Published online May 14, 2019. doi: 10.3748/wjg.v25.i18.2251
Peer-review started: January 24, 2019
First decision: March 14, 2019
Revised: March 22, 2019
Accepted: May 2, 2019
Article in press: May 3, 2019
Published online: May 14, 2019
Processing time: 110 Days and 16.1 Hours
The role of prophylactic clipping for the prevention of delayed polypectomy bleeding (DPB) remains unclear and conclusions from prior meta-analyses are limited due to the inclusion of variety of resection techniques and polyp sizes.
To conduct a meta-analysis on the effect of clipping on DPB following endoscopic mucosal resection (EMR) of colorectal lesions ≥ 20 mm.
We performed a search of PubMed and the Cochrane library for studies comparing the effect of clipping vs no clipping on DPB following endoscopic resection. The Cochran Q test and I2 were used to test for heterogeneity. Pooling was conducted using a random-effects model.
Thirteen studies with a total of 7794 polyps were identified, of which data was available on 1701 cases of EMR of lesions ≥ 20 mm. Prophylactic clipping was associated with a lower rate of DPB (1.4%) when compared to no clipping (5.2%) (pooled OR: 0.24, 95%CI: 0.12-0.50, P < 0.001) following EMR of lesions ≥ 20 mm. There was no significant heterogeneity among the studies (I2 = 0%, P = 0.67).
Prophylactic clipping may reduce DPB following EMR of large colorectal lesions. Future trials are needed to further identify risk factors and stratify high risk cases in order to implement a cost-effective preventive strategy.
Core tip: The role of prophylactic clipping for the prevention of delayed polypectomy bleeding (DPB) remains unclear and conclusions from prior meta-analyses are limited due to the inclusion of variety of resection techniques and polyp sizes. We conducted a meta-analysis that included 7794 polyps in 1701 cases of endoscopic mucosal resection (EMR) and found that prophylactic clipping may reduce DPB following EMR of large colorectal lesions. Future trials are needed to further identify risk factors and stratify high risk cases in order to implement a cost-effective preventive strategy.
- Citation: Ayoub F, Westerveld DR, Forde JJ, Forsmark CE, Draganov PV, Yang D. Effect of prophylactic clip placement following endoscopic mucosal resection of large colorectal lesions on delayed polypectomy bleeding: A meta-analysis. World J Gastroenterol 2019; 25(18): 2251-2263
- URL: https://www.wjgnet.com/1007-9327/full/v25/i18/2251.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i18.2251
Colonoscopy has been shown to decrease the risk of death from colorectal cancer through the early identification and removal of pre-malignant or early stage cancerous lesions[1]. Endoscopic resection (ER) is the preferred first-line treatment for most of these superficial neoplasms and is associated with lower costs, morbidity, and mortality when compared to surgery[2,3]. Most colonic polyps are less than 10 mm and can be safely and effectively resected with conventional snare polypectomy. Conversely, larger lateral spreading lesions (LSLs) or sessile polyps, particularly those ≥ 20 mm in size, are usually removed by endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). While ESD continues to gain traction as an alternative for lesions with suspected superficial invasion or subtypes of non-granular LSLs[4,5], its definitive role in Western clinical practice is yet to be defined. Hence, wide-field EMR remains the preferred therapy for large non-cancerous colorectal lesions.
Bleeding is the most common adverse event following ER of colorectal lesions. Bleeding can be immediate (during the procedure) or delayed (post-operatively), and has been estimated to occur in 1%-6% of cases[6,7]. In the absence of coagulopathy, the risk of delayed polypectomy bleeding (DPB) is nearly negligible for the resection of small polyps < 10 mm. Conversely, the incidence of DPB increases with polyp size[8-10]. Several studies have evaluated the effect of prophylactic clipping on DPB following ER, with mixed results[11-13]. The inclusion of small polyps and different ER techniques (i.e., conventional polypectomy, EMR, ESD) significantly limits the interpretability of the data. The primary aim of this study was to conduct a meta-analysis on the effect of prophylactic clipping on DPB following EMR of colorectal lesions ≥ 20 mm. A secondary aim was to evaluate the effect of clipping on the incidence of adverse events following colorectal ER.
We identified studies through a literature search of two databases (MEDLINE through PubMed and the Cochrane Library) with the last search performed in January 2018. The PubMed search strategy was constructed by using the following string of search terms: (“clip” OR “clipping”) AND (“colon” OR “colorectal” OR “colonic”) AND (“endoscopic”). The search of the Cochrane library was conducted using similar search terms. A review of the reference list of included studies was performed to identify any relevant articles missed through the original search strategy. Titles and abstracts were screened by two investigators (F.A. and D.R.W) for relevance to the study. The full text of potentially eligible studies was subsequently reviewed by the two investigators (F.A and D.R.W). Disagreements were resolved by consensus or by consulting with a third investigator (D.Y).
Studies eligible for inclusion were: (1) Prospective or retrospective, case-control, or cohort studies and clinical trials; (2) studies reporting incidence of DPB following ER; and (3) those that included outcomes on both patients with prophylactic clipping vs non-clipping after resection. Exclusion criteria were: (1) Case reports; (2) single arm retrospective or prospective case series; (3) studies not reporting incidence of DPB; (4) reviews, commentaries, surveys; and (5) duplicate studies.
Data from each eligible study were extracted using a standardized data extraction sheet. The extracted data included: (1) Study authors; (2) year of publication; (3) setting (location); (4) study period; (5) patient demographics (age, gender); (6) number of patients/lesions; (7) lesion characteristics (size, location, morphology); (8) type of ER (conventional polypectomy, EMR, ESD); (9) incidence of adverse events, including DPB and perforation; and (10) follow-up period.
The aim of this study was to conduct a meta-analysis studying the effect of prophylactic clipping on DPB following EMR of colorectal lesions ≥ 20 mm. A secondary aim was to evaluate the effect of prophylactic clipping on the incidence of adverse events following colorectal ER. Prophylactic clipping was defined as endoscopic clipping performed with the aim of reducing the risk of delayed (post-operative) adverse events. DPB was defined as bleeding occurring post-operatively (upon conclusion of the ER and after scope withdrawal from the patient). Conventional polypectomy was defined as removal of a colorectal lesion with a forceps or snare without prior submucosal injection. In contrast, EMR was defined as resection achieved by first lifting the target lesion with a submucosal injection followed by snare polypectomy. ESD was defined as any resection in which submucosal dissection was performed.
For prospective trials, the quality of each study was assessed using the risk-of-bias tool as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0). The methodologic quality of retrospective studies was assessed using the Newcastle-Ottawa scale[14]. The quality of all studies was assessed by 3 investigators (F.A, D.R.W, J.J.F). Funnel plots were generated to evaluate for any potential publication bias. Visual inspection of the funnel plot was used detect significant publication bias when less than 10 studies were available for meta-analysis as recommended by the Cochrane Handbook. Egger’s regression test was used when more than 10 studies were included in the meta-analysis.
We obtained or calculated the proportions and 95%CI for each categorical variable and the mean or median for continuous data when possible. The pooled means and OR were calculated utilizing a random effects model. The random effects model was used regardless of underlying statistical testing of heterogeneity since it provides more conservative estimations of the pooled effects that are more likely to contain the true effect. The Cochran Q test and I2 were used to assess heterogeneity of included studies. I2 values of < 25%, 25%-50% and > 50% were considered to represent low, moderate and high heterogeneity, respectively. P values < 0.05 were considered significant and all tests were two tailed. The study was performed in accordance with the PRISMA recommendations for reporting systematic reviews and meta-analyses. Analysis was conducted using Stata, version 15 (Stata Corp, College Station, TX, United States) and RevMan 5.3 (The Cochrane Collaboration, Copenhagen).
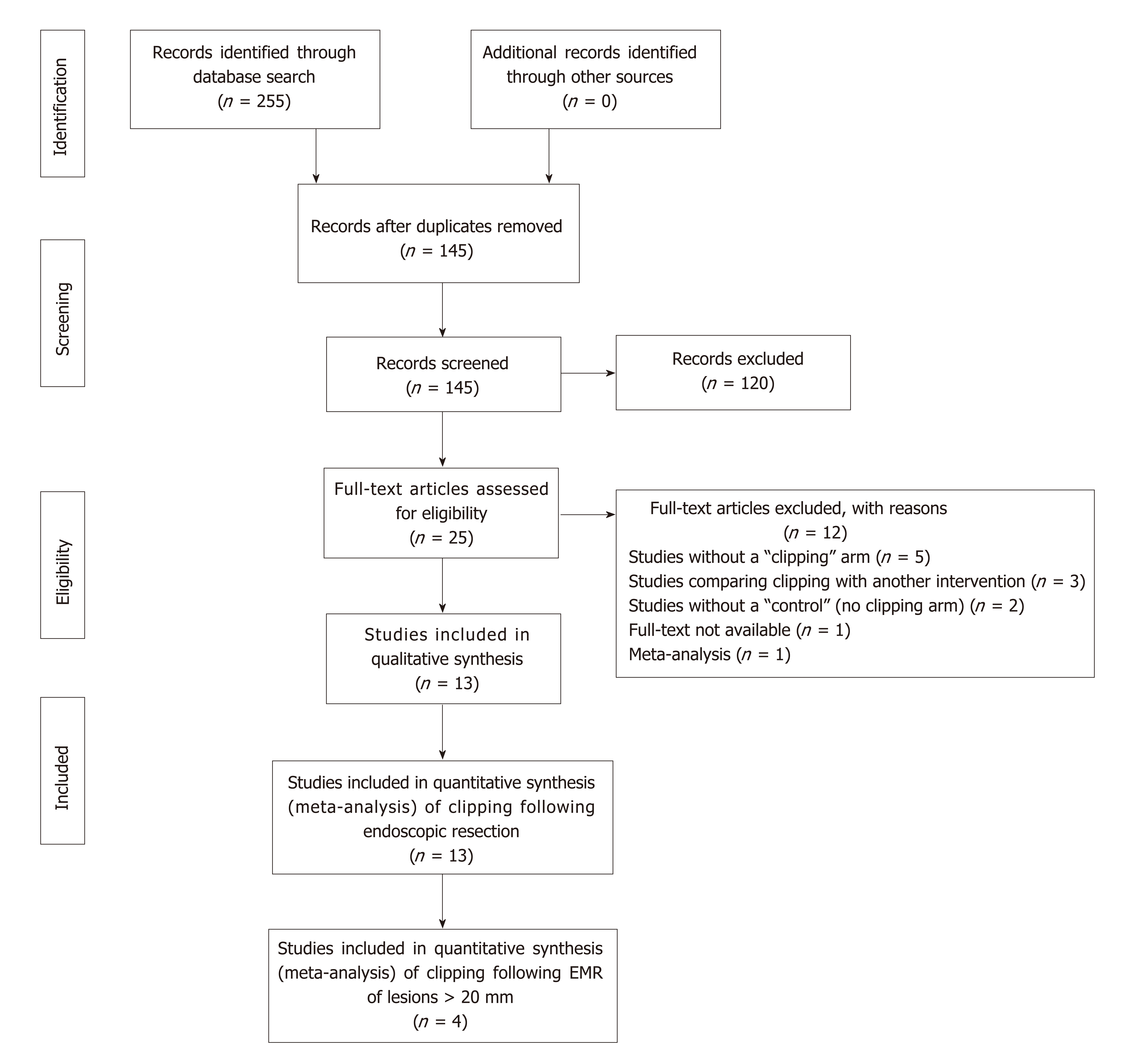
Figure 1 depicts the study selection flow diagram. Overall, 255 studies were identified using our search strategy, of which 110 were duplicates. Of the remaining 145 studies, 120 were excluded after screening titles and abstracts. Full text review was then performed on 25 studies using the predefined inclusion and exclusion criteria, after which 13 studies were retained. Of the 13 studies, 7 were randomized control trials (RCTs)[13,15-21] and 6 were cohort studies (2 prospective, 4 retrospective)[11,12,22-25]. Studies were published between 2003 and 2017. Nine studies were conducted in Asia, 2 in Europe, and 2 in the United States. These 13 studies were included in the meta-analysis evaluating the impact of prophylactic clipping on adverse events following colorectal ER. Of these, 4 studies with available data on specific parameters (lesion size, type of ER, clipping vs no clipping, incidence of DPB) were included in the analysis on the effects of prophylactic clipping on DPB after EMR of lesions ≥ 20 mm.
