Published online May 21, 2019. doi: 10.3748/wjg.v25.i19.2279
Peer-review started: March 8, 2019
First decision: March 20, 2019
Revised: March 27, 2019
Accepted: April 10, 2019
Article in press: April 10, 2019
Published online: May 21, 2019
Processing time: 73 Days and 11.2 Hours
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver. It is the second leading cause of cancer-related deaths worldwide, with a very poor prognosis. In the United States, there has been only minimal improvement in the prognosis for HCC patients over the past 15 years. Details of the molecular mechanisms and other mechanisms of HCC progression remain unclear. Consequently, there is an urgent need for better understanding of these mechanisms. HCC is often diagnosed at advanced stages, and most patients will therefore need systemic therapy, with sorafenib being the most common at the present time. However, sorafenib therapy only minimally enhances patient survival. This review provides a summary of some of the known mechanisms that either cause HCC or contribute to its progression. Included in this review are the roles of viral hepatitis, non-viral hepatitis, chronic alcohol intake, genetic predisposition and congenital abnormalities, toxic exposures, and autoimmune diseases of the liver. Well-established molecular mechanisms of HCC progression such as epithelial-mesenchymal transition, tumor-stromal interactions and the tumor microenvironment, cancer stem cells, and senescence bypass are also discussed. Additionally, we discuss the roles of circulating tumor cells, immunomodulation, and neural regulation as potential new mechanisms of HCC progression. A better understanding of these mechanisms could have implications for the development of novel and more effective therapeutic and prognostic strategies, which are critically needed.
Core tip: The overall prognosis for hepatocellular carcinoma patients remains poor, as there has only been minimal improvement over the past 15 years. Details of the mechanisms of hepatocellular carcinoma progression remain unclear. This review discusses a summary of both well-established and newly proposed mechanisms of hepatocellular carcinoma progression. A better understanding of these mechanisms is critical to the development of novel and more effective therapeutic strategies likely to improve hepatocellular carcinoma patient outcomes.
- Citation: Ogunwobi OO, Harricharran T, Huaman J, Galuza A, Odumuwagun O, Tan Y, Ma GX, Nguyen MT. Mechanisms of hepatocellular carcinoma progression. World J Gastroenterol 2019; 25(19): 2279-2293
- URL: https://www.wjgnet.com/1007-9327/full/v25/i19/2279.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i19.2279
Hepatocellular carcinoma (HCC) is the most common primary liver cancer comprising 75%-85% of cases of liver cancer[1]. It is the sixth most common cancer and the second leading cause of cancer deaths worldwide[1]. The incidence of HCC in the United States has been increasing over the past two decades[1-3]. While the overall prognosis for HCC patients in the United States has improved somewhat in the past 15 years, it still remains poor. In fact, in the United States, the 2-year survival for HCC is less than 50% and 5-year survival is only 10%[4].
In Asia, chronic hepatitis B virus (HBV) infection is the primary cause of HCC. While in the Western world, chronic hepatitis C virus (HCV), alcoholic cirrhosis and non-alcoholic steatohepatitis (NASH) are the main causes[5]. Other known risk factors of HCC include heavy alcohol consumption, nonalcoholic fatty liver disease, consumption of aflatoxins, obesity, type 2 diabetes and tobacco smoking[6,7].
Early diagnosis and effective treatment of HCC remain a challenge. While some patients can be symptomatic, including symptoms such as right upper abdominal quadrant pain, anorexia, early satiety, weight loss, obstructive jaundice, fever, watery diarrhea, lethargy, and bone pain (from metastases)[6,7], most patients remain asymptomatic, and clinical presentation occurs at advanced stages of the disease.
If detected very early, HCC can actually be cured with an excellent long-term prognosis[7], where the principal treatment options would be surgical resection or liver transplantation if the patient is a suitable transplant candidate[8]. However, for the vast majority of HCC patients, their cancer is detected at an advanced stage where surgical cure is no longer an option[7]. Most patients will therefore need chemotherapy, which works by destroying cancer cells and inhibiting the proliferation of new cancer cells via the use of chemical agents. Sorafenib, a small multi-tyrosine kinase inhibitor that blocks Raf kinase, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) receptor activities, is the most commonly used chemotherapeutic agent to treat HCC[4]. Although a targeted chemotherapeutic agent, its use has been shown to minimally enhance patient survival[9] by only about 7-10 months[10]. Other drugs such as sunitinib, brivanib, and other angiogenic inhibitors are currently still under development and hold promise in targeting the extensive angiogenic network that is present in the liver[11,12]. Additional multi-kinase inhibitors recently approved for HCC treatment include regorafenib (for secondary treatment after sorafenib), as well as levatinib (another first-line drug to treat HCC besides sorafenib). However, neither provide much more additional benefit than sorafenib treatment[13,14]. As such, better treatment options are still needed.
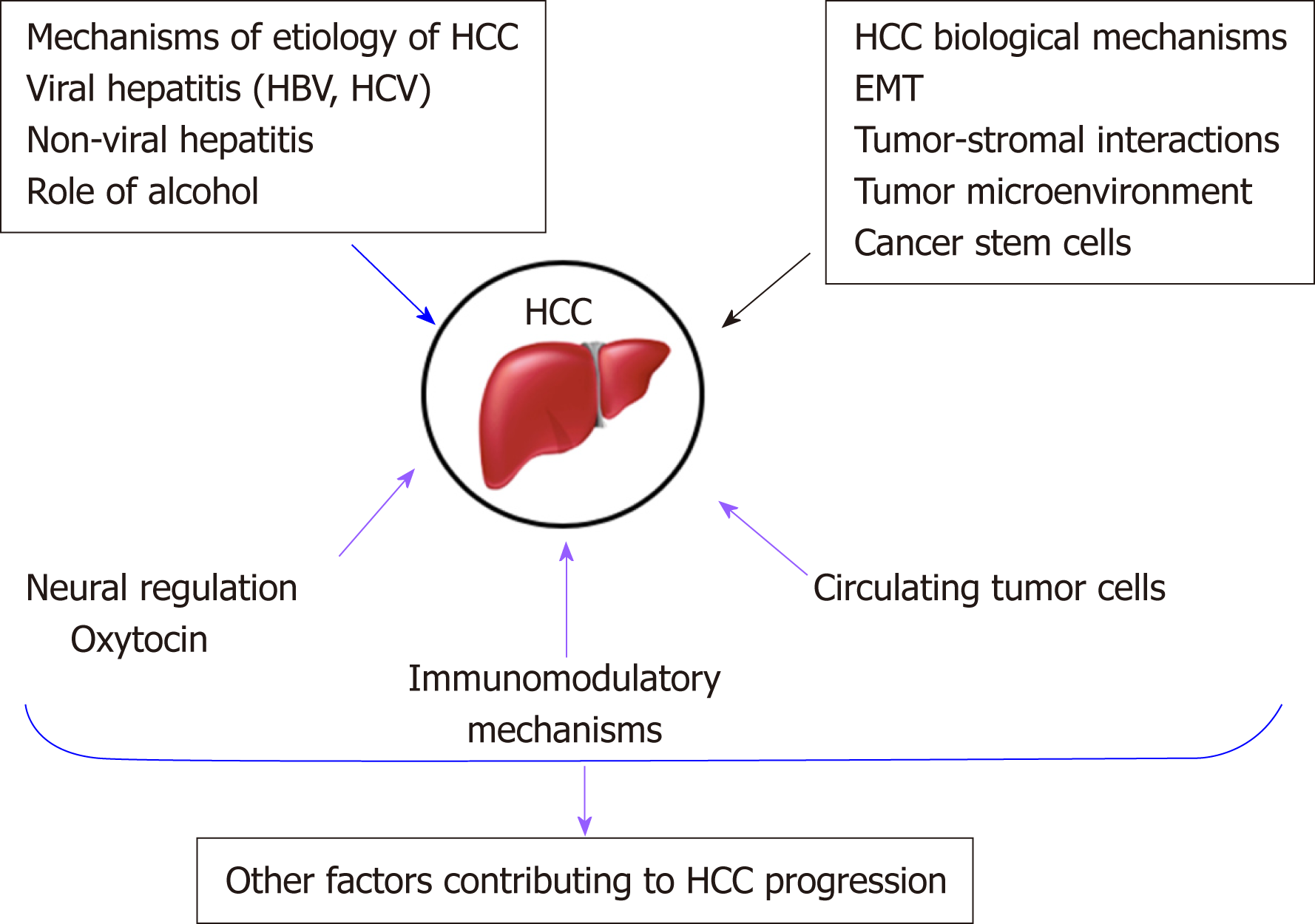
To address this unmet need, researchers are trying to identify different mechanisms that may be involved in HCC progression to find alternative therapeutic strategies[8]. There have been various signaling pathways and molecules implicated in HCC progression. Some of these will be discussed in this review article and are summarized in Figure 1.
Several risk factors have been implicated in the development and progression of HCC, notably chronic viral hepatitis, non-viral hepatitis, chronic alcohol intake, certain disease states (obesity and diabetes), and consumption of toxin-contaminated staples[15]. The epidemiologic distribution of these risk factors varies according to geographic location and host-specific factors.
HBV and HCV are major causes of viral hepatitis that lead to the development of cirrhosis and HCC. The pathogenesis of HBV-induced HCC is thought to involve several mechanisms, including HBV-DNA integration into host genetic machinery, DNA methylation, oxidative stress, and HBx protein[16]. The risk of developing HCC has been shown to be proportional to HBV-DNA level in liver cells. HBV gains entry into liver cells through a receptor mediated pathway. Chronic illness results from persistence of the virus in the host cells via various mechanisms that include infection of immune defense control centers, viral inhibition of antigen presentation, selective immune suppression, down-regulation of viral gene expression, and viral mutations that functionally incapacitate virus-specific T cells from recognizing HBV antigen[17]. Immune response and inflammatory reactions induce cytokine and chemokine mobilization, causing oxidative stress. This, in turn, promotes constant activation of several genes that cause cirrhosis, including TERT, MLL4, RARβ, CCNE1, Cyclin A2, FN1, ROCK1, SENP5, ANGPT1, PDGF receptor, calcium signaling-related genes, ribosomal protein genes, epidermal growth factor receptor (commonly known as EGFR), and mevalonate kinase carboxypeptidase[15].
HBV and HCV viral proteins may be involved in hijacking the cellular machinery. Viral attack can also directly cause cirrhotic tissue development through the release of proinflammatory cytokines (e.g., interleukin (IL)6, tumor necrosis factor (TNF)-α, IL1 and IL18)[18].
HCV hijacks host cellular machinery to increase cellular proliferation, steatosis, inflammatory processes, mitochondrial dysfunction, insulin resistance, all leading to oxidative stress, genetic instability and DNA damage with cirrhosis and HCC as a likely outcome[19].
HCC risk drastically increases at the cirrhotic liver stage, suggesting a close association.
The corresponding interplay of inflammatory responses, gene activation, and viral clearance suppression creates a conditioned environment that promotes cellular mutations leading to HCC.
Even though viral hepatitis from HBV and HCV are strongly associated with liver cancer, there are non-viral risk factors that can induce the development of HCC[20]. Diabetes mellitus, alcohol abuse, cardiovascular disease, liver inflammation, obesity, dyslipidemia and non-alcoholic fatty liver disease (NAFLD) are some other major contributors to HCC development.
Accumulation of iron in the liver of NASH and HCC patients[21,22] is correlated with progression of fibrosis and HCC[23]. In this context, a possible tumor biomarker may be serum ferritin rather than iron. However, because there is no exact correlation between iron inside the liver and iron in the blood, it is difficult to clarify the pathological features of ferritin on the poor prognosis of non-viral HCC (nvHCC)[24]. Results of a cohort study of 93 patients with nvHCC, 62 of whom had alcohol abuse problems, showed an increase in ferritin level in non-diabetics[24]. However, further research needs to be done to assess the correlation between the impacts of alcohol and ferritin on NAFLD[24].
On the other hand, HCC is associated with obesity. Obesity impairs metabolism, induces inflammation and is an etiological factor for NAFLD, steatosis, NASH, hepatic fibrosis, cirrhosis, and ultimately HCC. Caused partly by a sedentary lifestyle and obesity, impaired lipid metabolism and deregulation of energy equilibrium in the liver contributes to the correlation between type 2 diabetes and NAFLD. In fact, several studies have shown that high BMI, waist circumference, and type II diabetes mellitus are associated with higher risks of liver cancer[25,26]. They have also suggested that the association may vary depending on the status of viral hepatitis infection[25]. Conversely, NAFLD provides the metabolic environment to induce insulin resistance[27], a known etiological factor for HCC.
Chronic alcohol intake is detrimental to our health. It leads to liver cirrhosis, and subsequently HCC. Alcoholic liver disease is one of the leading causes of HCC[28]. According to case studies from all over the world, alcohol abuse is related to up to 2-fold increased risk of HCC[29]. Moreover, studies performed on mice fed an alcohol diet have shown exacerbation of inflammation, epithelial-mesenchymal transition (EMT) and fibrosis, and consequent progression to HCC[28].
Pure ethanol does not directly cause inflammation and liver damage, however, toxic by-products of alcohol catabolism such as accumulation of acetaldehyde and free radicals can influence oxidative stress, apoptotic cell death, necrosis and necroptosis[29]. Reactive oxygen species (ROS) generation is the result of increased inflammatory cytokine secretion caused by constant inflammatory pathways[19]. ROS-induced DNA damage, genomic vulnerability of hepatocytes and T-lymphocyte suppression contribute to HCC development[19].
Also, alcohol catabolism impacts several steps of lipid metabolism, which leads to liver steatosis and inhibition of fatty acid oxidation[29].
Reversibility of gene expression via epigenetic alteration is an important biological phenomenon that often plays a role in tumorigenesis. Epigenetic mechanisms affected by excessive alcohol consumption lead to altered DNA methylation and acetylation. For instance, altered acetylation is associated with hepatic steatosis alcohol-induced HCC[29]. Overexpression of c-Met and hepatocyte growth factor is directly associated with promoter hypomethylation in circulating tumor cells (CTCs) of HCC in a syngeneic BALB/c mouse tumor model[30].
Moreover, alcohol abuse is associated with HCC via impaired metabolism, such as accumulation of acetaldehyde, hypomethylation, lack of antioxidants and retinoic acid, together with inflammation, oxidative stress, hypoxia and genetic instability[28].
In addition to the role of viral hepatitis and alcohol in the development of HCC, other possible risk factors include genetic predisposition and congenital abnormalities, toxic exposures (aflatoxin or arsenic contaminated food), and autoimmune diseases of the liver.
Several congenital abnormalities have been shown to predispose patients to liver cirrhosis and HCC. These include hereditary tyrosinemia, Wilson’s disease, alpha-1-antitrypsin deficiency, and hemochromatosis[31].
The pathogenesis of aflatoxin B1 (AFB1) - induced HCC includes several mechanisms, including the formation of mutagenic and carcinogenic intermediates and adducts. Aflatoxins are released from food contaminated by the fungi, Aspergillus flavus and Aspergillus parasiticus. A series of chemical transformations occur that result in the conversion of AFB1 to established mutagenic or carcinogenic compounds: aflatoxin-B1 → aflatoxin B1-8,9 exo-epoxide → 8,9-dihydroxy-8-(N7) guanyl-9-hydroxy aflatoxin B1 adduct → aflatoxin B1 formaminopyrimidine adduct. These adducts and intermediates can also directly induce a mutation at codon 249 of the p53 tumor suppressor gene. This replaces arginine with serine, a change that reverses the tumor suppressing ability of the gene. There are reports that suggest that AFB1 acts synergistically[32] with HBV to induce HCC. Additive interactions have also been reported[33].
In a systematic review, Tansel et al[34] demonstrated a relationship between increased risk of developing HCC in patients with liver cirrhosis as a result of autoimmune hepatitis (AIH). The risk of liver cirrhosis from AIH was found to be lower than that of liver cirrhosis secondary to HBV and HCV infection or primary biliary cholangitis. Nevertheless, the risk of liver cirrhosis and HCC from AIH is clinically significant.
There are several established biological mechanisms involved in the progression of HCC. These include EMT, tumor-stromal interactions, tumor microenvironment, cancer stem cells, and dysregulation of microRNAs and well-known signaling pathways[35,36]. Some of these are discussed below.
EMT is a biological process that occurs normally during development and wound healing, but is hijacked by cancer cells. During this process, epithelial cells, which are normally attached to a basement membrane and closely adhered to one another, lose their cell adhesive properties and become migratory in nature[37-39]. This endowed mesenchymal behavior permits the successful migration of cells, which if usurped by cancer cells, can promote their dissemination and spread throughout the body.
EMT has been recognized by many in the field to be important for cancer progression[40,41]. In HCC, there have been several reports of EMT effectors such as cadherins, fibronectin, vimentin, and integrins, being altered to permit a more mesenchymal phenotype. Furthermore, transcription factors promoting EMT, including Snail, Slug, Twist and Zeb, are also upregulated during HCC progression[42,43]. Additionally, there have been a number of studies on exosomes, microRNAs, long noncoding RNAs, and regulatory signaling pathways that have been associated with EMT and demonstrate consequences in HCC progression[30,41,44-49]. This is indicative of the important role that EMT plays in HCC progression. The molecular mechanisms of EMT may have diagnostic, prognostic, and therapeutic implications in HCC.
Metastasis is the most common cause of cancer-related deaths[30,50]. Worldwide, HCC is a leading cause of death from cancer[51]. However, the molecular mechanisms of HCC and metastasis are still being clarified[50].
Tumor development and malignant progression can be promoted by a constantly changing extracellular environment that is impacted by microenvironmental stimuli, immune cell cooperation, and inflammatory signals. There is communication between hepatic tumor cells and non-tumor stroma. The non-tumor stroma consists of components of the extracellular matrix (ECM) such as non-malignant fibroblasts, immune and endothelial cells, collectively known as the peri-tumoral microenvironment[52]. Major alterations to the hepatic microenvironment and cells in chronic liver disease influence cancer development[53]. For example, a hypoxic microenvironment in primary HCC is strongly associated with progression and angiogenesis. The consequent enhanced blood supply in the tumor mediates growth formation and metastasis[54].
According to previous studies, tumor cells cross-talk with the abnormal microenvironment, ECM, inflammatory cytokines, chemokines and upregulated growth factors, contributing to increased angiogenesis[55,56]. Although the molecular mechanisms of tumor-stromal interactions are still being clarified, existing evidence show an accumulation of hepatic stellate cells (HSCs), triggered by hypoxia-induced platelet-derived growth factor-BB (PDGF-BB), and proliferation in the tumor stroma, as well as an increase in VEGF-A expression in HSCs leads to HCC angiogenesis[54].
Interactions between normal tumor-suppressive microenvironment and hepatic stellate cells and normal liver fibroblasts have been reported[53]. One of the major factors in liver fibrosis and cirrhosis is activated HSCs[53]. The important paracrine interactions between activated HSCs and hepatocytes impact HCC proliferation and metastasis[57-59]. HSCs (also known as peri-sinusoidal cells), one of the components of the cellular tumor microenvironment in HCC, are responsible for collagen synthesis in the liver[51]. As liver damage occurs, activated HSCs accumulate in the ECM and induce hepatic fibrosis and hepatocarcinogenesis[51].
The exact molecular mechanisms of interactions between non-tumor stromal constituents (specifically macrophages) and hepatic cancer cells are unclear. Studies in mice have shown induced macrophage infiltration of alternatively activated phenotype M2 pro-tumor monocyte-derived macrophages into tumors developed in the chronically damaged livers of mice injected with carbon tetrachloride (CCl4) for 7 weeks[52]. Therefore, an inflamed liver background is favorably associated with increased cancer development[52].
Liver lineage studies have uncovered four maturational levels of cells that allow the liver to strike a perfect balance between cell gain and cell loss. These include mature hepatocytes, oval cells, bone marrow cells and hepato-pancreas stem cells[60]. These different levels of stem cells integrate to respond to loss of liver cells in the body in several ways, and are thus implicated in liver cirrhosis and HCC.
The cancer stem cell (CSC) theory has been proposed as an explanatory mechanism of HCC metastasis, progression and aggressiveness. CSCs, like regular stem cells, have self-renewing features and are capable of differentiating into tumor cells of varying phenotypes and through several pathways, partly accounting for the heterogeneous clinical presentation of HCC[61]. Previous research has successfully demonstrated that liver cells are directly involved in hepatocarcinogenesis[62], and transformation of these cells may give rise to CSCs. Some reports also suggest that cancer cells in HCC develop from dedifferentiation of mature hepatocytes rather than from uncontrolled proliferation of liver stem cells[63], with intrinsic factors (genetics, autoimmune diseases) contributing, and extrinsic factors (HBV, HCV, alcohol, AFB1) accounting for 70%-90% of the transformation of small hepatocyte-like progenitor cells to cancer cells of HCC[64]. Nevertheless, the correlation between stem-cell division and cancer risk cannot distinguish the effect of intrinsic factors from that of extrinsic factors.
Stem cells originating from the bone marrow, known as bone marrow-derived stem cells, have been demonstrated to be involved in the progression of HCC. Yavorkovsky et al[65] observed the biomarkers when liver trauma simulating HCC was induced with allyl alcohol and demonstrated that only bone marrow-derived stem cells were activated to respond to the trauma.
Stem cells originating from the canal of Hering (oval cells) are mobilized in chronic liver injury[66]. Oval cell biomarkers include γ-glutamyl transpeptidase, glutathione-S-transferase, OV6, α-fetoprotein, neural cell adhesion molecule 1, and chromogranin A[67]. The normal compensatory mechanisms that mobilize stem cells during liver injury are altered in HCC in such a way that promotes progression of the carcinogenic process.
Various models are being used to explain cancer development and intra-tumoral heterogeneity in HCC. These include CSCs, cancer cell plasticity and the clonal evolution model, to mention a few[68]. While the majority of heterogeneous tumor cells stay inactive[69], a small subgroup comprised of CSCs and cancer initiating cells, facilitate tumor development and growth[70-72]. Phenotypic plasticity of cancer cells, which allows conversion from cancer stem cell to non-CSC and vice versa, is one of the proposed mechanisms that may be responsible for the intra-tumoral heterogeneity found in solid tumors[73]. According to previous studies, underlying molecular mechanisms of EMT and CSCs were found to be associated with a high risk for poor prognosis of cancer patients[68].
During normal development, EMT plays a crucial role in organogenesis[74]. At the time of early embryogenesis, through EMT, cell-cell adhesive epithelial cells undergo trans-differentiation and become mobile mesenchymal cells that can migrate, and invade into neighboring tissues and have increased resistance to apoptosis[73-75]. On the other hand, mesenchymal cells can transform back to epithelial cells via the process of mesenchymal-to-epithelial transition, or MET. These reprogramming processes emphasize the epithelial cell plasticity[73] facilitating metastasis to distant and local anatomical sites via increased invasive and migratory functions[68,73,74].
The CSC hypothesis in cancer remains controversial. While some studies have demonstrated the CSC hypothesis in brain, skin, and colon cancers, others have suggested that tumor-initiating cells (TICs, CSC-like cells) exist instead of CSCs in other cancer types[69,76]. Some studies have demonstrated that HCC arises from either TICs or hepatocytes. According to previous research based on drug-treated HCC patients, TICs are the main trigger of tumor development and progression[61]. However, the exact origin of TICs is still not completely understood.
Liver CSCs (LCSCs) have many analogous characteristics to normal liver stem/progenitor cells. In addition to self-renewal and tumorigenesis abilities, LCSCs have been implicated in therapeutic drug resistance and relapse in patients[77]. Long-term inflammatory microenvironment, caused by HBV or HCV, chronic alcohol consumption or NASH, and progression of HCC[35] highly contribute to reprogramming of non-CSC into CSCs[78] and the acquisition of CSC-like properties by non-CSCs through carcinogenic dedifferentiation[79].
Identification of tumor - specific biomarkers and discovery of molecular mechanisms are crucial to establish effective therapeutic and early detection strategies for cancer[60,80]. Through the work of several investigators, we are now familiar with some of the putative surface markers for liver CSCs, including epithelial cell adhesion molecule (EpCAM)[81], CD90[82], CD133[83], CD44[84], and CD13[85]. However, there is still uncertainty as to which cell surface markers best identify CSCs in different cancers.
Therapeutic approaches involving inhibitor targeting of signaling pathways, such as Wnt, hedgehog (Hh), TGF-β and Notch signaling, have been shown to diminish LCSC self-reprogramming, metastasis and tumor proliferation[60]. Moreover, drugs designed to modulate cross-talk between CSCs and cancer cells and the tumor microenvironment may have success in inhibiting tumor growth[60]. Other efforts to target LCSC markers and epigenetic modulators could produce promising results.
Another established mechanism of HCC progression is senescence bypass. The liver cells have powerful regenerative abilities. Progenitor cells rapidly divide to restore the balance offset by tissue loss. However, these cells reach a Hayflick limit, a point where cell division is permanently arrested after a number of divisions. The cells are said to exhibit replicative senescence. Replicative senescence can be due to (1) shortening of telomeres in the absence of telomerase, thereby halting cell division; (2) telomeric-independent oncogene activation; and (3) elevated ROS. Telomere shortening triggers the DNA damage response, which is thought to activate several signaling pathways, including the p53-p21‐pRB pathway, bringing replication to a halt. Non-telomeric senescence utilizes both ATM/Chk/p53 and p16-pRB pathways. Oncogene-induced senescence is closely associated with DNA hyper-replication that succeeds oncogenic activation. Several oncogenic pathways have been reported to be involved in triggering oncogene-induced senescence, including activated Ras, c-myc or Wnt/β-catenin[86,87]. Given the tumor suppressing tendency of cell senescence, bypassing it can result in the proliferation of genetically mutated cells, further DNA instability and propagation of HCC. Researchers have been exploring cell senescence induction as a potential strategy in cancer therapeutics.
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) plays a critical role in how cells respond to stressful stimuli, including infections and ultraviolet radiation[88]. Inflammatory responses mediated by the NF-kB signaling pathway have been reported to be involved in perpetuating the malignant state. NF-kB activation suppresses apoptosis[89], activates EMT[90], represses maspin (a metastasis suppressor gene)[91], and targets VEGF and other angiogenic factors required in forming new blood vessels that supply HCC[90].
There has been considerable advancement in understanding the fundamental epigenetic mechanisms in gene expression, which is now allowing for the development of novel insights into chronic liver disease epigenetic control[92]. For example, loss of DNA methylation has been pointed to as potential diagnostic markers in HCC progression. Some studies have also suggested that non-coding RNAs (ncRNAs) such as microRNAs (miRNAs), small non-coding RNAs (sncRNAs), long non-coding RNAs (lncRNAs), RNA interference (RNAi), small interfering RNAs (siRNAs), and piwi-interacting RNAs (piRNAs), could serve as therapeutic strategies for HCC[93,94]. Several preclinical studies have shown that significant tumor suppression can be achieved by modulating ncRNAs[93,94].
Other interesting factors that have been shown to correlate with HCC patient prognosis are molecular stratification and mutational signatures[95,96]. There are different classes of liver cancer based on varying molecular features and cell of origin[96]. It has been shown that each stratification has a different implication on patient prognosis[95-97]. For example, proliferative subclasses result in a more aggressive phenotype and poorer patient outcomes[97].
In terms of mutational signature, there are several genetic alterations that are promising for therapeutic interventions. For instance, approximately 15% of HCCs harbor amplifications at 11q13 and 6p21[95]. Currently, a better understanding of how molecular stratification and mutational signatures affect HCC progression is still needed before they can be used as therapeutic strategies or biomarkers in a clinical setting.
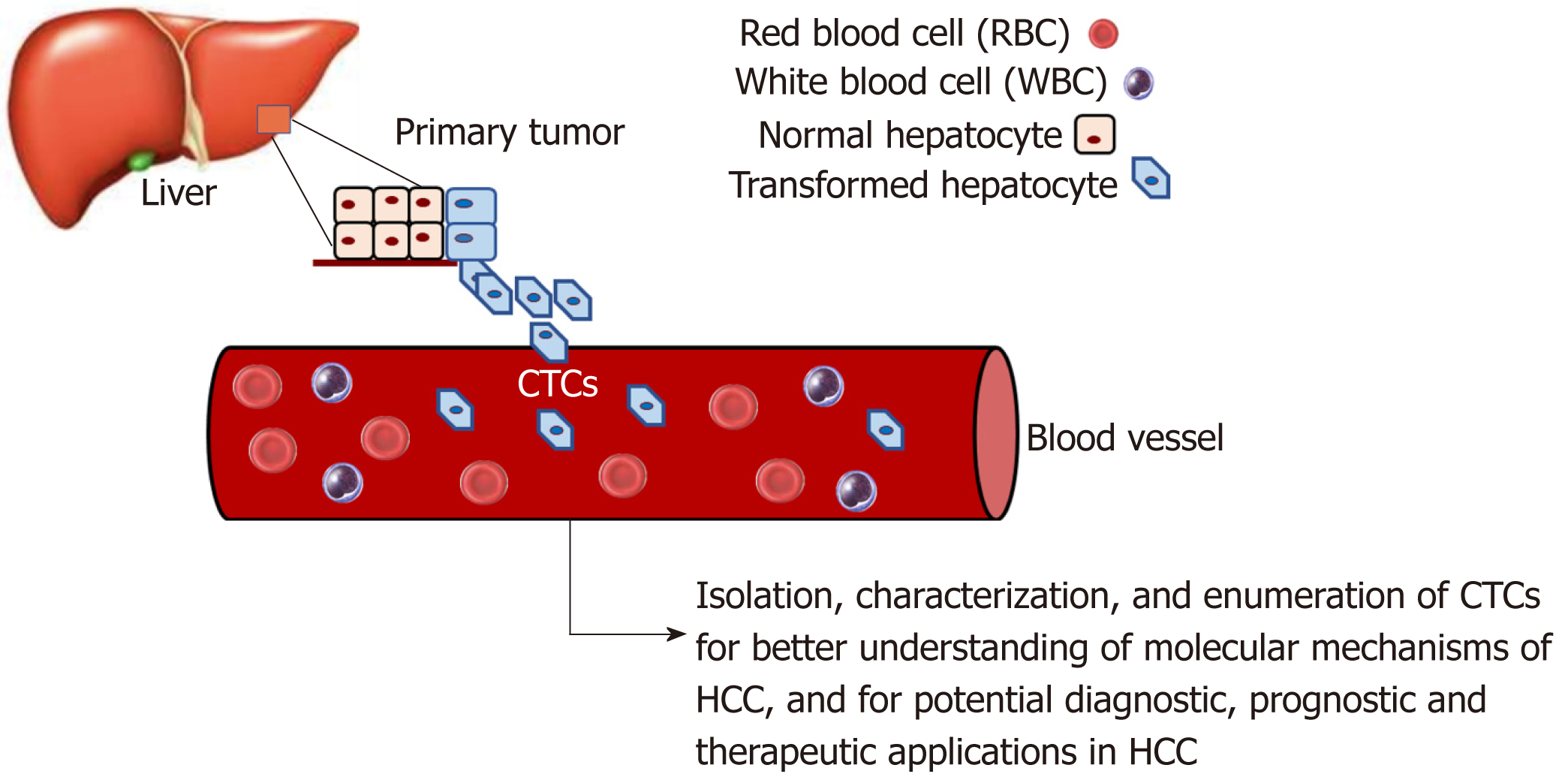
There is increasing evidence that CTCs play an important role in HCC progression. CTCs are considered an intermediate stage of metastasis. They are cancer cells that have dissociated from the primary tumor, enter circulation, and may subsequently form metastatic lesions[98,99]. There is strong interest in studying CTC biology to understand their molecular mechanisms and how they affect metastasis. Moreover, CTCs have clinical applications, such as diagnostic applications circumventing the need for invasive tissue biopsies[100].
As illustrated in Figure 2, a considerable amount of data has been and can be gathered through the study of CTCs in HCC. Through isolation, characterization and correlation of CTCs with pathological features, as well as disease stage, researchers have shown that a greater CTC count in patient blood is associated with poorer HCC prognosis[53,101-106]. As there is a current lack of reliable biomarkers for the early, non-invasive detection of HCC, a few studies have demonstrated the potential feasibility of using CTCs as a possible diagnostic marker[104,107-110]. Although CTCs are found in very low numbers in the blood[110,111], the advent of new single-cell sequencing technologies and methods to successfully expand CTCs in long-term cultures has enabled their molecular profiling and characterization[104,110,112-115], hence making CTCs promising diagnostic biomarkers in HCC.
Additionally, several immune mechanisms have been observed to be dysregulated during HCC progression[116]. For instance, HCC is a cancer arising against the backdrop of an inflammatory state in the liver. HBV, HCV, and many of the other etiological factors discussed earlier in this article give rise to chronic inflammation. In turn, this leads to the production of inhibitory cytokines such as IL-10 and transforming growth factor beta (TGFβ), which dampen the immune response and favor tumor growth[117-119]. During HCC progression, regulatory T cells and myeloid-derived suppressor cells are also recruited to the tumor site as a result of these cytokine secretions, adding to the already immunosuppressive environment[116,118,120]. Lastly, it has been found that several checkpoint inhibitor receptors such as CTLA4 and PD-1 are commonly upregulated in immune cells in the HCC setting. With more checkpoint inhibitor receptors being expressed on these immune cells, they are unable to become active and counterattack tumor cells for clearance from the body[119,121,122].
Ironically, a fundamental characteristic of the liver may also permit tumorigenesis. The liver is immunologically tolerant. This is because the liver is in constant contact with microbiota from the gut and therefore needs to have a tolerant immune response so that it does not become hyperactivated[116,119]. This, in conjunction with the supplementary immunosuppressive mechanisms that develop during HCC progression, enable tumors to grow. This irony makes exploration of immunotherapy for HCC a challenging but potentially exciting prospect to consider.
Indeed, several studies have shown that immune checkpoint inhibitors have had some efficacy in preclinical and early stage clinical trials of HCC. Additionally, the fact that sorafenib, the current first line treatment for advanced HCC, has been noted to exhibit some immunomodulatory effects, seems to suggest the potential efficacy of immunotherapeutic strategies in HCC[122-125].
Tumor cells and the cells in the tumor microenvironment are affected by stress physiology[126]. Neuroeffector molecules can reach the tumor microenvironment via the circulatory system or nerve fibers. During threatening or stressful life circumstances, there is an activation of the sympathetic nervous system, which mediates fight‐or‐flight stress responses. The hypothalamus-pituitary-adrenal axis is responsible for mediating withdrawal responses from more profound and overwhelming threats. The neurotransmitter norepinephrine is released by the sympathetic nervous system nerve fibers, while the major stress hormone cortisol is released into the blood by the adrenal gland upon hypothalamus-pituitary-adrenal activation[126]. Cortisol is secreted by the adrenal glands. However, its secretion is regulated by the pituitary gland. Under conditions of severe psychological stress, corticotropin-releasing factor upregulates the secretion of adrenocorticotropic hormone by the pituitary gland. The adrenocorticotropic hormone in turn upregulates the secretion of cortisol[127]. Cortisol can reach the tumor microenvironment via circulating blood, while norepinephrine can do so by being released from nerve fibers (carried by blood vessels), which are recruited in larger amounts by some tumors when these tumors secrete nerve growth factors. Cortisol and norepinephrine binding to the intracellular glucocorticoid receptor (located within the cell) or the beta‐adrenergic receptor (located on the cell surface) can trigger cellular responses[126].
It has long been recognized that psychosocial conditions affect the progression of some cancers[126]. In fact, epidemiological studies have shown that there is an accelerated progression of various cancers among patients with high stress levels or low social support[126]. While the relationship between stress and cancer development is not fully understood, some studies have shown that psychological stress causes abnormal immune responses, which are associated with cancer pathogenesis[128,129]. Cortisol release has been linked to the development and progression of, and survival from various cancers[130-134]. Cortisol inhibits immune responses, which allow cancer cells to evade the immune system[127,134].
Prostate cancer patients have also been shown to have high cortisol levels compared to low risk individuals[131], and breast cancer patients were reported to have high serum cortisol levels, which can be downregulated by emotional support[135].
Serum levels of cortisol have been shown to be higher in HCC patients than in healthy individuals[134]. Studies by Wu and colleagues have shown that exposing HCC cell cultures to cortisol represses p53 expression by upregulating expression of the p53 suppressor Bcl2L12. This suggests that cortisol is a factor that plays a role in the development of HCC[134]. Consequently, it has been suggested that cortisol may be a therapeutic target in HCC treatment[134].
Oxytocin is a neuropeptide hormone produced by hypothalamic neurons and has multiple roles in the central nervous system. While oxytocin is best known for its role in the female reproductive system (milk ejection), further research has shown that oxytocin also plays important roles in complex social behaviors, including stress and trust, anxiety, social interaction and bonding, and parental care, as well as in neuropsychiatric disorders linked to such social behaviors[136,137]. Oxytocin and its receptor have more recently been shown to play roles in some cancers[138-142].
Cortisol has also been linked to some functions of oxytocin[140]. Some studies have shown that higher oxytocin levels and increased social support (a known prognostic player in cancer) are associated with diminished effects of stress. In a study by Mankorious and colleagues, it was shown that there is a cross-talk network between oxytocin and cortisol at the molecular level, where the carcinogenic effect of cortisol was reversed by oxytocin via autophagy in human ovarian cancer cells in vitro[140].
It is known that the effects of oxytocin in cancer may depend on cell type, hormone concentration, its interactions with other hormones in the microenvironment, and the location of its receptor on the cell membrane[137]. Unpublished work from our laboratory analyzing data from sequenced HCC and pancreatic cancer cases in the TCGA dataset showed that genetic alterations in the oxytocin and oxytocin receptor genes were associated with lower median months of overall survival. It would be interesting to determine whether there could be an interaction between oxytocin and cortisol, which could be involved in a potential neural regulation of HCC as well as other gastrointestinal cancers.
There has been minimal improvement in the prognosis for HCC patients over the past two decades. The detailed molecular mechanisms of HCC progression remain unclear, and there is an urgent need to better understand the mechanisms underlying HCC progression so as to develop novel and effective therapeutic strategies and reliable prognostic biomarkers. Further, a better understanding of mechanisms of HCC development can further aid efforts at developing effective preventative strategies. This review provides a summary of some of the mechanisms of HCC etiology, and some of the well-established as well as a few recently proposed mechanisms of HCC progression.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo K, Huang YQ, Sun XY S-Editor: Ma RY L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55820] [Article Influence: 7974.3] [Reference Citation Analysis (132)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12187] [Article Influence: 1523.4] [Reference Citation Analysis (3)] |
| 3. | Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 532] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 4. | Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore). 2017;96:e5904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 5. | Medavaram S, Zhang Y. Emerging therapies in advanced hepatocellular carcinoma. Exp Hematol Oncol. 2018;7:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13166] [Article Influence: 1880.9] [Reference Citation Analysis (4)] |
| 7. | Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282-5294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (4)] |
| 8. | Daher S, Massarwa M, Benson AA, Khoury T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J Clin Transl Hepatol. 2018;6:69-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 9. | Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J. Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol. 2018;48:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Keating GM. Sorafenib: A Review in Hepatocellular Carcinoma. Target Oncol. 2017;12:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 11. | Sanz-Cameno P, Trapero-Marugán M, Chaparro M, Jones EA, Moreno-Otero R. Angiogenesis: from chronic liver inflammation to hepatocellular carcinoma. J Oncol. 2010;2010:272170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Sampat KR, O'Neil B. Antiangiogenic therapies for advanced hepatocellular carcinoma. Oncologist. 2013;18:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Personeni N, Pressiani T, Santoro A, Rimassa L. Regorafenib in hepatocellular carcinoma: latest evidence and clinical implications. Drugs Context. 2018;7:212533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Spallanzani A, Orsi G, Andrikou K, Gelsomino F, Rimini M, Riggi L, Cascinu S. Lenvatinib as a therapy for unresectable hepatocellular carcinoma. Expert Rev Anticancer Ther. 2018;18:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Tarocchi M, Polvani S, Marroncini G, Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol. 2014;20:11630-11640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 17. | Ortega-Prieto AM, Dorner M. Immune Evasion Strategies during Chronic Hepatitis B and C Virus Infection. Vaccines (Basel). 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 18. | Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R, Sarin SK. From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer. 2013;2:367-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Bartosch B, Thimme R, Blum HE, Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol. 2009;51:810-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Alzahrani B, Iseli TJ, Hebbard LW. Non-viral causes of liver cancer: does obesity led inflammation play a role? Cancer Lett. 2014;345:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Sorrentino P, D'Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Hann HW, Kim CY, London WT, Blumberg BS. Increased serum ferritin in chronic liver disease: a risk factor for primary hepatocellular carcinoma. Int J Cancer. 1989;43:376-379. [PubMed] |
| 23. | Asare GA, Bronz M, Naidoo V, Kew MC. Synergistic interaction between excess hepatic iron and alcohol ingestion in hepatic mutagenesis. Toxicology. 2008;254:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Siriwardana RC, Niriella MA, Dassanayake A, Ediriweera D, Gunetilleke B, Sivasundaram T, de Silva J. Association of Serum Ferritin with Diabetes and Alcohol in Patients with Non-Viral Liver Disease-Related Hepatocellular Carcinoma. Liver Cancer. 2017;6:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Campbell PT, Newton CC, Freedman ND, Koshiol J, Alavanja MC, Beane Freeman LE, Buring JE, Chan AT, Chong DQ, Datta M, Gaudet MM, Gaziano JM, Giovannucci EL, Graubard BI, Hollenbeck AR, King L, Lee IM, Linet MS, Palmer JR, Petrick JL, Poynter JN, Purdue MP, Robien K, Rosenberg L, Sahasrabuddhe VV, Schairer C, Sesso HD, Sigurdson AJ, Stevens VL, Wactawski-Wende J, Zeleniuch-Jacquotte A, Renehan AG, McGlynn KA. Body Mass Index, Waist Circumference, Diabetes, and Risk of Liver Cancer for U.S. Adults. Cancer Res. 2016;76:6076-6083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Wei L, Li N, Wang G, Feng X, Lyu Z, Li X, Wen Y, Chen Y, Chen H, Chen S, Wu S, Dai M, He J. Waist Circumference Might Be a Predictor of Primary Liver Cancer: A Population-Based Cohort Study. Front Oncol. 2018;8:607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Hu M, Phan F, Bourron O, Ferré P, Foufelle F. Steatosis and NASH in type 2 diabetes. Biochimie. 2017;143:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Yan G, Wang X, Sun C, Zheng X, Wei H, Tian Z, Sun R. Chronic Alcohol Consumption Promotes Diethylnitrosamine-Induced Hepatocarcinogenesis via Immune Disturbances. Sci Rep. 2017;7:2567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Ramadori P, Cubero FJ, Liedtke C, Trautwein C, Nevzorova YA. Alcohol and Hepatocellular Carcinoma: Adding Fuel to the Flame. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Ogunwobi OO, Puszyk W, Dong HJ, Liu C. Epigenetic upregulation of HGF and c-Met drives metastasis in hepatocellular carcinoma. PLoS One. 2013;8:e63765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Singh AK, Kumar R, Pandey AK. Hepatocellular Carcinoma: Causes, Mechanism of Progression and Biomarkers. Curr Chem Genom Transl Med. 2018;12:9-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013;22:305-310. [PubMed] |
| 33. | Wu HC, Santella R. The role of aflatoxins in hepatocellular carcinoma. Hepat Mon. 2012;12:e7238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Tansel A, Katz LH, El-Serag HB, Thrift AP, Parepally M, Shakhatreh MH, Kanwal F. Incidence and Determinants of Hepatocellular Carcinoma in Autoimmune Hepatitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1207-1217.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1872] [Article Influence: 208.0] [Reference Citation Analysis (4)] |
| 36. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1382] [Article Influence: 197.4] [Reference Citation Analysis (0)] |
| 37. | Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1104] [Cited by in RCA: 1460] [Article Influence: 208.6] [Reference Citation Analysis (0)] |
| 38. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6575] [Cited by in RCA: 7911] [Article Influence: 494.4] [Reference Citation Analysis (0)] |
| 39. | Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4715] [Cited by in RCA: 6230] [Article Influence: 566.4] [Reference Citation Analysis (0)] |
| 40. | Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25:675-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 41. | Huaman J, Bach C, Ilboudo A, Ogunwobi OO, Liu C. Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma. Liu C. Precision Molecular Pathology of Liver Cancer. Cham: Springer International Publishing 2018; 131-152. |
| 42. | Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 815] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 43. | Tiwari N, Gheldof A, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. 2012;22:194-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 398] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 44. | Chen L, Guo P, He Y, Chen Z, Chen L, Luo Y, Qi L, Liu Y, Wu Q, Cui Y, Fang F, Zhang X, Song T, Guo H. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9:513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 45. | Long L, Xiang H, Liu J, Zhang Z, Sun L. ZEB1 mediates doxorubicin (Dox) resistance and mesenchymal characteristics of hepatocarcinoma cells. Exp Mol Pathol. 2019;106:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Qu W, Wen X, Su K, Gou W. MiR-552 promotes the proliferation, migration and EMT of hepatocellular carcinoma cells by inhibiting AJAP1 expression. J Cell Mol Med. 2019;23:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Shi C, Chen Y, Chen Y, Yang Y, Bing W, Qi J. CD4+ CD25+ regulatory T cells promote hepatocellular carcinoma invasion via TGF-β1-induced epithelial-mesenchymal transition. Onco Targets Ther. 2018;12:279-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Xia C, Zhang XY, Liu W, Ju M, Ju Y, Bu YZ, Wang W, Shao H. LINC00857 contributes to hepatocellular carcinoma malignancy via enhancing epithelial-mesenchymal transition. J Cell Biochem. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Zhang B, Shi D, Zhang X, Liang G, Liu W, Qiao S. FK866 inhibits the epithelial-mesenchymal transition of hepatocarcinoma MHCC97-H cells. Oncol Lett. 2018;16:7231-7238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Das DK, Durojaiye V, Ilboudo A, Naidoo MK, Ogunwobi O. A "Patient-Like" Orthotopic Syngeneic Mouse Model of Hepatocellular Carcinoma Metastasis. J Vis Exp. 2015;e52858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 51. | Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21:35-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 52. | Delire B, Henriet P, Lemoine P, Leclercq IA, Stärkel P. Chronic liver injury promotes hepatocarcinoma cell seeding and growth, associated with infiltration by macrophages. Cancer Sci. 2018;109:2141-2152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Affo S, Yu LX, Schwabe RF. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu Rev Pathol. 2017;12:153-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 511] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 54. | Lu Y, Lin N, Chen Z, Xu R. Hypoxia-induced secretion of platelet-derived growth factor-BB by hepatocellular carcinoma cells increases activated hepatic stellate cell proliferation, migration and expression of vascular endothelial growth factor-A. Mol Med Rep. 2015;11:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Galiè M, Sorrentino C, Montani M, Micossi L, Di Carlo E, D'Antuono T, Calderan L, Marzola P, Benati D, Merigo F, Orlando F, Smorlesi A, Marchini C, Amici A, Sbarbati A. Mammary carcinoma provides highly tumourigenic and invasive reactive stromal cells. Carcinogenesis. 2005;26:1868-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 57. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2164] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 58. | Amann T, Bataille F, Spruss T, Mühlbauer M, Gäbele E, Schölmerich J, Kiefer P, Bosserhoff AK, Hellerbrand C. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 2009;100:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 59. | Faouzi S, Lepreux S, Bedin C, Dubuisson L, Balabaud C, Bioulac-Sage P, Desmoulière A, Rosenbaum J. Activation of cultured rat hepatic stellate cells by tumoral hepatocytes. Lab Invest. 1999;79:485-493. [PubMed] |
| 60. | Wang N, Wang S, Li MY, Hu BG, Liu LP, Yang SL, Yang S, Gong Z, Lai PBS, Chen GG. Cancer stem cells in hepatocellular carcinoma: an overview and promising therapeutic strategies. Ther Adv Med Oncol. 2018;10:1758835918816287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 61. | Fransvea E, Paradiso A, Antonaci S, Giannelli G. HCC heterogeneity: molecular pathogenesis and clinical implications. Cell Oncol. 2009;31:227-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 62. | Williams GM, Gebhardt R, Sirma H, Stenbäck F. Non-linearity of neoplastic conversion induced in rat liver by low exposures to diethylnitrosamine. Carcinogenesis. 1993;14:2149-2156. [PubMed] |
| 63. | Bralet MP, Pichard V, Ferry N. Demonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosamine-treated rats. Hepatology. 2002;36:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529:43-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 441] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 65. | Yavorkovsky L, Lai E, Ilic Z, Sell S. Participation of small intraportal stem cells in the restitutive response of the liver to periportal necrosis induced by allyl alcohol. Hepatology. 1995;21:1702-1712. [PubMed] |
| 66. | Zhang Y, Bai XF, Huang CX. Hepatic stem cells: existence and origin. World J Gastroenterol. 2003;9:201-204. [PubMed] |
| 67. | Mikhail S, He AR. Liver cancer stem cells. Int J Hepatol. 2011;2011:486954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Jayachandran A, Dhungel B, Steel JC. Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma. J Hematol Oncol. 2016;9:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 69. | Machida K. Existence of cancer stem cells in hepatocellular carcinoma: myth or reality? Hepatol Int. 2017;11:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1172] [Cited by in RCA: 1189] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 71. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [PubMed] |
| 72. | Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3316] [Cited by in RCA: 3387] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 73. | Cabrera MC, Hollingsworth RE, Hurt EM. Cancer stem cell plasticity and tumor hierarchy. World J Stem Cells. 2015;7:27-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 74. | Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 794] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 75. | Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4877] [Cited by in RCA: 5118] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 76. | Wang T, Shigdar S, Gantier MP, Hou Y, Wang L, Li Y, Shamaileh HA, Yin W, Zhou SF, Zhao X, Duan W. Cancer stem cell targeted therapy: progress amid controversies. Oncotarget. 2015;6:44191-44206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 77. | Vu NB, Nguyen TT, Tran LC, Do CD, Nguyen BH, Phan NK, Pham PV. Doxorubicin and 5-fluorouracil resistant hepatic cancer cells demonstrate stem-like properties. Cytotechnology. 2013;65:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Rao S, Zaidi S, Banerjee J, Jogunoori W, Sebastian R, Mishra B, Nguyen BN, Wu RC, White J, Deng C, Amdur R, Li S, Mishra L. Transforming growth factor-β in liver cancer stem cells and regeneration. Hepatol Commun. 2017;1:477-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 79. | Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer. 2017;16:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 80. | Yoon SK. The biology of cancer stem cells and its clinical implication in hepatocellular carcinoma. Gut Liver. 2012;6:29-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 958] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 82. | Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 927] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 83. | Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 922] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 84. | Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, Li J. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 85. | Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, Barnard GF, Doki Y, Mori M. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 86. | Merle P, Trepo C. Molecular mechanisms underlying hepatocellular carcinoma. Viruses. 2009;1:852-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre' M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d'Adda di Fagagna F. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1381] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 88. | Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 494] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 89. | Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787-789. [PubMed] |
| 90. | Bassères DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817-6830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 554] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 91. | Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 92. | Hardy T, Mann DA. Epigenetics in liver disease: from biology to therapeutics. Gut. 2016;65:1895-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 93. | Xiao Z, Shen J, Zhang L, Li M, Hu W, Cho C. Therapeutic targeting of noncoding RNAs in hepatocellular carcinoma: Recent progress and future prospects. Oncol Lett. 2018;15:3395-3402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Peng L, Yuan XQ, Zhang CY, Peng JY, Zhang YQ, Pan X, Li GC. The emergence of long non-coding RNAs in hepatocellular carcinoma: an update. J Cancer. 2018;9:2549-2558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 95. | Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 839] [Article Influence: 104.9] [Reference Citation Analysis (2)] |
| 96. | Letouzé E, Shinde J, Renault V, Couchy G, Blanc JF, Tubacher E, Bayard Q, Bacq D, Meyer V, Semhoun J, Bioulac-Sage P, Prévôt S, Azoulay D, Paradis V, Imbeaud S, Deleuze JF, Zucman-Rossi J. Mutational signatures reveal the dynamic interplay of risk factors and cellular processes during liver tumorigenesis. Nat Commun. 2017;8:1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 97. | Hagel M, Miduturu C, Sheets M, Rubin N, Weng W, Stransky N, Bifulco N, Kim JL, Hodous B, Brooijmans N, Shutes A, Winter C, Lengauer C, Kohl NE, Guzi T. First Selective Small Molecule Inhibitor of FGFR4 for the Treatment of Hepatocellular Carcinomas with an Activated FGFR4 Signaling Pathway. Cancer Discov. 2015;5:424-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 98. | Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 2017;31:1827-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 99. | Gallerani G, Fici P, Fabbri F. Circulating Tumor Cells: Back to the Future. Front Oncol. 2017;6:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | van de Stolpe A, Pantel K, Sleijfer S, Terstappen LW, den Toonder JM. Circulating tumor cell isolation and diagnostics: toward routine clinical use. Cancer Res. 2011;71:5955-5960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 101. | Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 869] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 102. | Fan JL, Yang YF, Yuan CH, Chen H, Wang FB. Circulating Tumor Cells for Predicting the Prognostic of Patients with Hepatocellular Carcinoma: A Meta Analysis. Cell Physiol Biochem. 2015;37:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 103. | Li J, Han X, Yu X, Xu Z, Yang G, Liu B, Xiu P. Clinical applications of liquid biopsy as prognostic and predictive biomarkers in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. J Exp Clin Cancer Res. 2018;37:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 104. | Okajima W, Komatsu S, Ichikawa D, Miyamae M, Ohashi T, Imamura T, Kiuchi J, Nishibeppu K, Arita T, Konishi H, Shiozaki A, Morimura R, Ikoma H, Okamoto K, Otsuji E. Liquid biopsy in patients with hepatocellular carcinoma: Circulating tumor cells and cell-free nucleic acids. World J Gastroenterol. 2017;23:5650-5668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 105. | Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, Riethdorf S, Wege H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013;133:2165-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 106. | von Felden J, Schulze K, Krech T, Ewald F, Nashan B, Pantel K, Lohse AW, Riethdorf S, Wege H. Circulating tumor cells as liquid biomarker for high HCC recurrence risk after curative liver resection. Oncotarget. 2017;8:89978-89987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 107. | Guo W, Sun YF, Shen MN, Ma XL, Wu J, Zhang CY, Zhou Y, Xu Y, Hu B, Zhang M, Wang G, Chen WQ, Guo L, Lu RQ, Zhou CH, Zhang X, Shi YH, Qiu SJ, Pan BS, Cao Y, Zhou J, Yang XR, Fan J. Circulating Tumor Cells with Stem-Like Phenotypes for Diagnosis, Prognosis, and Therapeutic Response Evaluation in Hepatocellular Carcinoma. Clin Cancer Res. 2018;24:2203-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 108. | Qi LN, Xiang BD, Wu FX, Ye JZ, Zhong JH, Wang YY, Chen YY, Chen ZS, Ma L, Chen J, Gong WF, Han ZG, Lu Y, Shang JJ, Li LQ. Circulating Tumor Cells Undergoing EMT Provide a Metric for Diagnosis and Prognosis of Patients with Hepatocellular Carcinoma. Cancer Res. 2018;78:4731-4744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 109. | Sun C, Liao W, Deng Z, Li E, Feng Q, Lei J, Yuan R, Zou S, Mao Y, Shao J, Wu L, Zhang C. The diagnostic value of assays for circulating tumor cells in hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2017;96:e7513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 110. | Yin CQ, Yuan CH, Qu Z, Guan Q, Chen H, Wang FB. Liquid Biopsy of Hepatocellular Carcinoma: Circulating Tumor-Derived Biomarkers. Dis Markers. 2016;2016:1427849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 111. | Alvarez Cubero MJ, Lorente JA, Robles-Fernandez I, Rodriguez-Martinez A, Puche JL, Serrano MJ. Circulating Tumor Cells: Markers and Methodologies for Enrichment and Detection. Methods Mol Biol. 2017;1634:283-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 112. | D'Avola D, Villacorta-Martin C, Martins-Filho SN, Craig A, Labgaa I, von Felden J, Kimaada A, Bonaccorso A, Tabrizian P, Hartmann BM, Sebra R, Schwartz M, Villanueva A. High-density single cell mRNA sequencing to characterize circulating tumor cells in hepatocellular carcinoma. Sci Rep. 2018;8:11570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 113. | Chen J, Cao SW, Cai Z, Zheng L, Wang Q. Epithelial-mesenchymal transition phenotypes of circulating tumor cells correlate with the clinical stages and cancer metastasis in hepatocellular carcinoma patients. Cancer Biomark. 2017;20:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 114. | Li YM, Xu SC, Li J, Han KQ, Pi HF, Zheng L, Zuo GH, Huang XB, Li HY, Zhao HZ, Yu ZP, Zhou Z, Liang P. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013;4:e831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 115. | Vona G, Estepa L, Béroud C, Damotte D, Capron F, Nalpas B, Mineur A, Franco D, Lacour B, Pol S, Bréchot C, Paterlini-Bréchot P. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004;39:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 116. | Lee S, Loecher M, Iyer R. Immunomodulation in hepatocellular cancer. J Gastrointest Oncol. 2018;9:208-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 117. | Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62:1420-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 118. | Nishida N, Kudo M. Immunological Microenvironment of Hepatocellular Carcinoma and Its Clinical Implication. Oncology. 2017;92 Suppl 1:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 119. | Sachdeva M, Chawla YK, Arora SK. Immunology of hepatocellular carcinoma. World J Hepatol. 2015;7:2080-2090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 120. | Zhao F, Korangy F, Greten TF. Cellular immune suppressor mechanisms in patients with hepatocellular carcinoma. Dig Dis. 2012;30:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Wellenstein MD, de Visser KE. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity. 2018;48:399-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 438] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 122. | Lugade AA, Kalathil S, Miller A, Iyer R, Thanavala Y. High immunosuppressive burden in advanced hepatocellular carcinoma patients: Can effector functions be restored? Oncoimmunology. 2013;2:e24679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 123. | Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 124. | Lin YY, Tan CT, Chen CW, Ou DL, Cheng AL, Hsu C. Immunomodulatory Effects of Current Targeted Therapies on Hepatocellular Carcinoma: Implication for the Future of Immunotherapy. Semin Liver Dis. 2018;38:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 125. | Mukaida N, Nakamoto Y. Emergence of immunotherapy as a novel way to treat hepatocellular carcinoma. World J Gastroenterol. 2018;24:1839-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 126. | Cole SW. New challenges in psycho-oncology: Neural regulation of the cancer genome. Psychooncology. 2018;27:2305-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 127. | Dong T, Zhi L, Bhayana B, Wu MX. Cortisol-induced immune suppression by a blockade of lymphocyte egress in traumatic brain injury. J Neuroinflammation. 2016;13:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 128. | Lang MJ, David V, Giese-Davis J. The Age Conundrum: A Scoping Review of Younger Age or Adolescent and Young Adult as a Risk Factor for Clinical Distress, Depression, or Anxiety in Cancer. J Adolesc Young Adult Oncol. 2015;4:157-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 129. | Idris SZ, Hassan N, Lee LJ, Md Noor S, Osman R, Abdul-Jalil M, Nordin AJ, Abdullah M. Increased regulatory T cells in acute lymphoblastic leukemia patients. Hematology. 2015;20:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 130. | Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30 Suppl:S163-S170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 131. | Fabre B, Grosman H, Gonzalez D, Machulsky NF, Repetto EM, Mesch V, Lopez MA, Mazza O, Berg G. Prostate Cancer, High Cortisol Levels and Complex Hormonal Interaction. Asian Pac J Cancer Prev. 2016;17:3167-3171. [PubMed] |
| 132. | Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 302] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 133. | Schrepf A, Thaker PH, Goodheart MJ, Bender D, Slavich GM, Dahmoush L, Penedo F, DeGeest K, Mendez L, Lubaroff DM, Cole SW, Sood AK, Lutgendorf SK. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology. 2015;53:256-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 134. | Wu W, Liu S, Liang Y, Zhou Z, Bian W, Liu X. Stress Hormone Cortisol Enhances Bcl2 Like-12 Expression to Inhibit p53 in Hepatocellular Carcinoma Cells. Dig Dis Sci. 2017;62:3495-3500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 135. | Webster S, Chandrasekaran S, Vijayaragavan R, Sethu G. Impact of Emotional Support on Serum Cortisol in Breast Cancer Patients. Indian J Palliat Care. 2016;22:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 136. | Scerbo MJ, Gerdes JM. Bonding With β-Cells-A Role for Oxytocin in Glucose Handling. Diabetes. 2017;66:256-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 137. | Lerman B, Harricharran T, Ogunwobi OO. Oxytocin and cancer: An emerging link. World J Clin Oncol. 2018;9:74-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 138. | Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Oxytocin inhibits the proliferation of MDA-MB231 human breast-cancer cells via cyclic adenosine monophosphate and protein kinase A. Int J Cancer. 1997;72:340-344. [PubMed] |
| 139. | Xu H, Fu S, Chen Q, Gu M, Zhou J, Liu C, Chen Y, Wang Z. The function of oxytocin: a potential biomarker for prostate cancer diagnosis and promoter of prostate cancer. Oncotarget. 2017;8:31215-31226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 140. | Mankarious A, Dave F, Pados G, Tsolakidis D, Gidron Y, Pang Y, Thomas P, Hall M, Karteris E. The pro-social neurohormone oxytocin reverses the actions of the stress hormone cortisol in human ovarian carcinoma cells in vitro. Int J Oncol. 2016;48:1805-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 141. | Lindblad M, García Rodríguez LA, Chandanos E, Lagergren J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br J Cancer. 2006;94:136-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Skinner HG, Michaud DS, Colditz GA, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev. 2003;12:433-438. [PubMed] |