Published online May 21, 2019. doi: 10.3748/wjg.v25.i19.2338
Peer-review started: March 14, 2019
First decision: March 27, 2019
Revised: April 17, 2019
Accepted: April 29, 2019
Article in press: April 29, 2019
Published online: May 21, 2019
Processing time: 67 Days and 5.5 Hours
Owing to the technical difficulty of pathological diagnosis, imaging is still the most commonly used method for clinical diagnosis of para-aortic lymph node metastasis (PALM) and evaluation of therapeutic effects in gastric cancer, which leads to inevitable false-positive findings in imaging. Patients with clinical PALM may have entirely different pathological stages (stage IV or not), which require completely different treatment strategies. There is no consensus on whether surgical intervention should be implemented for this group of patients. In particular, the value of D2 gastrectomy in a multidisciplinary treatment (MDT) approach for advanced gastric cancer with clinical PALM remains unknown.
To investigate the value of D2 gastrectomy in a MDT approach for gastric cancer patients with clinical PALM.
In this real-world study, clinico-pathological data of all gastric cancer patients treated at the Cancer Hospital, Chinese Academy of Medical Sciences between 2011 and 2016 were reviewed to identify those with clinically enlarged PALM. All the clinico-pathological data were prospectively documented in the patient medical record. For all the gastric cancer patients with advanced stage disease, especially those with suspicious distant metastasis, the treatment methods were determined by a multidisciplinary team.
In total, 48 of 7077 primary gastric cancer patients were diagnosed as having clinical PALM without other distant metastases. All 48 patients received chemotherapy as the initial treatment. Complete or partial response was observed in 39.6% (19/48) of patients in overall and 52.1% (25/48) of patients in the primary tumor. Complete response of PALM was observed in 50.0% (24/48) of patients. After chemotherapy, 45.8% (22/48) of patients received D2 gastrectomy, and 12.5% (6/48) of patients received additional radiotherapy. The postoperative major complication rate and mortality were 27.3% (6/22) and 4.5% (1/22), respectively. The median overall survival and progression-free survival of all the patients were 18.9 and 12.1 mo, respectively. The median overall survival of patients who underwent surgical resection or not was 50.7 and 12.8 mo, respectively. The 3-year and 5-year survival rates were 56.8% and 47.3%, respectively, for patients who underwent D2 resection. Limited PALM and complete response of PALM after chemotherapy were identified as favorable factors for D2 gastrectomy.
For gastric cancer patients with radiologically suspicious PALM that responds well to chemotherapy, D2 gastrectomy could be a safe and effective treatment and should be adopted in a MDT approach for gastric cancer with clinical PALM.
Core tip: The value of surgical resection in gastric cancer with radiologically overt para-aortic lymph node metastasis (PALM) is still not clear. Current controversial issues include the extent of resection (D1, D2, D2 + para-aortic lymph node metastasis dissection, or D3), surgical timing, and identification of optimal surgical candidates. This study confirmed the benefit of D2 gastrectomy after chemotherapy in select patients. Limited PALM at baseline and complete response of PALM after chemotherapy were proposed as criteria for selecting patients who will potentially benefit from D2 gastrectomy, which should be useful for future clinical trials.
- Citation: Zheng XH, Zhang W, Yang L, Du CX, Li N, Xing GS, Tian YT, Xie YB. Role of D2 gastrectomy in gastric cancer with clinical para-aortic lymph node metastasis. World J Gastroenterol 2019; 25(19): 2338-2353
- URL: https://www.wjgnet.com/1007-9327/full/v25/i19/2338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i19.2338
Gastric cancer is the fifth most common cancer and the third leading cause of mortality among all cancers worldwide. Gastric cancer with para-aortic lymph node metastasis (PALM) is considered a metastatic disease, and its prognosis remains poor after isolated surgical treatment. However, pathological diagnosis of enlarged para-aortic lymph nodes (PAN) is difficult. Certain methods, such as endoscopic ultrasound, B-ultrasound, or computed tomography (CT) guided fine needle aspiration, are theoretically feasible for pathological diagnosis of suspicious PALM. PAN biopsy is an invasive and technically difficult manipulation and thus is not typically used for clinical diagnosis of PALM in most institutes. In addition, positive lymph nodes will disappear or shrink after preoperative treatment, which makes it difficult to re-biopsy the original nodes during follow-up. Despite the inevitable false-positive findings, imaging is still the most commonly used noninvasive method for clinical diagnosis of PALM and preoperative evaluation of therapeutic effects.
However, due to the fact that suspicious lymph node enlargement can be the result of inflammatory lymphadenopathy or malignancy, patients with radiologically overt PALM may have entirely different pathological stages (stage IV or not), which will require completely different treatment strategies. And the best clinical practice for patients with clinical PALM remains controversial for over ten years. Early this century, Sasako et al[1] conducted prophylactic D3 resection in advanced stage gastric cancer patients without radiologically overt PALM, and according to their results published in 2008, extended resection is not necessary. At the same time, through retrospective studies, other researchers have shown that D2 gastrectomy plus para-aortic lymph node dissection (PAND) might result in satisfactory outcomes in a highly select group of patients with PAN enlargement. Results reported by Tokunaga et al[2] and Roviello et al[3] in 2010 further complicate this issue. Both studies showed that even after extended D3 resection, the 5-year survival rates of patients with pathologically positive PAN were as low as 13.0% and 17.0%, respectively, not to mention the extremely high complication rate. Moreover, the phase III clinical trial REGATTA, in which patients with clinical PALM were enrolled, showed that chemotherapy alone was better than D1 gastrectomy followed by chemotherapy[4]. The above studies indicate that D1, D2 plus PAND, or D3 with adjuvant chemotherapy all failed to prolong the survival of patients with pathological PALM.
Recently, as preoperative chemotherapy was adopted into studies, Japanese oncologists reported an encouraging 5-year survival rate of 53% in gastric cancer with PALM treated by D2 gastrectomy with PAND after neoadjuvant chemotherapy. However, developing a safe and standard D2 plus PAND protocol after chemotherapy was challenging, and to date, only a few surgeons worldwide can perform it expertly. In addition, only 10% of patients who underwent D2 plus PAND had a pathologically positive PAN. Therefore, whether their method is the best solution for radiologically evident PALM is up for debate. Wang et al[5] considered patients with a good response to chemotherapy and PAN shrinkage to < 1.0 cm for D2 gastrectomy without PAND, and the surgery group had a non-inferior outcome compared with the Japanese results. More recently, several small studies have also reported improved survival through resection without metastasectomy after conversional chemotherapy. These results indicate that extensive resection might not be the only way to improve prognosis and D2 gastrectomy can provide a choice for select patients[6,7].
In our center, management of suspicious stage IV gastric cancer is determined by a multidisciplinary team. After conversional chemotherapy, the subsequent treatment method for patients with enlarged PAN prior to treatment is decided according to the response to chemotherapy. However, D3 or D2 resection plus PAND is not routinely recommended due to high morbidity and mortality. For those with enlarged PALM that cannot be controlled by chemotherapy, additional radiotherapy is recommended. In this study, we sought to determine the value of D2 gastrectomy in a multidisciplinary treatment approach for patients with clinical PALM based on data from this single center.
In total, 7077 patients were diagnosed with gastric adenocarcinoma at the Cancer Hospital, Chinese Academy of Medical Sciences, from January 2011 to December 2016. We searched the clinico-pathological database for primary gastric adenocarcinoma patients with suspiciously enlarged lymph nodes in the para-aortic region documented in medical records prospectively. The inclusion criteria for this study were as follows: pathologically confirmed gastric adenocarcinoma with PAN enlargement; clinical T3-4 disease; no evidence of concurrent metastasis other than that in PAN, including distant hematogenous metastasis, distant lymph node metastasis, peritoneal metastasis and so on; esophageal invasion less than 3 cm; ECOG performance status of 0 or 1; sufficient oral intake and adequate organ function according to records at first visit; no previous malignancies; and pathologically confirmed HER2-negative gastric adenocarcinoma. In addition, patients who underwent reduction surgery or had positive lavage cytology were excluded, while palliative surgery to address severe uncontrollable complications during chemotherapy was allowed. This retrospective study was approved by the Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences, and the need for informed consent was waived.
Contrast-enhanced thoracic/abdominal/pelvic CT, upper gastrointestinal tract endoscopy, and endoscopic ultrasonography (EUS) with or without positron emission tomography and CT (PET-CT) were conducted as the pretreatment workup. Both the clinical tumor stage (cT) and the clinical nodal stage (cN) were diagnosed via EUS and enhanced CT. Classification of TNM stage was defined according to the 8th edition of the American Joint Committee on Cancer Staging Manual. The clinical stage was evaluated by a multidisciplinary team based on all the radiological results.
The major criterion for clinical positive nodes on CT and EUS was solitary nodes ≥ 8 mm in minor diameter. The supplementary criteria for clinical PALM on EUS were as follows: echo-poor, roundish, or well-demarcated nodes. The supplementary criteria for clinical PALM on CT were as follows: Marked enhancement in the portal venous phase; cluster nodes regardless of the enhancement pattern; certain metastasis-associated enhancement patterns, such as central necrosis and heterogeneous enhancement; and highly clinically suspicious lymph nodes that did not satisfy the above criteria. The nodal size and anatomic location (station numbers) of all the suspicious lymph nodes were recorded. The lymph node station was classified using the fifteenth edition of the Japanese Classification of Gastric Carcinoma.
The chemotherapy regimens for this cohort of patients included S-1 plus oxaliplatin (SOX), docetaxel/oxaliplatin/S-1 (DOS), docetaxel/capecitabine/oxaliplatin (DOX), docetaxel/cisplatin/S-1 (DCS), capecitabine and oxaliplatin (XELOX), S-1 monotherapy, paclitaxel monotherapy, 5-fluorouracil (5-FU)/leucovorin (LV)/oxaliplatin (FOLFOX), irinotecan/5-FU/LV/oxaliplatin (FOLFOXIRI), and taxane/oxaliplatin.
Patients began receiving four cycles of adjuvant chemotherapy within 45 d after D2 gastrectomy, under the same regimen used preoperatively. For patients who were not suitable or unwilling to receive surgical resection, chemotherapy was continued. Second-line chemotherapy was administered when disease progression or recurrence was observed. Radiotherapy was not routinely recommended by the multidisciplinary team unless the presence of acute symptoms indicated a need for radiotherapy during chemotherapy or patients had an incomplete response (CR) of PALM after perioperative chemotherapy.
All the enrolled patients were treated with chemotherapy initially and then subjected to CT after every two cycles of chemotherapy for the first six cycles and every 2 mo thereafter. Patients were reevaluated by the multidisciplinary team, and after evaluation, D2 gastrectomy was recommended to patients who had responded well to treatment. Clinical response was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and the response of the primary tumor was assessed according to the fifteenth edition of the Japanese Classification of Gastric Carcinoma[8,9]. After chemotherapy, PAN disappearance or shrinkage to < 8 mm on CT was regarded as CR of PALM. Unless otherwise specified, all the diameters in this study refer to the short-axis diameter. The largest PAN was recorded as the index node, and the index nodes in the short axis is recorded as the index diameter. If all the enlarged lymph nodes disappeared in imaging, the index diameter was documented as a default value (5 mm) according to the RECIST 1.1. Two experienced radiologists were asked to evaluate the CT scans to document the overall response, response of the primary tumor, and the metastatic sites. Adverse events were assessed according to the National Cancer Institute's Common Terminology Criteria for Adverse Events v 4.0.
All the patients were followed via contrast-enhanced thoracic/abdominal/pelvic CT and blood testing every 3 mo for the first 3 years and every 6 mo thereafter.
Exploration and lavage cytology examination were carried out to exclude patients with other non-curable factors before gastrectomy. Distal, proximal, or total gastrectomy with D2 dissection was performed based on the tumor location. The PAN were not removed intentionally. The pathological response grading was based on the Mandard tumor grading system (TRG). Tumor staging and dissection range were in accordance with the eighth edition of the AJCC Cancer Staging Manual[10]. Postoperative complications were recorded according to the Clavien-Dindo classification.
The primary outcome was overall survival (OS, survival time from diagnosis to death from any cause), and the secondary outcome was progression-free survival (PFS, time from diagnosis to disease progression). Categorical data are presented as absolute and relative frequencies calculated using a chi-square test. Differences were determined by a Wilcoxon rank-sum test for non-normally distributed continuous variable (the short axis diameter of lymph nodes). We constructed violin plots of index diameter to analyze the index diameter distribution according to clinical factors. The Kaplan-Meier method was used to generate survival curves. All statistical tests were two-sided, and a P-value less than 0.05 was considered statistically significant. Analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, United States).
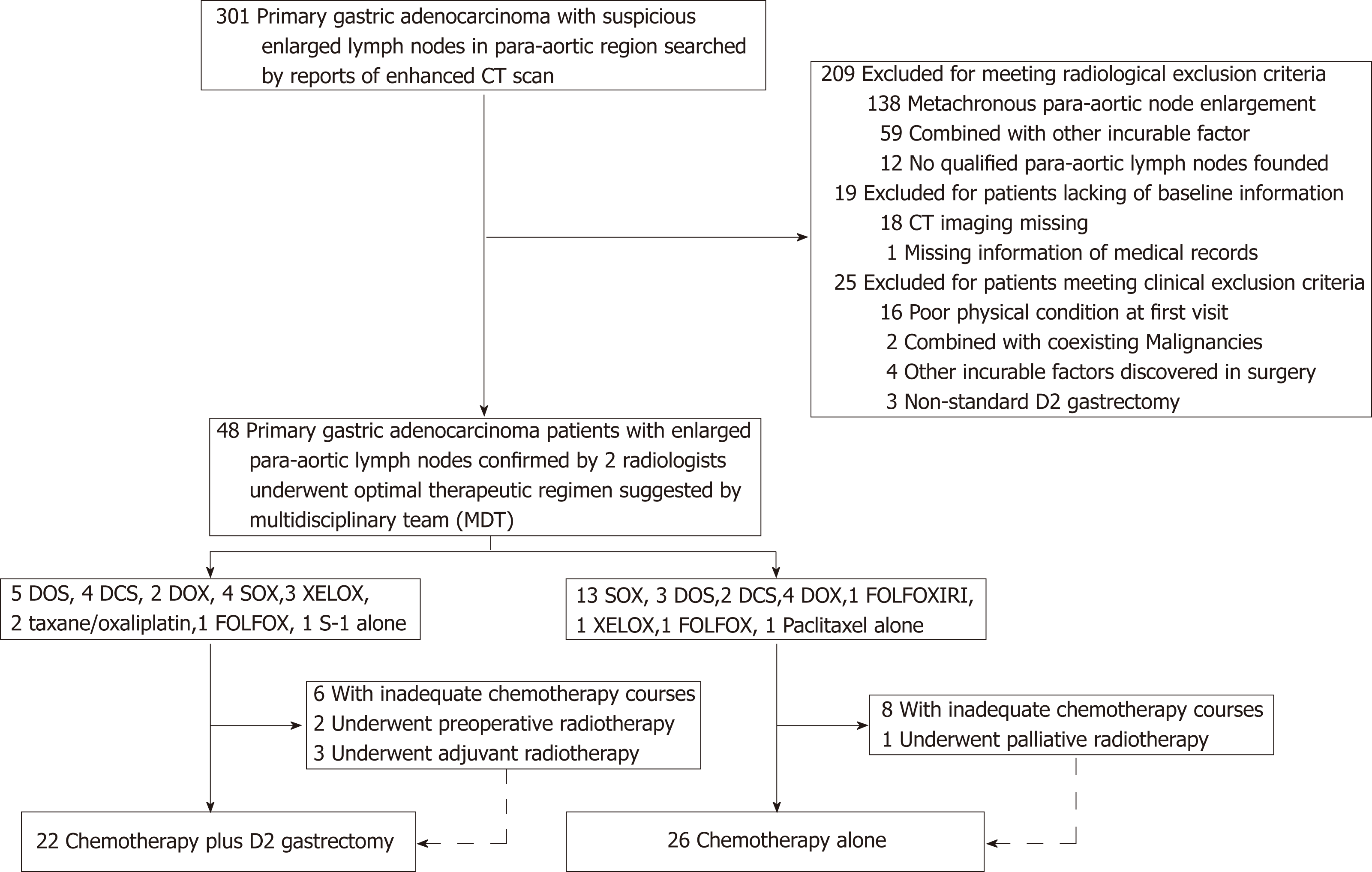
Between January 2011 and December 2016, 301 of 7077 gastric cancer patients were identified with PALM based on their medical history and were reevaluated by radiologists (Figure 1). A total of 209 patients were excluded because of a lack of concurrent PALM as the single non-curable factor. In addition, 19 patients with incomplete baseline information and 25 patients incompatible with the clinical inclusion criteria were also excluded. Finally, 48 patients with PALM as the single non-curable factor were included in this real-world study (Figure 1). Baseline information is shown in Table 1. The mean age at diagnosis was 57.2 years (range, 27-76 years), and male patients comprised the majority (81.3%). The common characteristics of the patients with radiological PAN enlargement were poor tumor differentiation and late tumor and nodal stage. In addition, major clinico-pathological characteristics were not significantly different between patients receiving or not receiving D2 gastrectomy.
| Variable | ≥ 60 yr old | < 60 yr old |
| Gender | ||
| Male | 21 (91.3) | 18 (72.0) |
| Female | 2 (8.7) | 7 (28.0) |
| Tumor location | ||
| Lower | 2 (8.7) | 4 (16.0) |
| Middle | 9 (39.1) | 12 (48.0) |
| Upper | 12 (52.2) | 9 (36.0) |
| Clinical tumor stage | ||
| T4 | 22 (95.7) | 23 (92.0) |
| T3 | 1 (4.3) | 2 (8.0) |
| Clinical nodal stage | ||
| N2-3 | 18 (78.3) | 21 (84.0) |
| N0-1 | 5 (21.7) | 4 (16.0) |
| Macroscopic type | ||
| 4 | 4 (17.4) | 3 (12.0) |
| 1-3 or 5 | 19 (82.6) | 22 (88.0) |
| Differentiation | ||
| Poorly differentiated | 18 (78.3) | 23 (92.0) |
| Well differentiated | 5 (21.7) | 2 (8.0) |
| Performance status | ||
| 0 | 6 (26.1) | 11 (44.0) |
| 1 | 17 (73.9) | 14 (56.0) |
Of the 48 patients included, 17 were treated with SOX, 8 with DOS, 6 with DOX, 6 with DCS, 4 with XELOX, 2 with FOLFOX, 2 with taxane/oxaliplatin, and 3 with other regimens (S-1, paclitaxel monotherapy, or FOLFOXIRI). Among the 22 patients who underwent D2 gastrectomy after perioperative chemotherapy, 5 received DOS, 4 received SOX, 4 received DCS, 3 received XELOX, 2 received DOX, 2 received taxane/oxaliplatin, 1 received FOLFOX, and 1 received S-1 monotherapy. Following resection, 18 patients received adjuvant chemotherapy using the same regimen that was used preoperatively, and the other 4 patients did not receive adjuvant chemotherapy. Respectively, 6 and 8 patients among the patients who underwent D2 gastrectomy or not received less than six cycles of chemotherapy in total (Figure 1).
Adverse events associated with chemotherapy are listed in Table 2. The most frequent adverse events were anorexia (68.8%) and nausea (68.8%), most of which occurred at grade 1 or 2. Neutropenia was observed, with the most frequent adverse events being grade 3 or higher. One treatment-related death was reported in a patient who died of acute pulmonary embolism during the first cycle of initial chemotherapy.
| Grade | Total | Grade ≥ 3 (%) | |||||
| Toxicity | 1 | 2 | 3 | 4 | 5 | ||
| Diarrhea | 4 | 2 | 2 | 0 | 0 | 8 | 4.2 |
| Malaise | 9 | 1 | 0 | 0 | 0 | 10 | 0.0 |
| Anorexia | 22 | 10 | 1 | 0 | 0 | 33 | 2.1 |
| Nausea | 20 | 11 | 2 | 0 | 0 | 33 | 4.2 |
| Vomiting | 6 | 4 | 0 | 0 | 0 | 10 | 0.0 |
| Peripheral sensory neuropathy | 13 | 4 | 0 | 0 | 0 | 17 | 0.0 |
| Rash | 1 | 0 | 1 | 0 | 0 | 2 | 2.1 |
| Thromboembolic event | 0 | 0 | 0 | 0 | 1 | 1 | 2.1 |
| Anemia | 11 | 2 | 3 | 0 | 0 | 16 | 6.3 |
| Thrombocytopenia | 7 | 6 | 4 | 1 | 0 | 18 | 10.4 |
| Leukopenia | 11 | 14 | 3 | 1 | 0 | 29 | 8.3 |
| Neutropenia | 7 | 5 | 9 | 4 | 0 | 25 | 27.1 |
| Febrile neutropenia | 4 | 0 | 0 | 0 | 0 | 4 | 0.0 |
Details related to lymph nodes at the first visit and at the time of best response during chemotherapy are listed in Table 3. The most common PAN station was No. 16b1 in 34 of 48 patients, followed by No. 16a2 (24/48). Overall, 27.1% (13/48) of patients had more than two para-aortic node stations involved. According to the RECIST 1.1 criteria, 26 patients had target lesions at baseline, while the other 22 patients had non-target lesions. The objective overall response rate in this group was 39.6% (19 of 48, Table 3). Response of the primary tumor was observed in 25 (52.1%) patients, and CR of metastatic sites was observed in 24 (50.0%) patients.
| Variable | No. of patients (%) |
| At baselinePAN station involved number | |
| 1-2 | 35 (72.9) |
| 3-4 | 13 (27.1) |
| PAN station involved | |
| n16a1 | 8 (16.7) |
| n16a2 | 24 (50.0) |
| n16b1 | 34 (70.8) |
| n16b2 | 9 (18.8) |
| Clinical response after chemotherapy | |
| Overall (RECIST) | |
| Target lesions | |
| CR | 2 (4.2) |
| PR | 16 (33.3) |
| SD | 6 (12.5) |
| PD | 2 (4.2) |
| Non-target lesions only | |
| CR | 1 (2.1) |
| Non-CR/Non-PD | 19 (39.6) |
| PD | 2 (4.2) |
| Primary lesions (JGCA) | |
| CR | 3 (6.3) |
| PR | 22 (45.8) |
| SD | 19 (39.6) |
| PD | 4 (8.3) |
| Metastatic lesions | |
| CR | 24 (50.0) |
| Non-CR | 24 (50.0) |
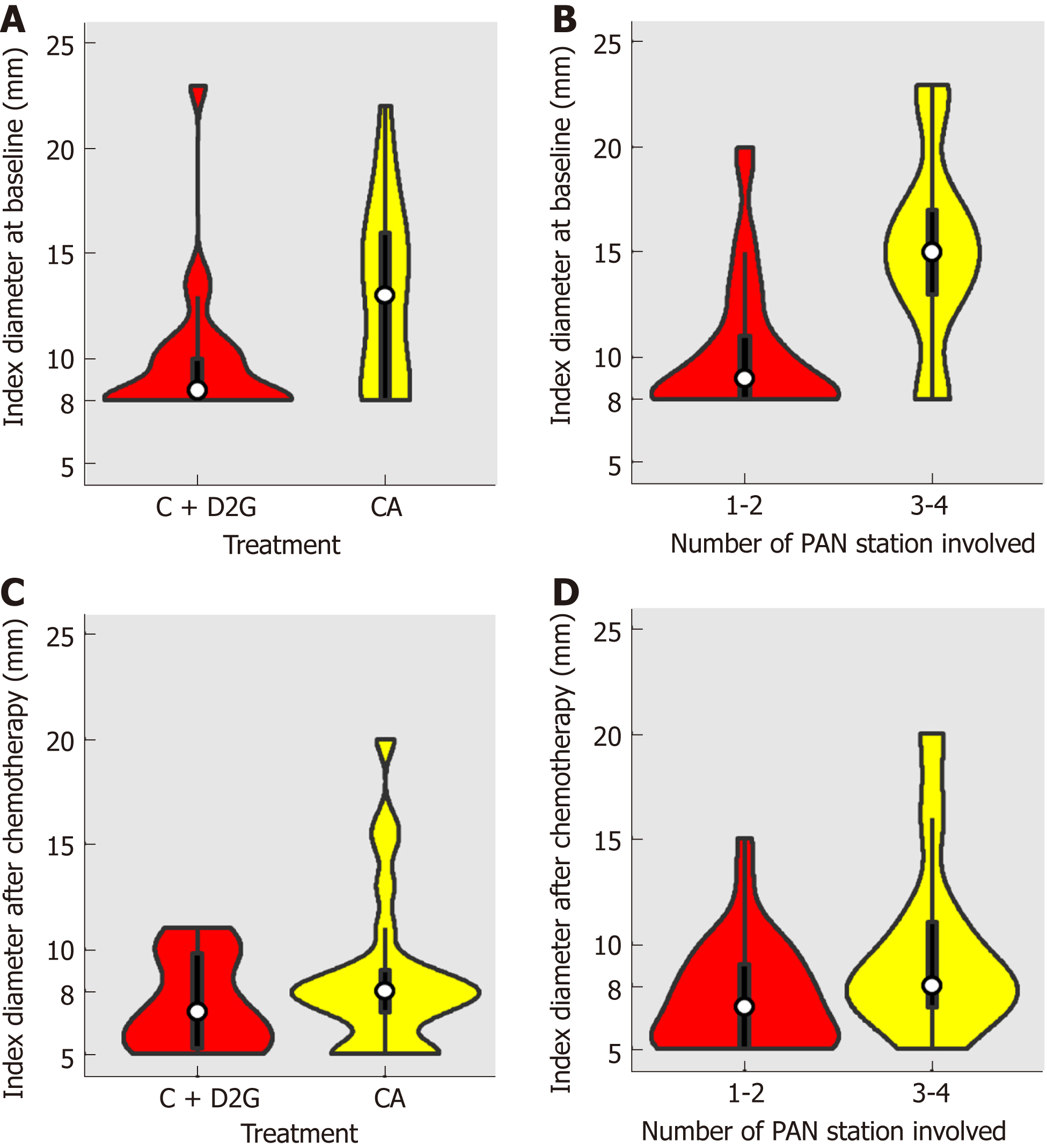
Violin plots of the distribution of the short axis diameter of the largest PAN distributed by whether the patient underwent D2 resection or not are shown in Figure 2A (baseline) and Figure 2C (after initial chemotherapy). Violin plots of the distribution of the short axis diameter of the largest PAN distributed by whether more than 2 PAN stations were involved or not are shown in Figure 2B (baseline) and Figure 2D (after initial chemotherapy). The distributions in both treatment groups and PAN stations significantly varied at baseline (chemotherapy vs chemotherapy plus D2 gastrectomy, P = 0.01, Figure 2A; PAN stations 1-2 vs 3-4, P = 0.001, Figure 2B) but were not significantly different after chemotherapy (chemotherapy vs chemotherapy plus D2 gastrectomy, P = 0.29, Figure 2C; PAN stations 1-2 vs 3-4, P = 0.06, Figure 2D). The correlation between CR of all clinical PALM and clinical characteristics is displayed in Table 4. The largest PAN in the short axis at baseline (≥15 mm vs < 15 mm), overall response (RECIST), and response of the primary lesion (JGCA) were correlated with CR of PALM. Considering the diameter of the index nodes, a CR was observed in 3 of 12 patients with PAN ≥ 15 mm (25%) and in 10 of 26 patients with PAN ≥ 10 mm (38.5%).
| Variable | n | Response of PAN | P-value | Treatment | P-value | ||
| Complete response | Residual tumor | With D2 resection | Without D2 resection | ||||
| Tumor location | 0.5647 | 0.0931 | |||||
| Upper | 21 | 10 (41.7) | 11 (45.8) | 7 (31.8) | 14 (53.8) | ||
| Middle | 21 | 12 (50.0) | 9 (37.5) | 10 (45.5) | 11 (42.3) | ||
| Lower | 6 | 2 (8.3) | 4 (16.7) | 5 (22.7) | 1 (3.8) | ||
| Clinical tumor stage | 0.5510 | 0.4545 | |||||
| T3 | 3 | 2 (8.3) | 1 (4.2) | 2 (9.1) | 1 (3.8) | ||
| T4 | 45 | 22 (91.7) | 23 (95.8) | 20 (90.9) | 25 (96.2) | ||
| Clinical nodal stage | 0.2673 | 0.1640 | |||||
| N0-1 | 9 | 6 (25.0) | 3 (12.5) | 6 (27.3) | 3 (11.5) | ||
| N2-3 | 39 | 18 (75.0) | 21 (87.5) | 16 (72.7) | 23 (88.5) | ||
| Macroscopic type | 0.2199 | 0.5158 | |||||
| 1-3 or 5 | 41 | 22 (91.7) | 19 (79.2) | 18 (81.8) | 23 (88.5) | ||
| 4 | 7 | 2 (8.3) | 5 (20.8) | 4 (18.2) | 3 (11.5) | ||
| No. of PAN stations involved | 0.1044 | 0.0012 | |||||
| 1-2 | 35 | 20 (83.3) | 15 (62.5) | 21 (95.5) | 14 (53.8) | ||
| 3-4 | 13 | 4 (16.7) | 9 (37.5) | 1 (4.5) | 12 (46.2) | ||
| Largest PAN in short-axis | 0.0822 | 0.0899 | |||||
| < 10 mm | 22 | 14 (58.3) | 8 (33.3) | 13 (59.1) | 9 (34.6) | ||
| ≥ 10 mm | 26 | 10 (41.7) | 16 (66.7) | 9 (40.9) | 17 (65.4) | ||
| Largest PAN in short-axis | 0.0455 | 0.0026 | |||||
| < 15 mm | 36 | 21 (87.5) | 15 (62.5) | 21 (95.5) | 15 (57.7) | ||
| ≥ 15 mm | 12 | 3 (12.5) | 9 (37.5) | 1 (4.5) | 11 (42.3) | ||
| Overall (RECIST) | 0.0109 | 0.1405 | |||||
| CR + PR | 19 | 10 (41.7) | 9 (37.5) | 7 (31.8) | 12 (46.2) | ||
| SD + PD | 10 | 1 (4.2) | 9 (37.5) | 3 (13.6) | 7 (26.9) | ||
| NE | 19 | 13 (54.2) | 6 (25.0) | 12 (54.5) | 7 (26.9) | ||
| Primary lesions (JGCA) | 0.0431 | 0.1405 | |||||
| CR + PR | 25 | 16 (66.7) | 9 (37.5) | 14 (63.6) | 11 (42.3) | ||
| SD + PD | 23 | 8 (33.3) | 15 (62.5) | 8 (36.4) | 15 (57.7) | ||
Of the 24 patients with CR of PALM, only 66.7% (16/24) achieved CR or partial response (PR) in the primary tumor. All 24 patients were recommended to receive surgical resection, and 14 patients with CR of PALM underwent D2 gastrectomy, while 8 patients with well-responded PALM also received D2 gastrectomy at the request of the patient. Among the 22 patients who received D2 gastrectomy, 2 exhibited CR, 5 exhibited PR, 2 exhibited stable disease (SD), 1 exhibited progressive disease (PD), and 12 were not evaluable considering the overall response; 2 exhibited CR, 12 exhibited PR, 7 exhibited SD, and 1 exhibited PD considering the response of the primary tumor. Among patients with an index node larger than 15 mm at the first visit, only 1 of 12 underwent D2 gastrectomy, and among patients with more than two PAN stations involved at baseline, only 1 of 13 underwent D2 gastrectomy.
In addition, six patients received radiotherapy as recommended by the multidisciplinary team in total. Among them, two patients received preoperative radiotherapy, three received adjuvant radiotherapy, and one received palliative radiotherapy.
Lavage cytology was routinely performed, and positive lavage cytology was considered an incurable factor. Therefore, patients with positive cytology were excluded. For the 22 patients who ultimately underwent D2 gastrectomy, the median number of preoperative chemotherapy cycles was 4 [interquartile range (IQR), 3-5]. The median blood loss was 150 mL (IQR, 100-200 mL), and the median surgery time was 195 min (IQR, 170-214 min). Surgical and pathological data are listed in Table 5. Postoperative complications occurred in 27.3% (6/22) of patients, including abdominal infection (2/22), lymphatic fistula (1/22), pneumonia (1/22), anastomotic leakage (1/22), and sudden cardiac death (1/22). One patient with a history of heart disease died of sudden cardiac death on postoperative day 28. Patients without CR of PALM were regarded as having an R1/R2 resection, and thus, R0 resection was achieved in 63.6% of patients. Three patients presented a pathological CR, and the pathological response rate was 68.2%.
| Variable | Chemotherapy plus surgery |
| Residual tumor | |
| R0 | 14 (63.6) |
| R1-R2 | 8 (36.4) |
| Surgery approach | |
| Laparoscopy | 8 (36.4) |
| Open | 14 (63.6) |
| Extent of gastric resection | |
| Distal | 11 (50.0) |
| Proximal | 3 (13.6) |
| Total | 7 (31.8) |
| Multiple organ resection | 1 (4.5) |
| Macroscopic type | |
| 1-3 or 5 | 18 (81.8) |
| 4 | 4 (18.2) |
| Histological type | |
| Intestinal or mixed | 11 (50.0) |
| Diffuse | 11 (50.0) |
| Mandard grade | |
| 1-2 | 2 (9.1) |
| 3 | 13 (59.1) |
| 4-5 | 7 (31.8) |
| Tumor depth | |
| ypT0 | 2 (9.1) |
| ypT1a | 1 (4.5) |
| ypT1b | 1 (4.5) |
| ypT2 | 1 (4.5) |
| ypT3 | 6 (27.3) |
| ypT4a | 10 (45.5) |
| ypT4b | 1 (4.5) |
| Lymph node metastases | |
| ypN0 | 7 (31.8) |
| ypN1 | 5 (22.7) |
| ypN2 | 2 (9.1) |
| ypN3a | 5 (22.7) |
| ypN3b | 3 (13.6) |
Overall, 9 patients experienced recurrence after surgery during the follow-up period, with 7 patients experiencing recurrence within 1 year. The progressive sites included four cases of PAN recurrence, one case of hepatic metastasis, one case of peritoneal metastasis, and one case of malignant ascites. Two patients relapsed after 1 year, including one with lung recurrence and one with mediastinal lymph node metastasis. Distant lymph node metastasis was the most common site of recurrence and occurred in 55.6% (5/9) of cases.
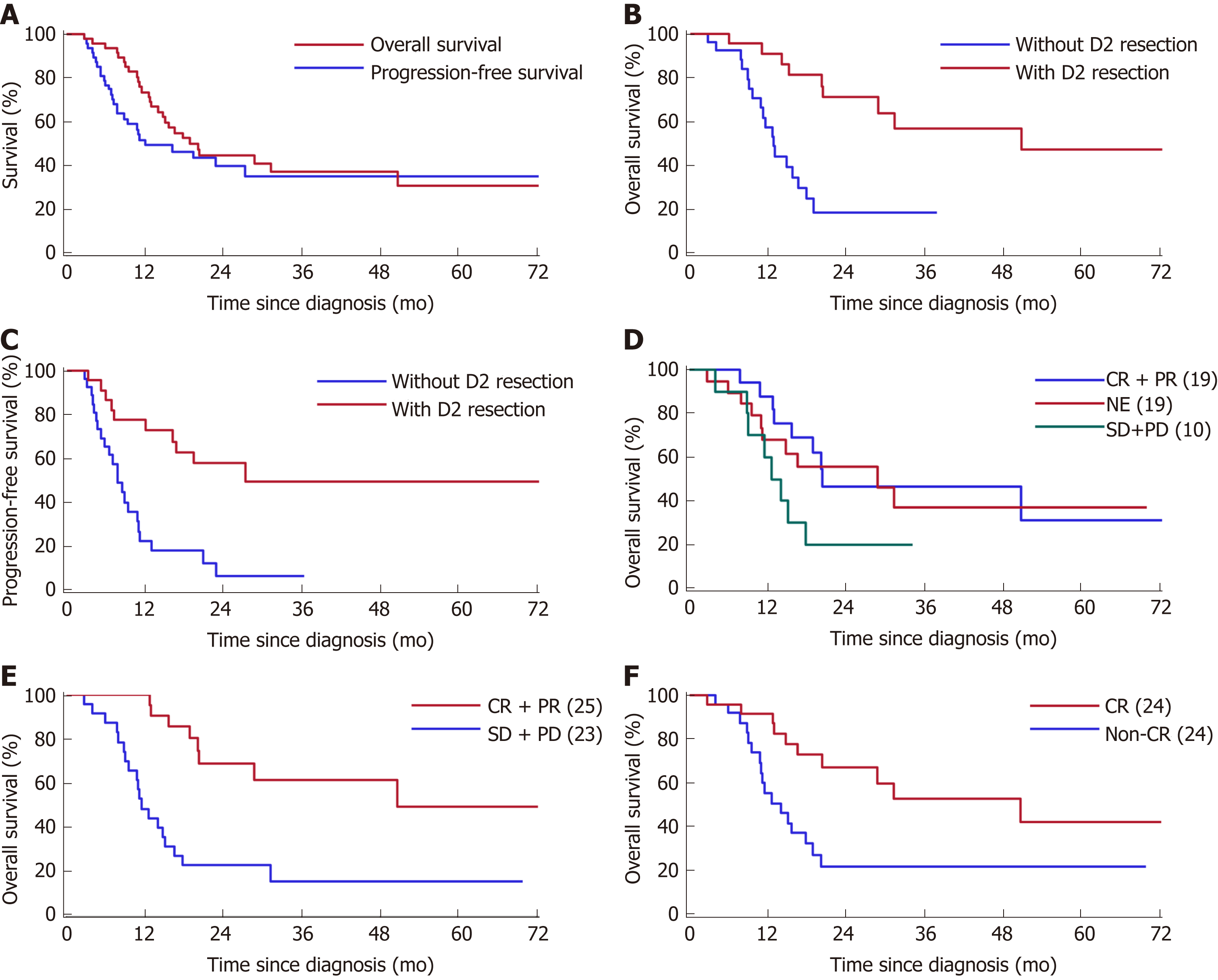
Survival plots are presented in Figure 3. The median follow-up period was 16.2 months (range, 2.8-72.4 mo). The 3-year OS rate for all patients was 36.9% [95% confidence interval (CI): 21.2-52.6], the 3-year PFS rate of all patients was 27.6% (95%CI: 13.5-41.6), and the median OS and PFS were 18.9 and 12.1 mo, respectively (Figure 3A). The survival time of those who received D2 gastrectomy was much longer than that of patients who did not undergo gastrectomy (median OS: 50.7 mo vs 12.8 mo, P = 0.0003, Figure 3B; median PFS: 27.4 mo vs 7.8 mo, P = 0.0002, Figure 3C; 3-year survival rate: 56.8% (95%CI: 33.2-80.4) vs 19.0% (95%CI: 0.02-35.9)). The 5-year survival rate for the D2 gastrectomy patients reached 47.3% (95%CI: 21.4-73.3). The survival difference according to overall response was not significant (Figure 4D). However, according to the response of the primary tumor, the median OS of patients who responded well was significantly better than that of those who responded poorly (50.7 mo vs 11.5 mo, P < 0.0001, Figure 3E), and according to the response of PALM, the median OS of patients with CR of PALM was much better than that of patients without CR of PALM (50.7 mo vs 14.0 mo, P = 0.0051, Figure 3F). Differences in survival according to the index diameter (≥ 15 mm vs < 15 mm) and the stations involved (total PAN stations involved: > 2 vs 1-2) at baseline and the pathological response (Mandard TRG: 1-3 vs 4-5) were not significant in univariate analyses (data not shown).
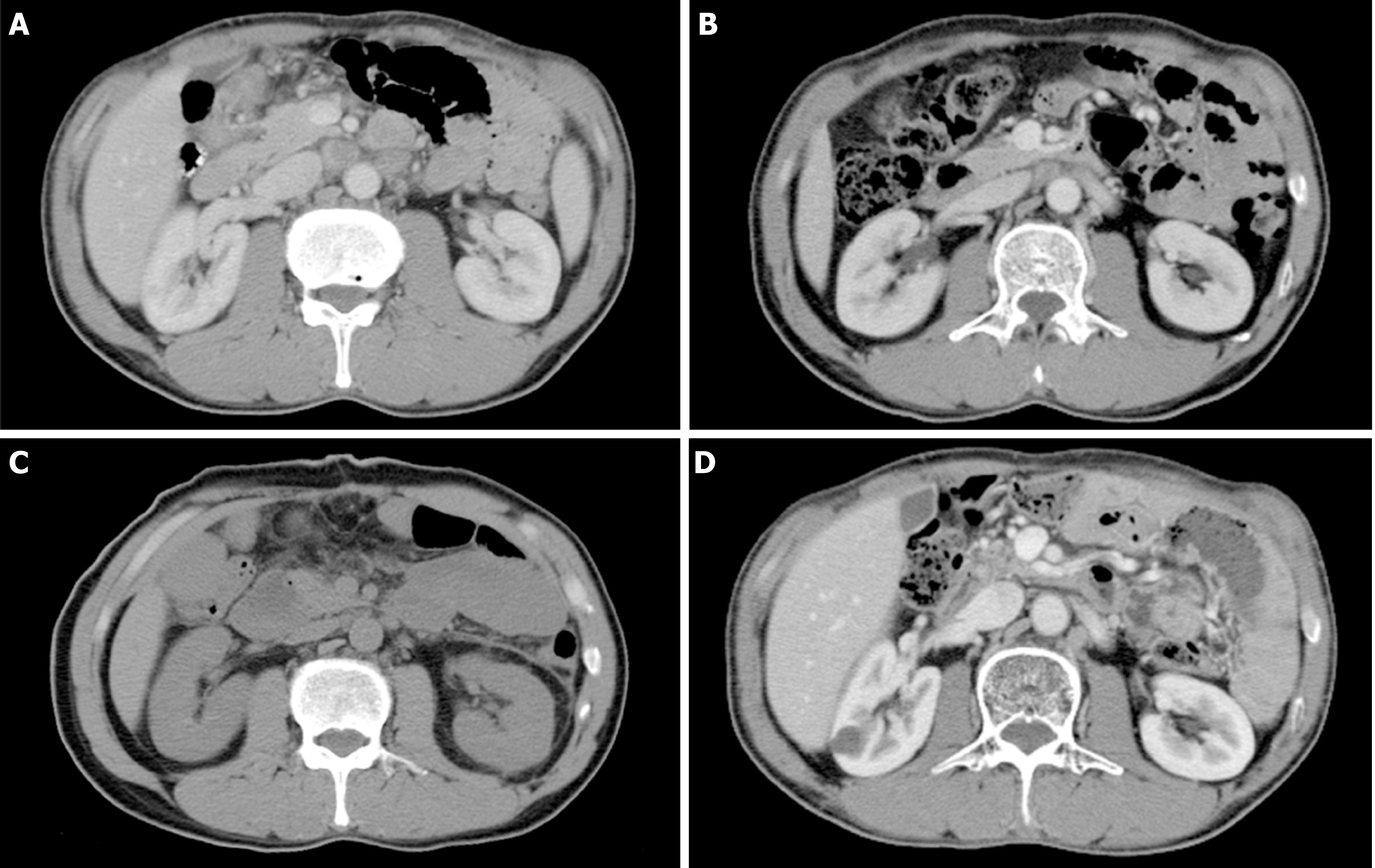
Data of patients who survived more than 3 years are listed in Table 6. Among them, two underwent chemotherapy alone, while the other six received interventions via D2 gastrectomy. The surgical groups were characterized as having non-target PAN (short diameter < 15 mm), no more than two PAN stations involved at baseline, and CR of PALM after chemotherapy (range, 2-11 cycles) with or without the aid of radiotherapy (Table 6). One patient underwent D2 gastrectomy with an 11 mm left PAN (R1 resection) and received adjuvant radiotherapy to control the enlarged PAN. As a result, the suspicious PAN diminished dramatically, and the patient has been alive for 68 months after surgery without recurrence (Figure 4).
| Therapy | PAN | Response | Survival | ||||||||
| Target | SN | Overall | Primary | PAN | SR | OS | PFS | Status | |||
| C+S+C | NT | 1 | NE | PR | CR | 65.1 | 65.1 | Alive | |||
| C+S+C | NT | 1 | PR | PR | CR | 72.4 | 72.4 | Alive | |||
| C+S+C | NT | 1 | NE | PR | CR | 62.1 | 62.1 | Alive | |||
| C+S+C | NT | 2 | CR | CR | CR | L | 52.8 | 16.8 | Alive | ||
| C+S+CRT | NT | 2 | NE | SD | NN | 70.1 | 70.1 | Alive | |||
| C+S+CRT | NT | 2 | PR | PR | CR | PAN | 50.7 | 16.2 | Dead | ||
| C | T | 3 | CR | CR | CR | NA | 37.8 | 20.9 | Alive | ||
| C | T | 3 | PR | PR | NN | NA | 36.3 | 36.3 | Alive | ||
Chemotherapy is considered the primary choice for treatment of stage IV gastric cancer, but the prognosis remains poor. Surgery is not routinely recommended, except for palliative reasons. Under some conditions, treatment of clinical stage IV gastric cancer with a single incurable factor, such as PALM, positive lavage cytology, and sole liver metastasis, can be controversial. Unlike other incurable factors, PAN lesions are difficult for a biopsy, and the diagnosis and follow-up primarily depend on CT or PET-CT scanning. Thus, there is confusion concerning clinico-pathological issues in gastric cancer with suspicious PALM.
Currently, except PET-CT, clinical PALM is primarily diagnosed based on the enlarged diameter in the short axis of PAN[8,9]. In previously published studies, different enrollment criteria and distribution bias have compromised the comparability of results[11-15]. Although the current criteria for clinically positive lymph nodes on imaging examination, such as CT or EUS, are mainly based on lymph node measurement in the short axis[16-20], the cut-off value varies dramatically across different studies[12,21]. In this study, we selected a minimal axial diameter of 8 mm or greater as the main criterion for diagnosis of clinical lymph node metastasis, which is widely accepted in several studies and has shown a sensitivity and specificity of up to 85% and 95%, respectively[13-15,22]. In addition, the diameter of index nodes (equal to the largest clinically positive lymph nodes) was used to help us determine clinically positive PALM during treatment, because a change in the short diameter has been shown to be significantly correlated with pathological outcomes[23].
The incidence of metastases in the PAN was found to be only 8.5% in the JCOG 9501 trial, and thus, for the majority of gastric cancer patients without radiologically positive PALM, curative D2 surgery is adequate[1]. However, whether this method is suitable for patients with CR of PALM after chemotherapy remains unknown. In the present study, we defined PALM disappearance or shrinkage to < 8 mm in the short axis as clinical CR. Moreover, the survival of patients with clinical CR of PALM exhibited better survival than patients with positive PALM after chemotherapy. These results confirmed that CR of PALM was associated with a good prognosis and was a favorable factor for D2 resection. In addition, according to our results, a short axis < 8 mm can be chosen as the cut-off value for clinically negative PAN after che-motherapy, which is a stricter criterion than that in previous studies[5,8].
Current response evaluation criteria also lead to difficulties in response evaluation of gastric cancer patients with isolated PALM. In this study, 26 advanced gastric cancer patients with isolated PALM were absent from the classification of target lesions in RECIST 1.1, which regards primary tumors and lymph nodes < 15 mm as non-measurable[8]. After chemotherapy, 19 patients were considered inevaluable leading to a response rate of only 39.6%. We further analyzed the response by stratifying the primary tumor and PALM separately. The response of primary tumor was evaluated based on the 15th edition of the Japanese Classification of Gastric Carcinoma. While for PALM, we considered lymph node disappearance or shrinkage to < 8 mm as clinical CR after chemotherapy. Under the adjusted response evaluation system, we found that a good response of the primary tumor or CR of PALM was significantly correlated with better survival (Figure 3E and F).
Whether surgical resection is needed for stage IV gastric cancer remains controversial. PALM is classified as a relatively early type in stage IV gastric cancer, is associated with a lower tumor burden than other organ and peritoneal metastases[24], and could be considered as the most suitable type for surgery among all the types of stage IV gastric cancer[25,26]. In this group, the long-term OS of those who underwent D2 resection was much better than that of those who did not. The main reason was attributed to R0 resection and the difference in response to chemotherapy. Patients with a lower tumor burden or incurability de novo, which was characterized as a smaller tumor size, fewer metastatic lymph nodes, or fewer metastatic lymph node stations in gastric cancer with clinical PALM, are more prone to achieve CR of metastasis (Table 4); therefore, D2 gastrectomy was performed, resulting in a better prognosis. Kaito et al[27] found that involvement of a greater number of PAN stations was associated with a poorer prognosis. To date, most studies on surgical interventions in gastric cancer with clinical PALM have been limited to no more than two PAN stations (No. 16a2/16b1)[2,5,24,27-33]. Lymph node size was also found to be an independent prognostic factor for gastric cancer[21]. In the present study, we found that 58.3% (21/36) of patients with an index diameter less than 15 mm achieved CR after chemotherapy and then received surgical resection. We found confounding factors in both the station number and baseline lymph node size. Although patients with a higher metastatic burden, characterized as having a greater number of PAN stations involved and larger PAN size, did not show a significant impact on OS, they showed fewer chances of CR of PALM and fewer surgical decision made by the multidisciplinary team.
The extent of lymph node resection has long been a debated question. Japanese researchers tend to perform D2 resection plus PAND for advanced stage gastric cancer with overt PALM after chemotherapy; however, their results were not significantly better than those of the study that chose D2 gastrectomy. Many retrospective studies have reported a clinical benefit of curative D2 gastrectomy for patients with stage IV gastric cancer, who exhibited a CR of distant metastasis after chemotherapy without extensive resection[28,34,35].
We chose D2 resection as the surgical method for three reasons. First, no more than 10% of patients have radiologically occult metastasis in the para-aortic region, which indicates that D2 resection is adequate for most patients. Meanwhile, the most common recurrence site is para-aortic region even after PAND [27,30]. In this study, patients who underwent D2 surgery had a 22.7% lymph node recurrence rate, which is comparable to the 24.6%-30.0% lymph node recurrence rate of patients who underwent D2 gastrectomy plus PAND in previous studies. More importantly, the prognosis of pathologically positive patients was poor, therefore we did not think that PAND was necessary. Second, D3 or D2 plus PAND after chemotherapy has not been fully demonstrated in clinical studies, and is accompanied by a higher rate of morbidity and mortality even in the Japanese studies. Only a few gastrointestinal surgeons worldwide are experts at this complicated procedure[36-39]. Finally, with the development of radiotherapy, new techniques can provide excellent local control rates to limit lymph node metastasis.
A similar phase II study conducted by Wang et al[5] also chose D2 resection as the surgical method and achieved an encouraging 1-year PFS rate of 47.8%, indicating non-inferior survival compared with neoadjuvant therapy plus extended dissection[31]. However, in our real-world study, the survival outcome was much more aggressive. The 3- and 5-year survival rates for patients who underwent D2 resection were 56.8% and 47.3%, respectively. In this study, the chemotherapy regimens and the compliance of perioperative chemotherapy varied. We think that the individualized chemotherapy regimens and the necessary radiotherapy targeted to each individual also contributed to the remarkable survival outcomes. In contrast, in some clinical trials, it is compulsory for patients to receive two or four cycles of chemotherapy regardless of whether it is the best timing[31,33,40,41].
Para-aortic lymph node metastasis (PALM) is classified as stage IV gastric cancer with a dismal outcome after isolated surgical treatment. However, the treatment issues for patients with clinical para-aortic lymph node (PAN) enlargement are complex, as PAN enlargement can represent either inflammatory lymphadenopathy or malignant metastasis. In recent years, the role of surgery in multidisciplinary treatment (MDT) of gastric cancer with clinical PALM has been recognized. Nevertheless, the effect of D2 gastrectomy treatment has not yet been fully studied.
The benefit of addition of D2 gastrectomy to MDT and the unsettled clinico-pathological issues in gastric cancer with clinical PALM need to be discussed.
The present study aimed to determine whether D2 resection can be adopted for gastric cancer with radiologically overt PALM and to identify criteria of enrollment and response evaluation and find a best treatment strategy for this group of patients.
We collected clinical and pathological data of gastric cancer patients with clinically positive PALM, including detailed information on PAN and clinical response. The short axis diameter of the largest PAN in every individual patient was recorded, and clinical response in the primary tumor and the metastatic sites was evaluated separately. Surgical decision making in accordance with the status of PALM after chemotherapy and survival data were documented.
D2 gastrectomy improved the prognosis of select patients, especially those with complete response (CR) of PALM. Patients with long-term survival were characterized as having limited PALM at baseline and CR of PALM after chemotherapy. For patients without CR of clinical PALM, radiotherapy may be considered as an option to complement D2 resection.
Chemotherapy followed by D2 gastrectomy may be a promising strategy for treating select gastric cancer patients with radiologically suspicious PALM. Patients with limited PALM at baseline and CR of PALM after chemotherapy may be good candidates for D2 gastrectomy. Large-scale, multicenter, randomized studies are needed to confirm the feasibility of addition of D2 gastrectomy to a practical MDT plan for patients with clinical PALM.
Although we confirmed the benefit of D2 gastrectomy in gastric cancer patients with enlarged PALM, the problem of whether dissection of the para-aortic region is necessary remains unresolved. D2 gastrectomy has limitations as it greatly depends on good response of the metastatic lesions. Currently, a surgical strategy seems promising for gastric cancer with clinical PALM, but the best clinical practice should be identified in future research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Matowicka-Karna J, alebi Bezmin Abadi A, Tanabe S S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K; Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 753] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 2. | Tokunaga M, Ohyama S, Hiki N, Fukunaga T, Aikou S, Yamaguchi T. Can superextended lymph node dissection be justified for gastric cancer with pathologically positive para-aortic lymph nodes? Ann Surg Oncol. 2010;17:2031-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Roviello F, Pedrazzani C, Marrelli D, Di Leo A, Caruso S, Giacopuzzi S, Corso G, de Manzoni G. Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol. 2010;36:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, Yoshikawa T, Hahn S, Nakamura K, Park CH, Kurokawa Y, Bang YJ, Park BJ, Sasako M, Tsujinaka T; REGATTA study investigators. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 502] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Yu YY, Li W, Feng Y, Hou J, Ji Y, Sun YH, Shen KT, Shen ZB, Qin XY, Liu TS. A phase II trial of Xeloda and oxaliplatin (XELOX) neo-adjuvant chemotherapy followed by surgery for advanced gastric cancer patients with para-aortic lymph node metastasis. Cancer Chemother Pharmacol. 2014;73:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Okabe H, Ueda S, Obama K, Hosogi H, Sakai Y. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol. 2009;16:3227-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Beom SH, Choi YY, Baek SE, Li SX, Lim JS, Son T, Kim HI, Cheong JH, Hyung WJ, Choi SH, Jung M, Kim HS, Jeung HC, Chung HC, Rha SY, Noh SH. Multidisciplinary treatment for patients with stage IV gastric cancer: The role of conversion surgery following chemotherapy. BMC Cancer. 2018;18:1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, Eisenhauer EA. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21474] [Article Influence: 1342.1] [Reference Citation Analysis (1)] |
| 10. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing. 2017;XVII, 1032. [DOI] [Full Text] |
| 11. | Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: Criteria for normal size determined with CT. Radiology. 1991;180:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 231] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Fukuya T, Honda H, Hayashi T, Kaneko K, Tateshi Y, Ro T, Maehara Y, Tanaka M, Tsuneyoshi M, Masuda K. Lymph-node metastases: Efficacy for detection with helical CT in patients with gastric cancer. Radiology. 1995;197:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 139] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Joo I, Kim SH, Ahn SJ, Lee ES, Shin CI, Lee HJ, Yang HK. Preoperative tumor restaging and resectability assessment of gastric cancers after chemotherapy: Diagnostic accuracy of MDCT using new staging criteria. Abdom Radiol (NY). 2017;42:2807-2815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Marrelli D, Mazzei MA, Pedrazzani C, Di Martino M, Vindigni C, Corso G, Morelli E, Volterrani L, Roviello F. High accuracy of multislices computed tomography (MSCT) for para-aortic lymph node metastases from gastric cancer: A prospective single-center study. Ann Surg Oncol. 2011;18:2265-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Fairweather M, Jajoo K, Sainani N, Bertagnolli MM, Wang J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol. 2015;111:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Feng XY, Wang W, Luo GY, Wu J, Zhou ZW, Li W, Sun XW, Li YF, Xu DZ, Guan YX, Chen S, Zhan YQ, Zhang XS, Xu GL, Zhang R, Chen YB. Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer - results of a single institution study of 610 Chinese patients. PLoS One. 2013;8:e78846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, Jung SH, Kim NY, Kim YH, Lee KH, Kim HH, Park DJ, Lee HS, Jung HC, Song IS. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010;25:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Mehmedović A, Mesihović R, Saray A, Vanis N. Gastric cancer staging: EUS and CT. Med Arch. 2014;68:34-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: A meta-analysis. Gastrointest Endosc. 2011;73:1122-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Tokunaga M, Sugisawa N, Tanizawa Y, Bando E, Kawamura T, Terashima M. The impact of preoperative lymph node size on long-term outcome following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1598-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | De Manzoni G, Marrelli D, Baiocchi GL, Morgagni P, Saragoni L, Degiuli M, Donini A, Fumagalli U, Mazzei MA, Pacelli F, Tomezzoli A, Berselli M, Catalano F, Di Leo A, Framarini M, Giacopuzzi S, Graziosi L, Marchet A, Marini M, Milandri C, Mura G, Orsenigo E, Quagliuolo V, Rausei S, Ricci R, Rosa F, Roviello G, Sansonetti A, Sgroi G, Tiberio GA, Verlato G, Vindigni C, Rosati R, Roviello F. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer. 2017;20:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Lee SM, Kim SH, Lee JM, Im SA, Bang YJ, Kim WH, Kim MA, Yang HK, Lee HJ, Kang WJ, Han JK, Choi BI. Usefulness of CT volumetry for primary gastric lesions in predicting pathologic response to neoadjuvant chemotherapy in advanced gastric cancer. Abdom Imaging. 2009;34:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Park IH, Kim SY, Kim YW, Ryu KW, Lee JH, Lee JS, Park YI, Kim NK, Park SR. Clinical characteristics and treatment outcomes of gastric cancer patients with isolated para-aortic lymph node involvement. Cancer Chemother Pharmacol. 2011;67:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: The proposal of new biological categories of classification. Gastric Cancer. 2016;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 26. | Yamaguchi K, Yoshida K, Tanaka Y, Matsuhashi N, Tanahashi T, Takahashi T. Conversion therapy for stage IV gastric cancer-the present and future. Transl Gastroenterol Hepatol. 2016;1:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Kaito A, Kinoshita T, Tokunaga M, Sunagawa H, Watanabe M, Sugita S, Tonouchi A, Sato R, Abe I, Akimoto T. Prognostic Factors and Recurrence Pattern of Far-advanced Gastric Cancer with Pathologically-positive Para-aortic Lymph Nodes. Anticancer Res. 2017;37:3685-3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Sato Y, Ohnuma H, Nobuoka T, Hirakawa M, Sagawa T, Fujikawa K, Takahashi Y, Shinya M, Katsuki S, Takahashi M, Maeda M, Okagawa Y, Naoki U, Kikuch S, Okamoto K, Miyamoto H, Shimada M, Takemasa I, Kato J, Takayama T. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: A multi-institutional retrospective study. Gastric Cancer. 2017;20:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Kodera Y, Kobayashi D, Tanaka C, Fujiwara M. Gastric adenocarcinoma with para-aortic lymph node metastasis: A borderline resectable cancer? Surg Today. 2015;45:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Fujiwara Y, Omori T, Demura K, Miyata H, Sugimura K, Ohue M, Kobayashi S, Takahashi H, Doki Y, Yano M. A Multidisciplinary Approach for Advanced Gastric Cancer with Paraaortic Lymph Node Metastasis. Anticancer Res. 2015;35:6739-6745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 32. | He Q, Ma L, Li Y, Li G. A pilot study of an individualized comprehensive treatment for advanced gastric cancer with para-aortic lymph node metastasis. BMC Gastroenterol. 2016;16:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, Kawashima Y, Kinoshita T, Terashima M, Nashimoto A, Nakamori M, Onaya H, Sasako M. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer. 2017;20:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 34. | Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, Tanabe K, Ohdan H. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21:315-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Seo HS, Song KY, Jung YJ, Park SM, Jeon HM, Kim W, Chin HM, Kim JJ, Kim SK, Chun KH, Kim JG, Lee JH, Lee HH, Kim DJ, Yoo HM, Kim CH, Kim EY, Park CH; Catholic Gastric Cancer Study Group (CGCSG). Radical Gastrectomy After Chemotherapy May Prolong Survival in Stage IV Gastric Cancer: A Korean Multi-institutional Analysis. World J Surg. 2018;42:3286-3293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Günther K, Horbach T, Merkel S, Meyer M, Schnell U, Klein P, Hohenberger W. D3 lymph node dissection in gastric cancer: Evaluation of postoperative mortality and complications. Surg Today. 2000;30:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Bostanci EB, Kayaalp C, Ozogul Y, Aydin C, Atalay F, Akoglu M. Comparison of complications after D2 and D3 dissection for gastric cancer. Eur J Surg Oncol. 2004;30:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Morita S, Fukagawa T, Fujiwara H, Katai H. The clinical significance of para-aortic nodal dissection for advanced gastric cancer. Eur J Surg Oncol. 2016;42:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Tamura S, Takeno A, Miki H. Lymph node dissection in curative gastrectomy for advanced gastric cancer. Int J Surg Oncol. 2011;2011:748745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y, Fukushima N. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Yoshikawa T, Morita S, Tanabe K, Nishikawa K, Ito Y, Matsui T, Fujitani K, Kimura Y, Fujita J, Aoyama T, Hayashi T, Cho H, Tsuburaya A, Miyashita Y, Sakamoto J. Survival results of a randomised two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of S-1 plus cisplatin (SC) and paclitaxel plus cisplatin (PC) followed by D2 gastrectomy for resectable advanced gastric cancer. Eur J Cancer. 2016;62:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |