Published online Jan 14, 2019. doi: 10.3748/wjg.v25.i2.178
Peer-review started: November 1, 2018
First decision: November 22, 2018
Revised: December 6, 2018
Accepted: December 13, 2018
Article in press: December 13, 2018
Published online: January 14, 2019
Processing time: 78 Days and 23.5 Hours
Many upper gastrointestinal (GI) endoscopies worldwide are performed for inappropriate indications. This overuse of healthcare negatively affects healthcare quality and puts pressure on endoscopy services. Dyspepsia is one of the most common inappropriate indications for upper GI endoscopy as diagnostic yield is low. Reasons for untimely referral are: unfamiliarity with dyspepsia guidelines, uncertainty about etiology of symptoms, and therapy failure. Unfiltered open-access referrals feed upper GI endoscopy overuse. This review highlights strategies applied to diminish use of upper GI endoscopies for dyspepsia. First, we describe the impact of active guideline implementation. We found improved guideline adherence, but resistance was encountered in the process. Secondly, we show several forms of clinical assessment. While algorithm use reduced upper GI endoscopy volume, effects of referral assessment of individual patients were minor. A third strategy proposed Helicobacter pylori test and treat for all dyspeptic patients. Many upper GI endoscopies can be avoided using this strategy, but outcomes may be prevalence dependent. Lastly, empirical treatment with Proton pump inhibitors achieved symptom relief for dyspepsia and avoided upper GI endoscopies in about two thirds of patients. Changing referral behavior is complex as contributing factors are manifold. A collaboration of multiple strategies is most likely to succeed.
Core tip: Strategies to halt overuse of upper gastrointestinal (GI) endoscopies are called for. Dyspepsia represents the indication for the majority of inappropriate upper GI endoscopies and provides a target for intervention. In this review, we describe four strategies that can be used to reduce upper GI endoscopies. While all strategies individually impact the number of performed endoscopies, a collaboration of improved guideline adherence, decision-making assistance, symptom management and Helicobacter pylori screening is most likely to change referral practice.
- Citation: de Jong JJ, Lantinga MA, Drenth JP. Prevention of overuse: A view on upper gastrointestinal endoscopy. World J Gastroenterol 2019; 25(2): 178-189
- URL: https://www.wjgnet.com/1007-9327/full/v25/i2/178.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i2.178
Overuse of upper gastrointestinal (GI) endoscopy is emerging as a global concern[1-3]. It is estimated that up to 56% of diagnostic upper GI endoscopy procedures are inappropriate[4,5], i.e., not according to guidelines.
Inappropriate use is a major source of unnecessary costs, risk of complications, and are associated with reduced diagnostic yield[6]. One of the most frequent indications for upper GI endoscopy is dyspepsia but diagnostic yield is limited, especially for patients younger than 60 years of age and for those patients without ‘alarm symptoms’ such as unintended weight loss, hematemesis, melena, dysphagia, anemia and persistent vomiting[4]. However, physicians still rely on upper GI endoscopy to assign a suitable treatment for dyspeptic patients. Patients hope that endoscopic procedures will reveal (or exclude) a cause for their symptoms. Reassurance of negative endoscopy may have beneficial effects on symptoms in some, although no long-term improvement in health related quality of life is seen[7].
The open-access system, which allows upper GI endoscopy referral without prior specialist consultation, has fueled the increase in referrals. Despite curtailed criteria for upper GI endoscopy referral by evidence-based guidelines, recent studies continue to report overuse[8,9].
It is evident that mere publication of guidelines and highlighting overuse is not sufficient to change referral practice. In this review we discuss several strategies applied to reduce the overuse of upper GI endoscopies for dyspepsia. We review pitfalls and elements of success of these strategies in order to aid design of future interventions.
Clinical guidelines are recommendations that assist clinicians in decision-making and have the ability to improve quality of care[10]. Care given according to guidelines is more efficient and consistent[11,12]. Several up-to-date practice guidelines recommend diagnostic and therapeutic pathways for dyspepsia management. Widely used are the American Society for Gastrointestinal Endoscopy (ASGE) guideline, ‘the role of endoscopy in dyspepsia’ (2015)[13]; two NICE guidelines, ‘Dyspepsia and gastro-esophageal reflux disease in adults’ (2015)[14] and ‘suspected cancer: recognition and referral’ (2017)[15]; and the joint American College of Gastroenterology (ACG) and the Canadian Association of Gastroenterology (CAG) guideline, ‘ACG and CAG clinical guideline: management of dyspepsia’ (2017)[16]. Locally adapted versions of these guidelines may be used per region.
While the ASGE advises upper GI endoscopy for all with new onset dyspepsia after 50 years of age and all with alarm symptoms, the ACG&CAG refrain from recommending endoscopic investigation for those < 60 years of age, even in the presence of alarm symptoms, so long as epigastric pain is the predominant symptom. The NICE guideline recommends urgent endoscopy for dyspepsia for those under 55 years of age, only in the case of co-existent dysphagia. For patients over 55 years of age, endoscopy is justified in the presence of weight loss and should be considered in the case of therapy resistance, or anemia or raised platelet count combined with specific symptoms.
For those not eligible for endoscopy empirical Proton Pump Inhibitors (PPI) treatment is advised as first choice over Histamine2-antagonists (H2-antagonists) in all guidelines. Prokinetics or tricyclic antidepressants are second choice after acid-suppressive therapy in the ACG&CAG guideline and are considered for endoscopy-negative dyspeptics by the ASGE. H. pylori testing is advised dependent on prevalence (ASGE), when infection is suspected (NICE) or for all dyspeptic patients (ACG&CAG).
In several areas with high gastric cancer incidence, for example Japan and Korea, a screening program for gastric cancer exists, which is outside the scope of the current review.
Many, if not all, dyspepsia guidelines offer strict advice of when to use upper GI endoscopies. Despite these recommendations, there is a global overuse of upper GI endoscopies, which mirrors poor adherence to practice guidelines[1,3,4,17]. Reasons for non-adherence are manifold and include a disconnect between guidelines and local situation, failure to reach the target audience, or the absence of a specific implementation strategy. Specifically, the overuse of upper GI endoscopies continues because of a lack of a filter or specific feedback to the referring physicians.
This review highlights the measures and strategies that have been taken to improve guideline adherence to reduce inappropriate upper GI endoscopy for dyspepsia.
We identified four studies that assessed the effect of guideline implementation to reduce the volume of inappropriate referrals. Details of the included studies are summarized in Table 1.
| Study | Country | Sample | Strategy | Effect on UGIE referrals |
| Cardin et al[18], 2005 | Italy | 2098 patients | Distribution of a locally adapted international guideline, changing referral criteria. | Reduction: 63% (at first) and 42% (at repeat presentation) |
| Elwyn et al[20], 2007 | United Kingdom | 215 PCPh, 359 hospital physicians | Feedback on referrals after distribution of adapted guideline. | Reduction: PCPh 9% (NS), Hospital physicians 31% |
| Banait et al[21], 2003 | United Kingdom | 114 PCPr | Educational program, including workshops, hand-outs, and reinforcement visit (intervention), vs passive guideline dissemination (control) | Appropriateness of referrals: Intervention 73%, Control 50% |
| Shaw et al[22], 2006 | United Kingdom | 47 PCPr | Promotion of HP testing, serology service, and treatment advice (intervention), vs reserved approach to HP testing | Reduction: Intervention: 27%, Control: 4% |
An Italian field study examined the effects of implementation of an adapted version of the ‘European Society of Primary Care Gastroenterology (ESPCG) dyspepsia and H. pylori infection management guideline’[18]. This local version recommended prompt upper GI endoscopy for uninvestigated dyspepsia for patients with alarm symptoms or for patients > 45 years of age after repeat presentation. This contrasts with the original ESPCG guideline which additionally recommends endoscopy for the latter scenario at first presentation[19]. Implementation comprised three phases: selection of the most suitable guideline by a dedicated group, adaptation based on prospectively collected prescribing data, and assessment of prescribing behavior. Over six months, 2098 patients were treated for dyspepsia and 11.7% were ≤ 45 years of age. Referrals for this group fell significantly with 63% at first and with 42% at repeat presentation. There was no effect for the age group >45 years of age, explained by under-referral of this group prior to guideline implementation.
A British group designed a different strategy to improve guideline adherence[20]. They took the following steps: hospital based (n = 359) and primary care based (n = 215) physicians received the ‘All Wales Dyspepsia Management guideline’. Recipients were informed that specific feedback would be given on referrals outside the remit of the guideline. Referrals were processed, irrespective of appropriateness. After five months adherence rates to the dyspepsia guideline increased with 36% for primary care physicians and that coincided with a (non-significant) 9% decrease in average weekly referral rate. The opposite was true for hospital-based physicians. Adherence did not change, but weekly referral rate fell by 31%.
Effects of passive guideline dissemination on the appropriateness of open-access upper GI endoscopy referrals was compared with an educational program in a cluster randomized controlled trial[21]. First, the ‘British Society of Gastroenterology dyspepsia management guideline’ was adapted to local use and all 114 selected primary care practices received a copy[22]. Subsequently, an educational program containing educational workshops, hand-outs and a reinforcement visit after three months was compared with no education. After seven months, appropriateness of upper GI endoscopy referrals was higher in the group that attended the education workshop (73%) compared to controls (50%). Interestingly, participation in the education program did not influence the appropriateness of referrals within the intervention group. This might be explained by the observation that appropriateness was based on referral letters, which is less accurate than assessment of the indication through interview or questionnaires.
In a pragmatic randomized controlled study, 47 primary care practices received a summary of the ‘Maastricht consensus statement on management of H. pylori’[23,24]. In addition, the intervention group received information actively promoting H. pylori testing instead of upper GI endoscopy in patients < 55 years of age, serology service was made available, and therapy was advised for every serology positive result. The control group received information stating the lack of evidence for H. pylori testing for dyspepsia. After 1 year, upper GI endoscopy referrals had fallen by 27% in the intervention group compared to 4% in the control group.
Clinical assessment of upper GI endoscopy referrals by a gastroenterologist at the outpatient clinic is time consuming. Since endoscopy is considered a safe procedure, it was deemed safe to short-circuit the gastroenterologist from the referral pathway[25,26]. Hence, the open-access system was launched and is by now adopted by many health care organizations.
The concept of open-access referral was promoted for its potential to reduce costs and waiting lists and improve early cancer detection rates. Indeed, open-access led to a reduction of gastroenterologist consultations, but studies consistently failed to detect improved cancer detection rates[27]. Many studies have documented that the diagnostic yield of open-access upper GI endoscopies is low, while costs have increased[28,29]. In the absence of a filter, referrals through the open-access system are liable to be performed for inappropriate reasons.
Adding an evaluation of all referrals to the system requires an additional step, which abolishes the attractiveness of the refer-and-scope open-access system. On the other hand, a low-cost filter, effectively reducing inappropriate referrals, may serve to enhance rather than obstruct the existing system. Indeed, censoring is probably needed to lower the high proportion of negative endoscopies[4].
There are fine examples of efforts to reduce the number of (inappropriate) upper GI endoscopies using a method of clinical assessment.
Already in the late 90s of the last century, the idea arose that some form of censoring of open-access referrals was necessary. Two one-stop dyspepsia clinics in the UK assessed the appropriateness of referrals through history and physical examination, for 12 and 22 mo[30,31]. Both clinics found a high level of agreement with the original referral. Only 6%-14% of upper GI endoscopy referrals were cancelled which is considerably lower than recent data shows.
An Italian study evaluated 5192 referral letters and ~10% of referrals were judged as inappropriate[32]. Interestingly, this study used a biomarker panel in selected patients that indicates the presence of chronic atrophic gastritis. A recent meta-analysis of 27 studies found a good specificity which supports the use of this biomarker panel[33]. An additional 0.7% of initially judged inappropriate referrals were reselected for upper GI endoscopy because of presumed chronic atrophic gastritis. Six percent of referrals were deemed inappropriate in view of the absence of a referral letter.
A recent study introduced a ‘virtual clinic’ to decide on the best pathway for patients referred to the gastroenterology outpatient clinic for five common indications, including dyspepsia[34]. Based on age, symptoms and previous diagnostics and treatment, the system decided on the need for (fast-track) upper GI endoscopy or sent the primary care physician an advisory letter. While impact on upper GI endoscopy volume was not described, the strategy is a good example of a self-supporting clinical assessment system. Of all 14245 patients assessed, 32% were managed without a face-to-face appointment.
A different approach of clinical assessment is ‘self-assessment’ through use of decision trees. A randomized controlled trial demonstrated the use of such an algorithm to distinguish patients with appropriate indications for upper GI endoscopy according to locally adapted clinical guidelines, from those that would benefit from PPI treatment or H. pylori testing, based on presenting symptoms[35]. The use of an algorithm resulted in a reduction of 35% of referrals for upper GI endoscopy compared to usual care.
Details of the included studies are summarized in Table 2.
| Study | Country | Sample | Strategy | Effect on UGIE referrals |
| Rutter et al[30], 1998 | United Kingdom | 485 visits | Patient assessment in one-stop dyspepsia clinic | Cancelled: 6% |
| Mourad et al[31], 1998 | United Kingdom | 272 visits | Patient assessment in one-stop dyspepsia clinic | Cancelled: 14% |
| Baldasarre et al[32], 2016 | Italy | 5192 referrals | Assessment of referral letters and biomarker panel for atrophic gastritis | Cancelled: 10% |
| Pelitari et al[33], 2017 | United Kingdom | 14,245 patients | Virtual assessment system to decide on best pathway for dyspeptic patients | 32% no face-to-face appointment (not UGIE specific) |
| Horowitz et al[34], 2007 | Israel | 138 patients | Decision tree for symptom management, based on presenting symptoms | Reduction: 35% |
Dyspeptic symptoms such as postprandial fullness, early satiety and epigastric pain or burning, could be attributed to Helicobacter pylori (H. pylori) infection. H. Pylori elicits inflammation of the gastric mucosa, and chronic gastritis[36-38]. Mucosal damage reduces ghrelin levels, which leads to acid hypersecretion and deterioration of gastrointestinal motility. Several studies have documented higher prevalences of H. pylori infection in patients with dyspepsia[39,40]. It is reasonable to postulate the H. pylori eradication would result in improvement of symptoms in dyspepsia.
Disappointingly, meta-analyses of RCTs fail to show beneficial effects of H. pylori eradication in dyspepsia, although the risk of having symptoms after 12 mo reduces[41,42]. Still, in view of the potential beneficial effects of H. pylori on dyspeptic symptoms, the impact of test and treat strategies on the number of performed upper GI endoscopies is of interest. Several studies have attempted to quantify this impact.
We found nine randomized controlled trials (RCTs) reporting on reduction of upper GI endoscopies through testing for and treatment of H. pylori. Details of the included studies are summarized in Table 3. Two came from Asia (China[43] and Malaysia[44]), and seven were performed in Western Europe (two in the Netherlands[45,46], four in the UK[47-50], one in Denmark[51]). Reductions of upper GI endoscopies ranged from 68% to as high as 91.8%.
| Study | Country | Sample | Strategy | No endoscopy after HP test and treat |
| Hu et al[43], 2006 | China | 161 patients | UGIE vs HP testing (UBT) + eradication (HP+) or Cisapride (HP-). | Symptom relief: 44%. Endoscopy for all after 6 weeks. |
| Mahadeva et al[44], 2008 | Malaysia | 432 patients | UGIE vs HP testing (UBT) + eradication (HP+) or reassurance and/or empirical treatment (HP-) | 89% |
| Arents et al[45], 2003 | Netherlands | 270 patients | UGIE vs HP testing (serology) + eradication (HP+) or Cisapride (HP-) | 68% |
| Laheij et al[46], 2004 | Netherlands | 199 patients | UGIE vs omeprazole 2 wk followed by UGIE (no improvement) or HP serology testing (relapse) + eradication (HP+) | 94% |
| Duggan et al[47], 2008 | United Kingdom | 584 patients | UGIE vs HP test + endoscopy (HP+) vs HP testing (serology) + eradication and/or endoscopy (HP+) or Lanzoprazole (HP-). | 73% |
| Heaney et al[49], 1999 | United Kingdom | 104 patients | All received HP testing (UBT). Randomization of HP+ patients: UGIE or eradication therapy. | 73% |
| McColl et al[50], 2002 | United Kingdom | 708 patients | UGIE vs HP testing (UBT) + eradication (HP+). | 92% |
| Lassen et al[51], 2000 | Denmark | 500 patients | UGIE vs HP testing (UBT) + eradication (HP+), PPI (HP-, NSAID-, reflux+), or lifestyle advice (HP-, NSAID-, reflux-) | 60% |
| Fraser et al[48], 2003 | New Zealand | 173 patients | All received UBT and eradication if HP+ | 76% |
| Slade et al[53], 1999 | United Kingdom | 232 patients | All received HP serology testing + UGIE (HP equivocal), eradication (HP+), or usual care (HP-). | 73% |
| Patel et al[54], 1995 | United Kingdom | 183 patients | All received serology testing + UGIE for HP+, sinister symptoms, or NSAID use. | 37% (after HP-) |
In six RCTs, primary care practitioners were free to refer test and treat patients for upper GI endoscopy after randomization if symptoms persisted[44-47,50,52]. A considerable proportion (68%-94%) did not need additional upper GI endoscopy after either H. pylori eradication or, if uninfected, empirical treatment with PPIs, H2-antagonists, prokinetics or no treatment. Three RCTs used a time delimited period after which upper GI endoscopy was allowed, if symptoms persisted[43,49,51]. The predefined period varied between 2-6 wk and the proportion of avoided endoscopies was high (72%, 82% and 76%).
A Chinese study subjected all patients to upper GI endoscopy after 6 weeks, irrespective of presence or absence of symptoms[43]. Four gastric erosions and three duodenal ulcers were the only tissue abnormalities found in a cohort of 78 asymptomatic, post-eradication patients.
Keeping many doctors from deciding against endoscopy for dyspeptic patients is a fear of missing out upper gastrointestinal cancers. The prevalence of cancers in all RCTs was extremely low. Across all studies, together representing 1531 patients, only three (0.2%) cancers were found in test & treat patients. The majority (60%) of upper GI endoscopies performed for persistent symptoms, did not reveal any abnormalities.
In two prospective studies, all dyspeptic patients without alarm symptoms referred for upper GI endoscopy followed a test and treat strategy for H. pylori[48,53]. This avoided about 75% of upper GI endoscopies. A third prospective study similarly tested all dyspeptic patients for the presence of H. pylori and treated those with positive tests[54]. Upper GI endoscopies were avoided for all H. pylori negative, asymptomatic patients (37%), and all H. pylori positive patients or symptomatic patients were subjected to upper GI endoscopy. No upper gastrointestinal cancer was found in the 588 patients included in the three studies.
PPIs are being used to treat patients with dyspepsia, especially those with acid-related symptoms[55]. Indeed, a meta-analysis revealed that PPIs are more effective than placebo to ameliorate symptoms in functional dyspepsia[56].
Prokinetic drugs might be helpful for patients with impaired gastric emptying[57]. Beneficial effects of prokinetics for dyspepsia were seen across studies, but a robust relationship with specific dysmotility-like symptoms is absent[58].
Currently, PPIs are the therapeutic front runners in dyspepsia. Achieving symptom relief is an important factor to battle inappropriate use of upper GI endoscopies, and there is evidence that empirical treatment may be effective.
Three studies have examined the concept of empirical drug treatment against prompt upper GI endoscopy. Details of the included studies are summarized in Table 4.
| Study | Country | Sample | Strategy | UGIE avoided |
| Laheij et al[59], 1998 | Netherlands | 80 patients | 2-wk PPI treatment. HP test and treat for persistent symptoms. | 69% |
| Kjeldsen et al[60], 2007 | Denmark | 184 patients | UGIE vs 2-wk PPI treatment and HP testing + UGIE (no improvement, recurrence at age ≥ 45, or HP- and age < 45 yr), eradication (HP+ and age < 45 yr), or PPI 2 additional weeks (HP-, age < 45 yr, and reflux+) | 63% |
| Bytzet et al[62], 1994 | Denmark | 206 patients | 4 weeks H2RA treatment | 34% |
One study treated 80 patients with empirical PPI for 2 wk and offered those with persistent symptoms, subsequent H. pylori screening[59]. Upper GI endoscopies were avoided in 69%. This result accords with a Danish study using a similar treatment regime and prevented 63% (n = 184) of listed upper gastrointestinal endoscopies[60]. The Dutch study revisited their cohort after 1 year and found that 6.5% (n = 77) of empirically treated patients were scoped[61]. One patient had developed gastric cancer within the first year of follow-up.
Another Danish study offered 206 patients with dyspepsia 4 weeks of H2-antagonists as initial empirical treatment[62]. After one year, upper GI endoscopy was avoided in only 34% of patients.
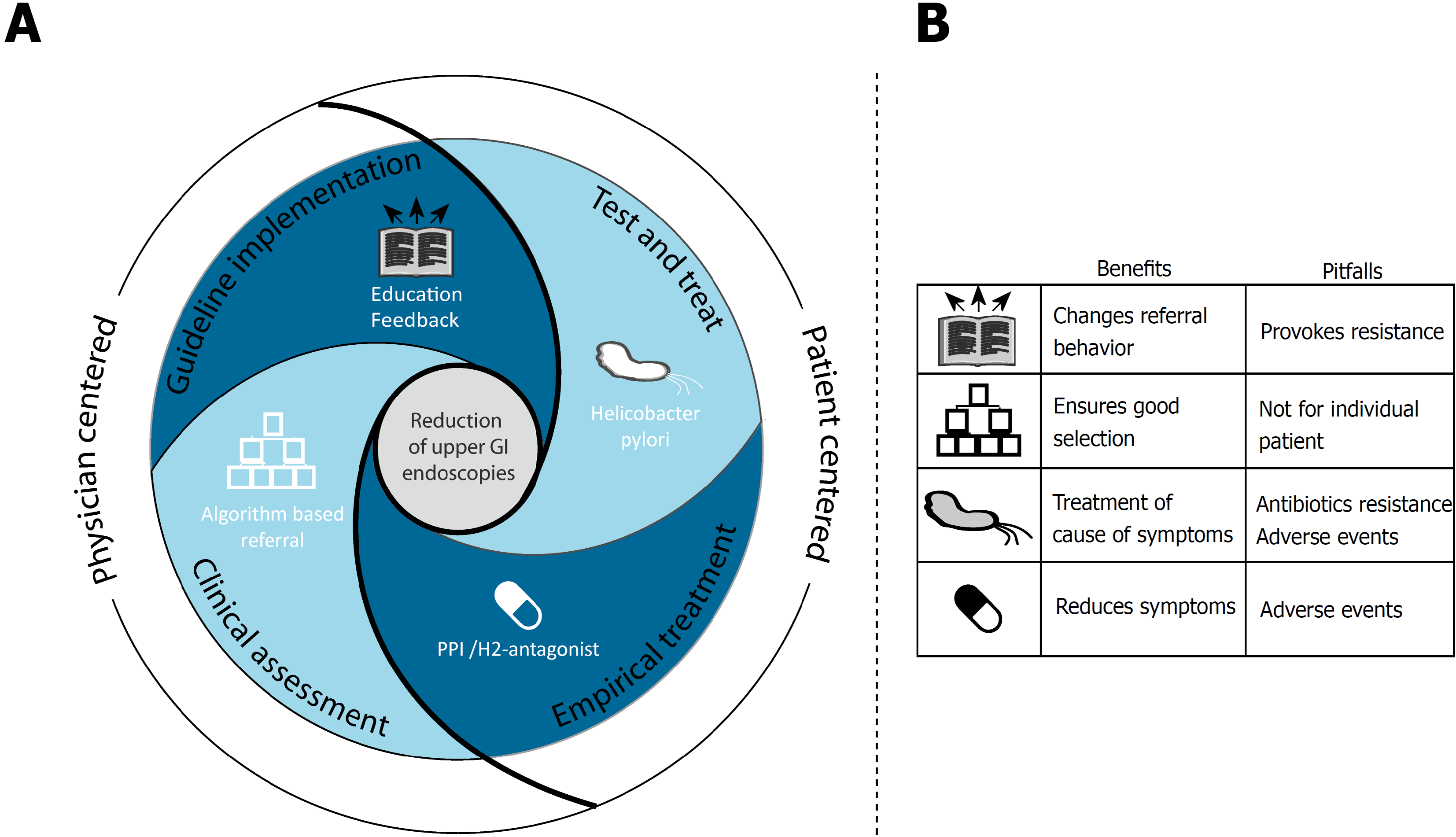
Four strategies reveal four important targets in the trajectory of dyspepsia management that can be addressed to reduce inappropriate upper GI endoscopies (see Figure 1). Whereas guideline implementation strategies focus on changing physicians’ behavior, a second strategy intervenes further in the referral process and assesses correctness of made referrals. A third strategy aims at detection and treatment of H. pylori, and lastly a strategy attempts to relieve symptoms through empirical treatment.
Guideline implementation is a low-cost intervention using existing infrastructure and resources. A lasting effect can be expected, due to its educational nature. Adjusting guidelines to local situations prior to implementation may be time consuming, but is of major importance for guideline acceptance[10].
Behavioral change is the basis for improvement in healthcare quality, but its delicate nature complicates strategy implementation. Indeed, in the studies highlighted in this review, resistance was encountered when feedback on referrals was given. Disagreement with guidelines and fear of losing clinical freedom were two most frequently used arguments[20]. One study notes that 15% of physicians cited lack of fit with clinical practice as a cause of non-adhere to guidelines[18]. And nearly 40% of primary care physicians failed to attend educational workshops organized in the context of guideline implementation[21]. Despite this disappointing data, active conveying of information about appropriate clinical behavior, including feedback, educational meetings and reminders, increases guideline adherence and results in a modest decline in upper GI endoscopy referrals.
Guideline implementation strategies are entirely directed at changing physicians’ behavior, which means that patient, institution, and finance-related factors are not taken into account. Amongst others, these are factors which often play a role in the level of guideline adherence.
All in all, strategies that actively implement guidelines improve appropriateness of upper GI endoscopies referrals for dyspepsia. However, this strategy will meet resistance among referring physicians and, alone, is unlikely to be able to achieve the desired effect on referral behavior. Comprehensive, inclusive, and multilevel approaches will be needed to truly affect appropriateness of referrals.
Strategies involving clinical assessment ensure proper selection of patients with indication for upper GI endoscopy. However, most clinical assessment strategies have logistical challenges, as new steps are introduced within a well-established open-access system, available in most healthcare settings. Also, extra costs and working hours of a one-stop clinic or clinical assessment by an endoscopist neutralizes the originally anticipated effects of an open-access system.
Low levels of inappropriateness were seen in studies that involved face-to-face assessment of patients referred for upper GI endoscopy[30,31]. However, these studies date from 1998, and different criteria for appropriateness of referrals were used at that time. Also, patients’ fear of serious disease or lack of confidence in conservative treatment may have played a role[63]. In a face-to-face dyspepsia clinic, these factors may not be changed, leading to upper GI endoscopy although not strictly appropriate. Arguing against patients’ influence is the equally low reduction of upper GI endoscopies found in the study evaluating referral letters[32].
Algorithms follow a more rigid approach, purely based on presence of variables, such as symptoms. Algorithms are useful in decision-making and to guide clinicians through the clutter of available evidence, as is the case for dyspepsia management. Leaving out individual patient factors using an algorithm, resulted in a considerable reduction of upper GI endoscopies compared to usual care[34]. However, the rigidity of such aids is an important drawback as it negates sound clinical judgment.
The use of an extensive virtual assessor to select the right pathway for patients effectively reduces the number of referrals to outpatient clinics[33]. Standardized feedback was given, aiding physicians in their decision making, and patients in management of their disease. Key elements for success in this study were patient involvement and the use of feedback in combination with the liberty of physicians to overrule the system, if deemed appropriate.
Future studies should explore combinations of clinical assessment strategies. An algorithm integrated in the referral process that gives feedback on, but does not reject, referrals would be an effective form of clinical assessment, while maintaining patient centered care.
H. pylori testing is a simple procedure that can easily be performed in primary care. Cost effectiveness of H. pylori test and treat is dependent on several local factors. Overall, test and treat after failure of acid-suppression therapy is more cost effective than prompt endoscopy, providing that H. pylori prevalence is at least ≥10%, and availability of endoscopy is good and costs > $200[64-66].
The prevalence of H. pylori is subject to considerable regional variation[67,68]. Highest prevalence is generally found in Africa, Latin America and the Caribbean, and Asia. Northern America features the lowest prevalence. However, even in low prevalence countries, the bacteria affects a substantial population and should always be considered when upper GI symptoms arise. Additionally, H. pylori is known to cause non-cardia gastric cancer, and peptic ulcers, emphasizing the benefits of H. pylori eradication[69,70].
Substantial numbers of upper GI endoscopies were avoided in trials that adopted a test and treat strategy for dyspepsia, both in high and low H. pylori prevalence countries. Upper GI endoscopies performed for persistent symptoms usually resulted in normal results and upper GI cancers were only incidentally found. This confirms that with this strategy the probability of missing significant abnormalities is low.
Arguing against widespread use of a test and treat strategy is the disappointing reduction of dyspeptic symptoms after eradication as reported by several clinical trials[42]. H. pylori treatment, traditionally consisting of dual antibiotics with a PPI, may result in novel on-treatment symptoms and carries the risk of antibiotic resistance[41]. It is advisable to take these factors into account when screening for H. pylori is considered in patients at low risk of infection.
In view of the ability to reduce the volume of upper GI endoscopies, H. pylori test and treat should be part of the diagnostic work-up of dyspepsia.
Most guidelines recommend empirical treatment with PPI or H2-antagonists. These drugs are well-suited for primary care-based use, as they are safe, especially with short-term use, and costs are low[71,72]. Monitoring of drug-use duration is often integrated in primary care systems, preventing undesired prolonged use.
A pre-emptive treatment of dyspepsia patients avoids two-thirds of upper GI endoscopies[59,60]. Risk of missing malignancies is extremely low using this approach. Which strategy (PPI, H2-antagonists) avoids most upper GI endoscopies is still unclear, as there have not been any head-to-head trials studying this outcome. A superior effect of PPI over H2-antagonists for symptom reduction is consistently shown.
Concerns have been raised about long-term safety of PPI use. However, these were based on weak evidence, concerning solely observational studies[73]. Robust, randomized studies are needed to establish whether a causal relationship exists. Benefits of PPI treatment often outweigh the potential risk of adverse effects and patients in need of acid-suppressive therapy should not be denied treatment. As with any other drug, PPIs should be prescribed in the lowest possible dose for the shortest possible time. Frequent re-evaluation of the appropriateness of use and, if not appropriate, careful stepwise discontinuation to prevent a rebound effect is paramount.
Empirical treatment of uninvestigated dyspepsia has been feared to ‘mask’ gastric cancer[74]. Based on the declining incidence of gastric cancer in the Western world it is unlikely that this is the case in low to moderate (e.g. < 15 per 100.000) prevalence countries. In particular, when adequate H. pylori testing is performed, and risk factors are observed.
Empirical treatment with PPI and H. pylori test and treat strategies greatly reduces the need for upper GI endoscopy. Individual choices for a suitable drug may be made and switched if the initial choice fails.
In this review we report four strategies that reduce the volume of upper GI endoscopies for dyspepsia. Dyspepsia is a multifactorial disorder and requires a matching multi-level approach. Therefore, no single best strategy is the panacea for all dyspepsia patients. Each intervention has its benefits and drawbacks and the key to success are multiple rather than individual strategies.
A randomized clinical trial (TRIODe) is currently ongoing that compares a multifactorial strategy involving e-learning with upper GI endoscopy (http://www.clinicaltrials.gov NCT03205319). The aim of this pragmatic study is to reduce the volume of upper GI endoscopies through a combination of increased patient and physician awareness and lifestyle interventions. The e-learning is a home-based tool that addresses the limited value of upper GI endoscopies for dyspepsia, explains etiology of dyspepsia and guides patients through lifestyle interventions. We will enroll 119 patients from four district general hospitals in the Netherlands. With this trial, we aim to provide a framework that can be used to improve appropriateness of upper GI endoscopy use.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Eleftheriadis NP, Jonaitis LV, Lanas A S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
| 1. | Aljebreen AM, Alswat K, Almadi MA. Appropriateness and diagnostic yield of upper gastrointestinal endoscopy in an open-access endoscopy system. Saudi J Gastroenterol. 2013;19:219-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Hassan C, Bersani G, Buri L, Zullo A, Anti M, Bianco MA, Di Giulio E, Ficano L, Morini S, Di Matteo G, Loriga P, Pietropaolo V, Cipolletta L, Costamagna G. Appropriateness of upper-GI endoscopy: an Italian survey on behalf of the Italian Society of Digestive Endoscopy. Gastrointest Endosc. 2007;65:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Tahir M. Appropriateness of Upper Gastrointestinal Endoscopy: Will the Diagnostic Yield Improve by the use of American Society of Gastroenterology Guidelines? Euroasian J Hepatogastroenterol. 2016;6:143-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Manes G, Balzano A, Marone P, Lioniello M, Mosca S. Appropriateness and diagnostic yield of upper gastrointestinal endoscopy in an open-access endoscopy system: a prospective observational study based on the Maastricht guidelines. Aliment Pharmacol Ther. 2002;16:105-110. [PubMed] |
| 5. | O’Sullivan JW, Albasri A, Nicholson BD, Perera R, Aronson JK, Roberts N, Heneghan C. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open. 2018;8:e018557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Di Giulio E, Hassan C, Marmo R, Zullo A, Annibale B. Appropriateness of the indication for upper endoscopy: a meta-analysis. Dig Liver Dis. 2010;42:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | van Kerkhoven LA, van Rossum LG, van Oijen MG, Tan AC, Laheij RJ, Jansen JB. Upper gastrointestinal endoscopy does not reassure patients with functional dyspepsia. Endoscopy. 2006;38:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Bohara TP, Laudari U, Thapa A, Rupakheti S, Joshi MR. Appropriateness of Indications of Upper Gastrointestinal Endoscopy and its Association With Positive Finding. JNMA J Nepal Med Assoc. 2018;56:504-509. [PubMed] |
| 9. | Crouwel F, Meurs-Szojda MM, Klemt-Kropp M, Fockens P, Grasman ME. The diagnostic yield of open-access endoscopy of the upper gastrointestinal tract in the Netherlands. Endosc Int Open. 2018;6:E383-E394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318:527-530. [PubMed] |
| 11. | Chassin MR, Brook RH, Park RE, Keesey J, Fink A, Kosecoff J, Kahn K, Merrick N, Solomon DH. Variations in the use of medical and surgical services by the Medicare population. N Engl J Med. 1986;314:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 352] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Shapiro DW, Lasker RD, Bindman AB, Lee PR. Containing costs while improving quality of care: the role of profiling and practice guidelines. Annu Rev Public Health. 1993;14:219-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Appropriate use of gastrointestinal endoscopy. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 2000;52:831-837. [PubMed] |
| 14. | National Institute for Health and Care Excellence: Dyspepsia and gastro-oesophageal reflux disease in adults. Available from: https://www.nice.org.uk/guidance/qs96.2015. |
| 15. | National Institute for Health and Care Excellence: Suspected cancer: recognition and referral. Available from: https://www.nice.org.uk/guidance/ng12.2017. |
| 16. | Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am J Gastroenterol. 2017;112:988-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 378] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 17. | Ching HL, Hale MF, Sidhu R, McAlindon ME. Reassessing the value of gastroscopy for the investigation of dyspepsia. Frontline Gastroenterol. 2018;9:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Cardin F, Zorzi M, Bovo E, Guerra C, Bandini F, Polito D, Bano F, Grion AM, Toffanin R. Effect of implementation of a dyspepsia and Helicobacter pylori eradication guideline in primary care. Digestion. 2005;72:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Rubin G, Meineche-Schmidt V, Roberts A, de Wit N. The use of consensus to develop guidelines for the management of Helicobacter pylori infection in primary care. European Society for Primary Care Gastroenterology. Fam Pract. 2000;17 Suppl 2:S21-S26. [PubMed] |
| 20. | Elwyn G, Owen D, Roberts L, Wareham K, Duane P, Allison M, Sykes A. Influencing referral practice using feedback of adherence to NICE guidelines: a quality improvement report for dyspepsia. Qual Saf Health Care. 2007;16:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Banait G, Sibbald B, Thompson D, Summerton D, Hann M, Talbot S; Salford and Trafford Ulcer Research Network. Modifying dyspepsia management in primary care: a cluster randomised controlled trial of educational outreach compared with passive guideline dissemination. Br J Gen Pract. 2003;53:94-100. [PubMed] |
| 22. | British Society of Gastroenterology: Dyspepsia management guidelines. 1996. |
| 23. | Shaw IS, Valori RM, Charlett A, McNulty CA. Limited impact on endoscopy demand from a primary care based ‘test and treat’ dyspepsia management strategy: the results of a randomised controlled trial. Br J Gen Pract. 2006;56:369-374. [PubMed] |
| 24. | Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut. 1997;41:8-13. [PubMed] |
| 25. | Keren D, Rainis T, Stermer E, Lavy A. A nine-year audit of open-access upper gastrointestinal endoscopic procedures: results and experience of a single centre. Can J Gastroenterol. 2011;25:83-88. [PubMed] |
| 26. | Chandrasekhara V, Eloubeidi MA, Bruining DH, Chathadi K, Faulx AL, Fonkalsrud L, Khashab MA, Lightdale JR, Muthusamy VR, Pasha S, Saltzman JR, Shaukat A, Wang A, Cash B, DeWitt JM; ASGE Standards of Practice Committee. Open-access endoscopy. Gastrointest Endosc. 2015;81:1326-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Paterson HM, McCole D, Auld CD. Impact of open-access endoscopy on detection of early oesophageal and gastric cancer 1994 - 2003: population-based study. Endoscopy. 2006;38:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Di Giulio E, Hassan C, Pickhardt PJ, Zullo A, Laghi A, Kim DH, Iafrate F. Cost-effectiveness of upper gastrointestinal endoscopy according to the appropriateness of the indication. Scand J Gastroenterol. 2009;44:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Froehlich F, Burnand B, Pache I, Vader J-P, Fried M, Schneider C, Kosecoff J, Kolodny M, DuBois RW, Brook RH, Gonvers JJ. Overuse of upper gastrointestinal endoscopy in a country with open-access endoscopy: a prospective study in primary care. Gastrointest Endosc. 1997;45:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Rutter MD, Michie AF, Trewby PN. The one-stop dyspepsia clinic--an alternative to open-access endoscopy for patients with dyspepsia. J R Soc Med. 1998;91:524-527. [PubMed] |
| 31. | Mourad FH, Taylor TM, Fairclough PD, Farthing MJ. General practitioner access to gastroscopy: is ‘censorship’ valuable? Br J Gen Pract. 1998;48:1165-1166. [PubMed] |
| 32. | Baldassarre G, Messina O, Panozzo MP, Tomba F, Sella D, Ferronato A, Vanzetto E, Toffanin R, Di Mario F, Antico A, Franceschi M. Improvement of appropriateness and reduction of waiting lists concerning upper GI endoscopy outpatients: A single-centre prospective study. Digest Liver Dis. 2016;2:e206. |
| 33. | Syrjänen K. A Panel of Serum Biomarkers (GastroPanel®) in Non-invasive Diagnosis of Atrophic Gastritis. Systematic Review and Meta-analysis. Anticancer Res. 2016;36:5133-5144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Pelitari S, Hathaway C, Gritton D, Smith A, Bush D, Menon S, McKaig B. Impact and cost-effectiveness of formal gastroenterology outpatient referral Clinical Assessment Service. Frontline Gastroenterol. 2018;9:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Horowitz N, Moshkowitz M, Leshno M, Ribak J, Birkenfeld S, Kenet G, Halpern Z. Clinical trial: evaluation of a clinical decision-support model for upper abdominal complaints in primary-care practice. Aliment Pharmacol Ther. 2007;26:1277-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Osawa H. Ghrelin and Helicobacter pylori infection. World J Gastroenterol. 2008;14:6327-6333. [PubMed] |
| 37. | Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 38. | Zullo A, Hassan C, De Francesco V, Repici A, Manta R, Tomao S, Annibale B, Vaira D. Helicobacter pylori and functional dyspepsia: an unsolved issue? World J Gastroenterol. 2014;20:8957-8963. |
| 39. | Khedmat H, Karbasi-Afshar R, Agah S, Taheri S. Helicobacter pylori Infection in the general population: A Middle Eastern perspective. Caspian J Intern Med. 2013;4:745-753. [PubMed] |
| 40. | Armstrong D. Helicobacter pylori infection and dyspepsia. Scand J Gastroenterol Suppl. 1996;215:38-47. [PubMed] |
| 41. | Du LJ, Chen BR, Kim JJ, Kim S, Shen JH, Dai N. Helicobacter pylori eradication therapy for functional dyspepsia: Systematic review and meta-analysis. World J Gastroenterol. 2016;22:3486-3495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 42. | Laine L, Schoenfeld P, Fennerty MB. Therapy for Helicobacter pylori in patients with nonulcer dyspepsia. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;134:361-369. [PubMed] |
| 43. | Hu WH, Lam SK, Lam CL, Wong WM, Lam KF, Lai KC, Wong YH, Wong BC, Chan AO, Chan CK, Leung GM, Hui WM. Comparison between empirical prokinetics, Helicobacter test-and-treat and empirical endoscopy in primary-care patients presenting with dyspepsia: a one-year study. World J Gastroenterol. 2006;12:5010-5016. [PubMed] |
| 44. | Mahadeva S, Chia YC, Vinothini A, Mohazmi M, Goh KL. Cost-effectiveness of and satisfaction with a Helicobacter pylori “test and treat” strategy compared with prompt endoscopy in young Asians with dyspepsia. Gut. 2008;57:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Arents NL, Thijs JC, van Zwet AA, Oudkerk Pool M, Gotz JM, van de Werf GT, Reenders K, Sluiter WJ, Kleibeuker JH. Approach to treatment of dyspepsia in primary care: a randomized trial comparing “test-and-treat” with prompt endoscopy. Arch Intern Med. 2003;163:1606-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Laheij RJ, Hermsen JT, Jansen JB, Horrevorts AM, Rongen RJ, Van Rossum LG, Witteman E, de Koning RW. Empirical treatment followed by a test-and-treat strategy is more cost-effective in comparison with prompt endoscopy or radiography in patients with dyspeptic symptoms: a randomized trial in a primary care setting. Fam Pract. 2004;21:238-243. [PubMed] |
| 47. | Duggan AE, Elliott CA, Miller P, Hawkey CJ, Logan RF. Clinical trial: a randomized trial of early endoscopy, Helicobacter pylori testing and empirical therapy for the management of dyspepsia in primary care. Aliment Pharmacol Ther. 2009;29:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Fraser A, Williamson S, Lane M, Hollis B. Nurse-led dyspepsia clinic using the urea breath test for Helicobacter pylori. N Z Med J. 2003;116:U479. [PubMed] |
| 49. | Heaney A, Collins JS, Watson RG, McFarland RJ, Bamford KB, Tham TC. A prospective randomised trial of a “test and treat” policy versus endoscopy based management in young Helicobacter pylori positive patients with ulcer-like dyspepsia, referred to a hospital clinic. Gut. 1999;45:186-190. [PubMed] |
| 50. | McColl KE, Murray LS, Gillen D, Walker A, Wirz A, Fletcher J, Mowat C, Henry E, Kelman A, Dickson A. Randomised trial of endoscopy with testing for Helicobacter pylori compared with non-invasive H pylori testing alone in the management of dyspepsia. BMJ. 2002;324:999-1002. [PubMed] |
| 51. | Lassen AT, Pedersen FM, Bytzer P, Schaffalitzky de Muckadell OB. Helicobacter pylori test-and-eradicate versus prompt endoscopy for management of dyspeptic patients: a randomised trial. Lancet. 2000;356:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Jones R, Tait C, Sladen G, Weston-Baker J. A trial of a test-and-treat strategy for Helicobacter pylori positive dyspeptic patients in general practice. Int J Clin Pract. 1999;53:413-416. [PubMed] |
| 53. | Slade PE, Davidson AR, Steel A, Cox RA, Blackburn PA. Reducing the endoscopic workload: does serological testing for Helicobacter pylori help? Eur J Gastroenterol Hepatol. 1999;11:857-862. [PubMed] |
| 54. | Patel P, Khulusi S, Mendall MA, Lloyd R, Jazrawi R, Maxwell JD, Northfield TC. Prospective screening of dyspeptic patients by Helicobacter pylori serology. Lancet. 1995;346:1315-1318. [PubMed] |
| 55. | Lee KJ, Vos R, Janssens J, Tack J. Influence of duodenal acidification on the sensorimotor function of the proximal stomach in humans. Am J Physiol Gastrointest Liver Physiol. 2004;286:G278-G284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Pinto-Sanchez MI, Yuan Y, Hassan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;11:CD011194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1195] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 58. | Veldhuyzen van Zanten SJ, Jones MJ, Verlinden M, Talley NJ. Efficacy of cisapride and domperidone in functional (nonulcer) dyspepsia: a meta-analysis. Am J Gastroenterol. 2001;96:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Laheij RJ, Severens JL, Van de Lisdonk EH, Verbeek AL, Jansen JB. Randomized controlled trial of omeprazole or endoscopy in patients with persistent dyspepsia: a cost-effectiveness analysis. Aliment Pharmacol Ther. 1998;12:1249-1256. [PubMed] |
| 60. | Kjeldsen HC, Bech M, Christensen B. Cost-effectiveness analysis of two management strategies for dyspepsia. Int J Technol Assess Health Care. 2007;23:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Laheij RJ, van Rossum LG, Heinen N, Jansen JB. Long-term follow-up of empirical treatment or prompt endoscopy for patients with persistent dyspeptic symptoms? Eur J Gastroenterol Hepatol. 2004;16:785-789. [PubMed] |
| 62. | Bytzer P, Hansen JM, Schaffalitzky de Muckadell OB. Empirical H2-blocker therapy or prompt endoscopy in management of dyspepsia. Lancet. 1994;343:811-816. [PubMed] |
| 63. | Cardin F, Andreotti A, Zorzi M, Terranova C, Martella B, Amato B, Militello C. Usefulness of a fast track list for anxious patients in a upper GI endoscopy. BMC Surg. 2012;12 Suppl 1:S11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Delaney BC, Qume M, Moayyedi P, Logan RF, Ford AC, Elliott C, McNulty C, Wilson S, Hobbs FD. Helicobacter pylori test and treat versus proton pump inhibitor in initial management of dyspepsia in primary care: multicentre randomised controlled trial (MRC-CUBE trial). BMJ. 2008;336:651-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Ford AC, Qume M, Moayyedi P, Arents NL, Lassen AT, Logan RF, McColl KE, Myres P, Delaney BC. Helicobacter pylori “test and treat” or endoscopy for managing dyspepsia: an individual patient data meta-analysis. Gastroenterology. 2005;128:1838-1844. [PubMed] |
| 66. | Gisbert JP, Calvet X. Helicobacter Pylori “Test-and-Treat” Strategy for Management of Dyspepsia: A Comprehensive Review. Clin Transl Gastroenterol. 2013;4:e32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2049] [Article Influence: 256.1] [Reference Citation Analysis (0)] |
| 68. | Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, Derakhshan MH. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 494] [Article Influence: 70.6] [Reference Citation Analysis (1)] |
| 69. | Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347-353. [PubMed] |
| 70. | Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 71. | Attwood SE, Ell C, Galmiche JP, Fiocca R, Hatlebakk JG, Hasselgren B, Långström G, Jahreskog M, Eklund S, Lind T, Lundell L. Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: data from the SOPRAN and LOTUS studies. Aliment Pharmacol Ther. 2015;41:1162-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Corleto VD, Festa S, Di Giulio E, Annibale B. Proton pump inhibitor therapy and potential long-term harm. Curr Opin Endocrinol Diabetes Obes. 2014;21:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterology. 2017;153:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |