Published online May 21, 2019. doi: 10.3748/wjg.v25.i19.2373
Peer-review started: March 14, 2019
First decision: March 27, 2019
Revised: March 28, 2019
Accepted: April 19, 2019
Article in press: April 20, 2019
Published online: May 21, 2019
Processing time: 68 Days and 3.4 Hours
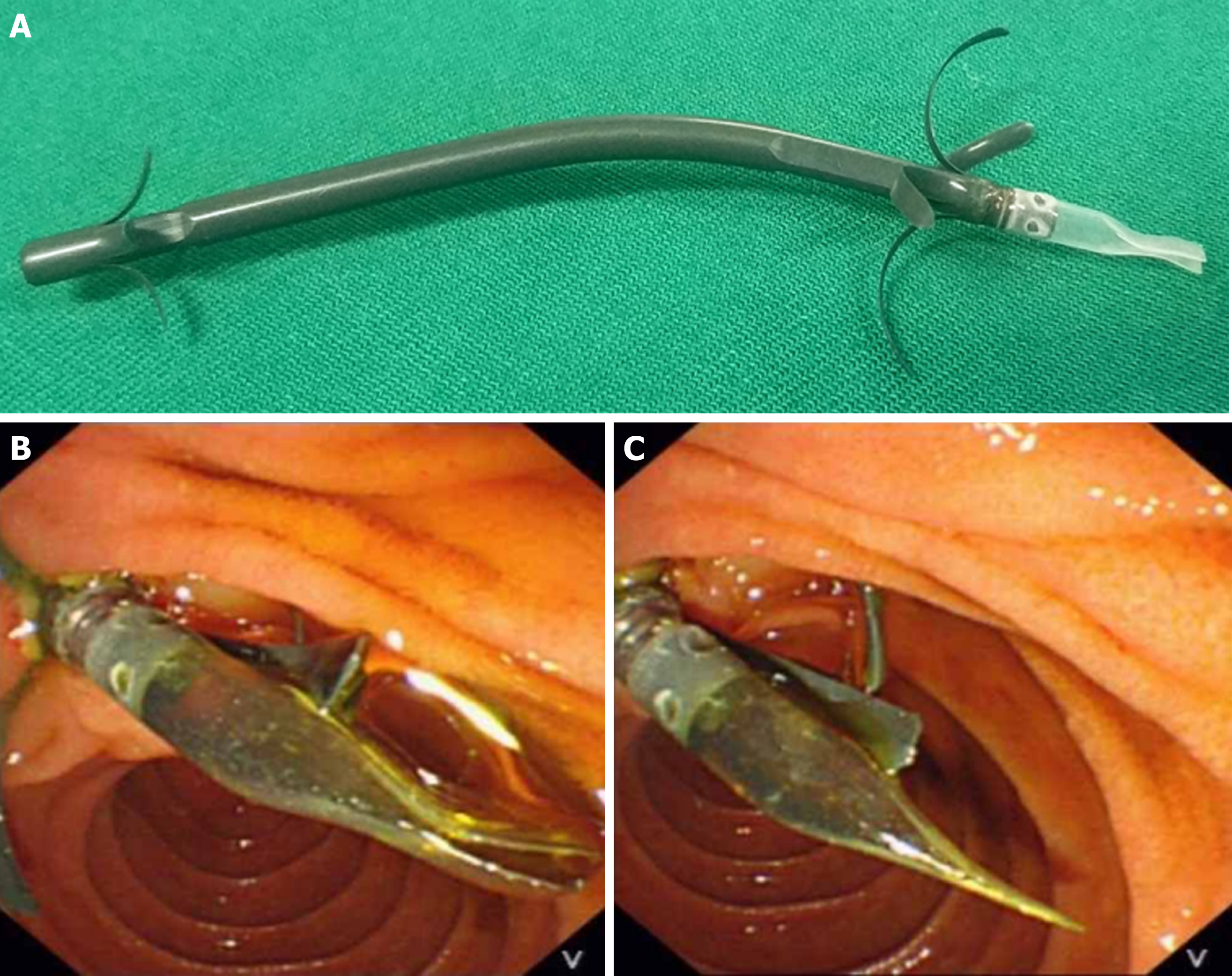
Endoscopic biliary stenting is a well-established palliative treatment for patients with unresectable distal malignant biliary obstruction (MBO). However, the main problem with stent placement is the relatively short duration of stent patency. Although self-expanding metal stents (SEMSs) have a longer patency period than plastic stents (PSs), the higher costs limit the wide use of SEMSs. A PS with an antireflux valve is an attractive idea to prolong stent patency, but no ideal design for an antireflux PS (ARPS) has been proposed. We developed a new ARPS with a “duckbilled” valve attached to the duodenal end of the stent.
To compare the patency of ARPSs with that of traditional PSs (TPSs) in patients with unresectable distal MBO.
We conducted a single-center, prospective, randomized, controlled, double-blind study. This study was conducted at the West China Hospital of Sichuan University. Consecutive patients with extrahepatic MBO were enrolled prospectively. Eligible patients were randomly assigned to receive either an ARPS or a TPS. Patients were followed by clinic visits or telephone interviews every 1-2 mo until stent exchange, death, or the final study follow-up in October 2018. The primary outcome was the duration of stent patency. Secondary outcomes included the rate of technical success, the rate of clinical success, adverse events, and patient survival.
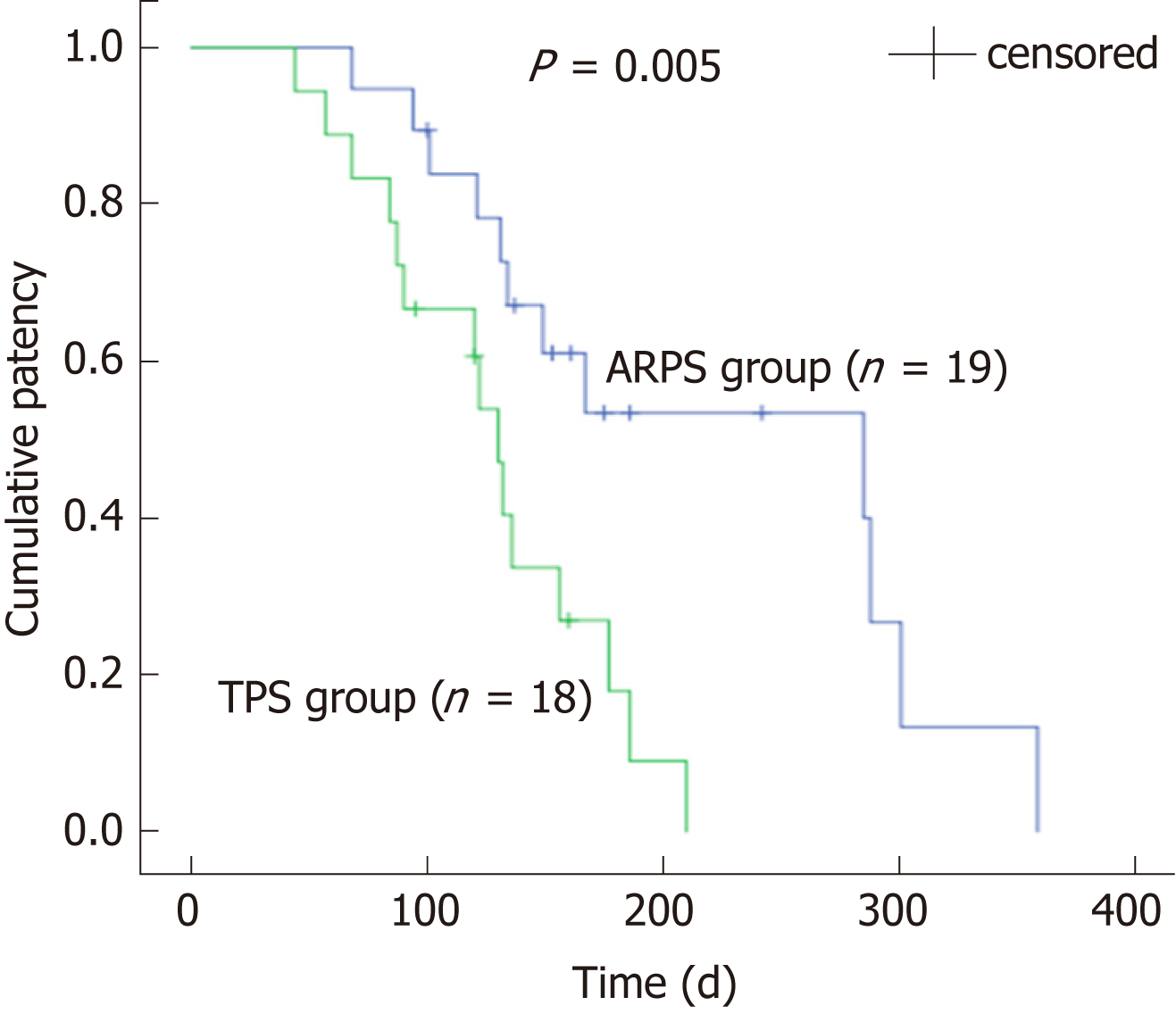
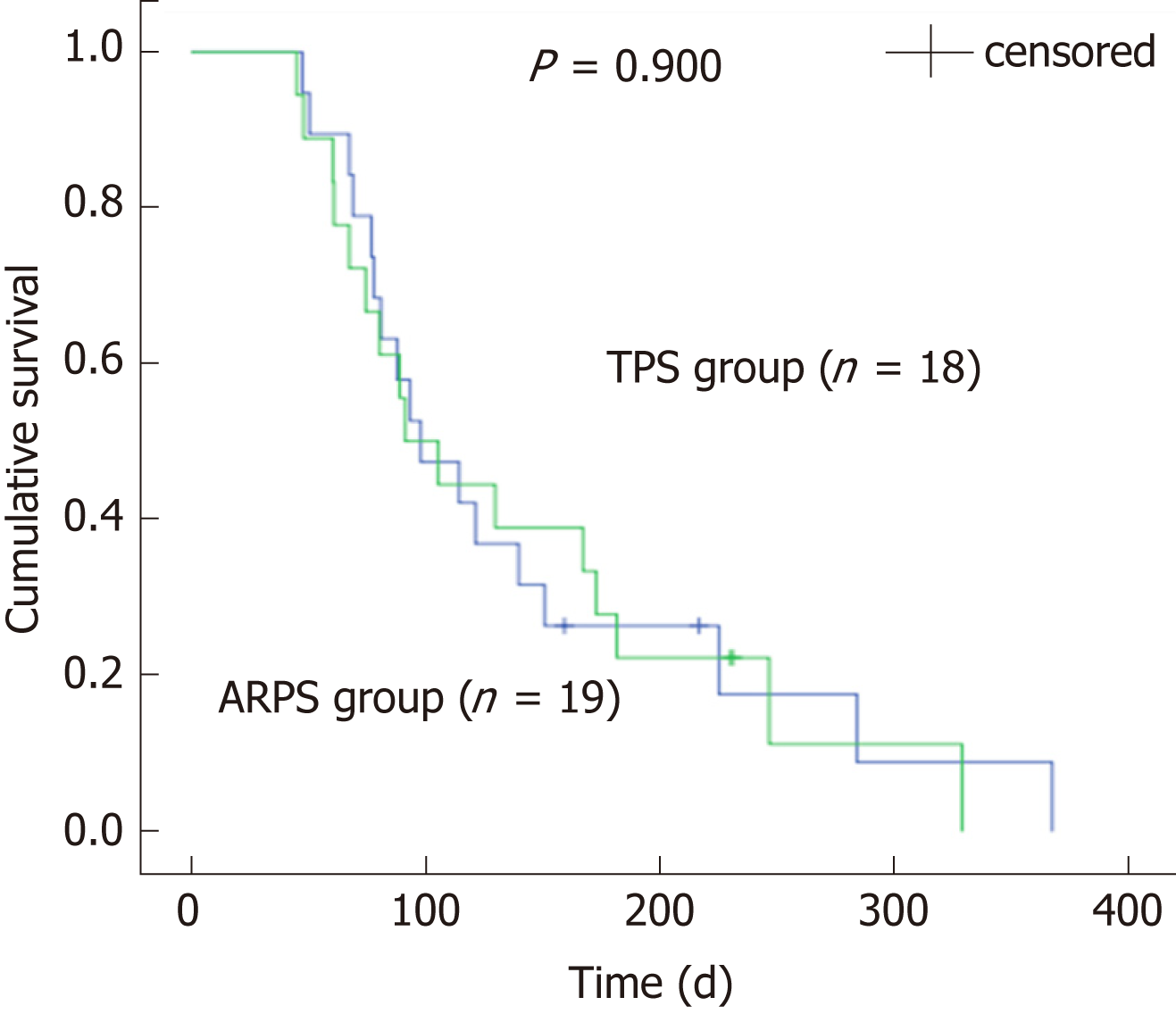
Between February 2016 and December 2017, 38 patients were randomly assigned to two groups, with 19 patients in each group, to receive ARPSs or TPSs. Stent insertion was technically successful in all patients. There were no significant differences between the two groups in the rates of clinical success or the rates of early or late adverse events (P = 0.660, 1.000, and 1.000, respectively). The median duration of stent patency in the ARPS group was 285 d [interquartile range (IQR), 170], which was significantly longer than that in the TPS group (median, 130 d; IQR, 90, P = 0.005). No significant difference in patient survival was noted between the two groups (P = 0.900).
The new ARPS is safe and effective for the palliation of unresectable distal MBO, and has a significantly longer stent patency than a TPS.
Core tip: There is no ideal design for an antireflux plastic stent for prolonging stent patency. In this study, a newly designed antireflux plastic stent with a “duckbilled” valve was successfully deployed in patients with unresectable distal malignant biliary obstruction. The median duration of stent patency in the antireflux plastic stent group was 285 d, which was significantly longer than that in the traditional plastic stent group (130 d).
- Citation: Yuan XL, Wei B, Ye LS, Wu CC, Tan QH, Yao MH, Zhang YH, Zeng XH, Li Y, Zhang YY, Hu B. New antireflux plastic stent for patients with distal malignant biliary obstruction. World J Gastroenterol 2019; 25(19): 2373-2382
- URL: https://www.wjgnet.com/1007-9327/full/v25/i19/2373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i19.2373
Distal malignant biliary obstruction (MBO) is mainly caused by cholangiocarcinoma, pancreatic cancer, and ampullary cancer. Since many of these tumors progress slowly and are usually detected at an advanced stage, curative surgical resection may not be feasible[1]. Endoscopic biliary stenting has become a well-established palliative treatment for patients with unresectable distal MBO[2,3]. However, the main problem with stent placement is the relatively short duration of stent patency[4,5]. Self-expanding metal stents (SEMSs) have longer patency periods than plastic stents (PSs). However, uncovered SEMSs are limited by their inability to be removed, and covered SEMSs are prone to migration[6-8]. Moreover, due to the problems of health insurance in China, the higher costs restrict the wide use of SEMSs[1]. PSs are still the main choice for patients in China because of their relatively low cost and easy replacement after stent dysfunction.
The actual mechanisms of PS occlusion remain largely unclear. Duodenobiliary reflux may be a major cause of stent occlusion[9,10]. In recent years, the design of PS with an antireflux valve at the duodenal end has been an attractive idea to eliminate retrograde flow from the duodenum, thereby prolonging stent patency. Some investigators have reported the effectiveness of these modified PSs[1,11,12], but no excellent results have been reported; thus, modified PSs have not been widely used in clinical practice. We developed a new antireflux PS (ARPS) with a “duckbilled” valve attached to the duodenal end of the stent (Figure 1). In this study, we aimed to compare the patency of this new ARPS with that of a traditional PS (TPS) in patients with unresectable distal MBO.
The study was a single-center, prospective, randomized, controlled, double-blind trial. The study protocol was approved by the China Ethics Committee of Registering Clinical Trials (Number: ChiECRCT-20150069; date of approval: December 13, 2015), and registered with the Chinese Clinical Trial Registry (Number: ChiCTR-IIR-16007869; date of registration: February 1, 2016). This study was conducted at West China Hospital of Sichuan University, a tertiary hospital. Informed consent for ARPS placement and use of clinical data was obtained from all patients involved in this study.
The ARPS (Micro-Tech (Nanjing) Co. Ltd., Nanjing, China) used in this study was made of polytetrafluoroethylene, the same material as a TPS, and had similar design (Tannenbaum design) as a TPS (Cook Ireland Ltd., Limerick, Ireland). The difference was that a 1.5 cm-long antireflux valve made of silicone rubber material was attached to the duodenal end of the ARPS. The bile flowed out when the valve was opened by increased common bile duct (CBD) pressure. Otherwise, the valve remained closed to prevent intestinal content regurgitation into the CBD when the duodenal pressure increased. The outer diameters of both types of stents were 10 Fr, and neither stent had any side holes. The length of both types of stents ranged from 5 cm to 9 cm, and the optimal length for each patient was determined by an endoscopist during the procedure.
Consecutive patients with extrahepatic MBO were prospectively enrolled. All patients were hospitalized for obstructive jaundice or elevated liver enzymes resulting from MBO. All of the patient lesions were surgically unresectable based on the stage of the tumors, the general condition of the patients, and consultations with the surgeons and anesthesiologists. Patients aged younger than 18 years old and those with a resectable tumor, hilar biliary stricture, or previous surgical drainage procedure were excluded. Patients with any contraindication to endoscopic procedures or who refused informed consent were also excluded.
Eligible patients were randomly assigned to receive either an ARPS or a TPS during the endoscopic procedure. Group allocation schemes generated randomly by a computer program at a ratio of 1:1 were placed into serially numbered sealed envelopes. After the biliary stricture was confirmed on cholangiography, an envelope was selected in sequence to determine the group allocation.
Patients were blinded to the stent assignment until a study endpoint was reached. Although blinding of the endoscopists was not possible, the endoscopists were not involved in the assessment of outcomes. The assessments were performed by reviewing physicians blinded to the randomization process. The data manager and statistician were not blinded.
All endoscopic retrograde cholangiopancreatography (ERCP) procedures were performed by one of four experienced endoscopists (≥300 ERCPs per year). Preoperative preparation was similar to that for general ERCPs. Prophylactic antibiotics and nonsteroidal anti-inflammatory drugs were not used before the procedure. All patients were placed in the prone position with conscious sedation, and a standard duodenoscope (TJF-260 V; Olympus Medical systems, Tokyo, Japan) was used. The endoscopist determined if sphincterotomy was necessary. Under the guidance of a guidewire (Jagwire; Boston Scientific, Natick, MA, United States), according to the group allocation, a single 10 Fr ARPS or TPS with an appropriate length was advanced into the bile duct approximately 1-2 cm above the proximal end of the stricture, leaving the distal end of the stent approximately 1 cm outside of the duodenal papilla. The flow of bile was confirmed before withdrawal of the duodenoscope (Video 1, Supplementary material).
Clinical evaluation and liver function tests were performed for all patients within one month after stent insertion. Subsequently, patients were followed by clinic visits or telephone interviews every 1-2 mo until stent exchange, death, or the final study follow-up period ended in October 2018. Patients lost to follow-up were excluded from the analysis.
The primary outcome was the duration of stent patency, which was recorded in days from stent placement to stent dysfunction requiring exchange. Stent dysfunction was considered present if recurrent obstructive jaundice and/or symptoms of cholangitis were observed along with biliary dilation on imaging studies or re-ERCP findings, and these abnormalities were resolved after insertion of a new stent. Secondary outcomes included the rate of technical success, the rate of clinical success, adverse events, and patient survival. Technical success was defined as successful insertion of the stent into the bile duct above the proximal end of the stricture and in an appropriate position based on fluoroscopic confirmation. Clinical success was defined as the resolution of obstructive symptoms and normalization of serum bilirubin within one month after stent placement. Adverse events were categorized as early (within 30 d) and late (after 30 d). Patient survival was measured as the duration from stent placement to death.
The sample size calculation was based on a previous study[11]. Under the assumption of a relative difference of 40% with an assumed standard deviation (SD) of 40 d in stent patency between the ARPS and TPS groups and an attrition rate of 10% for patients lost to follow-up, a sample size of 19 patients in each group would result in a power of 80% for a targeted significance level of 5% with a two-tailed test.
Continuous variables are characterized as the mean and SD or the median and interquartile range (IQR). Categorical variables are expressed as a frequency or proportion. Student’s t-test, Fisher’s exact test, Mann-Whitney U-test, and log-rank test were used whenever appropriate. Stent patency and patient survival were analyzed using the Kaplan-Meier method. A P-value < 0.05 was considered to be significant. Statistical analyses were performed using SPSS Statistics v. 23.0 (IBM Corp, Armonk, NY, United States).
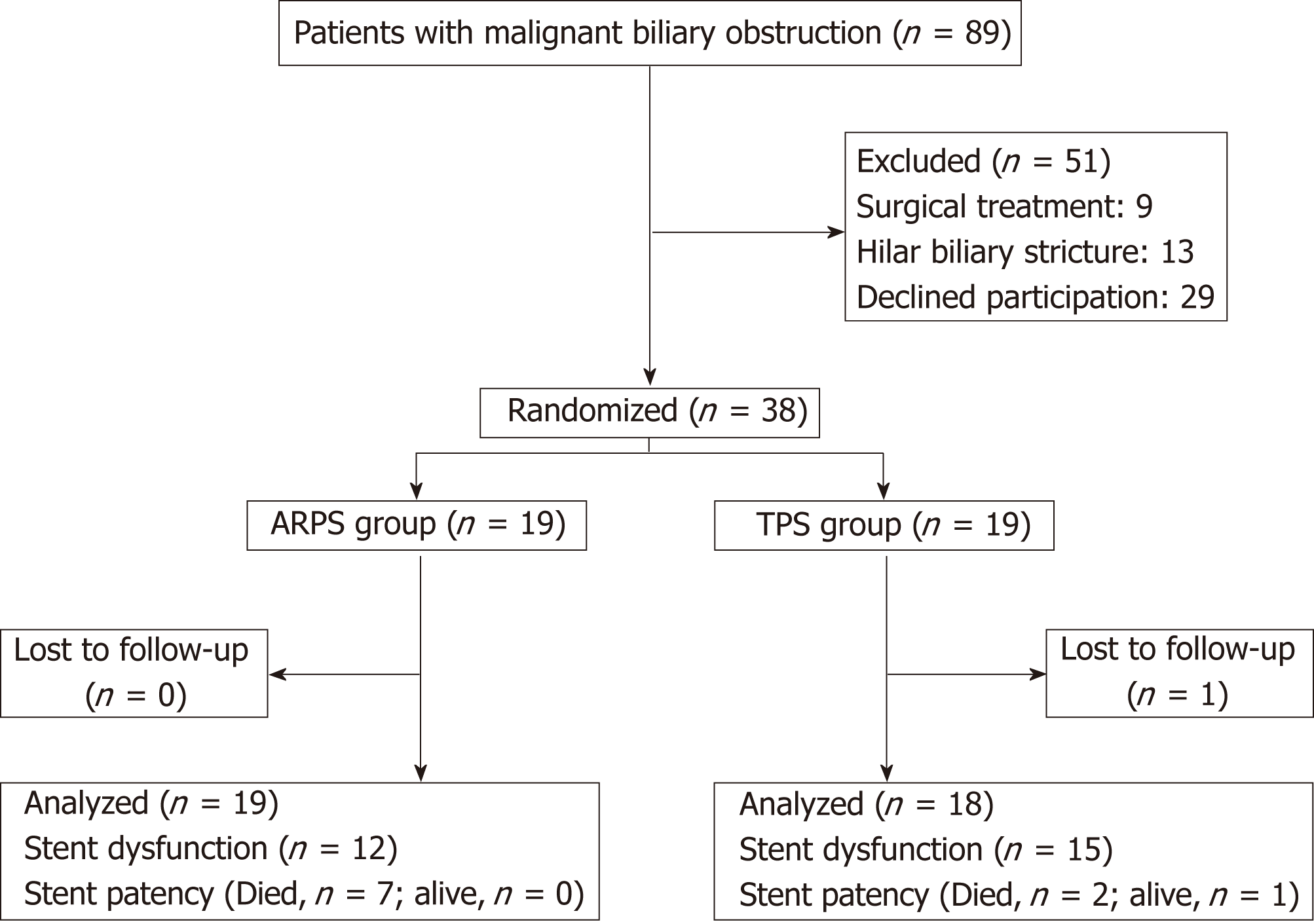
Between February 2016 and December 2017, 89 patients were screened for eligibility. Of these, 51 patients were excluded due to surgical treatment (n = 9), hilar biliary stricture (n = 13), and declined participation (n = 29). Finally, a total of 38 patients were randomized to receive an ARPS or a TPS (Figure 2). One patient in the TPS group was lost to follow-up after discharge. Thus, clinical information was available for 19 patients [mean (SD) age, 70.3 (13.1) years; male/female, 12/7] in the ARPS group and 18 patients [mean (SD) age, 73.8 (14.6) years; male/female, 14/4] in the TPS group. Table 1 shows the baseline patient characteristics and clinical information of the patients for each group. There was no significant difference between the two groups.
| ARPS group (n = 19) | TPS group (n = 18) | P-value | |
| Age, mean (SD), years | 70.3 (13.1) | 73.8 (14.6) | 0.452a |
| Sex, male/female, n | 12/7 | 14/4 | 0.476b |
| Diagnosis, n (%) | |||
| Pancreatic cancer | 9 (47.4) | 6 (33.3) | 0.753b |
| Cholangiocarcinoma | 7 (36.8) | 9 (50) | |
| Ampullary cancer | 3 (15.8) | 3 (16.7) | |
| Distant metastasis, n (%) | 9 (47.4) | 7 (38.9) | 0.743b |
| Comorbidity, n (%)d | 12 (63.2) | 14 (77.8) | 0.476b |
| Adjuvant therapy, n (%) | 6 (31.6) | 7 (38.9) | |
| Chemotherapy | 3 (50.0) | 2 (28.6) | 0.790b |
| Radiotherapy | 1 (16.7) | 3 (42.9) | |
| Radiochemotherapy | 2 (33.3) | 2 (28.6) | |
| Initial laboratory results | |||
| Total bilirubin, median (IQR), µmol/L | 234.6 (284.4) | 206.7 (215.9) | 0.761c |
| Direct bilirubin, median (IQR), µmol/L | 210.2 (232.6) | 191.30 (192.1) | 0.671c |
| Length of stricture, median (IQR), cm | 3 (2) | 3 (2) | 0.975c |
| Length of stent, median (IQR), cm | 7 (2) | 7 (3) | 0.585c |
| Sphincterotomy, yes, n (%) | 10 (52.6) | 5 (27.8) | 0.184b |
| Lost to follow–up, n (%) | 0 | 1 (5.6) | 1.000b |
Stent insertion was technically successful with a single attempt in 37 patients. Clinical success was achieved in 32 patients, and no significant difference was noted between the ARPS group (n = 17) and the TPS group (n = 15) (89.5% vs 83.3%, P = 0.660). Early adverse events were observed in four patients, including two cases of post-ERCP cholangitis and two cases of post-ERCP mild pancreatitis. Adverse events were all successfully controlled with conservative management. One patient in the ARPS group presented a late adverse event, mild pancreatitis, on day 94; she responded well to the conservative treatment. There were no significant differences in the rates of early or late adverse events between the two groups (Table 2).
| ARPS group(n = 19) | TPS group(n = 18) | P-value | |
| Technical success, n (%) | 19 (100) | 18 (100) | - |
| Clinical success, n (%) | 17 (89.5) | 15 (83.3) | 0.660a |
| Early adverse events, n (%) | 2 (10.5) | 2 (11.1) | |
| Cholangitis | 1 | 1 | 1.000a |
| Mild pancreatitis | 1 | 1 | |
| Late adverse event, n (%) | |||
| Mild pancreatitis | 1 (5.3) | 0 | 1.000a |
| Stent dysfunction, n (%) | 12 (63.2) | 15 (83.3) | 0.269a |
| Stent patency, median (IQR), d | 285 (170) | 130 (90) | 0.005b |
| Mortality, n (%) | 17 (89.5) | 16 (88.9) | 0.677a |
| Patient survival, median (IQR), d | 195 (297) | 182 (229) | 0.900b |
During the follow-up period, stent dysfunction was noted in 12 (63.2%) patients in the ARPS group and 15 (83.3%) patients in the TPS group. All dysfunctional stents were successfully removed endoscopically using a snare or biopsy forceps, and a new TPS or SEMS was inserted. Although there was no significant difference in the duration between stent placement and the occurrence of stent dysfunction between the two groups, a trend of later occurrence of stent dysfunction was observed in the ARPS group (median, 183 vs 119 d, P = 0.102). In the remaining patients, stent patency was maintained until death or the final study follow-up in October 2018. The median patency period in the ARPS group was 285 d (IQR, 170), which was significantly longer than that in the TPS group (median, 130 d; IQR, 90, P = 0.005) (Table 2, Figure 3). By the time of analysis, 33 patients had died, namely, 17 in the ARPS group (89.5%) with a median (IQR) survival time of 195 d (297) and 16 in the TPS group (88.9%) with a median (IQR) survival time of 182 d (229). There was no significant difference in patient survival (P = 0.900) (Table 2, Figure 4).
Although endoscopic placement of SEMS is considered the recommended treatment for palliative drainage of unresectable distal MBO[13,14], PSs were used more frequently than SEMSs in our center. The factors for our preference for PSs in patients with unresectable distal MBO are as follows: First, tumor ingrowth via metal mesh may result in the uncovered SEMSs being embedded into the bile duct wall, making them impossible to remove even if stent dysfunction occurs[6,7]. Second, covered SEMSs are prone to migration, leading to stent dysfunction[6-8]. Third, Sawas et al[15] observed that the incidence of ascending cholangitis was similar between patients with distal MBO who received TPSs and SEMSs. However, SEMSs have a larger lumen than TPSs, and duodenobiliary reflux and cholangitis are more likely to occur in patients treated with SEMSs[16]. Finally, the most important point is the higher costs of SEMSs due to health insurance problems in China. Thus, many patients choose TPSs, and many endoscopists also tend to insert TPSs in such patients, especially those with a life expectancy of shorter than 6 months[1]. The major problem with TPSs is the relatively short duration of stent patency; therefore, prolonging stent patency was the focus of the current study.
The exact mechanisms of TPS occlusion remain largely unclear. Previous studies[17-19] have indicated that the initial TPS occlusion event is caused by biofilm formation by the adherence of proteins and bacteria to the inner wall of the stent. Then, β−glucuronidase and phospholipase that are secreted by bacteria act on biliary components. Bacterial products, calcium bilirubinate, and calcium fatty acid soaps precipitate, leading to biliary sludge formation and stent occlusion. Several studies[20-23] have compared PSs of different materials or special coatings that may prevent bacterial adherence and biofilm formation. However, a discrepancy in the results between the in vitro and clinical studies was noted[19]. Although hydrophilic-coated stents or sliver-coated stents prevented biofilm formation on the surface of the stent, this ability was not be maintained for a long time duration[20,22,23]. Therefore, there was no definite conclusion on the superiority of one material or special coating over another in terms of stent patency.
Prior studies[9,10] have also revealed that large plant fibers refluxed from the duodenum have been found in occluded TPSs. This provided further evidence that duodenobiliary reflux may play another important role in stent occlusion. To our knowledge, only three studies[1,11,12] in the published English literature have focused on changing the design of PSs to eliminate retrograde flow from the duodenum, prolonging the duration of stent patency. Dua et al[11] in 2007 initially reported an ARPS with a 4 cm windsock-shaped tubular valve made of expanded polytetrafluoroethylene material attached to the duodenal end. Their results showed that the median stent patency was prolonged from 101 d to 145 d when using this specialized stent. However, the clinical relevance of an increase in median stent patency of 44 d could be questioned[24]. Vihervaara et al[12] also conducted a clinical study using the same ARPS; however, their study was prematurely terminated owing to early stent occlusion in the ARPS group. An unplanned interim analysis was performed and showed that the median stent patency in the ARPS group was 34 d, which was significantly shorter than that in the TPS group (167 d). Thus, they suggested that this ARPS should not be used in clinical practice. In a study by Leong et al[1], an ARPS with a collapsible antireflux sleeve made of polytetrafluoroethylene was analyzed; however, a trend of early ARPS malfunction or failure was noted. All their ARPSs were occluded within 30 d, which may be attributed to the collapse or fold of the antireflux valve.
To date, there is no ideal design for an ARPS. In this study, we developed a new ARPS with a “duckbilled” valve. We presumed that this valve could simulate the opening and closing function of a duck’s bill. When bile drainage does not impair the antegrade flow, the valve closes as the intestinal pressure increases, thereby preventing the reflux of the duodenal contents. This hypothesis was preliminarily confirmed by our study comparing stent patency between the ARPS group and the TPS group. In patients with stent dysfunction, the median stent patency in the ARPS group was 64 d longer than that in the TPS group. Although the difference was not statistically significant due to the limited sample size, the difference was impressive. In all patients enrolled in this study, the median patency of this new ARPS was 285 d, which was significantly longer than 130 d observed for the TPS. Moreover, the median patency was also better than previously reported median ARPS patency times[1,11,12]. There were no side holes in either ARPS or TPS; this avoided the possibility of duodenal contents entering the bile duct to bypass the valve. Malignant ingrowth rarely played a role in PS occlusion[10] and sludge was noted in occluded ARPSs; thus, in this study, ARPS dysfunction may have been due to sludge occlusion, but the exact causes were not clear. Further studies are needed to address the mechanisms of occlusion.
In the present study, a similar delivery system was used to deploy ARPSs or TPSs. Although ARPSs had a valve, there was no additional difficulty in placing such a stent. All patients presented technical success with a single placement attempt. After ARPS placement, bile flowed easily through the valve. No significant difference was noted in clinical success between the two groups, suggesting that the ARPS had good efficacy for palliation of jaundice caused by extrahepatic MBO. Some studies[11,16] have reported that duodenal contents may enter the bile duct from the side of the stent in patients with sphincterotomy; thus, no routine sphincterotomy was performed in these studies. In our experience, the placement of a 10 Fr stent in some patients without sphincterotomy is technically challenging; therefore, in this study, the endoscopist determined if sphincterotomy was necessary. No significant difference was noted between the two groups.
There were several limitations to our study. The main limitation was the small sample size. The calculation of sample size was based on a previous study. Although the sample size was small, the patency of ARPS was significantly longer than that of TPS. Another limitation was that microscopic examination of dysfunctional stents was not performed, and the exact causes of ARPS dysfunction were unclear. In addition, many cases were censored due to patient death without stent dysfunction, or patient survival with stent patency until the day of last follow-up. The accurate duration of stent patency might be underestimated. Although this new stent showed good and promising results in this study, further studies with larger samples are required to evaluate its safety and efficacy.
In conclusion, this new ARPS is safe and effective for the palliation of unresectable distal MBO, and has the potential advantage of prolonging stent patency markedly. Additional multicenter studies involving larger samples are needed to confirm and strengthen our results.
Endoscopic biliary stenting has become an established palliative treatment for patients with unresectable malignant biliary obstruction (MBO). However, stent occlusion is considered to be the most frequent delayed adverse event of stent placement. Since duodenobiliary reflux is discussed to be a major risk factor of stent occlusion, in recent years, the design of plastic stents with an anti-reflux valve has been an attractive idea for prolonging stent patency, unfortunately without convincing results and therefore limiting their use in clinical practice.
To reduce duodenobiliary reflux and thereby prolonging stent patency, we developed a new antireflux plastic stent (ARPS) with a “duckbilled” valve attached to the duodenal end of the stent. We presumed that this valve could simulate the opening and closing function of the duck’s bill. This geometry allows unimpaired antegrade bile flow into the duodenum, while it closes instantly when the intestinal pressure increases, thereby preventing the reflux of duodenal contents.
In this study, we compared the patency of ARPSs with that of traditional plastic stents (TPSs) in patients with unresectable distal MBO. The results of the study will guide the treatment of unresectable distal MBO in the future.
From February 2016 to December 2017, consecutive patients with extrahepatic MBO were recruited in our randomized clinical trial. Eligible patients were assigned to receive either an ARPS or a TPS in a randomized manner. Patients were followed by clinic visits or telephone interviews every 1-2 mo until stent exchange, death, or the final study follow-up in October 2018. The duration of stent patency, the rates of technical and clinical success, adverse events, and patient survival were documented. All data were analyzed statistically to evaluate the efficacy and safety of this new ARPS.
During the study period, 89 patients were screened for eligibility. Of these, 51 patients were excluded; thus, 38 patients were randomized to receive ARPSs or TPSs (19 per group). Stent insertion was technically successful in all patients. No significant differences were noted in the rates of clinical success, the rates of early or late adverse events, or patient survival. There was a significant difference when comparing the duration of stent patency, which was significantly longer in the ARPS group than in the TPS group.
This new ARPS is safe and effective for the palliation of unresectable distal MBO, and has a significantly longer stent patency compared with TPS. This ARPS may be an alternative option for the treatment of unresectable distal MBO.
Multiple center studies with larger samples are expected in the future to confirm and strengthen our results.
The abstract of this article has been accepted for a lecture presentation at Digestive Disease Week®, held in San Diego, California, USA, May 18-21, 2019.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hara K, Hillman LC S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Leong QW, Shen ML, Au KW, Luo D, Lau JY, Wu JC, Chan FK, Sung JJ. A prospective, randomized study of the patency period of the plastic antireflux biliary stent: An interim analysis. Gastrointest Endosc. 2016;83:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Andersen JR, Sørensen SM, Kruse A, Rokkjaer M, Matzen P. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30:1132-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 393] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 554] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Tringali A, Mutignani M, Perri V, Zuccalà G, Cipolletta L, Bianco MA, Rotondano G, Philipper M, Schumacher B, Neuhaus H, Schmit A, Devière J, Costamagna G. A prospective, randomized multicenter trial comparing DoubleLayer and polyethylene stents for malignant distal common bile duct strictures. Endoscopy. 2003;35:992-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321-327.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Chen MY, Lin JW, Zhu HP, Zhang B, Jiang GY, Yan PJ, Cai XJ. Covered Stents versus Uncovered Stents for Unresectable Malignant Biliary Strictures: A Meta-Analysis. Biomed Res Int. 2016;2016:6408067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Jang S, Stevens T, Parsi M, Lopez R, Zuccaro G, Dumot J, Vargo JJ. Association of covered metallic stents with cholecystitis and stent migration in malignant biliary stricture. Gastrointest Endosc. 2018;87:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | van Berkel AM, van Marle J, Groen AK, Bruno MJ. Mechanisms of biliary stent clogging: Confocal laser scanning and scanning electron microscopy. Endoscopy. 2005;37:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Weickert U, Venzke T, König J, Janssen J, Remberger K, Greiner L. Why do bilioduodenal plastic stents become occluded? A clinical and pathological investigation on 100 consecutive patients. Endoscopy. 2001;33:786-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Dua KS, Reddy ND, Rao VG, Banerjee R, Medda B, Lang I. Impact of reducing duodenobiliary reflux on biliary stent patency: An in vitro evaluation and a prospective randomized clinical trial that used a biliary stent with an antireflux valve. Gastrointest Endosc. 2007;65:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Vihervaara H, Grönroos JM, Hurme S, Gullichsen R, Salminen P. Antireflux Versus Conventional Plastic Stent in Malignant Biliary Obstruction: A Prospective Randomized Study. J Laparoendosc Adv Surg Tech A. 2017;27:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Dumonceau JM, Tringali A, Blero D, Devière J, Laugiers R, Heresbach D, Costamagna G; European Society of Gastrointestinal Endoscopy. Biliary stenting: Indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 483] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 15. | Sawas T, Al Halabi S, Parsi MA, Vargo JJ. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: A meta-analysis. Gastrointest Endosc. 2015;82:256-267.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 16. | Hu B, Wang TT, Wu J, Shi ZM, Gao DJ, Pan YM. Antireflux stents to reduce the risk of cholangitis in patients with malignant biliary strictures: A randomized trial. Endoscopy. 2014;46:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Leung JW, Ling TK, Kung JL, Vallance-Owen J. The role of bacteria in the blockage of biliary stents. Gastrointest Endosc. 1988;34:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Coene PP, Groen AK, Cheng J, Out MM, Tytgat GN, Huibregtse K. Clogging of biliary endoprostheses: A new perspective. Gut. 1990;31:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Kwon CI, Gromski MA, Sherman S, Easler JJ, El Hajj II, Watkins J, Fogel EL, McHenry L, Lehman GA. Time Sequence Evaluation of Biliary Stent Occlusion by Dissection Analysis of Retrieved Stents. Dig Dis Sci. 2016;61:2426-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | van Berkel AM, Bruno MJ, Bergman JJ, van Deventer SJ, Tytgat GN, Huibregtse K. A prospective randomized study of hydrophilic polymer-coated polyurethane versus polyethylene stents in distal malignant biliary obstruction. Endoscopy. 2003;35:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Costamagna G, Mutignani M, Rotondano G, Cipolletta L, Ghezzo L, Foco A, Zambelli A. Hydrophilic hydromer-coated polyurethane stents versus uncoated stents in malignant biliary obstruction: A randomized trial. Gastrointest Endosc. 2000;51:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Yamabe A, Irisawa A, Wada I, Shibukawa G, Fujisawa M, Sato A, Igarashi R, Maki T, Hoshi K. Application of a silver coating on plastic biliary stents to prevent biofilm formation: An experimental study using electron microscopy. Endosc Int Open. 2016;4:E1090-E1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Jansen B, Goodman LP, Ruiten D. Bacterial adherence to hydrophilic polymer-coated polyurethane stents. Gastrointest Endosc. 1993;39:670-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Kahaleh M. Antireflux biliary stents: Is it time to go with the flow? Gastrointest Endosc. 2007;65:829-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |