Published online May 7, 2019. doi: 10.3748/wjg.v25.i17.2029
Peer-review started: March 19, 2019
First decision: March 27, 2019
Revised: April 3, 2019
Accepted: April 19, 2019
Article in press: April 20, 2019
Published online: May 7, 2019
Processing time: 48 Days and 17.9 Hours
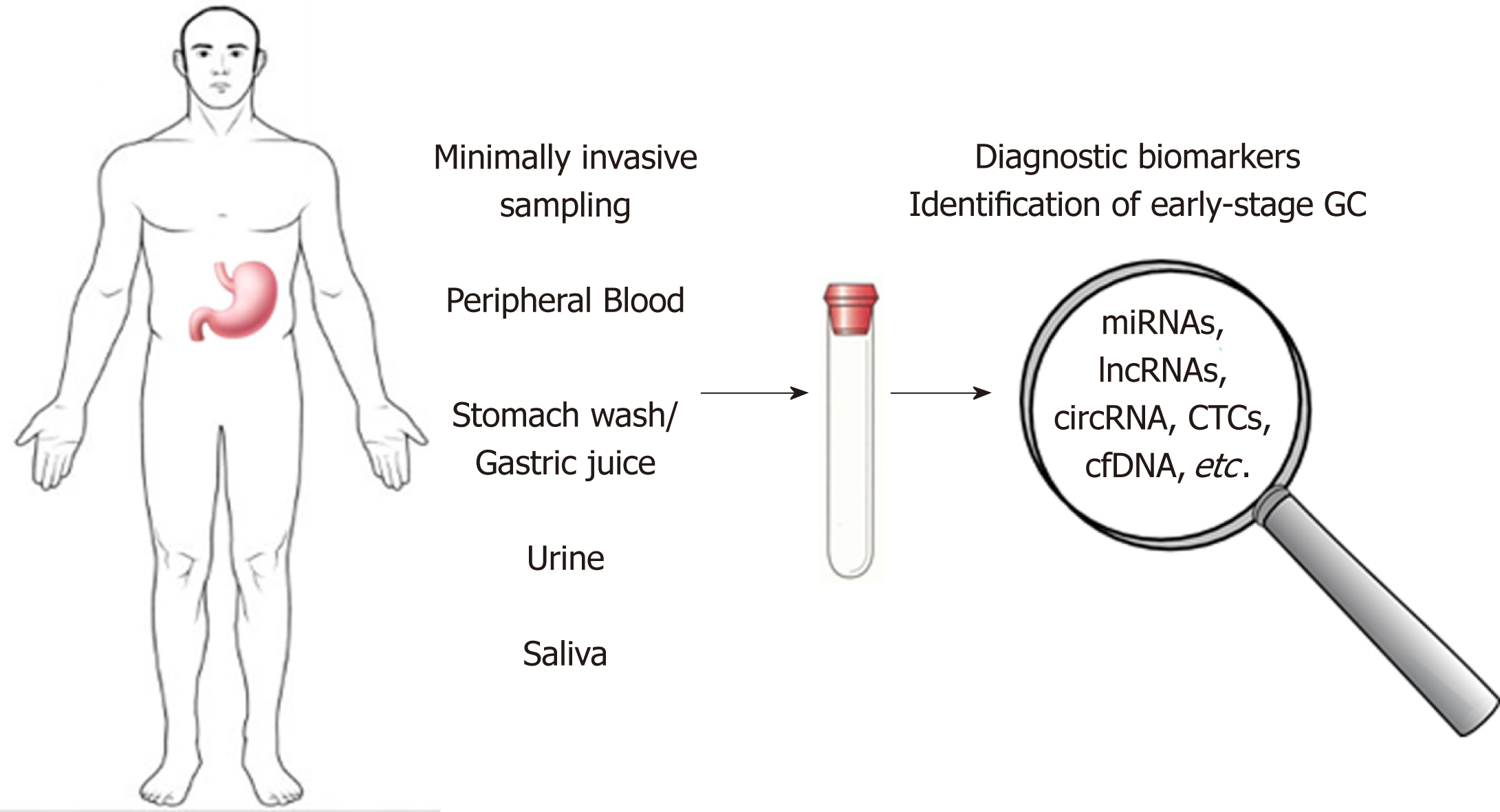
Gastric cancer (GC) remains an important cause of cancer death worldwide with a high mortality rate due to the fact that the majority of GC cases are diagnosed at an advanced stage when the prognosis is poor and the treatment options are limited. Unfortunately, the existing circulating biomarkers for GC diagnosis and prognosis display low sensitivity and specificity and the GC diagnosis is based only on the invasive procedures such as upper digestive endoscopy. There is a huge need for less invasive or non-invasive tests but also highly specific biomarkers in case of GC. Body fluids such as peripheral blood, urine or saliva, stomach wash/gastric juice could be a source of specific biomarkers, providing important data for screening and diagnosis in GC. This review summarized the recently discovered circulating molecules such as microRNAs, long non-coding RNAs, circular RNAs, which hold the promise to develop new strategies for early diagnosis of GC.
Core tip: Despite the fact that in the last decades, gastric cancer (GC) has shown a decreasing incidence, the five-year survival rate continues to remain poor mainly because most patients are asymptomatic until the disease progresses to advanced stages. Recent progress in molecular landscape of GC and improved detection methods may facilitate screening and diagnosis of GC in early stages. Numerous studies aim to identify specific non-invasive biomarkers from alternative sources such as peripheral blood, urine or saliva, stomach wash/gastric juice. This review summarized the recently discovered circulating molecules which hold the promise to develop new strategies for early diagnosis of GC.
- Citation: Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, Bleotu C, Diaconu CC, Chivu-Economescu M. Recent advances in gastric cancer early diagnosis. World J Gastroenterol 2019; 25(17): 2029-2044
- URL: https://www.wjgnet.com/1007-9327/full/v25/i17/2029.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i17.2029
Gastric cancer (GC) remains a challenge for oncology domain being the fifth most frequently diagnosed cancer (1033701 new cases in 2018) and the third leading cause of cancer death (782685 deaths) of all malignancies worldwide[1]. Although over the last decades GC has shown a decreasing incidence, the five-year survival rate continues to be poor, being estimated at 10% for patients with advanced GC. In the developed countries, like Japan, where early diagnosis of GC reaches 50%, the five-year survival rate attains 90%[2].
Currently, the most frequent tumor markers used in the clinic for early detection of GC comprise carcinoembryonic antigen (CEA), the carbohydrate antigens (CA) - CA19-9, CA72-4, CA125, CA24-2, CA50, and also pepsinogen and α-fetoprotein (AFP)[3]. However, the specificity and sensitivity of these serum biomarkers are poor and so far, none of them is unique for GC diagnosis[3,4]. Thereby, the development of improved detection method to diagnose CG in early stages is crucial, especially knowing that most patients are asymptomatic until the disease progresses to advanced stages. Moreover, GC is a complex, heterogeneous disease, involving multiple genetic and epigenetic alterations[5].
Recently, the use of high throughput technologies has brought new insights into the molecular pathogenesis, resulting in a new molecular classification of gastric adenocarcinoma into four subtypes, based on their genomic features. According to The Cancer Genome Atlas (TCGA), GCs are divided in Epstein-Barr virus (EBV)-infected tumors, microsatellite instability tumors (MSI), genomically stable tumors (GS), and chromosomally unstable tumors (CIN)[6]. The Asian Cancer Research Group (ACRG) categories GC into MSI tumors and Microsatellite Stable (MSS) tumors with either epithelial-to-mesenchymal transition (MSS/EMT), TP53 activity (MSS/TP53+), or TP53 inactivity (MSS/TP53-)[7,8]. This new classification opened the way for several clinical trials that are trying to define new therapeutic regimens combining immune checkpoint inhibitors with molecular targeted therapies, with promising results[9]. However, early diagnosis remains mandatory, and studies aiming to identify new biomarkers or genetic signatures are imperative.
Genetic alterations, including large chromosomal gain or loss, single nucleotide variations, and mutations, as well as epigenetic alterations, like aberrant DNA methylation, histone modification, microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) overexpression or down-regulation, were described as major aspects implicated in GC initiation and progression[10].
A better understanding of the molecular factors involved in gastric carcinogenesis can lead to the identification of novel biomarkers for early GC diagnosis or markers use for prognosis and for monitoring therapy response. This review aims to discuss the most important types of molecules secreted from the tumor tissues to the body fluids, which candidates as circulating biomarkers for early diagnosis of GC (Figure 1).
Although several circulating tumor-associated antigens have entered routine clinical practice for a long time their utility in early detection of GC remains elusive, due to the high incidence of false-positive and false-negative results[11,12]. CEA, CA19-9, and CA72-4 are the most frequently used conventional tumor markers in GC diagnosis, prognosis, therapeutic monitoring and detection of recurrences[13]. At diagnosis, both CEA and CA 19-9 levels can provide useful prognostic information regarding the depth of tumor invasion and the presence of metastases[14,15]. However, they do not represent effective tools for GC screening and early diagnosis as they do not display enough sensitivity and specificity under these circumstances[16,17]. CA72-4 was shown to exhibit higher sensitivity and accuracy than CEA, yet there are only few studies that investigated its relevance in GC screening[18]. Other tumor markers, such as AFP and CA125 proved to have very low positivity rates in early GC[19]. Also, CA50 is of limited diagnostic value[20].
To increase the diagnostic performance for GC different combinations of serological tumor markers were employed. In this respect, it was shown that by combining CEA, CA19-9, and CA72-4 with thymidine kinase 1 (TK1) - a biomarker of cell proliferation - a significant increase in sensitivity and specificity of GC detection was obtained, compared to the isolated use of the biomarkers[21]. Recently, a diagnostic model including the serum levels of CEA, CA72-4 and of three inflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8] was proposed for early GC detection. In the validation study, this model provided good discrimination between healthy controls, atypical hyperplasia of gastric mucosa, early-stage GC and advanced-stage GC groups[22].
Concerning the use of stomach-specific biomarkers, measurement of serum pepsinogens (PGs) is the most common non-invasive method employed for GC detection, although it identifies individuals with gastric precancerous lesions, rather than GC itself[23]. Thus, low levels of pepsinogen I (PGI) and a low pepsinogen-I to pepsinogen ratio (PGI/PGII) exhibit a good correlation with atrophic changes in the gastric corpus, while their accuracy for GC detection is low[24]. Additionally, gastrin-17 (G-17) was proposed as an indicator antral atrophy[25]. As shown recently, a biomarker panel comprising PGI, PGII, PGI/PGII, G-17 and IgG antibodies to Helicobacter pylori (H. pylori) represents a promising non-invasive tool to stratify individuals at high risk for GC development[26]. Also, serum levels of trefoil factor 3 (TFF3), a protein ectopically expressed in intestinal metaplasia of the stomach[27], was found to display better performance in GC detection than PGs[25], and the combination of TFF3 with PGs demonstrated even higher sensitivity for early GC[28].
Other potential circulating biomarkers for detecting early-stage GC include M2-pyruvate kinase, a tumor-associated metabolic marker; the adipocytokine leptin as an independent biomarker of intestinal metaplasia; p-53 autoantibody; the cell-cycle-related protein RegIV; the inflammatory signaling molecules olfactomedin 4 and vascular adhesion protein-1 (VAP-1)[29].
A different approach to identify early GC biomarkers has involved mass spectrometry for analyzing serological glycomic profiles in GC patients and non-cancer controls. Significant differences in serum N-glycans were observed between the two groups. Moreover, the decreased core fucose was validated as a potential biomarker for distinguishing early-stage GC patients from healthy controls[30].
The development of state-of-the-art techniques holds the promise of new molecular markers identification that are able to diagnose early, predict the disease outcome and help access the appropriate therapy. Numerous studies showed an increased level of expression of oncogenes in GC. They stimulate tumor cell growth and cell cycle and inhibit apoptosis.
Recent studies identified several genes whose elevated expression level proved to be associated with GC and might be useful in early detection, such as xpg, interferon-induced transmembrane protein 1 (iftim1), matrix metalloproteinase-9 (mmp-9), pituitary tumor-transforming gene-1 (pttg1), stc1[31].
XPG/ERCC5 (13q33) Xeroderma pigmentosum group G/excision repair cross-complementing group 5, an enzyme from NER (nucleotide excision repair) system, is involved in repairing of DNA lesions caused by genomic instability. The gene expression level of ercc5 was found to be significantly higher in GC compared to gastritis, and it was associated with tumor development and progression[32].
By microarray profiling methods, ifitm1 was identified as a gene upregulated in tumor cell lines and GC tissues. Moreover, important differences in expression level in intestinal vs diffuse type of GC were observed. Although the role of this gene in tumorigenesis is not clearly understood, ifitm1 raised expression was implicated in invasion and migration of GC cells[33] and was also related to increased inflammatory responses that may play a part in tumor progression.
MMP-9 is an enzyme that contributes to the degradation of the extracellular matrix, having a well-known role in tumor growth, invasion and metastasis in gastric carcinoma[34]. A study that evaluates both serum and tissue expression level of MMP-9, found out a correlation between serum concentration of MMP-9 before surgery and TNM staging. Although the mmp-9 expression level in gastric tumor was higher compared to healthy tissue, and positively associated with depth of invasion, it did not correlate significantly with MMP-9 serum level[35].
A recently discovered proto-oncogene, pttg1 can affect tumorigenesis, invasion, and metastasis of many cancer types. The expression of pttg1 is upregulated in gastric tumor tissue compared to gastric intraepithelial neoplasia and normal mucosa (both mRNA and protein level) and it is an independent factor for survival. PTTG1 might represent a potential diagnostic marker and a therapeutic target[36].
STC1 and STC2, members of STC (stanniocalcin) family, were highly expressed in numerous cancer types. In GC, both STC1 and STC2 expression is upregulated, STC1 being significantly associated with tumor staging, metastasis, and progression-free survival. Serum level of STC1 was significantly elevated in preoperative cancer patients compared to benign gastric cases and decreased 7-10 d after surgery[37]. Arigami et al[38] reported a significantly higher number of stc1 mRNA copies in the blood of GC patients vs. normal controls that correlates with tumor invasion and staging and has a greater sensitivity than CA 19-9 and CEA. These studies suggest the utility of serum STC1 as diagnosis and prognosis marker in GC.
Using gene microarray, our group also identified a panel of overexpressed genes associated with tumor progression: KRT17, COL10A1, KIAA1199, SPP1, IL11, S100A2, and MMP3. From these, COL10A1, KRT17, and SALL4 candidate as biomarkers for early detection having an increased expression in the early stages of gastric tumorigenesis[39]. COL10A1 was found elevated in serum of patients with colorectal cancer[40], proven to be a worthy circulating biomarker for early diagnosis. KRT17 was also demonstrated to be involved in tumor growth, motility, and invasion by in vitro and in vivo studies on gastric tumorigenesis[41].
Tumor suppressor genes can present loss of expression in GC patient samples that result in accelerated cell growth, the progression of the cell cycle, and decreased inhibition of the oncogene expression. These alterations were also studied in order to discover new diagnostic molecular markers for the early detection and progression of GC[31].
Using a gene microarray analysis, one study identified transmembrane protein with EGF like and two follistatin-like domains 2 (tmeff2) as a gene with significantly decreased expression in GC tissues, negatively correlated with the advanced cancer stage, large tumor size, and poor prognosis. The authors showed that the increase of tmeff2 expression decrease cell proliferation by increasing apoptosis and by blocking the cell cycle in GC cells[42]. Moreover, modification of tmeff2 expression in GC seems to be associated with H. pylori infection via STAT3 activation[43].
An interesting possible biomarker is gastrokine 1 (GKN1), a small protein significantly expressed in the surface lumen epithelial cell layer of gastric tissue, being involved in the maintenance of mucosal integrity and secreted into the stomach, but absent in GC[44]. It was also detected that GKN1 acts as a tumor suppressor and a modulator of apoptotic signals in GC, its lower expression might be considered an indicator of increased risk of gastric carcinogenesis[45].
Another study suggested the opportunity of detecting GC using the gene expression profile of the blood. In this study, a four-gene panel discriminated GC with an accuracy of 95%, sensitivity of 92% and specificity of 96%. This four-gene panel for detection of GC includes two overexpressed genes: purine-rich element binding protein B (purb) and structural maintenance of chromosomes 1A (smc1l1), and two underexpressed genes: DENN/MADD domain containing 1B (dennd1b) and programmed cell death 4 (pdcd4)[46].
Next-generation deep sequencing was used to evaluate mutations of tp53 in tumor biopsies, plasma and stomach fluids (gastric wash) obtained from GC patients. The results showed that tp53 mutations were identified in 15/46 biopsies (32.6%), 7/46 gastric wash - (15.2%) and 6/46 plasma samples (13%). The authors suggested that gastric wash could be useful to detect DNA alterations using a comprehensive gene-panel designed for GC diagnosis[47].
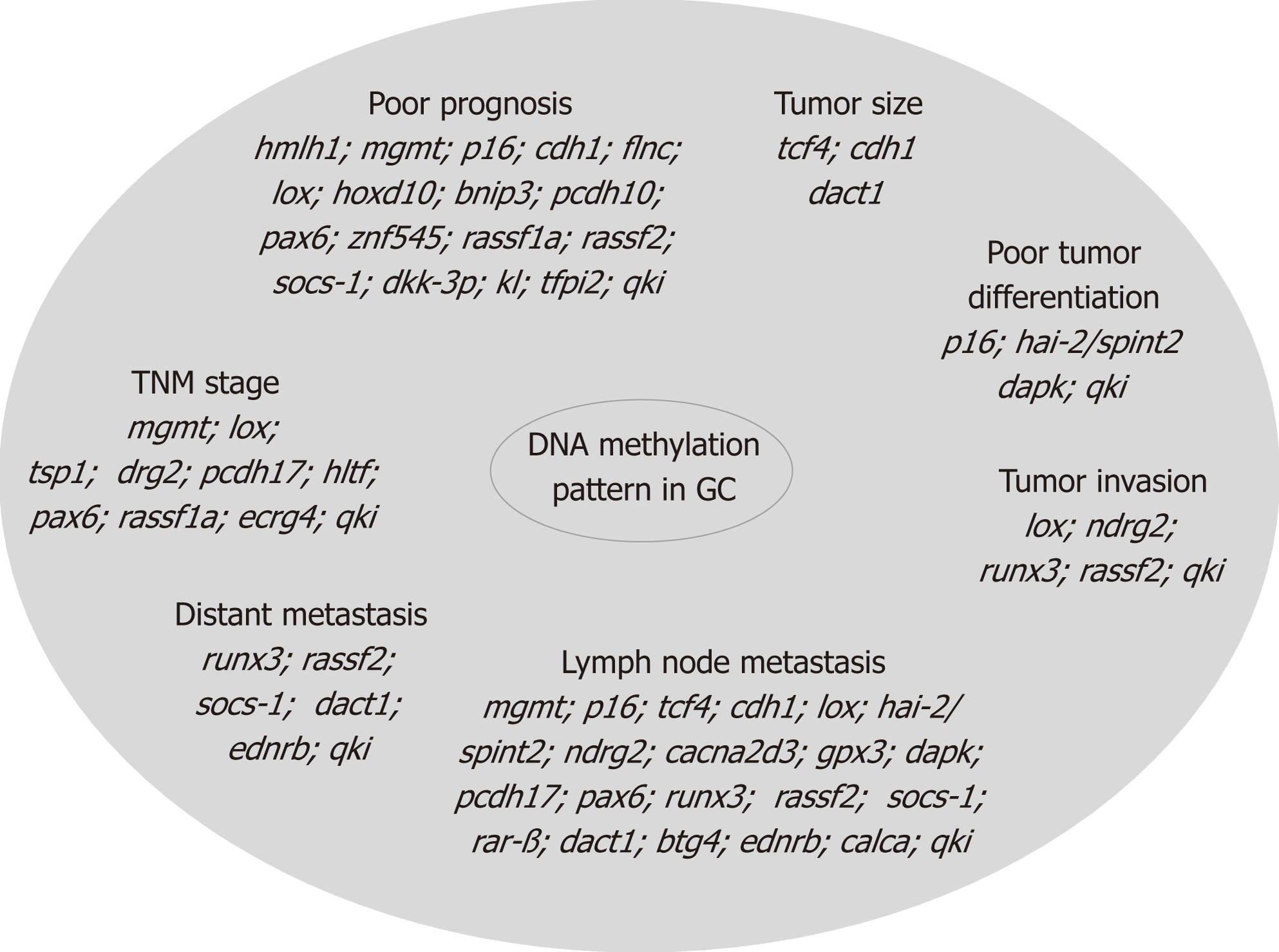
In GC, epigenetic alteration by methylation occurs in specific genes involved in various processes such as cell cycle regulation (p16nk4a, tcf4), DNA repair (hmlh1 and mgmt), cell growth/differentiation (hoxd10, hai-2/spint2, ndrg2), transcriptional regulation (hltf, pax6, znf545, runx3), cell adhesion/invasion/migration (cdh1, cdh4, apc, flnc, lox, timp3, tsp1), apoptosis (bnip3, xiap, bnip3, bcl2, cacna2d3, dapk, gpx3, pcdh10, pcdh17, casp8, xaf1), angiogenesis (thbs-1 and p73), STAT pathway (socs-1), Ras pathway (rassf1a, rassf2, hdab2ip, rkip), Wnt pathway (dkk-3, ctnnb1), as well as in multidrug resistance genes (mdr1, gstp1)[48,49] and in genes associated with Epstein-Barr virus-type tumors (pycard, bmpr1a, and pgr) or H. pylori positive tumors (brinp1, epha5, fli1, and sez6l)[50]. The correlation of these biomarkers with tumor size, localization, differentiation, invasion, lymph node metastasis, distant metastasis, TNM stage, and prognosis is presented in Figure 2.
It was demonstrated previously that, in the case of gastric tumors, aberrant DNA methylation occurs more frequently than mutations[51], making DNA methylation a more specific assay in detection of such disease. Therefore, researchers started looking for an easier and less invasive method for the collection of cells and detection of DNA originated from gastric tumors. In serum/plasma DNA obtained from GC patient was observed a significantly higher methylation level of some biomarkers, such as p16, cdh1, mgmt, rarb, and rnf180[52].
Previously it was considered that DNA is denatured by stomach acidity[53], later on, it was demonstrated that this process is true in case of normal cells, but incorrect in case of DNA from tumor cells[45]. Collection of samples from stomach wash during endoscopy demonstrated that cancer cells from mucosal layers are easier exfoliated than normal cells into gastric juice and also that DNA isolated from such tumor cells is less degraded due to acidity[45] making it easy to be studied, offering a sensitive and quantitative method of detection.
Several genes were found to be methylated with higher frequency in gastric neoplasia versus normal condition and therefore were analyzed as possible biomarkers. Among them six methylated genes were most specific and sensitive for GC: adam23, mint25, gdnf, prdm5, mlf1 and rora. The results have shown that the combination of the markers mint25 + adam23 + gdnf achieved a high sensitivity (95%) and specificity (92%). It was found that the methylation process is gene- and tumor stage-dependent during gastric carcinogenesis, some genes are highly methylated during dysplasia and early cancer phase compared with normal, but show lower methylation in advanced GC, similar with mechanism observed in ulcerative colitis-associated colon neoplasia[45].
But increased methylation process could have other causes as well, such as chronic inflammation of gastric mucosae, especially by H. pylori infection and aging. In order to test the effect of inflammation on methylation, the BarH-like 2 homeobox protein (barhl2) gene was chosen since is an H. pylori-independent biomarker. The barhl2 methylation analysis of exosomal DNA (exoDNA) derived from gastric juice proven that the process is not influenced by atrophy of the gastric mucosa or H. pylori infection and could be used as a biomarker for detection of both early and advanced GC[54].
MiRNAs represent a class of small non-coding RNAs (19-25 nucleotides) involved by epigenetic mechanisms in many cellular processes, such as differentiation, proliferation, and apoptosis. These molecules, that seem to present specific expression signatures in normal and tumor gastric tissue, can act as oncogenes and/or tumor suppressors depending on the role of the target mRNA/gene[55].
More studies suggested that miRNAs could be considered important potential biomarkers for gastric pathology as they are frequently found to be deregulated in gastric tissue in H. pylori infection, chronic gastritis, preneoplastic conditions such as atrophic gastritis and intestinal metaplasia, and also in early dysplasia and invasive cancer. Moreover, modifications of miRNA blood levels were also identified in GC patients supporting the development of new diagnostic and prognostic methods based on miRNA expression analysis[56].
A promising result was obtained by a study in which miRNA-21 levels, in serum and peripheral blood mononuclear cells, were found to be increased in GC patients with a positive prediction rate around 90%, while those of CA199 and CEA were around 50%. Moreover, circulating miR-21 levels can discriminate between stage I and stage IV of GC[57].
miR-376c was found to be up-regulated in tissue, plasma, and urine of GC patients, even from the early stage of the tumor. The increased expression of miR-376c was associated with the proliferation, migration and anchorage-independent growth of cancer cells, having as a direct target arid4a gene which is considerably down-regulated in tumor tissue[58].
Increased pre-operative circulating miR-196a and miR-196b levels were identified in GC patients compared to healthy controls, the expression level of these miRNAs being reduced after the surgical resection of the gastric tumor. Interestingly, higher circulating miR-196a/b levels were correlated with the metastatic potential of the tumor, advanced stages, and poorer survival. Moreover, the results of this study suggested that circulating miR-196a, miR-196b, and combined miR-196a and miR-196b can distinguish between GC patients and healthy controls with higher sensitivity and specificity compared to the CEA or CA19-9[59]. Another recent study analyzed circulating miRNA levels in GC patients and identified a four-miRNA panel (miR-501-3p, miR-143-3p, miR-451a, miR-146a) as possible noninvasive biomarkers for prediction and prognosis of lymph node metastasis (LNM). In addition, LNM patients with decreased levels of miR-451a and miR-146a presented worse overall survival[60]. A five-miRNA panel (miR-16, miR-25, miR-92a, miR-451, and miR-486-5p) was found to be differentially expressed in plasma of gastric non-cardia adenocarcinoma patients compared to healthy controls. This panel seems to be able to discriminate between early-stage of gastric non-cardia adenocarcinoma patients and cancer-free subjects[61]. Other panels containing up-regulated miRNAs (miR-200a-3p, miR-296-5p, miR-132-3p, miR-485-3p, and miR-22-5p)[62] and (miR10b-5p, miR132-3p, miR185-5p, miR195-5p, miR-20a3p, and miR296-5p)[63] were identified in serum of the GC patients compared to healthy controls. Based on the evidence that exosomes secreted by cancer and normal cells can be released into the circulatory system, a recent study identified overexpression of circulating exosomal miR-19b and miR-106a in GC patients compared to healthy controls. These increased levels were correlated with lymphatic metastasis and advanced stages of GC[64].
miR-146, miR-375, and Let-7 were found to be downregulated while miR-19 and miR-21 presented an increased expression in plasma of the GC patients with H. pylori infection. The study also identified overexpression of the genes involved in IRAK4 signaling and a decreased expression of pten gene in the GC patients with H. pylori infection compared to the control group, suggesting the potential of these molecules as biomarkers for early diagnosis of GC[65]. There are also other molecular potential biomarkers for screening GC identified in gastric juice: miR-421, miR-21, miR-106a and miR-129[66].
Table 1 summarized several miRNAs presenting modified circulating expression in GC patients compared to healthy controls.
| MicroRNA | Biological function | Type of biomarker | Origin of specimen | Sensitivity/specificity | Study |
| miR-21 | Upregulated; discriminates between stage I and stage IV of GC | Diagnostic, prognostic | Serum, PBMC | 88.4%/79.6% (serum) 81.3%/73.4% (PBMC) | Wu et al[57] |
| miR-196a/b | Upregulated; correlated with metastatic potential of the tumor, advanced stages, and poorer survival | Diagnostic, prognostic | Plasma | 69.5%/97.6% | Tsai et al[59] |
| miR-200c | Upregulated; predictor of progression and survival. | Diagnostic, prognostic | Blood | 65.4%/100% | Valladares-ayerbes et al[67] |
| miR-940 | Downregulated | Diagnostic | Plasma | 81.25 %/98.57 % | Liu et al[68] |
| miR-551b-5p | Upregulated | Diagnostic | Serum | 77.5%/80.0% | Jiang et al[69] |
| miR-19b, miR-106a | Upregulated; correlated with lymphatic metastasis and advanced stages | Diagnostic, prognostic | Circulating exosomes | 95%/90% | Wang et al[64] |
| miR-501-3p, miR-143-3p, miR-451a, miR-146a | Differentially expressed; prediction and prognosis of lymph node metastasis | Prognostic | Serum | 87.78%/63.33% | Jiang et al[60] |
| miR-16, miR-25, miR-92a, miR-451, miR-486-5p | Differentially expressed; discriminate between early-stage of GC and cancer-free subjects | Diagnostic | Plasma | 72.9%/89.2% | Zhu et al[61] |
| miR-200a-3p, miR-296-5p, miR-132-3p, miR-485-3p, miR-22-5p | Upregulated | Diagnostic | Serum | N/S | Wang et al[62] |
| miR10b-5p, miR132-3p, miR185-5p, miR195-5p, miR-20a3p, miR296-5p | Upregulated | Diagnostic | Serum | N/S | Huang et al[63] |
| miR-17-5p, miR-21, miR-106a, miR-106b, let-7a | Differentially expressed | Diagnostic | Plasma | 85.5%/80.0% | Tsujiura et al[70] |
| miR-146, miR-375, let-7, miR-19, miR-21 | Differentially expressed in GC patients with H. Pylori infection | Diagnostic | Plasma | N/S | Ranjbar et al[65] |
Even if these results need to be validated by independent groups or cohorts in prospective studies, circulating miRNAs could be considered a class of novel, non-invasive diagnostic biomarkers with sufficient diagnostic accuracy in detecting the early-stage GC.
LncRNAs are transcripts longer than 200 nucleotides with no or limited protein-coding potential. lncRNAs are implicated in the regulation of several biological processes like transcription and translation, cellular differentiation, gene expression, cell cycle, etc[71]. They are characterized by high stability while circulating in body fluids and their level in tumor tissue correlates with plasma levels. As such, lncRNAs can be used to distinguish tumor patients at early stages from healthy people, as well as to predict the prognostic, metastasis risks and recurrence after surgery[72,73].
In 2013, Cao et al[74] investigated the lncRNA expression in GC and detected 88 differentially expressed lncRNAs, 71 upregulated and 17 downregulated. Zhou et al[75] hypothesized that GC-related lncRNAs might be released into the circulation during tumor initiation and could be utilized to detect and monitor GC.
Highly upregulated in liver cancer (HULC) is a lncRNA implicated in the growth and tumorigenesis of human GC. In vitro overexpression of HULC in gastric cell lines stimulates proliferation and invasion, inhibits cell apoptosis and can induce autophagy patterns, while its silencing reverses the EMT phenotype[76]. Evaluated in plasma, HULC level is higher in preoperative patients compared with healthy control subjects[77].
Another candidate as a possible biomarker for early detection and prognosis prediction of GC is lncRNA PVT1 since the levels of PVT1 in gastric juice from gastric patients were significantly higher than those from normal subjects[78].
Zhou et al[75] proposed H19 (imprinted maternally expressed transcript) as a potential biomarker for diagnosis of GC, especially for early tumor screening. It stimulates cell proliferation and inhibits apoptosis[79]. H19 plasma level is significantly higher in GC patients compared with normal controls[75,80-82] and allows the discrimination of early stage GC[75]. On the other side, H19 plasma levels were significantly lower in postoperative samples than in preoperative ones[75,80]. Also, patients with smaller tumor sizes (< 5 cm) exhibit higher H19 level in their circulation compared with those with larger tumors (≥ 5 cm)[81].
Another abnormally expressed lncRNA in GC is long intergenic non-protein-coding RNA 152 (LINC00152), its plasma level being significantly elevated in GC patients compared with healthy controls[83,84] and presenting higher levels in postoperative plasma samples compared with preoperative ones[83]. This lncRNA allows differentiating GC patients from ones with benign gastric diseases and can be also detected in gastric juice[84]. Another lncRNA that can be detected in gastric juice is AA174084 characterized by higher levels in GC patients compared with healthy or other non-GC subjects. Its plasma level drops markedly in GC patients on day 15 post-surgery and is associated with invasion and lymphatic metastasis[85].
Hox transcript antisense intergenic RNA (HOTAIR) has been suggested to be implicated in GC tumorigenesis and progression[86]. It promotes cell proliferation and inhibits apoptosis[79]. HOTAIR plasma level is significantly higher in GC patients compared with healthy controls. Moreover, increased HOTAIR expression was associated with advanced tumor stages, higher grades, and metastasis[86]. Other up-regulated lncRNAs are human urothelial carcinoma associated 1 (UCA1), which is implicated in GC carcinogenesis and presents higher levels in GC patients[87], and ABHD11-AS, whose levels in gastric juice is significantly higher in GC patients, being also associated with clinicopathological factors[88].
Yang et al[89] investigated the diagnostic value of gastric cancer associated transcript 2 (GACAT2) in GC. In the evaluated cohort, the plasma GACAT2 levels in GC patients were significantly higher compared with healthy individuals, as well as in the preoperative group compared with the postoperative one. In addition, the individual relative changes of GACAT2 expression following surgery were significantly associated with lymphatic metastasis, distal metastasis, and perineural invasion.
Also, some lncRNAs panels were investigated for GC diagnosis. Zhang et al[90] identified a panel of five novel plasma lncRNAs (TINCR, CCAT2, AOC4P, BANCR, and LINC00857) using genome-wide lncRNA screening analysis which could distinguish GC patients from healthy controls and can help monitor tumor dynamics, tumor, depth of invasion, lymphatic metastasis and more advanced tumor stages. Also, Dong et al[91] identified a three-lncRNA signature, CUDR, LSINCT-5, and PTENP1, that allows distinguishing healthy controls from early GC patients.
However, to introduce lncRNAs as plasma biomarkers, further studies and improvements of extraction, quantification, probe enrichment, and evaluation methods should be performed.
CircRNAs are a new class of non-coding RNAs that form a closed loop, without 5’ and 3’ ends[92]. CircRNAs were first identified in RNA viruses, but later with the progress of new molecular techniques like high-throughput RNA sequencing and microarray analysis, circRNAs were found in all eukaryotic organisms as stable and conserved sequences that control gene expression through interactions with miRNAs[93]. New emerging data have confirmed that circRNAs are involved in the occurrence of many diseases, and also are strongly associated with tumor growth and metastasis[94]. These findings underline the potential of circRNAs to act as novel biomarkers and therapeutic targets for various human tumors.
Several recent studies have analyzed the aberrant expression of circRNA in GC compared with adjacent normal tissue and presented various lists with upregulated and downregulated circRNA[95-98] (Table 2). The study performed by Huang et al[96] identified circRNA0026 (hsa_circ_0000026) as having significantly downregulated expression 2.8fold change in GC. Sui et al[95] found six differentially expressed circRNA in GC tissue and managed to validate through qRT-PCR three of them (hsa_circRNA_400071, hsa_circRNA_000543, and hsa_circRNA_001959) as having a consistent expression with the differentially expressed gene. Through analysis of circRNA and mRNA differential expression profiles in GC tissues, the authors managed to identify the target mRNA and their respective genes for selected circRNAs, like cd44, cxxc5, myh9, malat1 and other genes with important implications in GC tumorigenesis and development.
| circRNA name | Biological function | Type of biomarker | Origin of specimen | Sensitivity/specificity | Study |
| hsa_circ_002059 | Downregulated in GC; correlated with TNM stage and metastasis | Diagnostic | Tissues; plasma | 81%/62% | Li et al[94] |
| hsa_circ_0000096 | Downregulated in GC; affects GC cell growth and migration | Diagnostic | Tissues | N/S | Li et al[99] |
| hsa_circ_0058246 | Upregulated in GC; associated with poor clinical outcomes | Prognostic | Tissues | N/S | Fang et al[100] |
| hsa_circ_0000745 | Downregulated in GC; correlated with tumor differentiation and tumor nodal metastasis | Prognostic | Tissues | 85.5%/45% | Huang et al[101] |
| hsa_circ_00000181 | Downregulated in GC; associated with TNM stage and metastasis | Prognostic | Plasma | 99%/85.2% | Zhao et al[102] |
| hsa_circ_0047905, has-circRNA7690-15, hsa_circ_0138960 | Substantially upregulated in GC; act as tumor promoters in the pathogenesis of GC | Diagnostic | Tissues | N/S | Lai et al[103] |
| hsa_circ_0014717 | Downregulated in GC; stably expressed in gastric juice; associated with TNM stage and metastasis | Prognostic | Tissues | 59.38%/81.25% | Shao et al[104] |
| hsa_circ_0001895 | Downregulated in both GC tissue and gastric precancerous lesions | Diagnostic | Tissues | 67.8%/85.7% | Shao et al[105] |
| has_circ_0000520 | Downregulated in GC; associated with TNM stage and in GC plasma linked with CEA expression | Diagnostic | Tissues; plasma | 53.57%/85.71% (tissue) 82.35%/84.44% (plasma) | Sun et al[106] |
| hsa_circ_0000190 | Downregulated in GC; associated with TNM stage and metastasis | Diagnostic | Tissues; plasma | 71.2%/75% | Chen et al[107] |
| hsa_circ_0001017 hsa_circ_0061276 | Downregulated in GC; associated with shorter overall survival | Prognostic | Tissues; plasma | 95.5%/95.7% | Li et al[108] |
One important finding related to circRNA in cancer was that they are not easily degraded by RNase and thus, are stably expressed in human cells, in plasma or in gastric juice[104,109]. These findings opened the way for plasma circRNA profiling studies, aiming to identify specific diagnostic and prognostic circRNA for GC patients. Li et al[108] performed circRNA microarray for three GC samples and plasma, to assess the differences of circRNA expression profiles. They found that 3 and 14 circRNA were upregulated and downregulated respectively, both in patients’ plasma and tumor tissue. Further, they analyzed through RT-droplet digital PCR (RT-ddPCR) the circRNA levels in plasma for 121 GC patients. Two circRNA: hsa_circ_0001017 (30.85-folds change) and hsa_circ_0061276 (121.54-folds change) were selected for their non-invasion diagnostic values. Results showed that patients with low level of hsa_circ_0001017 or hsa_circ_0061276 in plasma had shorter overall survival than those with high levels. Moreover, patients whose plasma levels of the two circRNA recovered to normal after the operation had longer disease-free survival.
Due to their documented correlation between tissue and plasma level, stability and presence as cell-free RNA in plasma, circRNA may be valuable blood-based biomarkers for GC screening, diagnosis, and prognosis.
GC diagnosis relies mostly on invasive procedures, which are rather expensive and may have sometimes serious adverse events[110]. In spite of being documented 150 years ago[111], only last years proved that analyzing circulating tumor cells (CTCs) in liquid biopsies, a blood-based diagnostic approach, as a substitute for tissue biopsies have emerged as real-time cancer development monitoring tool and management strategy[112].
CTCs are a very rare and heterogeneous population of cells circulating in peripheral blood, originating from either primary or metastatic tumors that express the antigenic or genetic characteristics of the specific tumor type[113]. CTCs were first described as expressing epithelial cell markers EpCAM, cytokeratin 8, 18, and 19 (CK8, CK18, CK19), and are CD45 negative[114]. Recently, EMT with potential overexpression of mesenchymal markers and decreased expression of epithelial cell markers or mesenchymal-epithelial transition (MET) that present mesenchymal and epithelial markers, were shown to characterize subpopulations of these cells[115,116]. Mesenchymal phenotypes have larger plasticity thus facilitating migration, invasion, and drug resistance[117]. Several studies revealed the presence of CTCs in circulating tumor microemboli (CTM), indicating poor prognosis and influencing disease progression[118].
This high heterogeneity of CTCs prompted researchers to develop different methodologies to enrich, isolate and/or enumerate them based on specific phenotypic or molecular characteristics. Basically, there are two general types of methods used in CTCs enrichment/isolation: biological and physical methods. Their combination is more likely to improve the efficiency of CTC detection. CellSearch™ platform (Veridex LLC, Huntingdon Valley, PA, United States) the only procedure approved for the enumeration and isolation of CTCs by the Food and Drug Administration (FDA) for clinical use, detect the adhesion molecule EpCAM, CK8, CK18 and CK19 and exclude CD45 cells but may overlook CTCs with predominantly mesenchymal phenotype. Using cell size - and phenotype-based systems, as centrifugal microfluidic system based on fluid-assisted separation technique (FAST), or Cascaded Inertial Focusing Microfluidic device, coupled with detection of an extended panel of markers might identify a different subpopulation of CTCs with higher efficiency[119,120].
Exploiting a frequent genetic abnormality reported in GC tumors, the aneuploidy of chromosome 8, Li et al[121] created an integrated subtraction enrichment (SET) and immunostaining-fluorescence in situ hybridization (iFISH) platform claimed to be more sensitive than the CellSearch™ to detect and characterize CTCs in advanced GC patients. Multiple studies showed that SET-iFISH method to enumerate CTCs with chromosome 8 aneuploidy is efficient in monitoring GC patient treatment response[113]. Expression of different other markers as vimentin, twist, MUC1, HER2, etc. proved to be very useful to evaluate therapeutic response and prognosis in patients with GC. However, irrespective of the detection method employed, there is weak evidence that detection of CTCs has the potential for early biomarker detection in GC but all data are consistent in supporting its utility in assessing the tumor heterogeneity, monitoring treatment responses and real-time cancer management[113].
Circulating tumor DNA (ctDNA) analysis refined the liquid biopsy to the level of identification of tumor molecular traces circulating in the body fluids and may give deeper insight on the cancer heterogeneity, early biomarker detection, therapeutic target detection, real-time evaluation of treatment response and possible resistance and prognosis. Originating from primary tumor cells, CTCs and/or distant metastasis, ctDNA give a broad cross-section of the disease offering information on methylation status, genetic alterations as mutations, amplifications, rearrangements, copy number variation (CNV), the latter being more difficult to analyze due to the short length and possibly unequal distribution of the ctDNA fragments[111].
Generally, ctDNA represents only a fraction of the cell-free circulating DNA (cfDNA), which is increased considerably in late-stage disease[122]. However, there is evidence that ctDNA can be detected in the plasma of cancer patients even in the early stages of their disease[123,124]. In GC, Fang et al[125] found that ctDNA levels were correlated with vascular invasion and the highest ctDNA detectable levels were associated with peritoneal recurrence and a poor prognosis. Balgkouranidou et al[126] showed that rassf1a and apc promoter hypermethylation in cfDNA represents a frequent epigenetic event in patients with early operable GC demonstrating a prognostic capacity for these patients. Another study suggested that cfDNA can identify EBV-associated gastric carcinoma (EBVaGC) subtype and monitor tumor progression as well as treatment response in patients with EBVaGC[127].
Being a rare event, ctDNA requires highly sensitive and reproducible analytical methods for proper investigation. Multiplex mass spectrometric SNP genotyping technology, real-time quantitative PCR (qRT-PCR), digital droplet PCR (ddPCR) with improved nucleic acid quantification, next-generation sequencing (NGS) were already employed for ctDNA analysis in GC patients[125,128-130] proving the usefulness in personalized treatment decisions. A panel of more than 70 genes and genomic biomarkers for MSI and blood tumor mutational burden (bTMB) by Foundation Medicine, the FoundationACT® assay, was granted breakthrough device designation by the FDA[131] and might become the first FDA-approved liquid biopsy assay to incorporate multiple companion diagnostics (CDx) and multiple biomarkers.
GC remains an important cause of cancer death worldwide with a high mortality rate due to the fact that the majority of GC cases are diagnosed at an advanced stage when the prognosis is poor and the treatment options are limited. Unfortunately, the existing circulating biomarkers for GC diagnosis and prognosis display low sensitivity and specificity and the GC diagnosis is based only on the invasive procedures such as upper digestive endoscopy. Therefore, most current GC studies are focused on the identification and validation of non-invasive cancer biomarkers released from the tumor tissues into the body fluids, such as blood and stomach juice. Many of these biomarkers are not specific for the early stages, being detected in advanced stages of GC, and cannot be used for early GC detection. However, some of recently discovered circulating molecules (miRNAs, lncRNAs, circRNA) hold the promise for developing new strategies for early diagnosis of GC, being able to discriminate between the early stage of GC and healthy subjects, with a sensitivity more than 77.5%. In order to improve the sensitivity and enlarge the early stage biomarkers list, further studies should be performed to optimize laboratory techniques such as extraction, quantification, probe enrichment, and evaluation methods. Moreover, these results need to be validated by independent groups or cohorts in prospective studies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoyagi K, Chiu CC, Kim BW, Tanabe S S-Editor: Ma RY L-Editor: A E-Editor: MaYJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Luo M, Li L. Clinical utility of miniprobe endoscopic ultrasonography for prediction of invasion depth of early gastric cancer: A meta-analysis of diagnostic test from PRISMA guideline. Medicine (Baltimore). 2019;98:e14430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Tsai MM, Wang CS, Tsai CY, Huang HW, Chi HC, Lin YH, Lu PH, Lin KH. Potential Diagnostic, Prognostic and Therapeutic Targets of MicroRNAs in Human Gastric Cancer. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 4. | Tong W, Ye F, He L, Cui L, Cui M, Hu Y, Li W, Jiang J, Zhang DY, Suo J. Serum biomarker panels for diagnosis of gastric cancer. Onco Targets Ther. 2016;9:2455-2463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Cisło M, Filip AA, Arnold Offerhaus GJ, Ciseł B, Rawicz-Pruszyński K, Skierucha M, Polkowski WP. Distinct molecular subtypes of gastric cancer: from Laurén to molecular pathology. Oncotarget. 2018;9:19427-19442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4848] [Article Influence: 440.7] [Reference Citation Analysis (2)] |
| 7. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1578] [Article Influence: 157.8] [Reference Citation Analysis (0)] |
| 8. | Katona BW, Rustgi AK. Gastric Cancer Genomics: Advances and Future Directions. Cell Mol Gastroenterol Hepatol. 2017;3:211-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Chivu-Economescu M, Matei L, Necula LG, Dragu DL, Bleotu C, Diaconu CC. New therapeutic options opened by the molecular classification of gastric cancer. World J Gastroenterol. 2018;24:1942-1961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Patel TN, Roy S, Ravi R. Gastric cancer and related epigenetic alterations. Ecancermedicalscience. 2017;11:714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 12. | Qian C, Ju S, Qi J, Zhao J, Shen X, Jing R, Yu J, Li L, Shi Y, Zhang L, Wang Z, Cong H. Alu-based cell-free DNA: a novel biomarker for screening of gastric cancer. Oncotarget. 2016;8:54037-54045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Kotzev AI, Draganov PV. Carbohydrate Antigen 19-9, Carcinoembryonic Antigen, and Carbohydrate Antigen 72-4 in Gastric Cancer: Is the Old Band Still Playing? Gastrointest Tumors. 2018;5:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Zhang YH, Li Y, Chen C, Peng CW. Carcinoembryonic antigen level is related to tumor invasion into the serosa of the stomach: study on 166 cases and suggestion for new therapy. Hepatogastroenterology. 2009;56:1750-1754. [PubMed] |
| 15. | Dilege E, Mihmanli M, Demir U, Ozer K, Bostanci O, Kaya C, Aksakal O, Sakiz D. Prognostic value of preoperative CEA and CA 19-9 levels in resectable gastric cancer. Hepatogastroenterology. 2010;57:674-677. [PubMed] |
| 16. | Jin Z, Jiang W, Wang L. Biomarkers for gastric cancer: Progression in early diagnosis and prognosis (Review). Oncol Lett. 2015;9:1502-1508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Dolscheid-Pommerich RC, Manekeller S, Walgenbach-Brünagel G, Kalff JC, Hartmann G, Wagner BS, Holdenrieder S. Clinical Performance of CEA, CA19-9, CA15-3, CA125 and AFP in Gastrointestinal Cancer Using LOCI™-based Assays. Anticancer Res. 2017;37:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Matsuoka T, Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J Gastroenterol. 2018;24:2818-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 230] [Cited by in RCA: 319] [Article Influence: 45.6] [Reference Citation Analysis (7)] |
| 19. | Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, Guo M, Lian X, Fan D, Zhang H. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 267] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 20. | Tian SB, Yu JC, Kang WM, Ma ZQ, Ye X, Cao ZJ, Yan C. Combined detection of CEA, CA 19-9, CA 242 and CA 50 in the diagnosis and prognosis of resectable gastric cancer. Asian Pac J Cancer Prev. 2014;15:6295-6300. [PubMed] |
| 21. | Ning S, Wei W, Li J, Hou B, Zhong J, Xie Y, Liu H, Mo X, Chen J, Zhang L. Clinical significance and diagnostic capacity of serum TK1, CEA, CA 19-9 and CA 72-4 levels in gastric and colorectal cancer patients. J Cancer. 2018;9:494-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Li J, Xu L, Run ZC, Feng W, Liu W, Zhang PJ, Li Z. Multiple cytokine profiling in serum for early detection of gastric cancer. World J Gastroenterol. 2018;24:2269-2278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Lansdorp-Vogelaar I, Kuipers EJ. Screening for gastric cancer in Western countries. Gut. 2016;65:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Kalniņa Z, Meistere I, Kikuste I, Tolmanis I, Zayakin P, Linē A. Emerging blood-based biomarkers for detection of gastric cancer. World J Gastroenterol. 2015;21:11636-11653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Leja M, You W, Camargo MC, Saito H. Implementation of gastric cancer screening - the global experience. Best Pract Res Clin Gastroenterol. 2014;28:1093-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Tu H, Sun L, Dong X, Gong Y, Xu Q, Jing J, Bostick RM, Wu X, Yuan Y. A Serological Biopsy Using Five Stomach-Specific Circulating Biomarkers for Gastric Cancer Risk Assessment: A Multi-Phase Study. Am J Gastroenterol. 2017;112:704-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Xiao P, Ling H, Lan G, Liu J, Hu H, Yang R. Trefoil factors: Gastrointestinal-specific proteins associated with gastric cancer. Clin Chim Acta. 2015;450:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Lee HS, Jeon SW, Nomura S, Seto Y, Kwon YH, Nam SY, Ishibashi Y, Ohtsu H, Ohmoto Y, Yang HM. Screening Biomarker as an Alternative to Endoscopy for the Detection of Early Gastric Cancer: The Combination of Serum Trefoil Factor Family 3 and Pepsinogen. Gastroenterol Res Pract. 2018;2018:1024074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Fu H, Cui D, Cui D. Human Serum Protein Markers for Gastric Cancer Detection. Cui D. Gastric Cancer Prewarning and Early Diagnosis System. Dordrecht: Springer Netherlands 2017; 11-36. |
| 30. | Qin R, Zhao J, Qin W, Zhang Z, Zhao R, Han J, Yang Y, Li L, Wang X, Ren S, Sun Y, Gu J. Discovery of Non-invasive Glycan Biomarkers for Detection and Surveillance of Gastric Cancer. J Cancer. 2017;8:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Kanda M, Kodera Y. Recent advances in the molecular diagnostics of gastric cancer. World J Gastroenterol. 2015;21:9838-9852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Deng N, Liu JW, Sun LP, Xu Q, Duan ZP, Dong NN, Yuan Y. Expression of XPG protein in the development, progression and prognosis of gastric cancer. PLoS One. 2014;9:e108704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Lee J, Goh SH, Song N, Hwang JA, Nam S, Choi IJ, Shin A, Kim IH, Ju MH, Jeong JS, Lee YS. Overexpression of IFITM1 has clinicopathologic effects on gastric cancer and is regulated by an epigenetic mechanism. Am J Pathol. 2012;181:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579-3583. [PubMed] |
| 35. | Chen SZ, Yao HQ, Zhu SZ, Li QY, Guo GH, Yu J. Expression levels of matrix metalloproteinase-9 in human gastric carcinoma. Oncol Lett. 2015;9:915-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Xu MD, Dong L, Qi P, Weng WW, Shen XH, Ni SJ, Huang D, Tan C, Sheng WQ, Zhou XY, Du X. Pituitary tumor-transforming gene-1 serves as an independent prognostic biomarker for gastric cancer. Gastric Cancer. 2016;19:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Fang Z, Tian Z, Luo K, Song H, Yi J. Clinical significance of stanniocalcin expression in tissue and serum of gastric cancer patients. Chin J Cancer Res. 2014;26:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 38. | Arigami T, Uenosono Y, Ishigami S, Hagihara T, Haraguchi N, Matsushita D, Yanagita S, Nakajo A, Okumura H, Hokita S, Natsugoe S. Expression of stanniocalcin 1 as a potential biomarker of gastric cancer. Oncology. 2012;83:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Chivu Economescu M, Necula LG, Dragu D, Badea L, Dima SO, Tudor S, Nastase A, Popescu I, Diaconu CC. Identification of potential biomarkers for early and advanced gastric adenocarcinoma detection. Hepatogastroenterology. 2010;57:1453-1464. [PubMed] |
| 40. | Solé X, Crous-Bou M, Cordero D, Olivares D, Guinó E, Sanz-Pamplona R, Rodriguez-Moranta F, Sanjuan X, de Oca J, Salazar R, Moreno V. Discovery and validation of new potential biomarkers for early detection of colon cancer. PLoS One. 2014;9:e106748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Chivu-Economescu M, Dragu DL, Necula LG, Matei L, Enciu AM, Bleotu C, Diaconu CC. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer. 2017;20:948-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Sun T, Du W, Xiong H, Yu Y, Weng Y, Ren L, Zhao H, Wang Y, Chen Y, Xu J, Xiang Y, Qin W, Cao W, Zou W, Chen H, Hong J, Fang JY. TMEFF2 deregulation contributes to gastric carcinogenesis and indicates poor survival outcome. Clin Cancer Res. 2014;20:4689-4704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Sun TT, Tang JY, Du W, Zhao HJ, Zhao G, Yang SL, Chen HY, Hong J, Fang JY. Bidirectional regulation between TMEFF2 and STAT3 may contribute to Helicobacter pylori-associated gastric carcinogenesis. Int J Cancer. 2015;136:1053-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Altieri F, Di Stadio CS, Federico A, Miselli G, De Palma M, Rippa E, Arcari P. Epigenetic alterations of gastrokine 1 gene expression in gastric cancer. Oncotarget. 2017;8:16899-16911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Watanabe Y, Kim HS, Castoro RJ, Chung W, Estecio MR, Kondo K, Guo Y, Ahmed SS, Toyota M, Itoh F, Suk KT, Cho MY, Shen L, Jelinek J, Issa JP. Sensitive and specific detection of early gastric cancer with DNA methylation analysis of gastric washes. Gastroenterology. 2009;136:2149-2158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Shi J, Cheng C, Ma J, Liew CC, Geng X. Gene expression signature for detection of gastric cancer in peripheral blood. Oncol Lett. 2018;15:9802-9810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Pizzi MP, Bartelli TF, Pelosof AG, Freitas HC, Begnami MD, de Abrantes LLS, Sztokfisz C, Valieris R, Knebel FH, Coelho LGV, da Costa WL, Coimbra FJF, da Silva IT, de Amorim MG, Nunes DN, Dias-Neto E. Identification of DNA mutations in gastric washes from gastric adenocarcinoma patients: Possible implications for liquid biopsies and patient follow-up. Int J Cancer. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 49. | Puneet, Kazmi HR, Kumari S, Tiwari S, Khanna A, Narayan G. Epigenetic Mechanisms and Events in Gastric Cancer-Emerging Novel Biomarkers. Pathol Oncol Res. 2018;24:757-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 50. | Sepulveda JL, Gutierrez-Pajares JL, Luna A, Yao Y, Tobias JW, Thomas S, Woo Y, Giorgi F, Komissarova EV, Califano A, Wang TC, Sepulveda AR. High-definition CpG methylation of novel genes in gastric carcinogenesis identified by next-generation sequencing. Mod Pathol. 2016;29:182-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 51. | Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5:121-125. [PubMed] |
| 52. | Tahara T, Arisawa T. DNA methylation as a molecular biomarker in gastric cancer. Epigenomics. 2015;7:475-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 53. | Ferrini AM, Mannoni V, Pontieri E, Pourshaban M. Longer resistance of some DNA traits from BT176 maize to gastric juice from gastrointestinal affected patients. Int J Immunopathol Pharmacol. 2007;20:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Yamamoto H, Watanabe Y, Oikawa R, Morita R, Yoshida Y, Maehata T, Yasuda H, Itoh F. BARHL2 Methylation Using Gastric Wash DNA or Gastric Juice Exosomal DNA is a Useful Marker For Early Detection of Gastric Cancer in an H. pylori-Independent Manner. Clin Transl Gastroenterol. 2016;7:e184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | da Silva Oliveira KC, Thomaz Araújo TM, Albuquerque CI, Barata GA, Gigek CO, Leal MF, Wisnieski F, Rodrigues Mello Junior FA, Khayat AS, de Assumpção PP, Rodriguez Burbano RM, Smith MC, Calcagno DQ. Role of miRNAs and their potential to be useful as diagnostic and prognostic biomarkers in gastric cancer. World J Gastroenterol. 2016;22:7951-7962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Link A, Kupcinskas J. MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: Current insights and future perspectives. World J Gastroenterol. 2018;24:3313-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 92] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 57. | Wu J, Li G, Wang Z, Yao Y, Chen R, Pu X, Wang J. Circulating MicroRNA-21 Is a Potential Diagnostic Biomarker in Gastric Cancer. Dis Markers. 2015;2015:435656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Hung PS, Chen CY, Chen WT, Kuo CY, Fang WL, Huang KH, Chiu PC, Lo SS. miR-376c promotes carcinogenesis and serves as a plasma marker for gastric carcinoma. PLoS One. 2017;12:e0177346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Tsai MM, Wang CS, Tsai CY, Huang CG, Lee KF, Huang HW, Lin YH, Chi HC, Kuo LM, Lu PH, Lin KH. Circulating microRNA-196a/b are novel biomarkers associated with metastatic gastric cancer. Eur J Cancer. 2016;64:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 60. | Jiang X, Wang W, Yang Y, Du L, Yang X, Wang L, Zheng G, Duan W, Wang R, Zhang X, Wang L, Chen X, Wang C. Identification of circulating microRNA signatures as potential noninvasive biomarkers for prediction and prognosis of lymph node metastasis in gastric cancer. Oncotarget. 2017;8:65132-65142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 61. | Zhu C, Ren C, Han J, Ding Y, Du J, Dai N, Dai J, Ma H, Hu Z, Shen H, Xu Y, Jin G. A five-microRNA panel in plasma was identified as potential biomarker for early detection of gastric cancer. Br J Cancer. 2014;110:2291-2299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 62. | Wang J, Zhang H, Zhou X, Wang T, Zhang J, Zhu W, Zhu H, Cheng W. Five serum-based miRNAs were identified as potential diagnostic biomarkers in gastric cardia adenocarcinoma. Cancer Biomark. 2018;23:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Huang Z, Zhu D, Wu L, He M, Zhou X, Zhang L, Zhang H, Wang W, Zhu J, Cheng W, Chen Y, Fan Y, Qi L, Yin Y, Zhu W, Shu Y, Liu P. Six Serum-Based miRNAs as Potential Diagnostic Biomarkers for Gastric Cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 64. | Wang N, Wang L, Yang Y, Gong L, Xiao B, Liu X. A serum exosomal microRNA panel as a potential biomarker test for gastric cancer. Biochem Biophys Res Commun. 2017;493:1322-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 65. | Ranjbar R, Hesari A, Ghasemi F, Sahebkar A. Expression of microRNAs and IRAK1 pathway genes are altered in gastric cancer patients with Helicobacter pylori infection. J Cell Biochem. 2018;119:7570-7576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Virgilio E, Giarnieri E, Giovagnoli MR, Montagnini M, Proietti A, D'Urso R, Mercantini P, Balducci G, Cavallini M. Gastric Juice MicroRNAs as Potential Biomarkers for Screening Gastric Cancer: A Systematic Review. Anticancer Res. 2018;38:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Valladares-Ayerbes M, Reboredo M, Medina-Villaamil V, Iglesias-Díaz P, Lorenzo-Patiño MJ, Haz M, Santamarina I, Blanco M, Fernández-Tajes J, Quindós M, Carral A, Figueroa A, Antón-Aparicio LM, Calvo L. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J Transl Med. 2012;10:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 68. | Liu X, Kwong A, Sihoe A, Chu KM. Plasma miR-940 may serve as a novel biomarker for gastric cancer. Tumour Biol. 2016;37:3589-3597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 69. | Jiang X, Jiang M, Xu M, Xu J, Li Y. Identification of diagnostic utility and molecular mechanisms of circulating miR-551b-5p in gastric cancer. Pathol Res Pract. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H, Okamoto K, Otsuji E. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 449] [Cited by in RCA: 509] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 71. | Wang J, Song YX, Wang ZN. Non-coding RNAs in gastric cancer. Gene. 2015;560:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 72. | Shi T, Gao G, Cao Y. Long Noncoding RNAs as Novel Biomarkers Have a Promising Future in Cancer Diagnostics. Dis Markers. 2016;2016:9085195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 73. | Bolha L, Ravnik-Glavač M, Glavač D. Long Noncoding RNAs as Biomarkers in Cancer. Dis Markers. 2017;2017:7243968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 290] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 74. | Cao WJ, Wu HL, He BS, Zhang YS, Zhang ZY. Analysis of long non-coding RNA expression profiles in gastric cancer. World J Gastroenterol. 2013;19:3658-3664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 144] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 75. | Zhou X, Yin C, Dang Y, Ye F, Zhang G. Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer. Sci Rep. 2015;5:11516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 76. | Zhao Y, Guo Q, Chen J, Hu J, Wang S, Sun Y. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigation. Oncol Rep. 2014;31:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 77. | Xian HP, Zhuo ZL, Sun YJ, Liang B, Zhao XT. Circulating long non-coding RNAs HULC and ZNFX1-AS1 are potential biomarkers in patients with gastric cancer. Oncol Lett. 2018;16:4689-4698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Yuan CL, Li H, Zhu L, Liu Z, Zhou J, Shu Y. Aberrant expression of long noncoding RNA PVT1 and its diagnostic and prognostic significance in patients with gastric cancer. Neoplasma. 2016;63:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 79. | Wang J, Sun J, Wang J, Song Y, Gao P, Shi J, Chen P, Wang Z. Long noncoding RNAs in gastric cancer: functions and clinical applications. Onco Targets Ther. 2016;9:681-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Shoda K, Kawaguchi T, Hirajima S, Nagata H, Kubota T, Fujiwara H, Okamoto K, Otsuji E. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185-3193. [PubMed] |
| 81. | Yörüker EE, Keskin M, Kulle CB, Holdenrieder S, Gezer U. Diagnostic and prognostic value of circulating lncRNA H19 in gastric cancer. Biomed Rep. 2018;9:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Hashad D, Elbanna A, Ibrahim A, Khedr G. Evaluation of the Role of Circulating Long Non-Coding RNA H19 as a Promising Novel Biomarker in Plasma of Patients with Gastric Cancer. J Clin Lab Anal. 2016;30:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 83. | Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L, Wang B, Ye G, Xiao B, Guo J. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36:2007-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 319] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 84. | Pang Q, Ge J, Shao Y, Sun W, Song H, Xia T, Xiao B, Guo J. Increased expression of long intergenic non-coding RNA LINC00152 in gastric cancer and its clinical significance. Tumour Biol. 2014;35:5441-5447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 85. | Shao Y, Ye M, Jiang X, Sun W, Ding X, Liu Z, Ye G, Zhang X, Xiao B, Guo J. Gastric juice long noncoding RNA used as a tumor marker for screening gastric cancer. Cancer. 2014;120:3320-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 86. | Elsayed ET, Salem PE, Darwish AM, Fayed HM. Plasma long non-coding RNA HOTAIR as a potential biomarker for gastric cancer. Int J Biol Markers. 2018;1724600818760244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 87. | Gao J, Cao R, Mu H. Long non-coding RNA UCA1 may be a novel diagnostic and predictive biomarker in plasma for early gastric cancer. Int J Clin Exp Pathol. 2015;8:12936-12942. [PubMed] |
| 88. | Yang Y, Shao Y, Zhu M, Li Q, Yang F, Lu X, Xu C, Xiao B, Sun Y, Guo J. Using gastric juice lncRNA-ABHD11-AS1 as a novel type of biomarker in the screening of gastric cancer. Tumour Biol. 2016;37:1183-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 89. | Tan L, Yang Y, Shao Y, Zhang H, Guo J. Plasma lncRNA-GACAT2 is a valuable marker for the screening of gastric cancer. Oncol Lett. 2016;12:4845-4849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Zhang K, Shi H, Xi H, Wu X, Cui J, Gao Y, Liang W, Hu C, Liu Y, Li J, Wang N, Wei B, Chen L. Genome-Wide lncRNA Microarray Profiling Identifies Novel Circulating lncRNAs for Detection of Gastric Cancer. Theranostics. 2017;7:213-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 91. | Dong L, Qi P, Xu MD, Ni SJ, Huang D, Xu QH, Weng WW, Tan C, Sheng WQ, Zhou XY, Du X. Circulating CUDR, LSINCT-5 and PTENP1 long noncoding RNAs in sera distinguish patients with gastric cancer from healthy controls. Int J Cancer. 2015;137:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 92. | Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 6021] [Article Influence: 501.8] [Reference Citation Analysis (0)] |
| 93. | Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1007] [Cited by in RCA: 1296] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 94. | Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 631] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 95. | Sui W, Shi Z, Xue W, Ou M, Zhu Y, Chen J, Lin H, Liu F, Dai Y. Circular RNA and gene expression profiles in gastric cancer based on microarray chip technology. Oncol Rep. 2017;37:1804-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 96. | Huang YS, Jie N, Zou KJ, Weng Y. Expression profile of circular RNAs in human gastric cancer tissues. Mol Med Rep. 2017;16:2469-2476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 97. | Vidal AF, Ribeiro-Dos-Santos AM, Vinasco-Sandoval T, Magalhães L, Pinto P, Anaissi AKM, Demachki S, de Assumpção PP, Dos Santos SEB, Ribeiro-Dos-Santos A. The comprehensive expression analysis of circular RNAs in gastric cancer and its association with field cancerization. Sci Rep. 2017;7:14551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Shen Y, Zhang J, Fu Z, Zhang B, Chen M, Ling X, Zou X. Gene microarray analysis of the circular RNAs expression profile in human gastric cancer. Oncol Lett. 2018;15:9965-9972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Li P, Chen H, Chen S, Mo X, Li T, Xiao B, Yu R, Guo J. Circular RNA 0000096 affects cell growth and migration in gastric cancer. Br J Cancer. 2017;116:626-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 100. | Fang Y, Ma M, Wang J, Liu X, Wang Y. Circular RNAs play an important role in late-stage gastric cancer: Circular RNA expression profiles and bioinformatics analyses. Tumour Biol. 2017;39:1010428317705850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Huang M, He YR, Liang LC, Huang Q, Zhu ZQ. Circular RNA hsa_circ_0000745 may serve as a diagnostic marker for gastric cancer. World J Gastroenterol. 2017;23:6330-6338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 102. | Zhao Q, Chen S, Li T, Xiao B, Zhang X. Clinical values of circular RNA 0000181 in the screening of gastric cancer. J Clin Lab Anal. 2018;32:e22333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 103. | Lai Z, Yang Y, Yan Y, Li T, Li Y, Wang Z, Shen Z, Ye Y, Jiang K, Wang S. Analysis of co-expression networks for circular RNAs and mRNAs reveals that circular RNAs hsa_circ_0047905, hsa_circ_0138960 and has-circRNA7690-15 are candidate oncogenes in gastric cancer. Cell Cycle. 2017;16:2301-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Shao Y, Li J, Lu R, Li T, Yang Y, Xiao B, Guo J. Global circular RNA expression profile of human gastric cancer and its clinical significance. Cancer Med. 2017;6:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 105. | Shao Y, Chen L, Lu R, Zhang X, Xiao B, Ye G, Guo J. Decreased expression of hsa_circ_0001895 in human gastric cancer and its clinical significances. Tumour Biol. 2017;39:1010428317699125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 106. | Sun H, Tang W, Rong D, Jin H, Fu K, Zhang W, Liu Z, Cao H, Cao X. Hsa_circ_0000520, a potential new circular RNA biomarker, is involved in gastric carcinoma. Cancer Biomark. 2018;21:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 107. | Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 108. | Li T, Shao Y, Fu L, Xie Y, Zhu L, Sun W, Yu R, Xiao B, Guo J. Plasma circular RNA profiling of patients with gastric cancer and their droplet digital RT-PCR detection. J Mol Med (Berl). 2018;96:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 109. | Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3481] [Cited by in RCA: 3415] [Article Influence: 284.6] [Reference Citation Analysis (0)] |
| 110. | Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol. 2016;30:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 111. | Neumann MHD, Bender S, Krahn T, Schlange T. ctDNA and CTCs in Liquid Biopsy - Current Status and Where We Need to Progress. Comput Struct Biotechnol J. 2018;16:190-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 112. | Pantel K, Alix-Panabières C. Liquid biopsy in 2016: Circulating tumour cells and cell-free DNA in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2017;14:73-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 113. | Uchôa Guimarães CT, Ferreira Martins NN, Cristina da Silva Oliveira K, Almeida CM, Pinheiro TM, Gigek CO, Roberto de Araújo Cavallero S, Assumpção PP, Cardoso Smith MA, Burbano RR, Calcagno DQ. Liquid biopsy provides new insights into gastric cancer. Oncotarget. 2018;9:15144-15156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-6904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1954] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 115. | Guan X, Ma F, Liu S, Wu S, Xiao R, Yuan L, Sun X, Yi Z, Yang H, Xu B. Analysis of the hormone receptor status of circulating tumor cell subpopulations based on epithelial-mesenchymal transition: a proof-of-principle study on the heterogeneity of circulating tumor cells. Oncotarget. 2016;7:65993-66002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Friedlander TW, Premasekharan G, Paris PL. Looking back, to the future of circulating tumor cells. Pharmacol Ther. 2014;142:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 117. | Liu H, Zhang X, Li J, Sun B, Qian H, Yin Z. The biological and clinical importance of epithelial-mesenchymal transition in circulating tumor cells. J Cancer Res Clin Oncol. 2015;141:189-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 118. | Chinen LTD, Abdallah EA, Braun AC, Flores BCTCP, Corassa M, Sanches SM, Fanelli MF. Circulating Tumor Cells as Cancer Biomarkers in the Clinic. Adv Exp Med Biol. 2017;994:1-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Kang HM, Kim GH, Jeon HK, Kim DH, Jeon TY, Park DY, Jeong H, Chun WJ, Kim MH, Park J, Lim M, Kim TH, Cho YK. Circulating tumor cells detected by lab-on-a-disc: Role in early diagnosis of gastric cancer. PLoS One. 2017;12:e0180251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 120. | Abdulla A, Liu W, Gholamipour-Shirazi A, Sun J, Ding X. High-Throughput Isolation of Circulating Tumor Cells Using Cascaded Inertial Focusing Microfluidic Channel. Anal Chem. 2018;90:4397-4405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 121. | Li Y, Zhang X, Ge S, Gao J, Gong J, Lu M, Zhang Q, Cao Y, Wang DD, Lin PP, Shen L. Clinical significance of phenotyping and karyotyping of circulating tumor cells in patients with advanced gastric cancer. Oncotarget. 2014;5:6594-6602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 122. | Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, Antonarakis ES, Azad NS, Bardelli A, Brem H, Cameron JL, Lee CC, Fecher LA, Gallia GL, Gallia GL, Gibbs P, Le D, Giuntoli RL, Goggins M, Hogarty MD, Holdhoff M, Hong SM, Jiao Y, Juhl HH, Kim JJ, Siravegna G, Laheru DA, Lim M, Lipson EJ, Marie SK, Netto GJ, Oliner KS, Olivi A. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2770] [Cited by in RCA: 3547] [Article Influence: 322.5] [Reference Citation Analysis (0)] |
| 123. | Alix-Panabières C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016;6:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 999] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 124. | Sumbal S, Javed A, Afroze B, Zulfiqar HF, Javed F, Noreen S, Ijaz B. Circulating tumor DNA in blood: Future genomic biomarkers for cancer detection. Exp Hematol. 2018;65:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 125. | Fang WL, Lan YT, Huang KH, Liu CA, Hung YP, Lin CH, Jhang FY, Chang SC, Chen MH, Chao Y, Lin WC, Lo SS, Fen-Yau Li A, Wu CW, Chiou SH, Shyr YM. Clinical significance of circulating plasma DNA in gastric cancer. Int J Cancer. 2016;138:2974-2983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 126. | Balgkouranidou I, Matthaios D, Karayiannakis A, Bolanaki H, Michailidis P, Xenidis N, Amarantidis K, Chelis L, Trypsianis G, Chatzaki E, Lianidou ES, Kakolyris S. Prognostic role of APC and RASSF1A promoter methylation status in cell free circulating DNA of operable gastric cancer patients. Mutat Res. 2015;778:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 127. | Shoda K, Ichikawa D, Fujita Y, Masuda K, Hiramoto H, Hamada J, Arita T, Konishi H, Kosuga T, Komatsu S, Shiozaki A, Okamoto K, Imoto I, Otsuji E. Clinical utility of circulating cell-free Epstein-Barr virus DNA in patients with gastric cancer. Oncotarget. 2017;8:28796-28804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 128. | Shoda K, Masuda K, Ichikawa D, Arita T, Miyakami Y, Watanabe M, Konishi H, Imoto I, Otsuji E. HER2 amplification detected in the circulating DNA of patients with gastric cancer: a retrospective pilot study. Gastric Cancer. 2015;18:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 129. | Shoda K, Ichikawa D, Fujita Y, Masuda K, Hiramoto H, Hamada J, Arita T, Konishi H, Komatsu S, Shiozaki A, Kakihara N, Okamoto K, Taniguchi H, Imoto I, Otsuji E. Monitoring the HER2 copy number status in circulating tumor DNA by droplet digital PCR in patients with gastric cancer. Gastric Cancer. 2017;20:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 130. | Kato S, Okamura R, Baumgartner JM, Patel H, Leichman L, Kelly K, Sicklick JK, Fanta PT, Lippman SM, Kurzrock R. Analysis of Circulating Tumor DNA and Clinical Correlates in Patients with Esophageal, Gastroesophageal Junction, and Gastric Adenocarcinoma. Clin Cancer Res. 2018;24:6248-6256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 131. | Foundation Medicine’s New Liquid Biopsy Assay Granted Breakthrough Device Designation by US. Food and Drug Administration 2018. [cited Feb 28 2019]. Available from: http://investors.foundationmedicine.com/news-releases/news-release-details/foundation-medicines-new-liquid-biopsy-assay-granted. |