Study characteristics are summarized in Table 1. Colorectal ER was performed in 7794 polyps, of which 3567 (45.8%) underwent prophylactic clipping. Out of the 13 studies identified, 7 studies excluded all pedunculated polyps whereas 1 study did not report details on polyp morphology[23]. Of the remaining six studies, 3772 out of 5225 polyps (72%) were reported as pedunculated. Eleven studies specified that the lesion located in the right colon (2695 out of 6309; 42.7%). Overall, 7 studies included data on EMR only, 3 studies reported outcomes on both EMR and conventional polypectomy, 2 on ESD alone, and 1 on both ESD and EMR. Most lesions (82%; 6377) were removed by EMR, followed by conventional polypectomy (14%; 1118), and ESD (4%; 299). While several studies reported the number of patients in each group (clipping vs non-clipping), a few studies only described the number of lesions in each arm[11,12,15,18,22-25]; hence, the number of lesions was used in the analysis.
| Study | Study design | Country | Endoscopic Resection (n) | Intervention | Age, mean ± SD | Gender (M/F) | Patients (n) | Lesions (n) | Lesion Size in mm, mean ± SD | Pedunculated (n) | Right colon (n) | ||
| CP | EMR | ESD | |||||||||||
| Shioji et al[20], 2003 | RCT | Japan | ----- | 413 | ---- | Clip | 64 ± 9 | 118/38 | 156 | 205 | 7.8 ± 3.9 | 67 | 97 |
| Non-Clip | 63 ± 12 | 130/37 | 167 | 208 | 7.8 ± 4.1 | 65 | 90 | ||||||
| Kaltenbach et al[25], 2007 | Cohort | United States | ----- | 125 | ---- | Clip | 68 ± 9 | 100/0 | Not reported | 49 | 16.7 ± 7 | Excluded | 49 |
| Non-clip | 76 | 0 | |||||||||||
| Dior et al[23], 2012 | Cohort | France | ------ | 139 | ---- | Clip | 66 (23-90)1 | 76/62 | Not reported | 75 | Not reported | Not reported | 63 |
| Non-clip | 64 | ||||||||||||
| Liaquat et al[11], 2012 | Cohort | United States | ------ | 472 | ---- | Clip | 67.1 ± 10.9 | 250/213 | Not reported | 225 | 31 (20-100)1 | Excluded | 273 |
| Non-clip | 247 | ||||||||||||
| Matsumoto et al[12], 2012 | Cohort | Japan | 403 | ---- | Clip | 63 ± 12 | 140/135 | Not reported | 174 | 27.1 ± 9.6 | Excluded | Not reported | |
| Non-clip | 229 | ||||||||||||
| Mori et al[18], 2014 | RCT | Japan | ------ | 148 | ---- | Clip | Not reported | Not reported | Not reported | 73 | 15.3 ± 2.84 | 24 | 9 |
| Non-clip | 75 | 15.5 ± 2.60 | 24 | 10 | |||||||||
| Tominaga et al[21], 2014 | RCT | Japan | ------ | 801 | ---- | Clip | 67 (22-88)1 | 151/60 | 211 | 385 | 7.7 (5-30)1 | 229 | 79 |
| Non-clip | 66.6 (15-94)1 | 148/68 | 216 | 416 | 8.5 (5-35)1 | 245 | 114 | ||||||
| Dokoshi et al[15], 2015 | RCT | Japan | 54 | 234 | ---- | Clip | 67.1 ± 82 | 109/45 | Not reported | 154 | < 10 mm: 98, 10-20 mm: 48, > 20 mm: 8 | 41 | 73 |
| Non-clip | 67.8 ± 112 | 99/35 | 134 | < 10 mm: 86, 10-20mm: 48, > 20 mm: 6 | |||||||||
| Zhang et al[13], 2015 | RCT | China | ---- | 286 | 62 | Clip | 67.9 ± 12.6 | 112/62 | 174 | 174 | 10-20 mm: 111, 20-40 mm: 63 | Excluded | 22 |
| Non-clip | 64.2 ± 9.8 | 107/67 | 174 | 174 | 10-20 mm: 107, 20-40 mm: 67 | 27 | |||||||
| Albéniz et al[22], 2016 | Cohort | Spain | ---- | 1056 | ---- | Clip | 67.9 ± 10.9 | 770/444 | Not reported | 281 | 30.5 ± 11.8 | Excluded | Not reported |
| Non-clip | 775 | ||||||||||||
| Matsumoto et al[16], 2016 | RCT | Japan | 1064 | 2300 | ---- | Clip | 65 (25-87) | 534/218 | 752 | 1636 | < 5 mm: 388, > 5 mm: 1248 | 1467 | 823 |
| Non-clip | 66 (25-88) | 513/234 | 747 | 1728 | < 5 mm: 447, > 5 mm: 1281 | 1595 | 845 | ||||||
| Osada et al[19], 2016 | RCT | Japan | ---- | ---- | 26 | Clip | 68.8 ± 8.7 | 9/4 | 13 | 13 | 677.2 ± 3063 | Excluded | Not reported |
| Non-clip | 66.2 ± 10.4 | 7/6 | 13 | 13 | 790 ± 2203 | ||||||||
| Harada et al[24], 2017 | Cohort | Japan | ---- | ---- | 211 | Clip | 70.7 ± 9.2 | 124/87 | Not reported | 123 | < 30 mm: 65, 30-60 mm: 58, > 60 mm: 2 | 14 | 50 |
| Non-Clip | 88 | < 30 mm: 23, 30-60 mm: 53, > 60 mm: 12 | |||||||||||
The risk of bias in the 6 nonrandomized studies was evaluated according to the Newcastle-Ottawa assessment scale (Supplementary Table 1). The average quality score was 8 out of the highest possible score of 9. Five of the 6 included cohort studies were of high methodological quality (score 8-9/9), and 1 was of low quality (score 4-5/9). The risk of bias for the 7 RCTs is shown in Supplementary Table 2. Blinding of participants and personnel was not performed in any of the included RCTs. Methods for random sequence generation and allocation concealment were described by 5 studies. All RCTs were found to have adequate assessment of incomplete outcomes and avoided selective reporting.
| Author/Year | Endoscopic resection (n) | Intervention | Polyps | DPB | Perforation | ||
| CP | EMR | ESD | |||||
| Shioji et al[20], 2003 | ----- | 413 | ---- | Clip | 205 | 2 | 0 |
| No clip | 208 | 2 | 0 | ||||
| Kaltenbach et al[25], 2007 | ----- | 125 | ----- | Clip | 49 | 0 | 0 |
| No clip | 76 | 0 | 0 | ||||
| Dior et al[23], 2012 | ------ | 139 | ------ | Clip | 75 | 0 | Not reported |
| No clip | 64 | 3 | Not reported | ||||
| Liaquat et al[11], 2012 | ------ | 472 | ----- | Clip | 225 | 4 | 1 |
| No clip | 247 | 24 | 1 | ||||
| Matsumoto et al[12], 2012 | 403 | ------ | Clip | 174 | 3 | Not reported | |
| No clip | 229 | 14 | Not reported | ||||
| Mori et al[18], 2014 | ------ | 148 | ------- | Clip | 73 | 2 | 0 |
| No clip | 75 | 0 | 0 | ||||
| Tominaga et al[21], 2014 | ------- | 801 | ------- | Clip | 385 | 4 | Not reported |
| No clip | 416 | 9 | Not reported | ||||
| Dokoshi et al[15], 2015 | 54 | 234 | ------- | Clip | 154 | 4 | 0 |
| No clip | 134 | 3 | 0 | ||||
| Zhang et al[13], 2015 | ------ | 286 | 62 | Clip | 174 | 2 | 1 |
| No clip | 174 | 12 | 1 | ||||
| Albéniz et al[22], 2016 | ------ | 1056 | ------ | Clip | 281 | 4 | Not reported |
| No clip | 775 | 30 | Not reported | ||||
| Matsumoto et al[16], 2016 | 1064 | 2300 | ------ | Clip | 1636 | 18 | Not reported |
| No clip | 1728 | 15 | Not reported | ||||
| Osada et al[19], 2016 | ------ | ------ | 26 | Clip | 13 | 0 | 0 |
| No clip | 13 | 0 | 0 | ||||
| Harada et al[24], 2017 | ------ | ------ | 211 | Clip | 123 | 3 | 0 |
| No clip | 88 | 2 | 0 | ||||
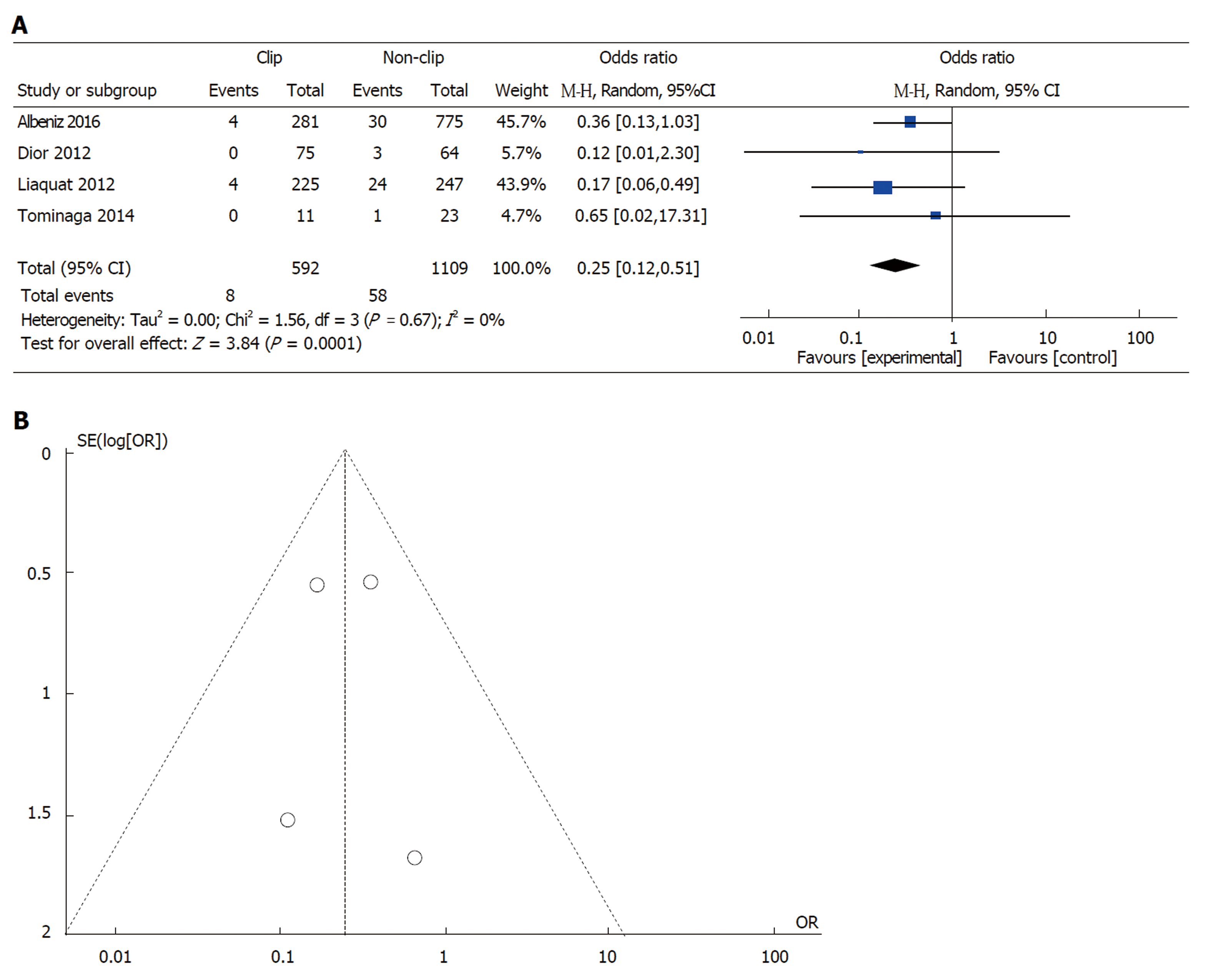
Effect of prophylactic clipping on DPB following EMR of colorectal lesions ≥ 20 mm: Of the 13 studies on colorectal ER, data from 4 studies were available to evaluate the incidence of DPB after EMR of lesions ≥ 20 mm[11,21-23]. In all, clipping was performed in 592 (34.8%) cases of the 1701 EMRs of lesions ≥ 20 mm. Clipping was associated with a lower incidence of DPB (8 out of 592; 1.4%) when compared to no clipping (58 out of 1109; 5.2%) (pooled OR: 0.24, 95%CI: 0.12-0.50, P < 0.001). There was little heterogeneity among the included studies (I2 = 0%, P = 0.67) (Figure 2A). There was no evidence of substantial publication bias based on visual inspection of the funnel plot (Figure 2B).
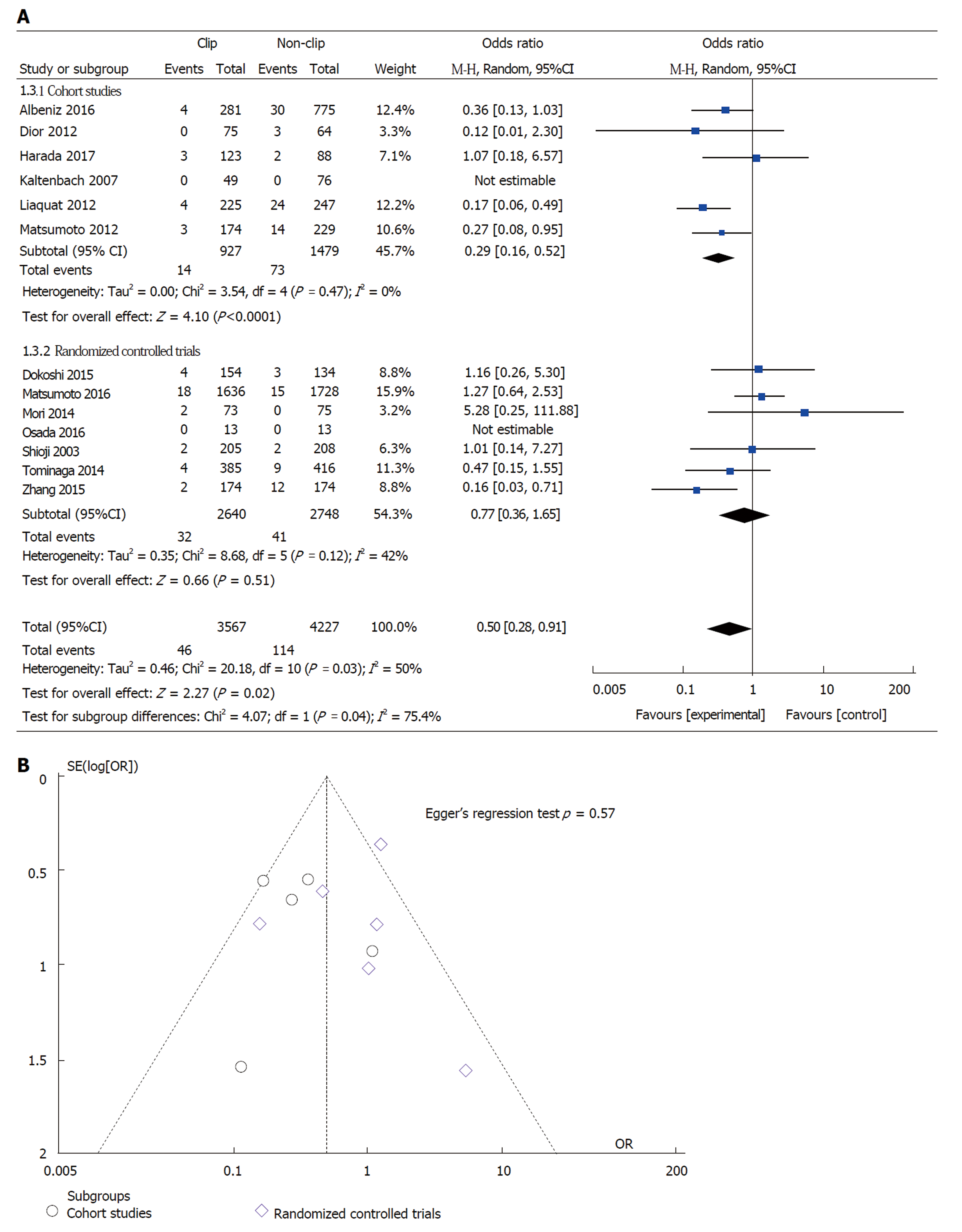
Effect of prophylactic clipping on the incidence of adverse events following colorectal ER: DPB, the incidence of DPB was reported in all 13 studies included in the meta-analysis. The overall pooled incidence of DPB was 2.1% (160 out of 7794 lesions) (Table 2). DPB was reported in 46 (1.3%) cases with prophylactic clipping as compared to 114 (2.7%) in the non-clipping arm (pooled OR: 0.50; 95%CI: 0.25-0.91, P = 0.02) (Figure 3A). A sensitivity analysis was performed by using patient instead of lesion numbers when available and this did not alter the overall pooled outcome (pooled OR 0.49; 95%CI: 0.27-0.89, P = 0.02). There was significant heterogeneity among the included studies (I2 = 50%, P = 0.03). When only RCTs were included in the analysis, compared with no clipping, the pooled OR for DPB with clipping was 0.77 (95%CI: 0.36-1.65, P = 0.51), suggesting no significant difference between the two groups (Figure 3A). However, there was moderate heterogeneity among these RCT results (I2 = 42%, P = 0.12). In all, there was no evidence of substantial publication bias based on the visual inspection of the funnel plot and Egger’s regression test (P = 0.57) (Figure 3B).
Perforation following Colorectal ER. Eight studies evaluated the rate of perforation following ER. No cases of perforation were reported in six studies, whereas the remaining two observed a total of 2 cases of perforation in each group (clipping vs non-clipping). Hence, the overall pooled rate for perforation was 0.19% (4 out of 2031 lesions), with no significant difference between the two groups (pooled OR: 1.05; 95%CI: 0.15-7.48, P = 0.96).
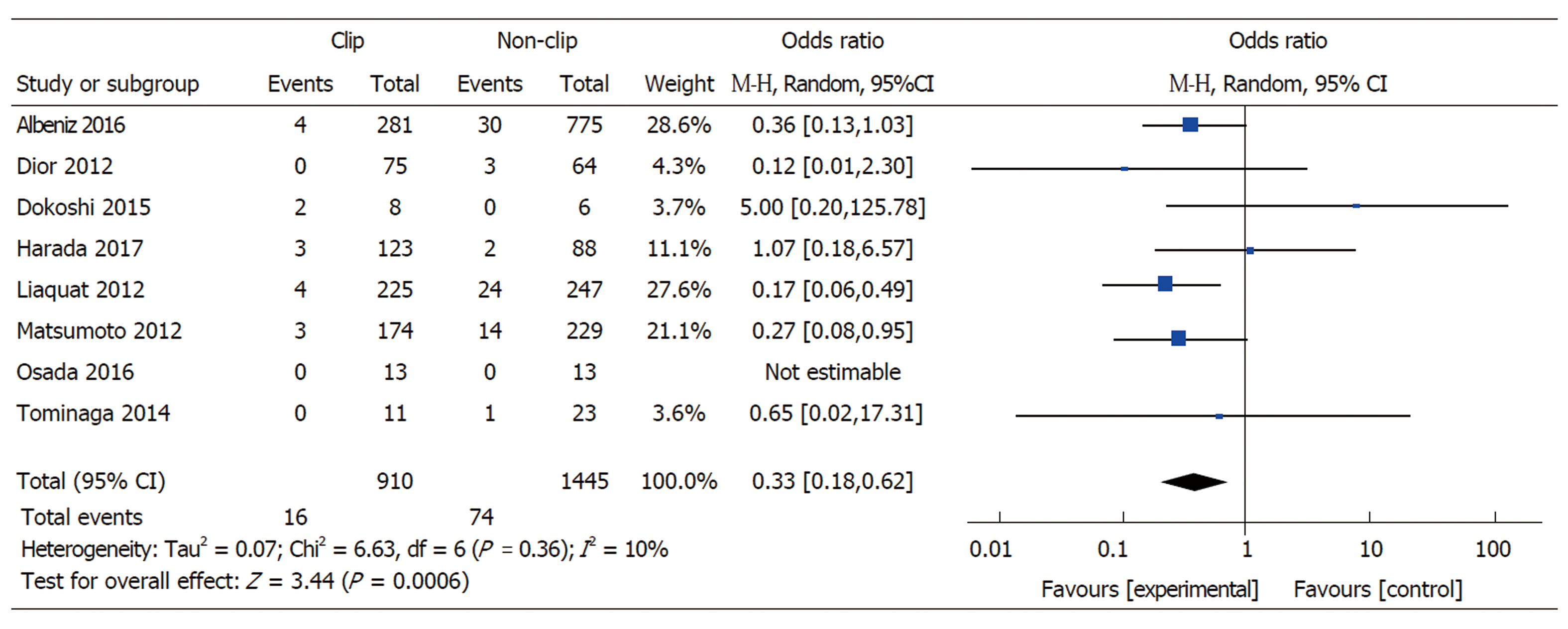
Lesion Size ≥ 20 mm: Eight studies with available data on outcomes for lesions ≥ 20 mm included 910 cases with clipping and 1445 without clipping following ER (EMR or ESD). The overall pooled rate of DPB was 3.8% for lesions ≥ 20 mm. Prophylactic clipping of lesions ≥ 20 mm was associated with a lower rate of DPB when compared to no clipping (1.8% vs 5.1%) (pooled OR: 0.33, 95%CI: 0.18-0.62, P < 0.001), with no significant heterogeneity among the available studies (I2 = 10%, P = 0.36) (Figure 4).
Polyp morphology (pedunculated) and right-colon location: Out of the 13 studies included in the meta-analysis, only two studies specified outcomes on DBP for pedunculated polyps[16,21]. The pooled incidence for DPB in pedunculated polyps from these two studies did not show a difference between clipping (1.1%) vs no clipping (1.1%) (pooled OR: 0.77, 95%CI: 0.17-3.46, P = 0.73). Only 1 study specified the incidence of DPB in right-sided colonic lesions. The authors did not report a significant difference in the rate of DPB between the two groups (1.3% with clipping vs 6% without clipping; OR: 2.28, 95%CI: 0.79-6.58, P = 0.13)[16].
DPB is the most common adverse event following ER of colorectal lesions. Prophylactic clipping has been suggested as a strategy for the prevention of DPB, although prior data has been marred by conflicting findings. The results from this meta-analysis suggests that endoscopic clipping may be associated with a lower occurrence of DPB after colorectal EMR of lesions ≥ 20 mm in size.
Nishizawa et al[26] recently reported the results of their meta-analysis on the effect of prophylactic clipping after colorectal ER. A total of 7 RCTs with 3059 cases were included. In their study, the rate of DPB was similar between cases with clipping (2.1%) vs no clipping (2.7%) (OR 0.76; 95%CI: 0.39-1.47; P = 0.414). Similarly, when only RCTs were included in our meta-analysis, clipping did not affect the rate of DPB when compared to no clipping after ER (OR 0.77; 95%CI: 0.36-1.65, P = 0.51). However, it is important to highlight that nearly all of the cases included in these RCTs (2847 out of 3059; 93%) involved polyps < 20 mm in size. DPB is a rare occurrence following ER of small colorectal lesions. Indeed, most if not all of these lesions can be safely and completely excised with conventional cold snare polypectomy with no risk for DPB[27,28]. Hence, it is not surprising that prophylactic clipping did not impact the rate of postoperative bleeding in patients included in those trials.
It is well known that the incidence of DPB is directly associated with lesion size, and has been more frequently reported after the resection of lesions ≥ 20 mm[8,29,30]. Nonetheless, the study by Nishizawa et al[26] did not report a difference in postoperative bleeding for lesions ≥ 20 mm with clipping vs no clipping (pooled OR 0.78; 95%CI: 0.23-2.68). The small number of cases with lesions ≥ 20 mm included in their study (97 with clipping and 115 without clipping) may have underpowered their analysis to detect any meaningful differences. In contrast, in effort to specifically evaluate the risk of DPB in lesions of clinically significant size, we included a total of 2355 polyps ≥ 20 mm in size. Our results demonstrated that clipping following the ER of lesions ≥ 20 mm was associated with a reduction in the risk of DPB when compared to no clipping (1.8% vs 5.1%, pooled OR: 0.33, 95%CI: 0.18-0.62, P < 0.001), with little heterogeneity among the studies (I2 = 10%, P = 0.36). Furthermore, given that colorectal lesions ≥ 20 mm are primarily removed with EMR, we specifically evaluated the risk of DPB in this group. Similarly, our meta-analysis demonstrated that clipping after EMR of lesions ≥ 20 mm significantly reduced the risk of bleeding when compared to no clipping (1.4% vs 5.2%; pooled OR: 0.24, 95%CI: 0.12-0.50, P < 0.001). When compared to conventional polypectomy, EMR, particularly when performed for the removal of larger lesions, inherently results in an extended residual mucosal defect[31]. Prophylactic clip closure of the defect reduces exposure of the submucosal tissue to the colonic luminal milieu, which may in turn reduce the risk of DPB and other adverse events, including abdominal pain and post-polypectomy syndrome[13].
Several issues remain to be addressed before this practice can be fully advocated. It is important to note that prophylactic clipping is not without its limitations. From a health economics standpoint, a prophylactic clipping strategy may not be cost effective and justifiable for all colorectal lesions removed by EMR[32]. Certainly, the added cost of clips and lengthier procedure should be weighed against the potential incremental expenditures associated with DPB (i.e., emergency room visits, readmissions, need for transfusions, repeat therapeutic interventions). Given the above limitations, a strategy of clipping targeted to patient and/or lesion characteristics would likely prove most efficient. Patient characteristics that may warrant prophylactic clipping may include those requiring resumption of anti-coagulant or anti-thrombotic therapy following resection, those with a high comorbidity burden who may not hemodynamically tolerate significant hemorrhage or patients with low likelihood of post-procedural follow up and access to care[11,33]. Lesion characteristics that may benefit from clipping may include those that are larger than 20 mm, pedunculated, located in the right colon or a combination of the aforementioned factors. Future well-designed RCTs are needed to further define the role of prophylactic clipping in the prevention of DPB in select lesions, specifically after EMR of large colonic lesions.
This study has several strengths. Given that DPB often occurs following ER of larger lesions, we specifically evaluated the efficacy of prophylactic clipping with respect to lesion size. Furthermore, many studies on prophylactic closure for DPB do not differentiate between the types of endoscopic intervention (i.e., EMR vs ESD), which significantly limits the interpretability of the results as both of these approaches are technically distinct and carry inherently different risks for post-procedural adverse events[34,35]. In this meta-analysis, we demonstrate that prophylactic clipping reduces the risk of DPB in arguably the most clinically significant group: lesions ≥ 20 mm removed with EMR. These observations have direct clinical implications as vast majority of these lesions in the West are approached with EMR.
We also acknowledge the limitations of this study. All available studies reporting the effect of clipping on DPB were included in this meta-analysis in efforts to capture sufficient cases for subgroup analyses. The inclusion of cohort studies, in addition to RCTs, potentially introduces selection bias. Nonetheless, the overall quality of the included cohort studies was satisfactory based on the Newcastle-Ottawa scale and there was little heterogeneity among the studies. Furthermore, given that the main aim of the study was to evaluate DPB in lesions ≥ 20 mm following EMR, only a few studies were available, and thereby these results should be interpreted with caution and underscores the need of additional well-designed trials. Secondly, the lack of data on polyp morphology, location in the colon, and management of anti-coagulant/anti-thrombotic medications prior to ER in many of the included studies limited our ability to perform additional sub-analyses or draw any meaningful conclusions on these important subgroups.
In summary, this meta-analysis suggests that prophylactic clipping may reduce DPB after ER of colorectal lesions. Clip closure was associated with a significant reduction in the incidence of DPB in lesions ≥ 20 mm following EMR. Future trials are needed to further identify risk factors for DPB and help implement a cost-effective preventive strategy.
The role of prophylactic clipping in the prevention of delayed polypectomy bleeding (DPB) is unclear.
Previous meta-analyses included a variety of polyp resection methods and all polyp sizes, our analysis used a more focused approach.
To assess the effect of prophylactic clip placement on DPB after endoscopic mucosal resection (EMR) of colorectal lesions 20mm or larger.
We performed a systematic search of Medline through PubMed and the Cochrane Library database for studies investigating the effect of prophylactic clipping on DPB following EMR of colorectal lesions. We used the PRISMA protocol for our analysis and assessed the quality of included articles using the Newcastle-Ottawa scale. We used RevMan version 5 for the statistical analysis, using the random-effects model (DeSimonian-Laird method).
A total of 7794 polyps in 13 studies were analyzed, including 1701 cases of EMR of lesions ≥ 20 mm. We found that prophylactic clipping following EMR of lesions ≥ 20 mm was associated with a lower rate of DPB (1.4%) compared to no clipping (5.2%).
Placement of clips prophylactically following EMR of colorectal lesions ≥ 20 mm may reduce rates of DPB and its associated morbidity and should be considered by practicing endoscopists in select patients.
Future prospective studies on the effect of clipping for DPB after EMR should focus on lesions ≥ 20 mm since those represent the highest risk. Cost analyses must also be conducted to implement the most cost-effective strategies for DPB prevention.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bustamante-Balen M, Chiu CC S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
| 1. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2287] [Article Influence: 175.9] [Reference Citation Analysis (2)] |
| 2. | Jayanna M, Burgess NG, Singh R, Hourigan LF, Brown GJ, Zanati SA, Moss A, Lim J, Sonson R, Williams SJ, Bourke MJ. Cost Analysis of Endoscopic Mucosal Resection vs Surgery for Large Laterally Spreading Colorectal Lesions. Clin Gastroenterol Hepatol. 2016;14:271-278; e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (2)] |
| 3. | Ahlenstiel G, Hourigan LF, Brown G, Zanati S, Williams SJ, Singh R, Moss A, Sonson R, Bourke MJ; Australian Colonic Endoscopic Mucosal Resection (ACE) Study Group. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. 2014;80:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 928] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 5. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara K, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 437] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 6. | Rosen L, Bub DS, Reed JF, Nastasee SA. Hemorrhage following colonoscopic polypectomy. Dis Colon Rectum. 1993;36:1126-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Gibbs DH, Opelka FG, Beck DE, Hicks TC, Timmcke AE, Gathright JB. Postpolypectomy colonic hemorrhage. Dis Colon Rectum. 1996;39:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Sawhney MS, Salfiti N, Nelson DB, Lederle FA, Bond JH. Risk factors for severe delayed postpolypectomy bleeding. Endoscopy. 2008;40:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Zhang Q, An Sl, Chen Zy, Fu FH, Jiang B, Zhi Fc, Bai Y, Gong W. Assessment of risk factors for delayed colonic post-polypectomy hemorrhage: a study of 15553 polypectomies from 2005 to 2013. PLoS One. 2014;9:e108290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Ma MX, Bourke MJ. Complications of endoscopic polypectomy, endoscopic mucosal resection and endoscopic submucosal dissection in the colon. Best Pract Res Clin Gastroenterol. 2016;30:749-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Liaquat H, Rohn E, Rex DK. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointest Endosc. 2013;77:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Matsumoto M, Fukunaga S, Saito Y, Matsuda T, Nakajima T, Sakamoto T, Tamai N, Kikuchi T. Risk factors for delayed bleeding after endoscopic resection for large colorectal tumors. Jpn J Clin Oncol. 2012;42:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Zhang QS, Han B, Xu JH, Gao P, Shen YC. Clip closure of defect after endoscopic resection in patients with larger colorectal tumors decreased the adverse events. Gastrointest Endosc. 2015;82:904-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12667] [Article Influence: 844.5] [Reference Citation Analysis (0)] |
| 15. | Dokoshi T, Fujiya M, Tanaka K, Sakatani A, Inaba Y, Ueno N, Kashima S, Goto T, Sasajima J, Tominaga M, Ito T, Moriichi K, Tanabe H, Ikuta K, Ohtake T, Kohgo Y. A randomized study on the effectiveness of prophylactic clipping during endoscopic resection of colon polyps for the prevention of delayed bleeding. Biomed Res Int. 2015;2015:490272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Matsumoto M, Kato M, Oba K, Abiko S, Tsuda M, Miyamoto S, Mizushima T, Ono M, Omori S, Takahashi M, Ono S, Mabe K, Nakagawa M, Nakagawa S, Kudo T, Shimizu Y, Sakamoto N. Multicenter randomized controlled study to assess the effect of prophylactic clipping on post-polypectomy delayed bleeding. Dig Endosc. 2016;28:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Sakamoto N, Beppu K, Matsumoto K, Shibuya T, Osada T, Mori H, Shimada Y, Konno A, Kurosawa A, Nagahara A, Otaka M, Ohkusa T, Ogihara T, Watanabe S. "Loop Clip", a new closure device for large mucosal defects after EMR and ESD. Endoscopy. 2008;40 Suppl 2:E97-E98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Mori H, Kobara H, Nishiyama N, Fujihara S, Matsunaga T, Ayaki M, Chiyo T, Masaki T. Simple and reliable treatment for post-EMR artificial ulcer floor with snare cauterization for 10- to 20-mm colorectal polyps: a randomized prospective study (with video). Surg Endosc. 2015;29:2818-2824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Osada T, Sakamoto N, Ritsuno H, Murakami T, Ueyama H, Matsumoto K, Shibuya T, Ogihara T, Watanabe S. Closure with clips to accelerate healing of mucosal defects caused by colorectal endoscopic submucosal dissection. Surg Endosc. 2016;30:4438-4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Shioji K, Suzuki Y, Kobayashi M, Nakamura A, Azumaya M, Takeuchi M, Baba Y, Honma T, Narisawa R. Prophylactic clip application does not decrease delayed bleeding after colonoscopic polypectomy. Gastrointest Endosc. 2003;57:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Tominaga N, Tanaka Y, Higuchi T, Yamaguchi D, Watanabe A, Ogata S, Kajiwara T. The effect of hemostasis clipping post endoscopic mucosal resection of colorectal polyps. Gastroenterol Endosc. 2014;56:15–20. [DOI] [Full Text] |
| 22. | Endoscopic Mucosal Resection Endoscopic Spanish Society Group; Albéniz E, Fraile M, Ibáñez B, Alonso-Aguirre P, Martínez-Ares D, Soto S, Gargallo CJ, Ramos Zabala F, Álvarez MA, Rodríguez-Sánchez J, Múgica F, Herreros de Tejada A, Redondo E, Pin N, León-Brito H, Pardeiro R, López-Roses L, Rodríguez-Téllez M, Jiménez A, Martínez-Alcalá F, García O, de la Peña J, Ono A, Alberca de Las Parras F, Pellisé M, Rivero L, Saperas E, Pérez-Roldán F, Pueyo Royo A, Eguaras Ros J, Zúñiga Ripa A, Concepción-Martín M, Huelin-Álvarez P, Colán-Hernández J, Cubiella J, Remedios D, Bessa I Caserras X, López-Viedma B, Cobian J, González-Haba M, Santiago J, Martínez-Cara JG, Valdivielso E, Guarner-Argente C, Nogales Ó. A Scoring System to Determine Risk of Delayed Bleeding After Endoscopic Mucosal Resection of Large Colorectal Lesions. Clin Gastroenterol Hepatol. 2016;14:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Dior M, Coriat R, Tarabichi S, Leblanc S, Polin V, Perkins G, Dhooge M, Prat F, Chaussade S. Does endoscopic mucosal resection for large colorectal polyps allow ambulatory management? Surg Endosc. 2013;27:2775-2781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Harada H, Suehiro S, Murakami D, Nakahara R, Ujihara T, Shimizu T, Miyama Y, Katsuyama Y, Hayasaka K, Tounou S. Clinical impact of prophylactic clip closure of mucosal defects after colorectal endoscopic submucosal dissection. Endosc Int Open. 2017;5:E1165-E1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Kaltenbach T, Friedland S, Maheshwari A, Ouyang D, Rouse RV, Wren S, Soetikno R. Short- and long-term outcomes of standardized EMR of nonpolypoid (flat and depressed) colorectal lesions or = 1 cm (with video). Gastrointest Endosc. 2007;65:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Nishizawa T, Suzuki H, Goto O, Ogata H, Kanai T, Yahagi N. Effect of prophylactic clipping in colorectal endoscopic resection: A meta-analysis of randomized controlled studies. United European Gastroenterol J. 2017;5:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Paspatis GA, Tribonias G, Konstantinidis K, Theodoropoulou A, Vardas E, Voudoukis E, Manolaraki MM, Chainaki I, Chlouverakis G. A prospective randomized comparison of cold vs hot snare polypectomy in the occurrence of postpolypectomy bleeding in small colonic polyps. Colorectal Dis. 2011;13:e345-e348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Fujiya M, Sato H, Ueno N, Sakatani A, Tanaka K, Dokoshi T, Fujibayashi S, Nomura Y, Kashima S, Gotoh T, Sasajima J, Moriichi K, Watari J, Kohgo Y. Efficacy and adverse events of cold vs hot polypectomy: A meta-analysis. World J Gastroenterol. 2016;22:5436-5444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Macrae FA, Tan KG, Williams CB. Towards safer colonoscopy: a report on the complications of 5000 diagnostic or therapeutic colonoscopies. Gut. 1983;24:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 316] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Sorbi D, Norton I, Conio M, Balm R, Zinsmeister A, Gostout CJ. Postpolypectomy lower GI bleeding: descriptive analysis. Gastrointest Endosc. 2000;51:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | ASGE Technology Committee. Hwang JH, Konda V, Abu Dayyeh BK, Chauhan SS, Enestvedt BK, Fujii-Lau LL, Komanduri S, Maple JT, Murad FM, Pannala R, Thosani NC, Banerjee S. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 32. | Bahin FF, Rasouli KN, Williams SJ, Lee EY, Bourke MJ. Prophylactic clipping for the prevention of bleeding following wide-field endoscopic mucosal resection of laterally spreading colorectal lesions: an economic modeling study. Endoscopy. 2016;48:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | 2013 March (Vol. 77) GIE Author Interview Series-Douglas K. Rex-YouTube [Internet]. [cited 2018 Mar 2]; Available from: https://www.youtube.com/watch?v=S8igj1EFJqc. |
| 34. | Arezzo A, Passera R, Marchese N, Galloro G, Manta R, Cirocchi R. Systematic review and meta-analysis of endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal lesions. United European Gastroenterol J. 2016;4:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, Bhandari P, Boeri F, Conio M. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol. 2016;104:138-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |