BMJ Open. 2012;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e3837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Huang LT, Wu SL, Liao X, Ma SJ, Tan HZ. Adiponectin gene polymorphisms and risk of gestational diabetes mellitus: A meta-analysis. World J Clin Cases. 2019;7:572-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 20. | Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 21. | Lin ZN, Zuo YQ, Hu P. Association of Proton Pump Inhibitor Therapy with Hepatic Encephalopathy in Hepatitis B Virus-related Acute-on-Chronic Liver Failure. Hepat Mon. 2014;14:e16258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, Hou MC, Lee FY, Su TP, Lu CL. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology. 2017;152:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 23. | Bajaj JS, Cox IJ, Betrapally NS, Heuman DM, Schubert ML, Ratneswaran M, Hylemon PB, White MB, Daita K, Noble NA, Sikaroodi M, Williams R, Crossey MM, Taylor-Robinson SD, Gillevet PM. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol. 2014;307:G951-G957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Richardson AJ, McKain N, Wallace RJ. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 2013;13:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Parekh PJ, Balart LA. Ammonia and Its Role in the Pathogenesis of Hepatic Encephalopathy. Clin Liver Dis. 2015;19:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Yoshida N, Yoshikawa T, Tanaka Y, Fujita N, Kassai K, Naito Y, Kondo M. A new mechanism for anti-inflammatory actions of proton pump inhibitors--inhibitory effects on neutrophil-endothelial cell interactions. Aliment Pharmacol Ther. 2000;14 Suppl 1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Savarino V, Tosetti C, Benedetto E, Compare D, Nardone G. Appropriateness in prescribing PPIs: A position paper of the Italian Society of Gastroenterology (SIGE) - Study section "Digestive Diseases in Primary Care". Dig Liver Dis. 2018;50:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Savarino V, Marabotto E, Zentilin P, Furnari M, Bodini G, De Maria C, Pellegatta G, Coppo C, Savarino E. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev Clin Pharmacol. 2018;11:1123-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 31. | Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterology. 2017;153:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |