Published online May 14, 2019. doi: 10.3748/wjg.v25.i18.2162
Peer-review started: March 22, 2019
First decision: April 4, 2019
Revised: April 11, 2019
Accepted: April 19, 2019
Article in press: April 20, 2019
Published online: May 14, 2019
Processing time: 53 Days and 16.3 Hours
Spondyloarthropathies (SpA) include many different forms of inflammatory arthritis and can affect the spine (axial SpA) and/or peripheral joints (peripheral SpA) with Ankylosing spondylitis (AS) being the prototype of the former. Extra-articular manifestations, like uveitis, psoriasis and inflammatory bowel disease (IBD) are frequently observed in the setting of SpA and are, in fact, part of the SpA classification criteria. Bowel involvement seems to be the most common of these manifestations. Clinically evident IBD is observed in 6%-14% of AS patients, which is significantly more frequent compared to the general population. Besides, it seems that silent microscopic gut inflammation, is evident in around 60% in AS patients. Interestingly, occurrence of IBD has been associated with AS disease activity. For peripheral SpA, two different forms have been proposed with diverse characteristics. Of note, SpA (axial or peripheral) is more commonly observed in Crohn’s disease than in ulcerative colitis. The common pathogenetic mechanisms that explain the link between IBD and SpA are still ill-defined. The role of dysregulated microbiome along with migration of T lymphocytes and other cells from gut to the joint (“gut-joint” axis) has been recognized, in the context of a genetic background including associations with alleles inside or outside the human leukocyte antigen system. Various therapeutic modalities are available with monoclonal antibodies against tumour necrosis factor, interleukin-23 and interleukin-17, being the most effective. Both gastroenterologists and rheumatologists should be alert to identify the co-existence of these conditions and ideally follow-up these patients in combined clinics.
Core tip: Spondyloarthropathies (SpA) are subdivided to axial and peripheral SpA with ankylosing spondylitis (AS) being the prototype disease of the former. They have many extra-articular manifestations the most common of which is bowel involvement. Inflammatory bowel disease (IBD) (silent or clinically evident) occurs much more frequently in AS compared to the general population and associates with AS disease activity. Both axial and peripheral SpA occur more frequently in Crohn’s disease than ulcerative colitis. Pathogenetic mechanisms that have been proposed to explain the link between SpA and IBD include dysregulated microbiome and migration of T lymphocytes and other cells from gut to the joint.
- Citation: Fragoulis GE, Liava C, Daoussis D, Akriviadis E, Garyfallos A, Dimitroulas T. Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment. World J Gastroenterol 2019; 25(18): 2162-2176
- URL: https://www.wjgnet.com/1007-9327/full/v25/i18/2162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i18.2162
Under the term spondyloarthropathy (SpA) are classified many inflammatory arthropathies with similar clinical and imaging features. However, diagnostic laboratory or pathological findings with high specificity are lacking. SpA affects mainly the spine, but symptomatology from the peripheral joints as well as from entheses and other tissues might occur. SpA include: psoriatic arthritis (PsA), peripheral SpA, enteropathic [also known as inflammatory bowel disease (IBD)-related] arthritis, reactive arthritis, undifferentiated spondyloarthropathy and axial spondyloarthropathy (axSpA) which includes non-radiographic axial spondyloar-thropathy (nraxSpA), and Ankylosing spondylitis (AS)[1]. The latter is considered the prototype of these diseases[2]. Enthesitis (inflammation of the entheses which are the insertions of the ligaments and tendons into the bone) is thought to be one of the key manifestations in SpA, helping to distinguish from other inflammatory arthropathies[1]. Apart from the skeletal disease, extra-articular manifestations like uveitis, psoriasis and inflammatory bowel disease often occur[2], offering significant help in the diagnosis of these diseases and being part of their classification criteria[3,4]. Of note, IBD contributes to the diagnosis of axSpA, as it has a positive likelihood ratio of 4.3 for axSpA diagnosis in patients with chronic low back pain[5,6].
According to the latest Assessment of SpondyloArthritis Society (ASAS) criteria, SpA can be classified to axSpA or peripheral SpA. The former pertains to patients with low back pain for ≥ 3 mo and age of onset < 45 years old and requires either sacroiliitis on imaging and at least one other SpA feature (e.g., dactylitis, enthesitis) or positivity for HLA-B27 and at least two other SpA features (Table 1)[4]. Difference between nraxSpA and axSpA is the lack of radiographically confirmed sacroiliitis in the former. Under the term “peripheral SpA” are classified patients without current low back pain, with peripheral arthritis, or enthesitis or dactylitis plus at least one or two of the following SpA features: (uveitis, psoriasis, crohn´s disease/ulcerative colitis, preceding infection, positive HLA-B27, sacroiliitis on imaging) or (arthritis, enthesitis, dactylitis, inflammatory back pain in the past, family history of SpA), respectively[3].
| Axial Spondyloarthropathy | ||
| Patients with back pain ≥ 3 months and age at onset <45 years | ||
| Sacroiliitis on imaging1 plus ≥ 1 | HLA-B27 plus ≥ 2 other | |
| Spondyloarthropahty feature | OR | Spondyloarthropahty features |
| (Imaging arm) | (Clinical arm) | |
| Spondyloarthropahty features | ||
| Inflammatory back pain | ||
| Arthritis | ||
| Enthesitis (heel) | ||
| Uveitis | ||
| Dactylitis | ||
| Psoriasis | ||
| Crohn’s Disease/Ulcerative colitis | ||
| Good response to NSAIDs | ||
| Family history of spondyloarthropahty | ||
| HLA-B27 | ||
| Elevated CRP | ||
| Peripheral Spondyloarthropathy | ||
| Arthritis OR Dactylitis OR Enthesitis2 | ||
| PLUS | ||
| ≥ 1 | ≥ 2 | |
| Uveitis | Arthritis | |
| Psoriasis | Enthesitis | |
| Inflammatory bowel disease | OR | Dactylitis |
| Preceding infection | IBP (past) | |
| HLA-B27 | Family history of Spondyloarthropahty | |
| Sacroiliitis on imaging | ||
It is well known that there is a close association between IBD and SpA[7]. Purpose of our review was to present in detail the existing epidemiological data and treatment approaches to these patients and to delineate the current diagnostic challenges. Also, we aimed to describe the underlying pathogenetic mechanisms that have been suggested to link these two entities.
IBD [including Crohn’s disease (CD) and ulcerative colitis (UC)] is not rare in AS, with its prevalence ranging from 6%-14%[2,6,8,9]. In detail, in a large population, control-matched study, including 4101 patients with AS, Stolwijk et al[6] found that at the time of AS diagnosis, the cumulative incidence was 4%. Additionally, in a French large, prospective study for early inflammatory back pain, IBD occurred in 7.2% of patients with newly diagnosed AS[10]. Furthermore, in an early axSpA cohort frequency of IBD was calculated to be 2.6% (1.7% for AS and 0.9% for nraxSpA, difference was not significant)[11]. In fact, it has been suggested that the risk for IBD is more pronounced in the first years of AS diagnosis and falls at baseline levels approximately 10 years after[6]. However, this has not been confirmed by a SLR and meta-analysis[2]. In that, Stolwijk et al[6] found that prevalence of IBD in AS was 6.8% (95%CI: 6.1%-7.7%), which is much higher than the percentages observed in the general population (0.01% to 0.5%). Likewise, in a large population study was shown that the incidence rate of IBD was 5.3-fold increased to the AS patients compared to healthy controls. For nraxSpA the results seem to be largely similar. In a meta-analysis addressing the prevalence of extra-articular disease in nraxSpA versus AS, it was found that IBD was almost equally frequent (pooled prevalence difference of 1.4% in favour of AS) between these two entities[8].
The question remains open whether we can predict which SpA patients suffer from or will develop IBD. Stolwijk et al[6] 2014 found that IBD was in general more common in males and that its frequency decreases with age, in AS. In a multi-centre AS study with a long follow-up, no differences were recorded between patients who had a history of IBD at baseline and those who did not[9]. On the other hand, development of IBD was associated with disease activity and spinal pain scores at baseline and worse physical function and patient well-being, at the time of IBD diagnosis[9]. Additionally, in a case control study[12] it was found that anterior uveitis was less frequent in patients with IBD-related spondyloarthropathy compared to those with SpA without bowel involvement[12]. Interestingly, in a sub-analysis of the GIANT cohort, it was shown that in patients with axSpA, there is a link between bone marrow edema of the sacroiliac joints and the gut inflammation. For this, SPARCC (Spondyloarthritis Research Consortium of Canada) scores which is a tool to measure MRI-defined sacroiliitis and ileocolonoscopy were used, respectively. It was found that SPARCC scores were higher in patients with chronic gut inflammation, compared to those without gut lesions[13].
Despite clinically evident IBD in the context of AS is observed in less than 15%, it has been suggested that clinically silent macroscopic and microscopic gut inflammation occurs in about 60% of AS patients[14-16]. From them, 5%-20% will develop CD within 5 years[17,18]. Microscopic gut inflammation, in axSpA, has been associated with younger age, male gender, progressive disease, early disease onset, radiologic sacroiliitis, high disease activity as assessed by the BASDAI and restricted spinal mobility measured by the Bath Ankylosing Spondylitis Metrology Index[9,16]. No association was identified with other extra-articular features or with the status of HLA-B27. Results were comparable between nraxSpA and AS[16].
Seeing the opposite flip of the coin, SpA is encountered in about 10-39% of patients with IBD, being the most frequent extra-intestinal manifestation in these individuals[16,19-25]. SpA is more commonly observed in patients with CD compared to those with UC[26-28]. Axial/arthritis symptomatology usually follows IBD diagnosis, but in about 20% the opposite is the case[19,23] especially for axial disease[20]. In general, AS and sacroiliitis (symptomatic or not) is estimated to occur in about 2%-16% and 12%-46% of IBD patients, respectively[19,20,22,23,27,29], both being more common in CD than in UC[19,30]. In a recent meta-analysis, it was shown that prevalence of AS and sacroiliitis in IBD were 3% (95%CI: 2%-4%) and 10% (95%CI: 8%-12%), respectively.
Comparing CD patients with and without AS, in a small single centre study, Liu et al did not observe any differences between these two groups[31]. Of note, they demonstrated that there was a significant correlation between disease activities of these two entities. These were measured by CD activity index for CD and with BASDAI for AS. They also showed that activity of CD significantly correlated with functional disability in AS, as assessed by Bath AS functional index - BASFI. All these possibly imply that there is a tight connection in the pathogenetic mechanisms of these conditions.
On the other hand, in a study examining possible associations between clinical and other characteristics with the occurrence of AS or SI in patients with CD, it was found that there was an association between SI and peripheral arthritis as well as between AS and uveitis, in these patients[32]. Besides, it has been suggested that in CD patients, colitis is more commonly associated with arthritic involvement compared with patients suffering from ileitis, while regarding UC, it seems that isolated proctitis is rarely combined with rheumatic manifestations[20,23].
Finally, patients with IBD-related ankylosing spondylitis and IBD-related isolated sacroiliitis are HLA-B27 positive in about 25%-78% and 7%-15%, respectively[20,22,32,33], possibly suggesting that isolated sacroiliitis is of different nature compared to AS in the setting of IBD[23]. These percentages are also lower compared to the prevalence of HLA-B27 observed in patients with AS which range from 80%-90%[1,20,34-36].
Peripheral SpA is also common in IBD with its prevalence ranging from 0.4% to 34.6%[19,28,37]. A recent systematic review and meta-analysis found that the pooled prevalence of peripheral arthritis, in the context of IBD was 13% (95%CI: 12%-15%) with its prevalence being much higher in the younger ages: 25% (95%CI: 19%-32%) and 2% (95%CI: 1%-5%) for age groups between 20-30 and 50-60 years old, respectively[30]. As observed for axial disease, peripheral SpA is more common in CD compared to UC[28,30,38]. A large retrospective study in the IBD Oxford clinics, had shown that peripheral arthritis occurred in 10% and 6% of patients with CD and UC, respectively[38,39]. This study, led to identification of two major groups of peripheral arthritis in the context of IBD, namely: oligoarticular (< 5 joints are affected) and polyarticular (≥ 5 joints are affected)[20]. Some authors suggest that in the first group, which is more frequent than the second[39], arthritis is usually asymmetrical, non-erosive, affects lower limbs[20] and is associated with IBD activity and positivity for HLA-B27[23,39]. Patients belonging in the second group tend to have a more chronic course and be destructive and unrelated with IBD activity and HLA-B27 status[38]. Furthermore, Yüksel et al[28], examining the characteristics of peripheral arthritis in patients with IBD, they found that erythema nodosum and pyoderma gangrenosum were more commonly observed in IBD patients who also had peripheral arthritis, compared to those without. Various risk factors have been reported for peripheral arthritis in the context of IBD including: family history of IBD, appendicectomy, smoking and presence of other extra-intestinal manifestations[19,40,41].
Finally, some IBD patients might exhibit clinical features of SpA (e.g., dactylitis) without fulfilling diagnostic criteria for SpA[20,42]. The frequency of dactylitis in patients with SpA in the context of IBD varies from 0% to 15.5%, but it seems to be around 5%[12,30] and therefore less common than in patients with SpA without IBD[12]. Incidence of enthesitis also varies largely, among different studies, in these patients[12,30]. A case control study[12] found that enthesitis was also less frequent in IBD-SpA patients compared to SpA individuals without IBD. For both dactylitis and enthesitis, no differences in their frequency were detected between CD and UC patients, while they occurred more frequently in patients with IBD and psoriasis compared to the IBD patients without skin disease[12].
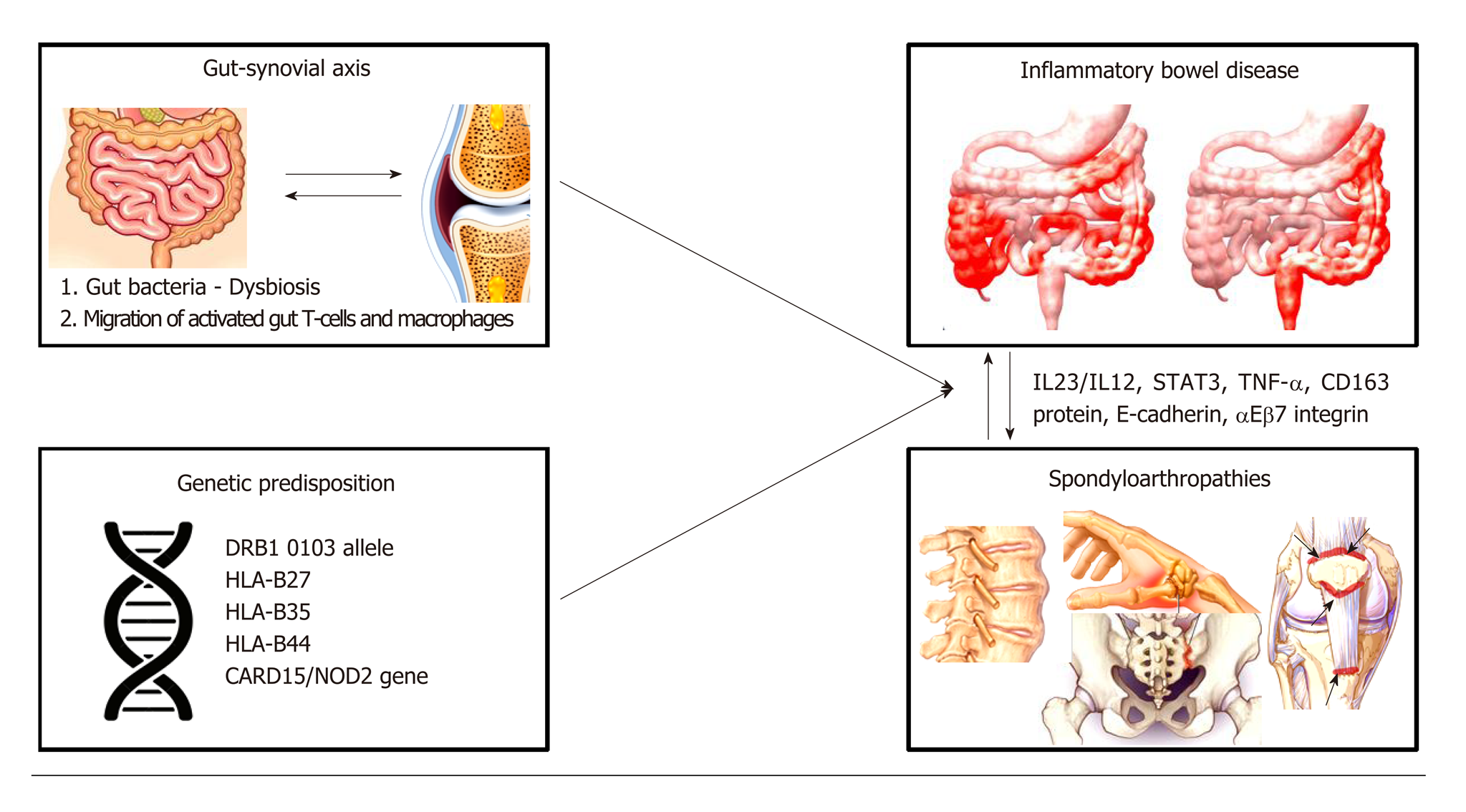
The pathophysiology of spondylathropathies associated with IBD involves the so-called ‘’gut-synovial axis’’ hypothesis, which implicates environmental and host factors. Many of them act as triggers leading to initiation of inflammation in genetically predisposed individuals (Figure 1). Several studies have confirmed the link between joint and gut inflammation. It seems likely that both bacterial antigens and reactive T-cell clones, activated into the gut home the joint. However, the exact immunological mechanisms linking gut and joint inflammation are not fully understood[43,44].
Genetic predisposition seems to carry a significant role in linking these conditions. In a large genotyping study, investigating risk variants for AS, it was shown that many of these were also linked with CD and UC[45]. Additionally, in a genealogic study in Iceland it was shown that first and second-degree relatives of patients with AS had increased risk (3.0 and 2.1, respectively) for IBD and vice versa[46].
Genetic factors play an important role, through alterations in both the adaptive and innate immune pathways[43,44]. Certain human leukocyte antigen (HLA) alleles have been recognized in patients with IBD who are at higher risk for having SpA. As mentioned, 25%-78% of patients with AS and IBD are positive for HLA class I molecule B27 (HLA-B27)[23,43,44]. Furthermore, MHC class II allele DRB1 0103 along with HLA-B35 and HLA-B27 are frequently associated with type I peripheral arthritis[23,47,48], while approximately 38% of patients with active UC or CD have been identified as carrying the allele DRB1 0103. On the other hand, type II peripheral arthritis is associated with HLA-B44[44,49].
Genetic factors outside the HLA system, have also been described. Variations of CARD15 gene (which encodes the protein product NOD2) increase the risk of CD about 4-40 times and has been linked to the development of sacroiliitis in IBD patients[50-52]. In addition, patients with AS and CARD15 mutations are at higher risk for subclinical intestinal inflammation[44,51,53]. NOD2 is an intracellular receptor for bacterial molecules and is expressed in the surface of macrophages, lymphocytes, paneth cells and intestinal epithelial cells. This receptor plays a role in the innate immune response by activating nuclear factor-κB (NFκB) which is a transcriptional regulator of a large variety of genes encoding pro-inflammatory cytokines, adhesion molecules, cytokines, growth factors and enzymes)[43,44,48,51,54]. As a result, NOD2 protein is responsible for positive regulation of immune defense in the gut and induction of a pro-inflammatory state[54]. However, though NOD2 gene mutations are associated with the clinical expression of CD in 20%-30% of patients there is no established association between presence of NOD2 mutations and development of SpA in IBD patients[44,51,55].
Furthermore, CD, AS and PsA have been associated with polymorphisms in some common genes like IL-23R, IL-12B, STAT3, and CARD9, all of them implicated in the anti-IL-23/IL-17 axis[56-60].
Having said that, IL-23/-17 axis seems to play an important role in both axSpA and IBD (regarding the latter, the evidence mainly pertains to CD rather than UC)[61-63]. This axis is mainly regulated by IL-23, resulting in the production of IL-17, IL-22 and to a lesser extent of tumor necrosis factor (TNF)[1] by the so-called Th17 cells, which are a subgroup of T-helper cells. These cytokines are also produced from other cells like innate lymphoid cells[64]. In the gut of patients with CD or patients with SpA, it has been observed increased expression of IL-23[65]. Similarly, in the peripheral blood of AS patients there is increased number of Tγδ cells expressing IL-23R and producing IL-17; additionally, increased expression of IL-23 is noticed in patients’ facets. Interestingly, it seems that there are some cells able to produce IL-17 irrespective of the presence of IL-23. This, as discussed below, might has some implications in the therapeutic approach of these patients[66].
Several other findings also highlight the common underlying pathogenetic mechanisms between IBD and SpA. aEβ7 integrin which is expressed by intraepithelial T cells in the intestinal mucosa and binds to the glycoprotein E-cadherin expressed by gut epithelial cells, has been found to be upregulated on colonic T cells from AS patients and also from lymphocytes obtained from synovial tissue of SpA patients[67,68]. The E-cadherin molecules have been also observed to be up-regulated in the gut of patients with IBD and SpA individuals with subclinical gut inflammation[51,67-69].
In another study increased levels of macrophages expressing the protein CD163 have been reported in both gut mucosa of IBD patients with and without SpA and in the synovial tissue and gut from SpA patients[51,70,71]. Finally, animal models have shown that prolonged exposure to the pro-inflammatory cytokine TNF-α might lead to a phenotype resembling IBD-SpA[72]. In the last decade, it was recognized that a common target of this cytokine could be the synovial fibroblasts and the intestinal myofibroblasts[68,73].
Two - probably complementary- theories have been formulated to explain the development of SpA in patients with IBD. These theories include both alterations in gut bacteria and migration of gut lymphocytes to the joint[43,44]. Changes in the gut microbiome, which is also known as dysbiosis, have been associated with SpA. In detail, Faecalibacterium prausnitzii has been found to be in reduced numbers, in stools of SpA patients[18,74,75]. Also, in AS patients, increased numbers of Dialister microbes in ileal and colon biopsies have been correlated with Ankylosing Spondylitis Disease Activity Score (ASDAS)[18,75] and of Ruminococcus gnavus in the stools with Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)[18,76,77]. Furthermore, other studies have detected in the inflamed joints of these patients certain bacteria like Yersinia enterocolitica, Salmonella enteritidis and typhimurium, or antigens related to them[26,78,79]. Role of microbiome in the pathogenesis of SpA is also supported by data from animal models. For example, the arthritis and inflammatory colitis features developed in HLA-B27 transgenic rats are ameliorated when they are raised in germ-free conditions[18,80]. These observations suggest that gut and joint inflammation process depends on the presence of bacteria into the gastrointestinal tract, which emphasizes the role of autoimmunity and antigen mimicry[26,44].
The second hypothesis is based on experimental studies which showed that gut T-cells activated by antigens migrate to the joints and induce inflammation[43,44,51]. In state of inflammation alterations occur in the mucosal vasculature, such as vasodilation, hyperemia and increased vascular permeability which are induced by various inflammatory cytokines, resulting in enhanced extravasation of leukocytes. Furthermore, the migration pathways of lymphocytes are altered by aberrant expression patterns of adhesion molecules, inflammatory cytokines and receptors[51]. It is known that integrins α4β7 and αΕβ7 and MadCAM-1 mucosal vascular receptor play an important role in the lymphocytes’ gut homing. It has been shown that leukocytes populations from inflamed gut can bind to synovial vessels and home into the joint, using multiple adhesion molecules[81], such as αΕβ7 integrins, vascular adhesion protein-1 (VAP-1) and intracellular adhesion molecule-1 (ICAM-1/CD54)[26] . The increased number of T cells expressing αΕβ7 integrins in the synovial membrane is in favor of the mucosal origin of these cells, however this hypothesis remains to be proven[68].
Additionally, It has been shown that macrophages from the gut of IBD patients are able to adhere in endothelial cells of synovial tissue[81], further enhancing the activation of T cells locally[26]. Collectively, one could argue that gut T-cells are activated in the Peyer’s patches and mesenteric lymph nodes, express a pattern of adhesion molecules that under specific conditions leads to migration of these activated T-cells into the joint causing inflammation[43,44,51,82]. Further studies are needed to fully understand the pathogenic pathways linking IBD and SpA.
Given the common pathogenetic mechanisms underlying SpA and IBD, therapeutic approach to these entities is largely similar. However, there are some differences in the safety and efficacy of the various treatment modalities used.
Non-steroidal anti-inflammatory drugs (NSAIDs) although commonly used in SpA, should be generally avoided in IBD especially in the active ones, but short courses (e.g., 2 wk) do not seem to cause exacerbations[20]. On the other hand, short courses of systemic steroids, although demonstrate some efficacy for CD or UC are not effective for axSpA[20,83]. Local steroids injections and low doses of systemic steroids have been used for peripheral SpA[20].
The most common conventional Disease modifying antirheumatic drugs (DMARDs) used for treatment of SpA are methotrexate and sulfasalazine, both demonstrating some efficacy for peripheral but not for axial SpA[84,85]. Furthermore, methotrexate has proved to be helpful in inducing and maintaining remission in CD patients[86,87] but is not recommended as first line treatment of UC. Similarly, sulfasalazine has some efficacy in CD (but not for ileal CD)[20] and UC[88,89].
Anti-TNF regimes are the gold-standard treatment for the patients with co-existing IBD with SpA who are not controlled with conventional DMARDs. All of them are approved for the treatment of axSpA[90] while infliximab and adalimumab are the most well studied for patients with IBD, both having indication for CD and UC[87]. Etanercept, was not effective for IBD[91]. Many hypotheses have been made to explain its lack of efficacy, including that this might be related to its insufficiency to induce apoptosis in the T cells of the lamina propria[92]. Also, etanercept blocks both TNFa and TNFb. The latter seems to regulate, in the lamina propria, T-cell dependent IgA production, which in turn controls the intestinal microbiota composition[93,94].
Interestingly, new onset IBD in the context of AS, has been observed in patients who started treatment with anti-TNF reagents[95,96]. These cases were more frequently resembling CD rather than UC and have been associated with commencement of etanercept[97-99]. Of note, a large multicentre AS study examining the presence and development of extra-articular manifestations did not find any correlation between biologics use and development of IBD[9]. Whether there is a true association between treatment with anti-TNF drugs in AS patients and new-onset of IBD, remains to be defined.
The role of the IL-23/-IL-17 axis in the pathogenesis of SpA and IBD is supported by many studies of basic and clinical research[61]. Despite, monoclonal antibodies targeting the key cytokines of the axis (i.e., IL-23 and IL-17) were thought to be very effective, data from clinical trials did not fully support this notion. Ustekinumab, a monoclonal antibody against the common p40 subunit of IL-12 and IL-23, has proved to be effective for CD but does not appear to work for AS. Although data derived from post hoc analyses of phase 3 trials in patients with psoriatic spondylitis were promising, ustekinumab did not achieve the primary endpoint in phase 3 trials for AS and non-radiographic axSpA[100]. Similarly, risankizumab, which is an antibody specifically targeting the p19 subunit of IL-23 failed to show clinical and radiological efficacy in a phase 2 trial for AS[101]. Data from clinical trial about other antibodies against p19 subunit of IL-23 are eagerly awaited. To explain the differences observed in the efficacy of anti-IL-23 between psoriatic spondylitis and AS, it is not irrational to speculate that the pathogenetic mechanisms underlying AS are somewhat different to those of spondylitis in the context of PsA.
Secukinumab, which is a monoclonal antibody against IL-17 recently received approval for AS and therefore is another therapeutic option in patients with axSpA. Phase 3 trials are now underway for secukinumab in nraxSpA. One could expect, based on the underlying pathogenetic mechanisms that secukinumab would have good results in CD. However, results in phase 2 trials were negative with the drug being numerically worse than placebo. Many hypotheses have been formed to explain the failure of secukinumab in CD. Candida albicans proliferation has been proposed as a plausible explanation for the CD exacerbation given the role of IL-17 in fighting fungal infections[61,102]. Although new cases of IBD in axSpA patients treated with secukinumab have been described[103], a recently published study analysing data from 21 clinical trials from patients with psoriasis, psoriatic arthritis and ankylosing spondylitis, has shown that exposure adjusted incidence rates for IBD did not increase over time with secukinumab treatment[104]. Interestingly, a recent study provided some evidence supporting that suppression of IL-17F but not IL-17A was indeed protective for colitis by inducing T regulatory cells via modifications in colonic microbiota[105].
An obvious question is how ustekinumab, which blocks IL-23 and subsequently IL-17 works for CD but secukinumab does not? There is accumulated evidence that IL-17 can be produced also -to a lesser extent possibly- in an IL-23 independent manner from innate lymphoid, T γδ or other types of cells[64,106]. Therefore, blocking IL-23 leaves some “basal” levels of IL-17. Lee et al[107] have shown, that T γδ cells in the lamina propria are the producers of gut-protective IL-17, in an IL-23 independent way. Its effect is possibly mediated through regulation of the tight-junction protein “occludin” which maintain barriers integrity.
Vedolizumab, a gut selective α4β7 integrin antagonist, has shown to be effective in patients with CD[108,109] and for inducing or maintaining therapy in UC patients[110]. Results of this drug in articular symptoms are somewhat conflicting. Whether this drug is linked with exacerbation or new-onset arthralgias or inflammatory arthritis remains to be answered[87,111-113]. Of note, a recent post hoc analysis of the “Gemini” trials showed that vedolizumab was associated with decreased likelihood of new or worsening arthritis/arthralgia in CD patients while in UC the incidence was similar between patients treated with the active drug or with placebo[114].
JAK inhibitors are a new drug class category with promising results in various immune mediated diseases. Genome wide association studies have shown that there is association between CD and single nucleotide polymorphisms in the JAK-STAT pathway[115]. Results in a phase 2 trial for CD has shown that tofacitinib was not effective[116]. However, newer and more selective JAK-inhibitors, like filgotinib and upadacitinib have favorable results in achieving clinical remission in phase 2 trials for CD[117,118]. For UC, tofacitinib after the promising results with patients achieving higher rates of clinical remission and clinical response compared to placebo[119] received Food and Drug Administration (FDA) approval for patients with moderate to severely active UC.
As regards to the efficacy of JAK-inhibitors in SpA, tofacitinib has shown favorable results in phase 2 trials of AS with 80.8% of the patients treated with tofacitinib achieving ASAS20 improvement at week 8, compared to 41.2% of placebo-treated patients[120]. Recently published results from a phase 2 clinical trials showed also that filgotinib was effective for AS with patients experiencing significant clinical improvement, compared to placebo, at week 12. A phase 2b/3a clinical trial assessing the efficacy and safety of upadacitinib in patients with AS is currently underway (NCT03178487). Whether JAK-inhibitors could be another potential therapeutic option in patients with IBD and SpA remains to be defined from future studies.
Although colonoscopy is being considered as the gold-standard for IBD diagnosis, a recent study has shown that capsule endoscopy was superior to classical colonoscopy in diagnosing CD in the context of SpA. It was shown that small bowel inflammation was present in 42.2% and 10.9% of the patients who underwent capsule endoscopy and classical colonoscopy, respectively. Interestingly, positive findings were not associated with symptomatology from the gastrointestinal system but with elevated faecal calprotectin levels, confirming that many SpA patients have “silent” IBD[121]. Calprotectin measured in the serum or in the stools has been used to identify subclinical bowel inflammation in patients with SpA. Cypers et al[14] have found that elevated serum calprotectin levels have been associated with subclinical microscopic colitis in SpA patients. In detail, individuals who had both CRP and calprotectin elevated had a frequency of bowel inflammation of 64% compared to 25% in patients who had low levels of these proteins. Additionally, in patients who had high levels of either serum calprotectin or CRP, frequency of bowel inflammation was significantly higher in SpA patients with high faecal calprotectin compared to those with low[14]. In a recent study, Ostgard et al[122] confirmed that faecal calprotectin could serve as a biomarker to identify patients with subclinical bowel inflammation. It has to be noted however that faecal calprotectin levels can be influenced by NSAIDs use, which is quite common in SpA patients[123]. Interestingly, patients with elevated faecal calprotectin levels had more inflammation in the sacroiliac joints compared to those with low levels. Also, the former had better response to adalimumab as assessed by ASDAS. It has to be said however, that these patients received an extra loading dose of 80 mg adalimumab, at baseline[122]. The concept of calprotectin as biomarker of treatment response has been suggested also previously: In proof of concept trials for SpA, serum calprotectin has been found to be decreased after treatment of axSpA and peripheral SpA with infliximab and etanercept, respectively[124].
It is increasingly being recognized that there is a very close link between IBD and SpA. As outlined in this review, there are several hints for that: epidemiological, clinical, laboratory (i.e., positivity for HLA-B27) histopathologic and pathogenetic. Regarding the latter, it is very intriguing to define to which extent these are common between these entities and identify the diversities that lead to different clinical expressions. However, many limitations impede this venture. Firstly, over the last years, many different criteria have been used for the classification of SpA, which comprise a group of relatively heterogenous diseases. Besides, classification criteria in SpA do not mean necessarily a certain diagnosis and vice versa[125]. Secondly, these patients, depending on the cardinal manifestation, are followed up by a gastroenterologist or a rheumatologist that might overlook the articular or bowel manifestations of the disease, respectively. To that end, the effective communication between different professions and the interdisciplinary approach, through combined clinics for example, in imperative.
Treatment of this entities has progressed significantly over the last years. To the successful anti-TNF reagents, drugs targeting IL-23 and IL-17 as well as the JAK-inhibitors have been added to the clinician’s arsenal. However, treating patients with co-existing SpA and IBD, should not only include these manifestations but also considerate other extra-articular and extra-intestinal manifestations like skin disease or uveitis. Comprehensive algorithms, designed by clinicians of many disciplines are urgently needed, in light of the numerous emerging therapeutic modalities.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Horesh N, Ksel IY, M'Koma AE, Perse M S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
| 1. | Kiltz U, Siebert S, Frargoulis G, McInnes I. Spondyloarthritis: Pathogenesis, Clinical aspects and Diagnosis. In: Bijlsma JW, Hachulla E. EULAR Textbook on Rheumatic Diseases. BMJ Publishing group 2018; 338-364. |
| 2. | Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 289] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 3. | Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, Dougados M, Huang F, Gu J, Kirazli Y, Van den Bosch F, Olivieri I, Roussou E, Scarpato S, Sørensen IJ, Valle-Oñate R, Weber U, Wei J, Sieper J. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1130] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 4. | Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M, Huang F, Gu J, Khan MA, Kirazli Y, Maksymowych WP, Mielants H, Sørensen IJ, Ozgocmen S, Roussou E, Valle-Oñate R, Weber U, Wei J, Sieper J. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2694] [Cited by in RCA: 2478] [Article Influence: 154.9] [Reference Citation Analysis (0)] |
| 5. | Moltó A, Paternotte S, Comet D, Thibout E, Rudwaleit M, Claudepierre P, van der Heijde D, Dougados M. Performances of the Assessment of SpondyloArthritis International Society axial spondyloarthritis criteria for diagnostic and classification purposes in patients visiting a rheumatologist because of chronic back pain: results from a multicenter, cross-sectional study. Arthritis Care Res (Hoboken). 2013;65:1472-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Stolwijk C, Essers I, van Tubergen A, Boonen A, Bazelier MT, De Bruin ML, de Vries F. The epidemiology of extra-articular manifestations in ankylosing spondylitis: a population-based matched cohort study. Ann Rheum Dis. 2015;74:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Orlando A, Renna S, Perricone G, Cottone M. Gastrointestinal lesions associated with spondyloarthropathies. World J Gastroenterol. 2009;15:2443-2448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther. 2016;18:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 9. | Essers I, Ramiro S, Stolwijk C, Blaauw M, Landewé R, van der Heijde D, Van den Bosch F, Dougados M, van Tubergen A. Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatology (Oxford). 2015;54:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Dougados M, d'Agostino MA, Benessiano J, Berenbaum F, Breban M, Claudepierre P, Combe B, Dargent-Molina P, Daurès JP, Fautrel B, Feydy A, Goupille P, Leblanc V, Logeart I, Pham T, Richette P, Roux C, Rudwaleit M, Saraux A, Treluyer JM, van der Heijde D, Wendling D. The DESIR cohort: a 10-year follow-up of early inflammatory back pain in France: study design and baseline characteristics of the 708 recruited patients. Joint Bone Spine. 2011;78:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker-Hermann E, Zeidler H, Braun J, Sieper J. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009;60:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 519] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 12. | Cantini F, Niccoli L, Nannini C, Cassarà E, Kaloudi O, Rizzello F, Gionchetti P. Case-control Study on Dactylitis, Enthesitis, and Anterior Uveitis in Spondyloarthritis Associated with Inflammatory Bowel Diseases: Role of Coexistent Psoriasis. J Rheumatol. 2017;44:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Van Praet L, Jans L, Carron P, Jacques P, Glorieus E, Colman R, Cypers H, Mielants H, De Vos M, Cuvelier C, Van den Bosch F, Elewaut D. Degree of bone marrow oedema in sacroiliac joints of patients with axial spondyloarthritis is linked to gut inflammation and male sex: results from the GIANT cohort. Ann Rheum Dis. 2014;73:1186-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Cypers H, Varkas G, Beeckman S, Debusschere K, Vogl T, Roth J, Drennan MB, Lavric M, Foell D, Cuvelier CA, De Vos M, Delanghe J, Van den Bosch F, Elewaut D. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann Rheum Dis. 2016;75:1357-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Rudwaleit M, Baeten D. Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol. 2006;20:451-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Van Praet L, Van den Bosch FE, Jacques P, Carron P, Jans L, Colman R, Glorieus E, Peeters H, Mielants H, De Vos M, Cuvelier C, Elewaut D. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis. 2013;72:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 17. | De Vos M, Mielants H, Cuvelier C, Elewaut A, Veys E. Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology. 1996;110:1696-1703. [PubMed] |
| 18. | Gilis E, Mortier C, Venken K, Debusschere K, Vereecke L, Elewaut D. The Role of the Microbiome in Gut and Joint Inflammation in Psoriatic Arthritis and Spondyloarthritis. J Rheumatol Suppl. 2018;94:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Gionchetti P, Calabrese C, Rizzello F. Inflammatory Bowel Diseases and Spondyloarthropathies. J Rheumatol Suppl. 2015;93:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Olivieri I, Cantini F, Castiglione F, Felice C, Gionchetti P, Orlando A, Salvarani C, Scarpa R, Vecchi M, Armuzzi A. Italian Expert Panel on the management of patients with coexisting spondyloarthritis and inflammatory bowel disease. Autoimmun Rev. 2014;13:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Orchard TR, Holt H, Bradbury L, Hammersma J, McNally E, Jewell DP, Wordsworth BP. The prevalence, clinical features and association of HLA-B27 in sacroiliitis associated with established Crohn's disease. Aliment Pharmacol Ther. 2009;29:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Palm O, Moum B, Ongre A, Gran JT. Prevalence of ankylosing spondylitis and other spondyloarthropathies among patients with inflammatory bowel disease: a population study (the IBSEN study). J Rheumatol. 2002;29:511-515. [PubMed] |
| 23. | Salvarani C, Fries W. Clinical features and epidemiology of spondyloarthritides associated with inflammatory bowel disease. World J Gastroenterol. 2009;15:2449-2455. [PubMed] |
| 24. | Shivashankar R, Loftus EV, Tremaine WJ, Bongartz T, Harmsen WS, Zinsmeister AR, Matteson EL. Incidence of spondyloarthropathy in patients with Crohn's disease: a population-based study. J Rheumatol. 2012;39:2148-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Smale S, Natt RS, Orchard TR, Russell AS, Bjarnason I. Inflammatory bowel disease and spondylarthropathy. Arthritis Rheum. 2001;44:2728-2736. [PubMed] |
| 26. | Fantini MC, Pallone F, Monteleone G. Common immunologic mechanisms in inflammatory bowel disease and spondylarthropathies. World J Gastroenterol. 2009;15:2472-2478. [PubMed] |
| 27. | Turkcapar N, Toruner M, Soykan I, Aydintug OT, Cetinkaya H, Duzgun N, Ozden A, Duman M. The prevalence of extraintestinal manifestations and HLA association in patients with inflammatory bowel disease. Rheumatol Int. 2006;26:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Yüksel I, Ataseven H, Başar O, Köklü S, Ertuğrul I, Ulker A, Dağlı U, Saşmaz N. Peripheral arthritis in the course of inflammatory bowel diseases. Dig Dis Sci. 2011;56:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | de Vlam K, Mielants H, Cuvelier C, De Keyser F, Veys EM, De Vos M. Spondyloarthropathy is underestimated in inflammatory bowel disease: prevalence and HLA association. J Rheumatol. 2000;27:2860-2865. [PubMed] |
| 30. | Karreman MC, Luime JJ, Hazes JMW, Weel AEAM. The Prevalence and Incidence of Axial and Peripheral Spondyloarthritis in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis. 2017;11:631-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Liu S, Ding J, Wang M, Zhou W, Feng M, Guan W. Clinical features of Crohn disease concomitant with ankylosing spondylitis: A preliminary single-center study. Medicine (Baltimore). 2016;95:e4267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Peeters H, Vander Cruyssen B, Mielants H, de Vlam K, Vermeire S, Louis E, Rutgeerts P, Belaiche J, De Vos M. Clinical and genetic factors associated with sacroiliitis in Crohn's disease. J Gastroenterol Hepatol. 2008;23:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Steer S, Jones H, Hibbert J, Kondeatis E, Vaughan R, Sanderson J, Gibson T. Low back pain, sacroiliitis, and the relationship with HLA-B27 in Crohn's disease. J Rheumatol. 2003;30:518-522. [PubMed] |
| 34. | Alamanos Y, Papadopoulos NG, Voulgari PV, Karakatsanis A, Siozos C, Drosos AA. Epidemiology of ankylosing spondylitis in Northwest Greece, 1983-2002. Rheumatology (Oxford). 2004;43:615-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Fırat SN, Yazıcı A, Yılmazer B, Coşan F, Savlı H, Cefle A. Low frequency of HLA-B27 in ankylosing spondylitis and its relationship with clinical findings in patients from Turkey. Eur J Rheumatol. 2017;4:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Khan MA. Spondyloarthropathies. Curr Opin Rheumatol. 1994;6:351-353. [PubMed] |
| 37. | Peluso R, Di Minno MN, Iervolino S, Manguso F, Tramontano G, Ambrosino P, Esposito C, Scalera A, Castiglione F, Scarpa R. Enteropathic spondyloarthritis: from diagnosis to treatment. Clin Dev Immunol. 2013;2013:631408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Rodríguez-Reyna TS, Martínez-Reyes C, Yamamoto-Furusho JK. Rheumatic manifestations of inflammatory bowel disease. World J Gastroenterol. 2009;15:5517-5524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut. 1998;42:387-391. [PubMed] |
| 40. | Manguso F, Sanges M, Staiano T, Gargiulo S, Nastro P, Gargano D, Somma P, Mansueto G, Peluso R, Scarpa R, D'Armiento FP, Astarita C, Ayala F, Renda A, Mazzacca G, D'Arienzo A. Cigarette smoking and appendectomy are risk factors for extraintestinal manifestations in ulcerative colitis. Am J Gastroenterol. 2004;99:327-334. [PubMed] |
| 41. | Vavricka SR, Brun L, Ballabeni P, Pittet V, Prinz Vavricka BM, Zeitz J, Rogler G, Schoepfer AM. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 42. | Queiro R, Maiz O, Intxausti J, de Dios JR, Belzunegui J, González C, Figueroa M. Subclinical sacroiliitis in inflammatory bowel disease: a clinical and follow-up study. Clin Rheumatol. 2000;19:445-449. [PubMed] |
| 43. | Arvikar SL, Fisher MC. Inflammatory bowel disease associated arthropathy. Curr Rev Musculoskelet Med. 2011;4:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Sheth T, Pitchumoni CS, Das KM. Management of Musculoskeletal Manifestations in Inflammatory Bowel Disease. Gastroenterol Res Pract. 2015;2015:387891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | International Genetics of Ankylosing Spondylitis Consortium, Australo-Anglo-American Spondyloarthritis Consortium (TASC), Groupe Française d'Etude Génétique des Spondylarthrites (GFEGS), Nord-Trøndelag Health Study (HUNT), Spondyloarthritis Research Consortium of Canada (SPARCC), Wellcome Trust Case Control Consortium 2 (WTCCC2); Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, Leo P, Cremin K, Pryce K, Harris J, Lee S, Joo KB, Shim SC, Weisman M, Ward M, Zhou X, Garchon HJ, Chiocchia G, Nossent J, Lie BA, Tuomilehto J, Laiho K, Jiang L, Liu Y, Wu X, Bradbury LA, Elewaut D, Stebbings S, Appleton L, Farrah C, Lau J, Kenna TJ, Haroon N, Ferreira MA, Yang J, Mulero J, Deloukas P, Donnelly P, Bowness P, Gafney K, Gaston H, Gladman DD, Rahman P, Maksymowych WP, Xu H, Crusius JB, Chou CT, Hansen IM, Inman RD, Videm V, Martin J, Breban M, Reveille JD, Evans DM, Kim TH, Wordsworth BP, Brown MA, Burgos-Vargas R, Fernandez-Sueiro JL, Gonzalez-Gay MA, Lopez-Larrea C, Romero-Sánchez C, Pimentel-Santos FM, Valle-Oñate R, van der Horst-Bruinsma IE, Førre Ø. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet. 2013;45:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 671] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 46. | Thjodleifsson B, Geirsson AJ, Björnsson S, Bjarnason I. A common genetic background for inflammatory bowel disease and ankylosing spondylitis: a genealogic study in Iceland. Arthritis Rheum. 2007;56:2633-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Orchard TR, Thiyagaraja S, Welsh KI, Wordsworth BP, Hill Gaston JS, Jewell DP. Clinical phenotype is related to HLA genotype in the peripheral arthropathies of inflammatory bowel disease. Gastroenterology. 2000;118:274-278. [PubMed] |
| 48. | Voulgari PV. Rheumatological manifestations in inflammatory bowel disease. Ann Gastroenterol. 2011;24:173-180. [PubMed] |
| 49. | Jewell D. Do HLA antigens predict the occurrence of extraintestinal manifestations of IBD? Inflamm Bowel Dis. 2008;14 Suppl 2:S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1348] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 51. | Brakenhoff LK, van der Heijde DM, Hommes DW, Huizinga TW, Fidder HH. The joint-gut axis in inflammatory bowel diseases. J Crohns Colitis. 2010;4:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3555] [Cited by in RCA: 3478] [Article Influence: 144.9] [Reference Citation Analysis (1)] |
| 53. | Laukens D, Peeters H, Marichal D, Vander Cruyssen B, Mielants H, Elewaut D, Demetter P, Cuvelier C, Van Den Berghe M, Rottiers P, Veys EM, Remaut E, Steidler L, De Keyser F, De Vos M. CARD15 gene polymorphisms in patients with spondyloarthropathies identify a specific phenotype previously related to Crohn's disease. Ann Rheum Dis. 2005;64:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Abraham C, Cho JH. Functional consequences of NOD2 (CARD15) mutations. Inflamm Bowel Dis. 2006;12:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, Frenzel H, King K, Hasselmeyer A, MacPherson AJ, Bridger S, van Deventer S, Forbes A, Nikolaus S, Lennard-Jones JE, Foelsch UR, Krawczak M, Lewis C, Schreiber S, Mathew CG. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet. 2001;357:1925-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 769] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 56. | Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 57. | Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2428] [Cited by in RCA: 2310] [Article Influence: 121.6] [Reference Citation Analysis (1)] |
| 58. | Rahman P, Inman RD, Gladman DD, Reeve JP, Peddle L, Maksymowych WP. Association of interleukin-23 receptor variants with ankylosing spondylitis. Arthritis Rheum. 2008;58:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 59. | Danoy P, Pryce K, Hadler J, Bradbury LA, Farrar C, Pointon J; Australo-Anglo-American Spondyloarthritis Consortium, Ward M, Weisman M, Reveille JD, Wordsworth BP, Stone MA; Spondyloarthritis Research Consortium of Canada, Maksymowych WP, Rahman P, Gladman D, Inman RD, Brown MA. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn's disease. PLoS Genet. 2010;6:e1001195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 60. | Nanda S. Spondyloarthropathies: novel genetic variants link ankylosing spondylitis and Crohn disease: evidence of a shared pathogenesis? Nat Rev Rheumatol. 2011;7:70. [PubMed] |
| 61. | Fragoulis GE, Siebert S, McInnes IB. Therapeutic Targeting of IL-17 and IL-23 Cytokines in Immune-Mediated Diseases. Annu Rev Med. 2016;67:337-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 62. | Fries W. Inflammatory bowel disease-associated spondyloarthropathies. World J Gastroenterol. 2009;15:2441-2442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Liossis NS, Daoussis D. Is there a link between IL-23/IL-17 and developmental pathways such as the Wnt and Hedgehog pathway? Mediterr J Rheumatol. 2017;28:69-71. |
| 64. | Hasegawa E, Sonoda KH, Shichita T, Morita R, Sekiya T, Kimura A, Oshima Y, Takeda A, Yoshimura T, Yoshida S, Ishibashi T, Yoshimura A. IL-23-independent induction of IL-17 from γδT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J Immunol. 2013;190:1778-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 65. | Ciccia F, Bombardieri M, Principato A, Giardina A, Tripodo C, Porcasi R, Peralta S, Franco V, Giardina E, Craxi A, Pitzalis C, Triolo G. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 66. | Siebert S, Millar NL, McInnes IB. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann Rheum Dis. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 67. | Asquith M, Elewaut D, Lin P, Rosenbaum JT. The role of the gut and microbes in the pathogenesis of spondyloarthritis. Best Pract Res Clin Rheumatol. 2014;28:687-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 68. | Jacques P, Elewaut D. Joint expedition: linking gut inflammation to arthritis. Mucosal Immunol. 2008;1:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Demetter P, Baeten D, De Keyser F, De Vos M, Van Damme N, Verbruggen G, Vermeulen S, Mareel M, Elewaut D, Mielants H, Veys EM, Cuvelier CA. Subclinical gut inflammation in spondyloarthropathy patients is associated with upregulation of the E-cadherin/catenin complex. Ann Rheum Dis. 2000;59:211-216. [PubMed] |
| 70. | Baeten D, Demetter P, Cuvelier CA, Kruithof E, Van Damme N, De Vos M, Veys EM, De Keyser F. Macrophages expressing the scavenger receptor CD163: a link between immune alterations of the gut and synovial inflammation in spondyloarthropathy. J Pathol. 2002;196:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Demetter P, De Vos M, Van Huysse JA, Baeten D, Ferdinande L, Peeters H, Mielants H, Veys EM, De Keyser F, Cuvelier CA. Colon mucosa of patients both with spondyloarthritis and Crohn's disease is enriched with macrophages expressing the scavenger receptor CD163. Ann Rheum Dis. 2005;64:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387-398. [PubMed] |
| 73. | Armaka M, Apostolaki M, Jacques P, Kontoyiannis DL, Elewaut D, Kollias G. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med. 2008;205:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 74. | Stoll ML, Kumar R, Morrow CD, Lefkowitz EJ, Cui X, Genin A, Cron RQ, Elson CO. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis Res Ther. 2014;16:486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 75. | Tito RY, Cypers H, Joossens M, Varkas G, Van Praet L, Glorieus E, Van den Bosch F, De Vos M, Raes J, Elewaut D. Brief Report: Dialister as a Microbial Marker of Disease Activity in Spondyloarthritis. Arthritis Rheumatol. 2017;69:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 76. | Breban M, Tap J, Leboime A, Said-Nahal R, Langella P, Chiocchia G, Furet JP, Sokol H. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis. 2017;76:1614-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 77. | Vereecke L, Elewaut D. Spondyloarthropathies: Ruminococcus on the horizon in arthritic disease. Nat Rev Rheumatol. 2017;13:574-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Granfors K, Jalkanen S, Lindberg AA, Mäki-Ikola O, von Essen R, Lahesmaa-Rantala R, Isomäki H, Saario R, Arnold WJ, Toivanen A. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet. 1990;335:685-688. [PubMed] |
| 79. | Granfors K, Jalkanen S, von Essen R, Lahesmaa-Rantala R, Isomäki O, Pekkola-Heino K, Merilahti-Palo R, Saario R, Isomäki H, Toivanen A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med. 1989;320:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 316] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernández-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359-2364. [PubMed] |
| 81. | Salmi M, Jalkanen S. Human leukocyte subpopulations from inflamed gut bind to joint vasculature using distinct sets of adhesion molecules. J Immunol. 2001;166:4650-4657. [PubMed] |
| 82. | Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1624] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 83. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D'Haens G, D'Hoore A, Mantzaris G, Novacek G, Oresland T, Reinisch W, Sans M, Stange E, Vermeire S, Travis S, Van Assche G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 84. | Haibel H, Brandt HC, Song IH, Brandt A, Listing J, Rudwaleit M, Sieper J. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis. 2007;66:419-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 85. | van der Heijde D, Sieper J, Maksymowych WP, Dougados M, Burgos-Vargas R, Landewé R, Rudwaleit M, Braun J; Assessment of SpondyloArthritis international Society. 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70:905-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 86. | McDonald JW, Wang Y, Tsoulis DJ, MacDonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn's disease. Cochrane Database Syst Rev. 2014;CD003459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Pouillon L, Bossuyt P, Vanderstukken J, Moulin D, Netter P, Danese S, Jouzeau JY, Loeuille D, Peyrin-Biroulet L. Management of patients with inflammatory bowel disease and spondyloarthritis. Expert Rev Clin Pharmacol. 2017;10:1363-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F, European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 938] [Cited by in RCA: 867] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 89. | Lim WC, Wang Y, MacDonald JK, Hanauer S. Aminosalicylates for induction of remission or response in Crohn's disease. Cochrane Database Syst Rev. 2016;7:CD008870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 90. | van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, Sepriano A, Regel A, Ciurea A, Dagfinrud H, Dougados M, van Gaalen F, Géher P, van der Horst-Bruinsma I, Inman RD, Jongkees M, Kiltz U, Kvien TK, Machado PM, Marzo-Ortega H, Molto A, Navarro-Compàn V, Ozgocmen S, Pimentel-Santos FM, Reveille J, Rudwaleit M, Sieper J, Sampaio-Barros P, Wiek D, Braun J. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1038] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 91. | Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD, Tremaine WJ, Johnson T, Diehl NN, Zinsmeister AR. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121:1088-1094. [PubMed] |
| 92. | Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van Montfrans C, Hommes DW, Peppelenbosch MP, van Deventer SJ. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology. 2003;124:1774-1785. [PubMed] |
| 93. | Bazin T, Hooks KB, Barnetche T, Truchetet ME, Enaud R, Richez C, Dougados M, Hubert C, Barré A, Nikolski M, Schaeverbeke T. Microbiota Composition May Predict Anti-Tnf Alpha Response in Spondyloarthritis Patients: an Exploratory Study. Sci Rep. 2018;8:5446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 94. | Kruglov AA, Grivennikov SI, Kuprash DV, Winsauer C, Prepens S, Seleznik GM, Eberl G, Littman DR, Heikenwalder M, Tumanov AV, Nedospasov SA. Nonredundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science. 2013;342:1243-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 95. | Braun J, Baraliakos X, Listing J, Davis J, van der Heijde D, Haibel H, Rudwaleit M, Sieper J. Differences in the incidence of flares or new onset of inflammatory bowel diseases in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor alpha agents. Arthritis Rheum. 2007;57:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 96. | Fouache D, Martin A, Dernis E, Wendling D, Ansemant T, Berthelot JM, Kantelip B, Le CRI, Bader-Meunier B, Le Dantec P, Toussirot É, Houvenagel É, Goëb V. Development of inflammatory bowel disease during anti-TNF-α therapy for inflammatory rheumatic disease: a nationwide series. Joint Bone Spine. 2012;79:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 97. | Freeman HJ. Colitis associated with biological agents. World J Gastroenterol. 2012;18:1871-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 98. | Haraoui B, Krelenbaum M. Emergence of Crohn's disease during treatment with the anti-tumor necrosis factor agent etanercept for ankylosing spondylitis: possible mechanisms of action. Semin Arthritis Rheum. 2009;39:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | O'Toole A, Lucci M, Korzenik J. Inflammatory Bowel Disease Provoked by Etanercept: Report of 443 Possible Cases Combined from an IBD Referral Center and the FDA. Dig Dis Sci. 2016;61:1772-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 100. | Tahir H. Therapies in ankylosing spondylitis-from clinical trials to clinical practice. Rheumatology (Oxford). 2018;57:vi23-vi28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Baeten D, Østergaard M, Wei JC, Sieper J, Järvinen P, Tam LS, Salvarani C, Kim TH, Solinger A, Datsenko Y, Pamulapati C, Visvanathan S, Hall DB, Aslanyan S, Scholl P, Padula SJ. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018;77:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 294] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 102. | Colombel JF, Sendid B, Jouault T, Poulain D. Secukinumab failure in Crohn's disease: the yeast connection? Gut. 2013;62:800-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 103. | Fobelo Lozano MJ, Serrano Giménez R, Castro Fernández M. Emergence of Inflammatory Bowel Disease During Treatment with Secukinumab. J Crohns Colitis. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Schreiber S, Colombel JF, Feagan BG, Reich K, Deodhar AA, McInnes IB, Porter B, Das Gupta A, Pricop L, Fox T. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis. 2019;78:473-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 105. | Tang C, Kakuta S, Shimizu K, Kadoki M, Kamiya T, Shimazu T, Kubo S, Saijo S, Ishigame H, Nakae S, Iwakura Y. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 106. | Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1210] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 107. | Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, Cua DJ. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 592] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 108. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1568] [Article Influence: 130.7] [Reference Citation Analysis (1)] |
| 109. | Sands BE, Sandborn WJ, Van Assche G, Lukas M, Xu J, James A, Abhyankar B, Lasch K. Vedolizumab as Induction and Maintenance Therapy for Crohn's Disease in Patients Naïve to or Who Have Failed Tumor Necrosis Factor Antagonist Therapy. Inflamm Bowel Dis. 2017;23:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 110. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1865] [Article Influence: 155.4] [Reference Citation Analysis (1)] |
| 111. | Orlando A, Orlando R, Ciccia F, Renna S, Rizzo A, Cottone M, Macaluso FS. Clinical benefit of vedolizumab on articular manifestations in patients with active spondyloarthritis associated with inflammatory bowel disease. Ann Rheum Dis. 2017;76:e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 112. | Tadbiri S, Peyrin-Biroulet L, Serrero M, Filippi J, Pariente B, Roblin X, Buisson A, Stefanescu C, Trang-Poisson C, Altwegg R, Marteau P, Vaysse T, Bourrier A, Nancey S, Laharie D, Allez M, Savoye G, Gilletta C, Gagniere C, Vuitton L, Viennot S, Aubourg A, Pelletier AL, Bouguen G, Abitbol V, Fumery M, Claudepierre P, Bouhnik Y, Amiot A; GETAID OBSERV-IBD study group. Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther. 2018;47:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 113. | Varkas G, Thevissen K, De Brabanter G, Van Praet L, Czul-Gurdian F, Cypers H, De Kock J, Carron P, De Vos M, Hindryckx P, Arts J, Vanneuville I, Schoenaers P, Claerhout B, Abreu M, Van den Bosch F, Elewaut D. An induction or flare of arthritis and/or sacroiliitis by vedolizumab in inflammatory bowel disease: a case series. Ann Rheum Dis. 2017;76:878-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 114. | Feagan BG, Sandborn WJ, Colombel JF, Byrne SO, Khalid JM, Kempf C, Geransar P, Bhayat F, Rubin DT. Incidence of Arthritis/Arthralgia in Inflammatory Bowel Disease with Long-term Vedolizumab Treatment: Post Hoc Analyses of the GEMINI Trials. J Crohns Colitis. 2019;13:50-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 115. | Ferguson LR, Han DY, Fraser AG, Huebner C, Lam WJ, Morgan AR, Duan H, Karunasinghe N. Genetic factors in chronic inflammation: single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn's disease in a New Zealand population. Mutat Res. 2010;690:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 116. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W; Study A3921043 Investigators. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014;12:1485-1493.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 117. | Sandborn W, Feagan B, Panes J, D'Haens G, Colombel J, Zhou Q, Huang B, Enejosa J, Pangan A, Lacerda A. Safety and Efficacy of ABT-494 (Upadacitinib), an Oral Jak1 Inhibitor, as Induction Therapy in Patients with Crohn's Disease: Results from Celest. Gatroenterology. 2017;152 Suppl 1:1308-1309. |
| 118. | Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis A, Wisniewska-Jarosinska M, Baranovsky A, Sike R, Stoyanova K, Tasset C, Van der Aa A, Harrison P. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 119. | Sandborn WJ, Su C, Panes J. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;377:496-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 120. | van der Heijde D, Deodhar A, Wei JC, Drescher E, Fleishaker D, Hendrikx T, Li D, Menon S, Kanik KS. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2017;76:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 279] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 121. | Kopylov U, Starr M, Watts C, Dionne S, Girardin M, Seidman EG. Detection of Crohn Disease in Patients with Spondyloarthropathy: The SpACE Capsule Study. J Rheumatol. 2018;45:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 122. | Østgård RD, Deleuran BW, Dam MY, Hansen IT, Jurik AG, Glerup H. Faecal calprotectin detects subclinical bowel inflammation and may predict treatment response in spondyloarthritis. Scand J Rheumatol. 2018;47:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Klingberg E, Carlsten H, Hilme E, Hedberg M, Forsblad-d'Elia H. Calprotectin in ankylosing spondylitis--frequently elevated in feces, but normal in serum. Scand J Gastroenterol. 2012;47:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 124. | Turina MC, Yeremenko N, Paramarta JE, De Rycke L, Baeten D. Calprotectin (S100A8/9) as serum biomarker for clinical response in proof-of-concept trials in axial and peripheral spondyloarthritis. Arthritis Res Ther. 2014;16:413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 125. | van Tubergen A, Weber U. Diagnosis and classification in spondyloarthritis: identifying a chameleon. Nat Rev Rheumatol. 2012;8:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |