Published online Jan 14, 2019. doi: 10.3748/wjg.v25.i2.258
Peer-review started: October 31, 2018
First decision: December 5, 2018
Revised: December 9, 2018
Accepted: December 19, 2018
Article in press: December 19, 2018
Published online: January 14, 2019
Processing time: 75 Days and 14 Hours
Anastomotic leakage (AL) is a severe complication associated with high morbidity and mortality after radical gastrectomy (RG) for gastric cancer (GC). We hypothesized that a novel abdominal negative pressure lavage-drainage system (ANPLDS) can effectively reduce the failure-to-rescue (FTR) and the risk of reoperation, and it is a feasible management for AL.
To report our institution’s experience with a novel ANPLDS for AL after RG for GC.
The study enrolled 4173 patients who underwent R0 resection for GC at our institution between June 2009 and December 2016. ANPLDS was routinely used for patients with AL after January 2014. Characterization of patients who underwent R0 resection was compared between different study periods. AL rates and postoperative outcome among patients with AL were compared before and after the ANPLDS therapy. We used multivariate analyses to evaluate clinicopathological and perioperative factors for associations with AL and FTR after AL.
AL occurred in 83 (83/4173, 2%) patients, leading to 7 deaths. The mean time of occurrence of AL was 5.6 days. The AL rate was similar before (2009-2013, period 1) and after (2014-2016, period 2) the implementation of the ANPLDS therapy (1.7% vs 2.3%, P = 0.121). Age and malnourishment were independently associated with AL. The FTR rate and abdominal bleeding rate after AL occurred were respectively 8.4% and 9.6% for the entire period; however, compared with period 1, this significantly decreased during period 2 (16.2% vs 2.2%, P = 0.041; 18.9% vs 2.2%, P = 0.020, respectively). Moreover, the reoperation rate was also reduced in period 2, although this result was not statistically significant (13.5% vs 2.2%, P = 0.084). Additionally, only ANPLDS therapy was an independent protective factor for FTR after AL (P = 0.04).
Our experience demonstrates that ANPLDS is a feasible management for AL after RG for GC.
Core tip: A novel abdominal negative pressure lavage-drainage system (ANPLDS) can effectively reduce the failure-to-rescue and abdominal bleeding rate after anastomotic leakage (AL). Our experience demonstrates that ANPLDS is a feasible management for AL after radical gastrectomy for gastric cancer.
- Citation: Zheng ZF, Lu J, Zhang PY, Xu BB, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Huang CM. Novel abdominal negative pressure lavage-drainage system for anastomotic leakage after R0 resection for gastric cancer. World J Gastroenterol 2019; 25(2): 258-268
- URL: https://www.wjgnet.com/1007-9327/full/v25/i2/258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i2.258
Improvements in surgical techniques and perioperative management have resulted in reduced postoperative mortality after radical gastrectomy (RG) for gastric cancer (GC). However, anastomotic leakage (AL) remains relatively common and represents a major cause of postoperative morbidity after RG for GC. The reported incidence of AL varies between 0% and 15.0%[1], and AL is associated with high mortality[2-4].
For several decades, routine prophylactic placement of abdominal drains has been the standard procedure in abdominal surgery. However, since the introduction of the enhanced recovery after surgery (ERAS) program, routine drain placement after gastrectomy is not warranted anymore, since drains do not reduce postoperative complications after gastrectomy and prolong hospital stay and postoperative recovery[5-7]. Despite these recommendations, prophylactic drainage of the abdominal cavity is still widely performed, because drains are believed to remove intraperitoneal fluids, which can be a source of infection, and drainage fluid might serve as an early warning sign of early complications like AL[7-10].
In January 2014, a novel abdominal negative pressure lavage-drainage system (ANPLDS) was routinely used for patients with AL at our institution. We found that ANPLDS can effectively reduce the failure-to-rescue (FTR) and abdominal bleeding rate after AL, and it is a feasible management for AL. Therefore, in this report, we present our utilization of and experiences with ANPLDS for AL after RG for GC.
From a prospective database, clinical data of patients who underwent RG for primary gastric adenocarcinoma at Fujian Medical University Union Hospital (FMUUH) between June 2009 and December 2016 were identified. The case exclusion criteria eliminated the following from the study: distant metastasis, neoadjuvant chemotherapy, thoracoabdominal incision, and incomplete clinical and pathologic data. Finally, 4173 patients were included in this study. Laboratory blood test data were collected within 1 wk before surgery, including preoperative hemoglobin (HB) and albumin (ALB) levels. The type of surgical resection and the extent of lymph node dissection were selected according to the Japanese GC treatment guidelines[11]. The 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual was used to determine the disease stage[12]. This study was approved by the relevant institutional review board.
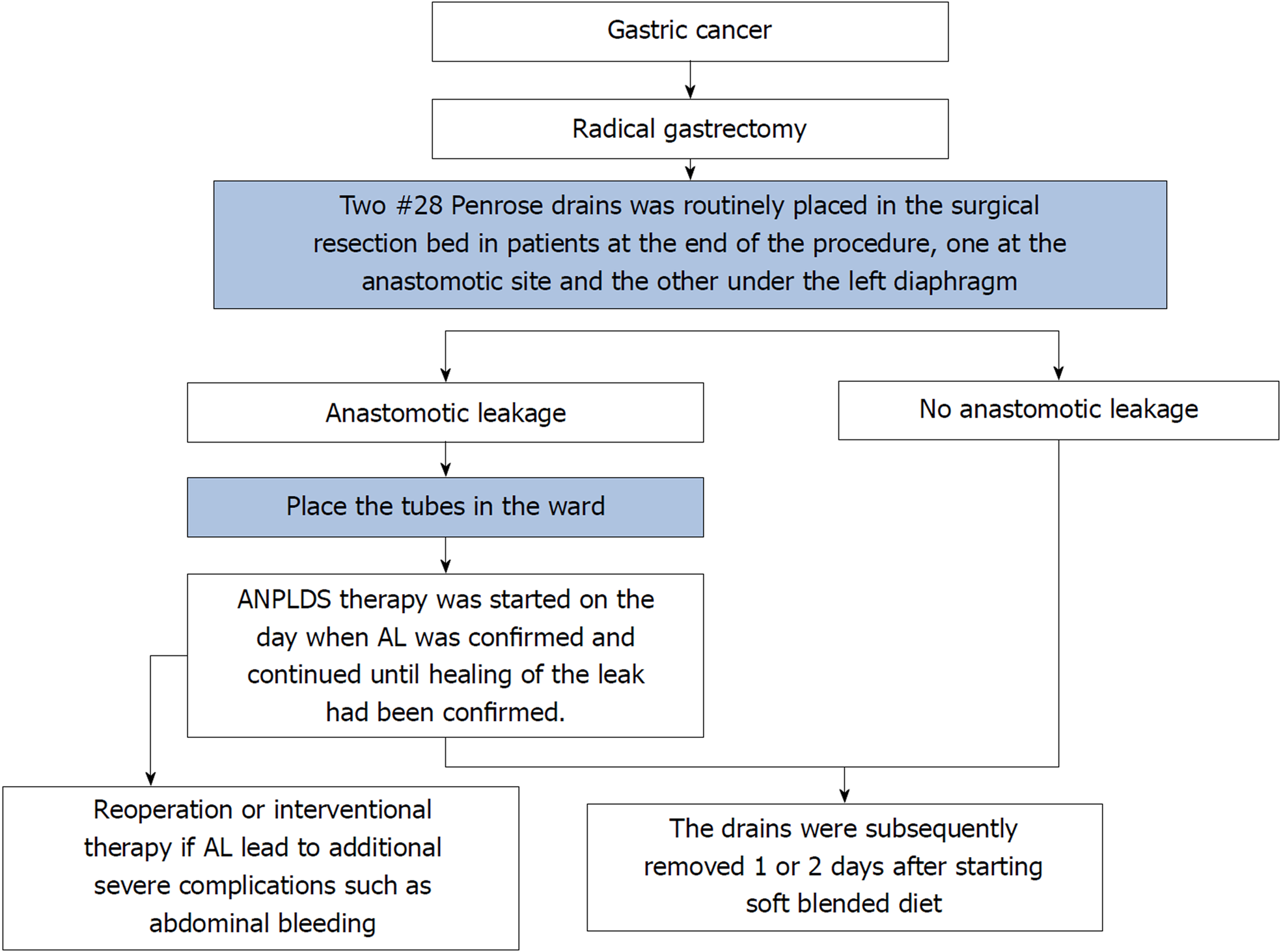
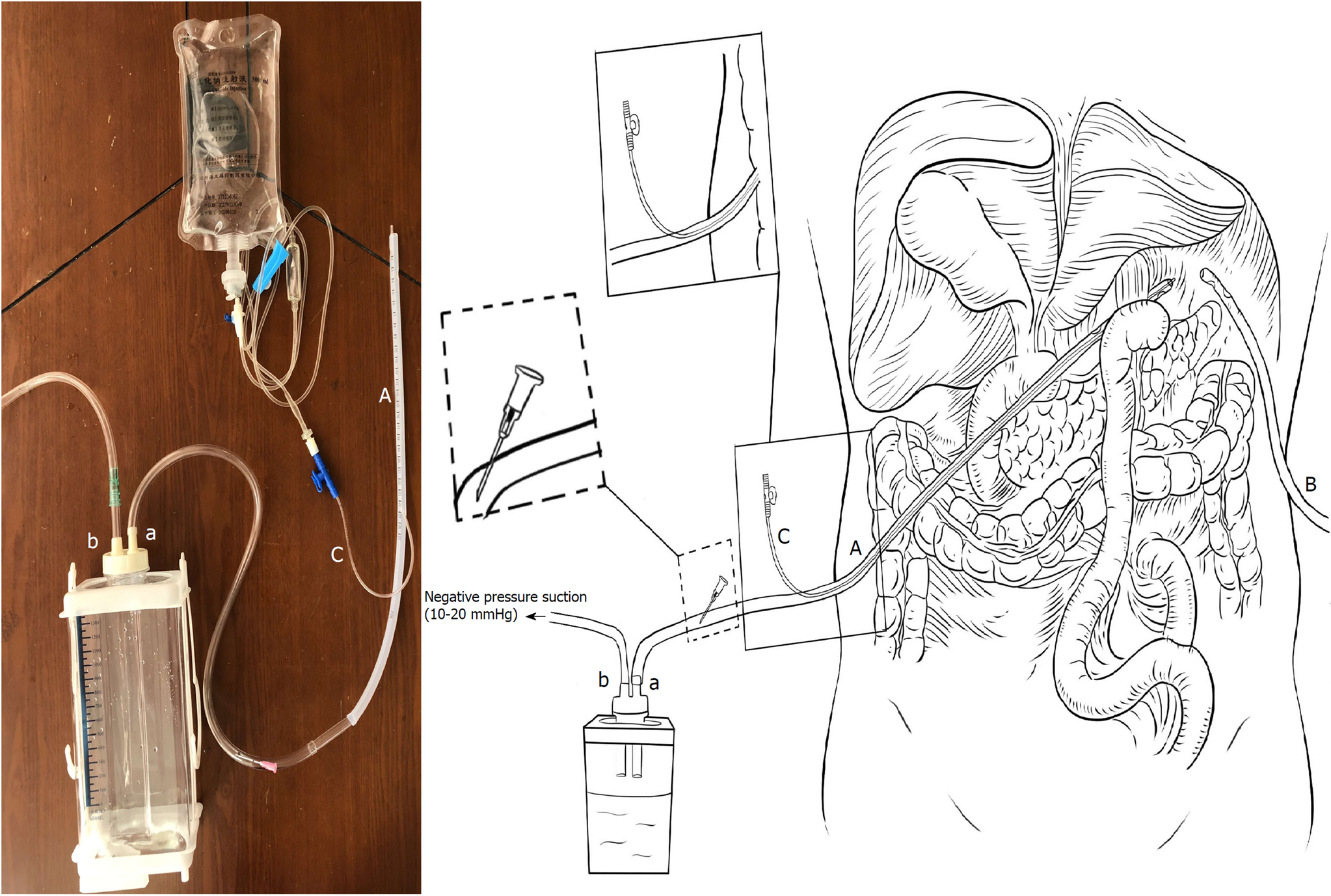
AL was defined as a complete intestinal wall defect at the anastomotic suture line that was confirmed via clinical findings, radiologic contrast medium assessment, abdominal computed tomography, a positive color test, or laparoscopic examination[13,14]. AL patients underwent reoperation or interventional therapy if AL led to additional severe complications such as abdominal bleeding, whereas other patients with AL underwent endoscopy or conservative treatment, including fasting, gastrointestinal decompression, drainage, anti-infection therapy, and nutritional support, among other therapeutic measures. During the entire study period, two #28 Penrose drains (Mingchuang Health, Suzhou, Jiangsu Province, China) were routinely placed in the surgical resection bed in patients at the end of the procedure, one at the anastomotic site (tube A) and the other under the left diaphragm (tube B) (Figure 1). A radiological contrast study was performed at the 4th or 5th postoperative day to assess anastomotic integrity. The drains were subsequently removed 1 or 2 d after starting a soft blended diet. At our institution, ANPLDS therapy (Figure 2) has been a supported first-line treatment for the management of these nonreoperation patients since January 2014. This therapy was started on the day when AL was confirmed and continued until healing of the leak had been confirmed. The healing of the leak was confirmed by the radiological contrast study. For patients with AL, a radiological contrast study was performed once a week.
In the ward, a #6 sputum suction tube (tube C) was placed next to the anastomotic stoma from the inside of tube A if AL occurred (Figure 1). The inner end of the sputum suction tube was exposed to 0.3-0.5 cm inside tube A (Figure 1), and saline was used for continuous irrigation (150-200 mL/h, 3000 mL/d). The position of tube C was confirmed via X- ray if necessary. Tube A was attached to the drainage bottle (hole a), and a needle was maintained on the outside end of tube A as a blowhole (Figure 1). A suction drain with a negative pressure of 10-20 mmHg was attached to hole b of the drainage bottle (Figure 1).
The primary outcome of interest was FTR after AL. FTR was defined as mortality after the complication of interest[15,16]. Other postoperative outcomes included other complications, reoperation, and length of stay. The secondary outcomes of interest were clinicopathological and perioperative factors for associations with AL and FTR after AL. In this study, malnourishment was defined by the presence of at least one of the following criteria according to the Guidelines of the European Society for Clinical Nutrition and Metabolism (ESPEN)[17]: weight loss 10%-15% within 6 mo, body mass index (BMI) < 18.5 kg/m2, Subjective Global Assessment Grade C, or serum albumin < 30 g/L. However, patients who only met the criterion of BMI < 18.5 kg/m2 were considered lean but well-nourished[17,18].
Continuous data are reported as the mean ± standard deviation, and categorical data are presented as the proportion percentage and were analyzed by the Chi square test or Fisher’s exact test. To identify factors that predicted AL and the FTR rate after AL, variables significant in the univariable analysis (P < 0.05) were included in a multivariate analysis. A binary logistic regression with the forward entry method for the covariates was used to perform a multivariate analysis. All tests were two-sided, and a P-value lower than 0.05 was used to define statistical significance. Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, United States).
Between June 2009 and December 2016, a total of 83 patients experienced AL among the 4173 identified patients. Data are compared between 2009 and 2013, which was the period before implementation of the ANPLDS therapy (period 1); as well as between 2014 and 2016, which was after the implementation (period 2). The incidence of AL before and after implementation of the ANPLDS therapy was similar [1.7% (37/2219) vs 2.3% (46/1958), P = 0.121]. Clinicopathological, preoperative, and operative data are reported in Table 1.
| Characteristic | All patients (n = 4173) | 2009-2013 (n = 2219) | 2014-2016 (n = 1954) | P-value |
| Mean age, yr | 0.148 | |||
| < 65 | 2701 (64.7) | 1414 (63.7) | 1287 (65.9) | |
| ≥ 65 | 1472 (35.3) | 805 (36.3) | 667 (34.1) | |
| Sex | 0.002 | |||
| Male | 3083 (73.9) | 1683 (75.8) | 1400 (71.6) | |
| Female | 1090 (26.1) | 536 (24.2) | 554 (28.4) | |
| Mean BMI, kg/m2 (SD) | 22.3 (5.2) | 22.0 (3.1) | 22.6 (3.7) | < 0.001 |
| History of abdominal surgery | 0.436 | |||
| No | 3543 (84.9) | 1875 (84.5) | 1668 (85.4) | |
| Yes | 630 (15.1) | 344 (15.5) | 286 (14.6) | |
| Tumor site | < 0.001 | |||
| Upper | 1136 (27.2) | 590 (26.6) | 546 (27.9) | |
| Middle | 892 (21.4) | 383 (17.3) | 509 (26.0) | |
| Lower | 1654 (39.6) | 958 (43.2) | 696 (35.6) | |
| ≥ 2 areas | 491 (11.8) | 288 (13.0) | 203 (10.4) | |
| Malnourished | 0.277 | |||
| No | 3730 (89.4) | 1919 (88.9) | 1811 (89.9)) | |
| Yes | 443 (10.6) | 240 (11.1) | 203 (10.1) | |
| Mean tumor size, cm (SD) | 4.4 (2.6) | 4.6 (2.6) | 4.0 (2.7) | < 0.001 |
| AJCC-TNM stage, 8th edition | < 0.001 | |||
| I | 1158 (27.7) | 598 (26.9) | 560 (28.7) | |
| II | 1052 (25.2) | 487 (21.9) | 565 (28.9) | |
| III | 1963 (47.0) | 1134 (51.1) | 829 (42.4) | |
| Comorbidities | 0.112 | |||
| No | 2876 (68.9) | 1553 (70.0) | 1323 (67.7) | |
| Yes | 1297 (31.1) | 666 (30.0) | 631 (32.3) | |
| HB, g/dL | 0.317 | |||
| ≥ 90 | 3718 (89.1) | 1967 (88.6) | 1751 (89.6) | |
| < 90 | 455 (10.9) | 252 (11.4) | 203 (10.4) | |
| ASA | 0.641 | |||
| I-II | 4000 (95.9) | 2130 (96.0) | 1870 (95.7) | |
| III-IV | 173 (4.1) | 89 (4.0) | 84 (4.3) | |
| Operation method | < 0.001 | |||
| Open | 670 (16.1) | 469 (21.1) | 201 (10.3) | |
| Laparoscopic | 3503 (83.9) | 1750 (78.9) | 1753 (89.7) | |
| Type of resection | 0.235 | |||
| Subtotal gastrectomy | 1873 (44.9) | 1015 (45.7) | 858 (43.9) | |
| Total gastrectomy | 2300 (55.1) | 1204 (54.3) | 1096 (56.1) | |
| Type of reconstruction | < 0.001 | |||
| Billroth I | 1100 (26.4) | 782 (35.2) | 318 (16.3) | |
| Billroth II | 540 (12.9) | 160 (7.2) | 380 (19.4) | |
| Roux-en-Y | 2468 (59.1) | 1225 (55.2) | 1243 (63.6) | |
| Other | 65 (1.6) | 52 (2.3) | 13 (0.7) | |
| Mean surgical duration, min (SD) | 184.7 (67.6) | 191.3 (67.8) | 177.1 (66.7) | < 0.001 |
| Mean intraoperative blood loss, mL (SD) | 94.7 (256.8) | 115.0 (335.3) | 71.5 (108.6) | < 0.001 |
| Mean length of stay, d (SD) | 13.5 (7.8) | 13.5 (7.4) | 13.4 (8.2) | 0.296 |
| AL | 83 (2.0) | 37 (1.7) | 46 (2.4) | 0.113 |
AL occurring in 83 patients resulted in 7 deaths. The mean time of occurrence of AL was 5.6 d. The mean length of postoperative stay was 30.2 d. These AL required no invasive treatment in 58 (69.9%) patients, CT-guided puncture drainage in 16 (19.3%) patients, endoscopy in 3 (3.6%) patients, and reoperation in 6 (7.2%) patients. Postoperative morbidity, mortality, and treatment are summarized in Table 2. Compared with period 1, the rates significantly decreased for FTR (16.2% vs 2.2%, P = 0.041) and abdominal bleeding (18.9% vs 2.2%, P = 0.020) in period 2, but the time of AL occurrence and postoperative hospital stay for patients with AL were similar for the two periods (5.4 d vs 5.7 d, P = 0.738; 31.6 d vs 28.4 d, P = 0.458, respectively). Moreover, the reoperation rate was also reduced in period 2, although this result was not statistically significant (13.5% vs 2.2%, P = 0.084).
| Postoperative condition | All patients (n = 83) | 2009-2013 (n = 37) | 2014-2016 (n = 46) | P-value |
| Death | 7 (8.4) | 6 (16.2) | 1 (2.2) | 0.041 |
| Time of occurrence of AL, d (mean) | 5.6 | 5.4 | 5.7 | 0.738 |
| Other complication | ||||
| Pneumonia | 41 (49.3) | 16 (43.2) | 25 (54.3) | 0.315 |
| Abdominal bleeding | 8 (9.6) | 7 (18.9) | 1 (2.2) | 0.020 |
| Pancreatic fistula | 3 (3.6) | 3 (8.1) | 0 (0) | 0.085 |
| Chylous leak | 4 (4.8) | 3 (8.1) | 1 (2.2) | 0.319 |
| Anastomotic bleeding | 2 (2.4) | 2 (5.4) | 0 (0) | 0.196 |
| Wound infection | 4 (4.8) | 2 (5.4) | 2 (4.3) | > 0.999 |
| ARF&RI | 2 (2.4) | 2 (5.4) | 0 (0) | 0.196 |
| Cardiac event | 2 (2.4) | 1 (2.7) | 1 (2.2) | > 0.999 |
| HF and hypohepatia | 2 (2.4) | 1 (2.7) | 1 (2.2) | > 0.999 |
| Treatment | ||||
| No invasive | 58 (69.9) | 18 (48.6) | 40 (87.0) | < 0.001 |
| CT-guided puncture drainage | 16 (19.3) | 11 (29.7) | 5 (10.9) | 0.030 |
| endoscopy | 3 (3.6) | 3 (8.1) | 0 (0) | 0.085 |
| Reoperation | 6 (7.2) | 5 (13.5) | 1 (2.2) | 0.084 |
| Length of stay, d (mean) | 30.2 | 31.6 | 28.4 | 0.458 |
The univariate analysis showed that AL were most significantly associated with age ≥ 65, malnourishment, comorbidities, HB < 90 g/dL, and total gastrectomy (Supplemental Table 1). The multivariate analysis showed that the two factors independently associated with major complications were age ≥ 65 and malnourishment (Table 3).
| Factor | Multivariate analysis results | |
| OR (95%CI) | P-value | |
| Age, yr | ||
| < 65 | Reference | 0.006 |
| ≥ 65 | 1.886 (1.195-2.975) | |
| Malnourished | ||
| No | Reference | 0.005 |
| Yes | 2.194 (1.267-3.797) | |
| Comorbidities | ||
| No | Reference | 0.171 |
| Yes | 1.377 (0.871-2.178) | |
| HB, g/dL | ||
| ≥ 90 | Reference | 0.221 |
| < 90 | 1.441 (0.803-2.586) | |
| Type of resection | ||
| Subtotal gastrectomy | Reference | 0.069 |
| Total gastrectomy | 1.534 (0.967-2.434) | |
The FTR rate after AL was 8.4% (7/83) over the whole study period. The univariate analysis showed that the only factor independently associated with the risk of FTR rate after AL was ANPLDS therapy [odds ratio (OR) = 0.103, 95%CI: 0.012-0.898, P = 0.040] (Table 4). The detailed clinical characteristics of the deaths with AL are presented in Supplemental Table 2.
| Factor | Univariate analysis | |
| OR (95%CI) | P-value | |
| ANPLDS therapy | ||
| No | Reference | 0.040 |
| Yes | 0.103 (0.012-0.898) | |
| Age, yr | ||
| < 65 | Reference | 0.532 |
| ≥ 65 | 0.607 (0.127-2.900) | |
| Sex | ||
| Female | Reference | 0.998 |
| Male | / | |
| BMI, kg/m2 | ||
| < 25 | Reference | 0.773 |
| ≥ 25 | 1.289 (0.230-7.219) | |
| History of abdominal surgery | ||
| No | Reference | 0.849 |
| Yes | 0.808 (0.090-7.286) | |
| Tumor site | ||
| Upper | Reference | 0.935 |
| Middle | / | 0.998 |
| Lower | 0.697 (0.106-4.578) | 0.707 |
| ≥ 2 areas | 1.394 (0.203-9.585) | 0.736 |
| Malnourished | ||
| No | Reference | 0.710 |
| Yes | 1.388 (0.247-7.802) | |
| Tumor size, cm | ||
| ≤ 5 | Reference | 0.700 |
| > 5 | 1.361 (0.283-6.535) | |
| AJCC-TNM stage, 8th edition | ||
| I-II | Reference | 0.115 |
| III | 5.692 (0.654-49.570) | |
| Comorbidities | ||
| No | Reference | 0.448 |
| Yes | 1.833 (0.383-8.765) | |
| HB, g/dL | ||
| ≥ 90 | Reference | 0.728 |
| < 90 | 0.678 (0.076-6.063) | |
| ASA | ||
| I-II | Reference | 0.567 |
| III-IV | 1.944 (0.200-18.920) | |
| Operation method | ||
| Open | Reference | 0.073 |
| Laparoscopic | 0.226 (0.044-1.149) | |
| Type of resection | ||
| Subtotal gastrectomy | Reference | 0.763 |
| Total gastrectomy | 1.300 (0.236-7.166) | |
| Type of reconstruction | ||
| Billroth I | Reference | 0.997 |
| Billroth II | 0.817 (0.148-4.520) | 0.817 |
| Roux-en-Y | / | > 0.999 |
| Other | / | 0.999 |
| Surgical duration, min (SD) | ||
| > 180 | Reference | 0.138 |
| ≤ 180 | 3.629 (0.661-19.914) | |
| Intraoperative blood loss, ml | ||
| > 50 | Reference | 0.997 |
| ≤ 50 | / | |
AL is a common serious complication after gastrectomy in patients with GC. It is also the most important cause of postoperative abdominal infection, abscess, and abdominal bleeding. Improper management of AL may also increase the risk of death, prolong the length of stay, increase the cost of hospitalization, and even affect long-term survival[3,4]. Therefore, it is essential to identify the risk factors for AL and to take effective treatment measures.
Several risk factors have been reported to be associated with AL, such as age, sex, smoking, malnutrition, longer operative time, tumor location, and tumor stage[19-22]. In this study, we found that age ≥ 65 and malnutrition were independent risk factors for AL after RG, which is consistent with previous studies by our center[23]. Elderly or malnourished patients often suffer from poor body conditions and insufficient blood and energy supply in the anastomotic area, which increases the risk of anastomotic fistula. Therefore, clinicians need to pay more attention to these patients and take appropriate measures to prevent postoperative AL, such as preoperative correction of malnutrition, intraoperative protection of perianastomotic tissue, perioperative supplemental oxygen administration[22], and appropriate use of antibiotics. Even with this, AL cannot be completely avoided. Therefore, it is the goal of clinical attention to select reasonable treatment methods and reduce the FTR rate in a situation of AL. However, the choice of treatment measures after the onset of AL is rarely reported.
When AL occurs, the most important treatment is effective and unobstructed drainage. However, traditional drainage techniques to treat AL depend on gravity and pressure in the cavity with AL. In addition, viscous secretions greatly affect drainage and may even clog the drainage tube. Moreover, secretions that fail to discharge in a timely manner can cause abdominal infection or abscess and may also corrode vascular stumps in the local area, resulting in anastomotic or abdominal bleeding.
For better drainage, negative pressure-flush is often used clinically. Lin et al[24] found that continuous negative pressure-flush through an extraperitoneal dual tube can increase the successful rate of conservative therapy, decrease the reoperation rate, and improve the quality of life when combined with the use of an intra-rectal dual tube. Jiang et al[25] achieved an early intervention for severe bile leakage and pancreatic fistula after pancreaticoduodenectomy using an enclosed passive infraversion lavage-drainage system (EPILDS). However, the application of negative pressure flush in the treatment of GC gastrointestinal fistula has not been reported. Therefore, we present our utilization of and experiences with ANPLDS for AL after RG for GC in this study. We believe that the key reasons for our success with ANPLDS therapy were the use of local continuous irrigation and negative pressure drainage. First, continuous irrigation dilutes secretions, which is beneficial for the discharge of secretions via the drainage tube. Second, liquid is actively drawn off using negative pressure. The sustained air flow in the tubes makes the pressure in the tube lower than that in the area to be rinsed. Thus, secretions and necrotic tissue can be removed in a timely manner. Therefore, ANPLDS reduces local inflammation and provides a good environment for the closing and healing of an AL-inducing rupture.
FTR or death after major complications has gained acceptance as an interesting metric evaluation of quality after surgery[26,27]. Although the definition of FTR varies widely in the previous literature, surgeons and researchers agree that the ability to rescue patients from severe postoperative complications, thus preventing mortality, is key to improve the quality and safety of surgery. We found that ANPLDS therapy was the only independent protective factor associated with FTR after AL. Our simple ANPLDS reduced the FTR, primarily due to decreases in reoperation and in the occurrence of severe complications such as abdominal bleeding. It is reasonable to believe that the decline in FTR is a direct external effect of this new treatment, because the FTR rate decreased suddenly after the implementation of ANPLDS. Additionally, our surgical team had performed more than 1000 cases and had sufficiently mastered the RG procedure for GC before the study period. Therefore, the impact of increasing experience on mortality that is expected to be progressive can be ignored. Of course, other factors that cannot be specifically measured in this study, such as the improvement of ICU care or postoperative care practices, may have an impact on FTR, but it does not seem likely to significantly reduce mortality in the short term. Therefore, we believe that this system is a potentially advantageous alternative to the treatment of AL.
This study had several limitations. First, it is a single-center retrospective study that needs to be verified by a multi-center, large sample prospective trial. Second, due to the limited number of AL cases, we analyzed all reconstruction methods instead of focusing solely on one type of anastomosis, which may increase the heterogeneity of the patient’s material. However, we first reported the feasible management of AL and the successful implementation of this system at our institution may serve as a model for treating AL at other centers.
In conclusion, ANPLDS can effectively reduce the FTR and abdominal bleeding rates after AL. Our experience demonstrates that ANPLDS is a feasible management for AL after RG for GC.
Anastomotic leakage (AL) remains relatively common and represents a major cause of postoperative morbidity after radical gastrectomy (RG) for gastric cancer (GC). AL is associated with high mortality.
Prophylactic drainage of the abdominal cavity is widely performed. The optimal creation of drainage in AL patients after RG remains controversial.
The novel abdominal negative pressure lavage-drainage system (ANPLDS) is a feasible management for AL. Therefore, we present our utilization of and experiences with ANPLDS for AL after RG for GC.
In January 2014, a novel ANPLDS was routinely used for patients with AL at our institution. AL rates and postoperative outcome were compared before and after the ANPLDS therapy.
The novel ANPLDS can effectively reduce the failure-to-rescue and abdominal bleeding rates after AL.
Our experience demonstrates that the novel ANPLDS is a feasible management for AL after RG for GC.
The successful implementation of the novel ANPLDS at our institution may serve as a model for treating AL after RG for GC at other centers.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Manuscript source: Unsolicited manuscript
P- Reviewer: Sikiric P S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Yin SY
| 1. | Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J Gastroenterol. 2015;21:9656-9665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Meyer L, Meyer F, Dralle H, Ernst M, Lippert H, Gastinger I; East German Study Group for Quality Control in Operative Medicine and Regional Development in Surgery. Insufficiency risk of esophagojejunal anastomosis after total abdominal gastrectomy for gastric carcinoma. Langenbecks Arch Surg. 2005;390:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Ichikawa D, Kurioka H, Yamaguchi T, Koike H, Okamoto K, Otsuji E, Shirono K, Shioaki Y, Ikeda E, Mutoh F, Yamagishi H. Postoperative complications following gastrectomy for gastric cancer during the last decade. Hepatogastroenterology. 2004;51:613-617. [PubMed] |
| 4. | Lang H, Piso P, Stukenborg C, Raab R, Jähne J. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol. 2000;26:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Wang Z, Chen J, Su K, Dong Z. Abdominal drainage versus no drainage post-gastrectomy for gastric cancer. Cochrane Database Syst Rev. 2015;CD008788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Kim J, Lee J, Hyung WJ, Cheong JH, Chen J, Choi SH, Noh SH. Gastric cancer surgery without drains: a prospective randomized trial. J Gastrointest Surg. 2004;8:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Lee J, Choi YY, An JY, Seo SH, Kim DW, Seo YB, Nakagawa M, Li S, Cheong JH, Hyung WJ, Noh SH. Do All Patients Require Prophylactic Drainage After Gastrectomy for Gastric Cancer? The Experience of a High-Volume Center. Ann Surg Oncol. 2015;22:3929-3937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Shrikhande SV, Barreto SG, Shetty G, Suradkar K, Bodhankar YD, Shah SB, Goel M. Post-operative abdominal drainage following major upper gastrointestinal surgery: single drain versus two drains. J Cancer Res Ther. 2013;9:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Liu HP, Zhang YC, Zhang YL, Yin LN, Wang J. Drain versus no-drain after gastrectomy for patients with advanced gastric cancer: systematic review and meta-analysis. Dig Surg. 2011;28:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Petrowsky H, Demartines N, Rousson V, Clavien PA. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 2004;240:1074-84; discussion 1084-5. [PubMed] |
| 11. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 12. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. New York: Springer 2017; . |
| 13. | Schardey HM, Joosten U, Finke U, Staubach KH, Schauer R, Heiss A, Kooistra A, Rau HG, Nibler R, Lüdeling S, Unertl K, Ruckdeschel G, Exner H, Schildberg FW. The prevention of anastomotic leakage after total gastrectomy with local decontamination. A prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann Surg. 1997;225:172-180. [PubMed] |
| 14. | Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, Bae BN, Son GM, Lee SI, Kang H. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 15. | Tevis SE, Carchman EH, Foley EF, Heise CP, Harms BA, Kennedy GD. Does Anastomotic Leak Contribute to High Failure-to-rescue Rates? Ann Surg. 2016;263:1148-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Passot G, Vaudoyer D, Villeneuve L, Wallet F, Beaujard AC, Boschetti G, Rousset P, Bakrin N, Cotte E, Glehen O. A Perioperative Clinical Pathway Can Dramatically Reduce Failure-to-rescue Rates After Cytoreductive Surgery for Peritoneal Carcinomatosis: A Retrospective Study of 666 Consecutive Cytoreductions. Ann Surg. 2017;265:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, Jauch KW, Kemen M, Hiesmayr JM, Horbach T, Kuse ER, Vestweber KH; DGEM (German Society for Nutritional Medicine), ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr. 2006;25:224-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 659] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 18. | Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Maeda S, Haraguchi N, Miyake M, Hama N, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M, Fujitani K, Tsujinaka T. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann Surg Oncol. 2015;22 Suppl 3:S778-S785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 19. | Deguchi Y, Fukagawa T, Morita S, Ohashi M, Saka M, Katai H. Identification of risk factors for esophagojejunal anastomotic leakage after gastric surgery. World J Surg. 2012;36:1617-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Kim CW, Baek SJ, Hur H, Min BS, Baik SH, Kim NK. Anastomotic Leakage After Low Anterior Resection for Rectal Cancer Is Different Between Minimally Invasive Surgery and Open Surgery. Ann Surg. 2016;263:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Migita K, Takayama T, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Nakajima Y. Risk factors for esophagojejunal anastomotic leakage after elective gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1659-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Schietroma M, Cecilia EM, Carlei F, Sista F, De Santis G, Piccione F, Amicucci G. Prevention of anastomotic leakage after total gastrectomy with perioperative supplemental oxygen administration: a prospective randomized, double-blind, controlled, single-center trial. Ann Surg Oncol. 2013;20:1584-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Tu RH, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Huang CM. Development of a nomogram for predicting the risk of anastomotic leakage after a gastrectomy for gastric cancer. Eur J Surg Oncol. 2017;43:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Lin C, Zhang Z, Wang Y, Huang S, Wang L, Wang B. [Continuous negative pressure-flush through extraperitoneal dual tube in the treatment and prevention for rectal cancer patients with anastomotic leakage after low anterior resection]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:469-472. [PubMed] |
| 25. | Jiang K, Zhang W, Feng Y, Su M, Dong J, Huang Z. Enclosed passive infraversion lavage-drainage system (EPILDS): a novel safe technique for local management of early stage bile leakage and pancreatic fistula Post Pancreatoduodenectomy. Cell Biochem Biophys. 2014;68:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Glance LG, Dick AW, Meredith JW, Mukamel DB. Variation in hospital complication rates and failure-to-rescue for trauma patients. Ann Surg. 2011;253:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Sheetz KH, Dimick JB, Ghaferi AA. Impact of Hospital Characteristics on Failure to Rescue Following Major Surgery. Ann Surg. 2016;263:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |