Published online Apr 21, 2019. doi: 10.3748/wjg.v25.i15.1879
Peer-review started: February 19, 2019
First decision: February 26, 2019
Revised: March 4, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 21, 2019
Processing time: 58 Days and 22 Hours
Due to the significant shortage of organs and the increasing number of candidates on the transplant waiting list, there is an urgent need to identify patients who are most likely to benefit from liver transplantation. The albumin-bilirubin (ALBI) grading system was recently developed to identify patients at risk for adverse outcomes after hepatectomy. However, the value of the pretransplant ALBI score in predicting outcomes after liver transplantation has not been assessed.
To retrospectively investigate the value of the pretransplant ALBI score in predicting outcomes after liver transplantation.
The clinical data of 272 consecutive adult patients who received donation after cardiac death and underwent liver transplantation at our centre from March 2012 to March 2017 were analysed in the cohort study. After the exclusion of patients who met any of the exclusion criteria, 258 patients remained. The performance of the ALBI score in predicting overall survival and postoperative complications after liver transplantation was evaluated. The optimal cut-off value of preoperative ALBI was calculated according to long-term survival status. The outcomes after liver transplantation, including postoperative complications and survival analysis, were measured.
The remaining 258 consecutive patients were included in the analysis. The median follow-up time was 17.30 (interquartile range: 8.90-28.98) mo. Death occurred in 35 patients during follow-up. The overall survival rate was 81.0%. The preoperative ALBI score had a significant positive correlation with the overall survival rate after liver transplantation. The calculated cut-off for ALBI scores to predict postoperative survival was -1.48. Patients with an ALBI score > -1.48 had a significantly lower survival rate than those with an ALBI score ≤ -1.48 (73.7% vs 87.6%, P < 0.05), and there were no statistically significant differences in survival rates between patients with a model for end stage liver disease score ≥ 10 and < 10 and different Child-Pugh grades. In terms of the specific complications, a high ALBI score was associated with an increased incidence of biliary complications, intraabdominal bleeding, septicaemia, and acute kidney injury after liver transplantation (P < 0.05 for all).
The ALBI score predicts overall survival and postoperative complications after liver transplantation. The ALBI grading system may be useful in risk-stratifying patients on the liver transplant waiting list.
Core tip: The albumin-bilirubin (ALBI) grading system was developed to identify patients at risk for poor outcomes after hepatectomy. The study showed the preoperative ALBI score had a significant positive correlation with the overall survival rate after liver transplantation. The calculated cut-off for ALBI scores to predict postoperative survival was -1.48. Patients with an ALBI score > -1.48 had a significantly lower survival rate than those with an ALBI score ≤ -1.48. A high ALBI score was also associated with an increased incidence of postoperative complications. Thus, the ALBI grading system may be useful in risk-stratifying patients on the liver transplant waiting list.
- Citation: Ma T, Li QS, Wang Y, Wang B, Wu Z, Lv Y, Wu RQ. Value of pretransplant albumin-bilirubin score in predicting outcomes after liver transplantation. World J Gastroenterol 2019; 25(15): 1879-1889
- URL: https://www.wjgnet.com/1007-9327/full/v25/i15/1879.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i15.1879
Advances in immunosuppression and improvements in surgical techniques and perioperative care have markedly improved the outcome of liver transplant recipients, and liver transplantation (LT) has become the only effective treatment for patients with end-stage liver disease[1-3]. Because of the significant shortage of organs and the increasing number of candidates on the transplant waiting list, there is an urgent need to identify patients who are most likely to benefit from LT[2,4,5].
The albumin-bilirubin (ALBI) score, as a simple assessment of liver function, is objectively calculated by only two variables (albumin and bilirubin)[6]. It was recently proposed by Johnson et al[6], Andreatos et al[7], and Zou et al[8] as a new method for preoperative risk evaluation to discern patients with the risk of adverse outcomes after hepatectomy. While the ALBI grading system has been closely related to in-hospital mortality in patients with chronic liver disease, its value to predict outcomes after LT has not been evaluated. Therefore, the purpose of this study was to explore the ability of the pretransplant ALBI score to predict outcomes after LT.
This single-centre, retrospective cohort study was conducted to investigate the relationship between pretransplant ALBI scores and outcomes after LT. From March 1, 2012 to March 31, 2017, 272 consecutive adult patients (age > 18 years) with end-stage liver disease who received donation after cardiac death (DCD) and underwent LT at the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China were included in this study. All clinical variables of these 272 patients, including demographic features and preoperative, intraoperative, and postoperative data, were obtained from a computerized clinical database from the hospital. In addition to the date of this study, available medical records, including follow-up data, met the inclusion criteria. This study was approved by the First Affiliated Hospital of Xi'an Jiaotong University Ethics Committee. Written informed consent from the patients was waived due to the retrospective nature of this study. All cases received follow-up care routinely until June 2017.
The ALBI score was calculated using the formula: (log10 bilirubin × 0.66) + (albumin × -0.085), where bilirubin is measured in μmol/L and albumin in g/L[6]. The primary outcome was overall survival. The secondary outcomes included total complications and the incidence of biliary complications, portal vein thrombosis, rejection, pneumonia, acute kidney injury (AKI), intraabdominal bleeding, and in-hospital mortality as well as length of postoperative hospital stay after LT.
To minimize bias, follow-ups and reviews were completed by two clinicians. Categorical variables are reported as numbers and percentages and were compared by a chi-squared analysis or Fisher’s exact test as appropriate. Normal and abnormal continuous variables are reported as the mean ± standard deviation (SD) and median [interquartile range (IQR)], and were compared by Student’s t-test and the Mann-Whitney rank-sum test, respectively. The optimal cut-off value of preoperative ALBI was calculated by receiver operating characteristic (ROC) curve analysis and utilizing the Youden index according to long-term survival status. The accuracy of ALBI for predicting outcomes was evaluated using the area under the ROC curve (AUC). The survival rates of recipients with high ALBI grades and low ALBI grades were compared using a Kaplan-Meier estimation and a log-rank test. Univariate and multivariate analyses of prognostic factors were performed using the Cox proportional hazards model. All statistical tests were two-sided, and P-values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS Statistics 22.0 software (IBM Corporation, Armonk, NY, United States).
A total of 272 patients underwent LT at our hospital from March 1, 2012 to March 31, 2017. Of these patients, 14 were excluded from this study: 12 were lost to follow-up and 2 were missing criteria for ALBI and model for end stage liver disease (MELD) score calculations. The remaining 258 consecutive patients were included in the analysis. The median follow-up time was 17.30 (IQR: 8.90-28.98) mo. Table 1 shows the demographics and baseline characteristics of these patients. Of these patients, 206 were male (79.8%), and 52 were female (20.2%). The median age of the patients was 47.0 (IQR: 39.0-56.0) years. The indications for LT were hepatocellular carcinoma (HCC) (33.7%), viral hepatitis-related cirrhosis (77.5%), alcoholic cirrhosis (3.1%), primary biliary cirrhosis and autoimmune hepatitis (8.2%), and others (11.2%), such as hepatolenticular degeneration, cryptogenic cirrhosis, drug-induced liver injury, upper biliary tract obstruction, and acute liver failure. The median preoperative ALBI score and MELD score were -1.78 (-2.40 to -1.33) and 15.5 (11.0-23.0), respectively. Death occurred in 35 patients during follow-up. The overall survival rate was 81.0%.
| Patients characteristic | n (%)/mean ± SD/median (IQR) |
| Demographic feature | |
| Age, yr | 47.0 (39.0-56.0) |
| Male, n (%) | 206 (79.8) |
| Coexisting condition | |
| Smoking, n (%) | 78 (30.2) |
| Drinking, n (%) | 44 (17.1) |
| Hypertension, n (%) | 19 (7.4) |
| Diabetes, n (%) | 27 (10.5) |
| Etiology | |
| Hepatocellular carcinoma, n (%) | 87 (33.7) |
| Viral hepatitis, n (%) | 200 (77.5) |
| Alcoholic cirrhosis, n (%) | 8 (3.1) |
| PBC and AIH, n (%) | 21 (8.2) |
| Other, n (%) | 29 (11.2) |
| Clinical feature | |
| ALBI score | -1.78 (-2.40 - -1.33) |
| MELD score | 15.5 (11.0-23.0) |
| Child-Pugh grade | |
| A, n (%) | 43 (16.7) |
| B, n (%) | 94 (36.4) |
| C, n (%) | 121 (46.9) |
| Operation time (min) | 390.0 (332.5-436.5) |
| Anhepatic phase (min) | 49 (44-58) |
| Blood loss (mL) | 1500 (900-3000) |
| Total input quantity (mL) | 6040 (4810-7810) |
| Warm ischemia time (min) | 9 (8-10) |
| Cold ischemia time (h) | 5 (4-6) |
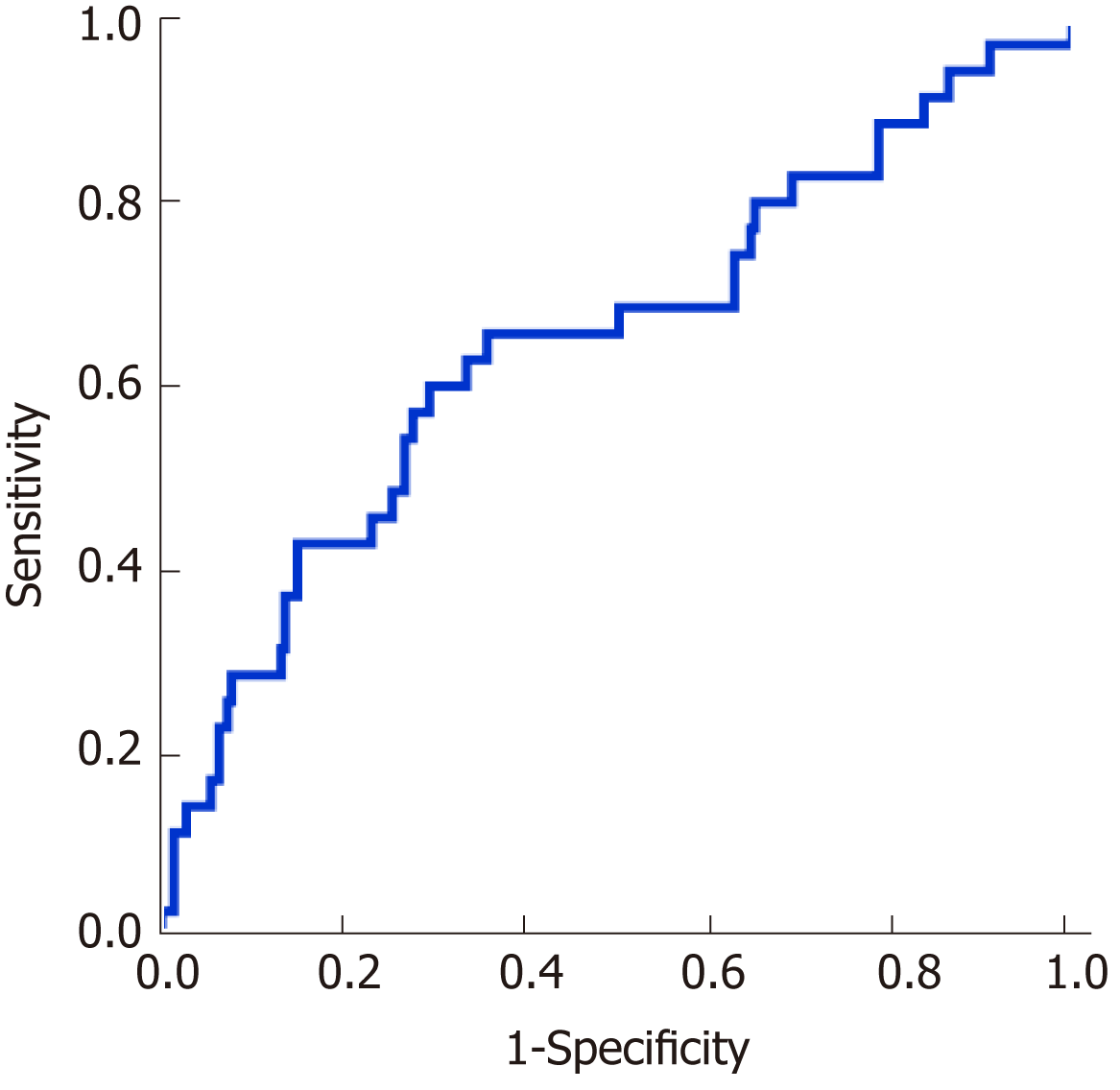
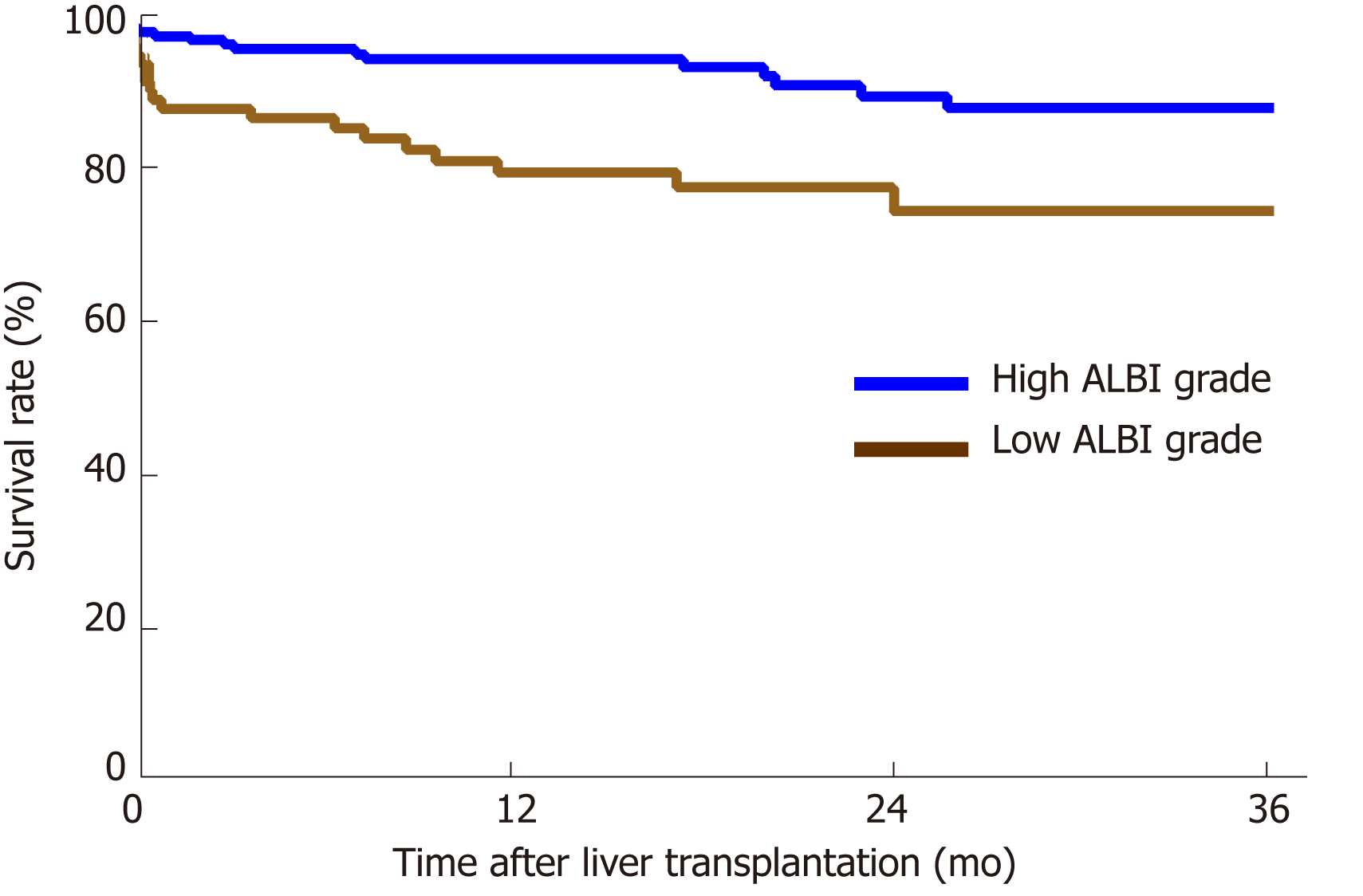
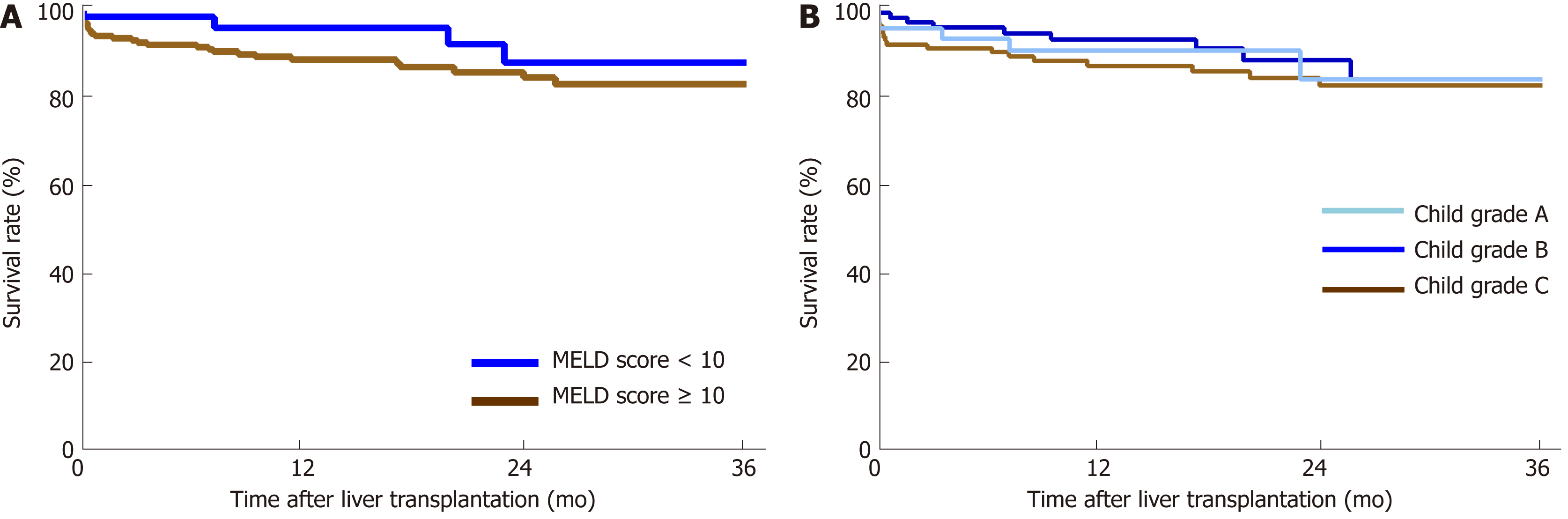
The performance of the ROC curve analysis was determined by the value of the pretransplant ALBI score to predict the overall survival after LT. Figure 1 shows that the pretransplant ALBI score had a significant positive relationship with the overall survival rate. The AUC was 0.647 with a 95% confidence interval (CI) of 0.540-0.753 and a P-value of 0.005. The cut-off for ALBI scores was calculated as -1.48 by predicting postoperative survival, with a Youden index of 0.304 (sensitivity = 60.0%, and specificity = 70.4%). Based on the cut-off value, 173 patients had a low ALBI score (ALBI ≤ -1.48, 67.1%) and 85 patients had a high ALBI score (ALBI > -1.48, 32.9%). As shown in Table 2, the pretransplant and demographic data were related to the ALBI grade. There was less likely to be HCC in patients with high ALBI scores than in patients with low ALBI scores. Patients in the high ALBI group also had higher preoperative MELD scores and higher Child-Pugh (C-P) grades. In terms of the preoperative laboratory values, patients in the high ALBI group had higher values for aspartate transaminase (AST), alpha-fetoprotein (ALT), total bilirubin (TBIL), direct bilirubin (DBIL), neutrophil granulocytes (NEUT), monocytes (MONO), prothrombin time (PT), activated partial thromboplastin time (APTT), and international normalized ratio (INR) (P < 0.05 for all) but lower levels of red blood cells (RBC), haemoglobin (HGB), platelets (PLT), albumin (ALB), and alpha-fetoprotein (AFP) (P < 0.05 for all). The 3-year survival after LT was analysed based on pretransplant ALBI scores using the Kaplan-Meier estimation. As shown in Figure 2, patients with high ALBI scores had a significantly lower survival rate than patients with low ALBI scores (73.7% vs 87.6%, P < 0.05). However, there were no statistically significant differences in 3-year survival rates between patients with MELD scores ≥ 10 and < 10 (Figure 3A). Similarly, no statistically significant differences were found in 3-year survival rates among patients with different C-P grades (Figure 3B). Univariable and multivariable analyses were performed to identify independent risk factors related to poor survival after LT. Univariate variables with P < 0.1 were included in the multivariate analysis. Table 3 shows that high pretransplant ALBI scores, high PLT, high serum levels of creatinine, and high APTT were independently associated with poor survival after LT in the multivariate analysis.
| Variable | ALBI ≤ -1.48 (n = 173) | ALBI > -1.48 (n = 85) | P-value |
| Demographic feature | |||
| Age (yr) | 47 (39-55) | 47 (38-56) | 0.926 |
| Male (Y/N) | 138/35 | 68/17 | 0.965 |
| Coexisting condition | |||
| Smoking (Y/N) | 52/121 | 26/59 | 0.931 |
| Drinking (Y/N) | 26/147 | 18/67 | 0.217 |
| Hypertension (Y/N) | 13/160 | 6/79 | 0.895 |
| Diabetes (Y/N) | 15/158 | 12/73 | 0.179 |
| Etiology | 0.025 | ||
| HCC (Y/N) | 71/102 | 16/69 | < 0.001 |
| Viral hepatitis | 143 | 57 | |
| Alcoholic cirrhosis | 4 | 4 | |
| PBC and AIH | 9 | 12 | |
| Other | 17 | 12 | |
| Hepatic feature | |||
| MELD score | 14 (10-18) | 23 (17.5-28) | < 0.001 |
| Child grade A/B/C | 42/80/51 | 1/14/70 | < 0.001 |
| Preoperative laboratory value | |||
| RBC (1012/L) | 3.40 (2.90-4.15) | 2.94 (2.49-3.32) | < 0.001 |
| HGB (g/L) | 105.0 (88.0-130.0) | 95.00 (86.5-107.0) | 0.001 |
| PLT (109/L) | 59.0 (38.5-103.0) | 45.0 (28.5-72.5) | 0.002 |
| WBC (109/L) | 3.71 (2.55-5.36) | 4.31 (2.84-7.38) | 0.084 |
| NEUT (109/L) | 2.33 (1.67-3.74) | 2.86 (1.80-5.90) | 0.044 |
| LYMPH (109/L) | 0.70 (0.45-1.15) | 0.60 (0.41-0.95) | 0.189 |
| MONO (109/L) | 0.28 (0.18-0.45) | 0.38 (0.22-0.57) | 0.011 |
| AFP (μg/L) | 4.74 (2.73-16.30) | 3.50 (2.22-6.23) | 0.032 |
| ALT (U/L) | 32.00 (22.00-47.00) | 38.00 (23.57-67.06) | 0.042 |
| AST (U/L) | 41.50 (29.00-59.00) | 54.52 (34.05-102.00) | 0.001 |
| TBIL (μmol/L) | 32.75 (17.67-54.83) | 105.48 (51.72-314.33) | < 0.001 |
| DBIL (μmol/L) | 11.70 (5.87-27.00) | 50.70 (19.70-192.23) | < 0.001 |
| ALB (g/L) | 37.20 (34.50-41.99) | 29.83 (26.95-32.11) | < 0.001 |
| BUN (mmol/L) | 4.39 (3.59-6.21) | 5.07 (3.87-7.32) | 0.077 |
| CRE (μmol/L) | 58.00 (48.00-68.85) | 61.88 (48.15-82.00) | 0.199 |
| GLU (mmol/L) | 5.60 (4.91-6.91) | 6.06 (5.03-8.40) | 0.062 |
| PT (s) | 17.20 (15.05-19.15) | 20.70 (18.05-24.30) | < 0.001 |
| APTT (s) | 42.40 (39.15-47.70) | 49.10 (43.25-54.75) | < 0.001 |
| INR | 1.41 (1.20-1.60) | 1.74 (1.49-2.27) | < 0.001 |
| Variable | Univariate | Multivariate | ||
| P-value | OR (95%CI) | P-value | OR (95%CI) | |
| ALBI grade | 0.002 | 3.923 (1.495-5.716) | 0.036 | 2.290 (1.057-4.963) |
| MELD grade | 0.192 | 2.002 (0.706-5.676) | ||
| Child-Pugh grade | ||||
| A | Reference | |||
| B | 0.713 | 0.815 (0.273-2.431) | ||
| C | 0.451 | 1.456 (0.548-3.868) | ||
| Age | 0.537 | 1.010 (0.978-1.044) | ||
| Sex | 0.233 | 0.629 (0.294-1.347) | ||
| Drinking | 0.266 | 1.565 (0.711-3.447) | ||
| Smoking | 0.931 | 0.968 (0.465-2.017) | ||
| Diabetes | 0.791 | 0.852 (0.261-2.785) | ||
| Hypertension | 0.576 | 1.402 (0.428-4.587) | ||
| HCC | 0.972 | 1.013 (0.504-2.035) | ||
| Disease time | 0.470 | 1.014 (0.977-1.051) | ||
| RBC | 0.282 | 0.794 (0.522-1.208) | ||
| HGB | 0.617 | 0.997 (0.987-1.008) | ||
| PLT | 0.054 | 1.004 (1.000-1.008) | 0.048 | 1.005 (1.000-1.011) |
| WBC | 0.002 | 1.097 (1.034-1.165) | 0.481 | 1.034 (0.942-1.134) |
| NEUT | 0.494 | 1.010 (0.981-1.041) | ||
| LYMPH | 0.615 | 1.071 (0.821-1.397) | ||
| MONO | 0.457 | 1.106 (0.849-1.441) | ||
| AFP | 0.085 | 1.000 (1.000-1.000) | 0.391 | 1.000 (1.000-1.000) |
| ALT | 0.002 | 1.001 (1.000-1.001) | 0.278 | 1.000 (1.000-1.001) |
| AST | < 0.001 | 1.001 (1.001-1.002) | 0.418 | 1.000 (0.999-1.002) |
| BUN | 0.395 | 1.022 (0.972-1.075) | ||
| CRE | 0.002 | 1.005 (1.002-1.008) | 0.027 | 1.005 (1.001-1.026) |
| GLU | 0.575 | 0.959 (0.830-1.109) | ||
| PT | 0.181 | 1.034 (0.985-1.086) | ||
| INR | 0.127 | 1.336 (0.921-1.937) | ||
| APTT | 0.028 | 1.013 (1.001-1.024) | 0.028 | 1.014 (1.001-1.026) |
| Operation time | 0.007 | 1.005 (1.001-1.009) | 0.182 | 1.003 (0.999-1.008) |
| Warm ischemia time | 0.750 | 0.970 (0.803-1.171) | ||
| Cold ischemia time | 0.145 | 1.192 (0.941-1.509) | ||
Table 4 shows postoperative complications stratified by pretransplant ALBI scores. A total of 189 patients developed various postoperative complications according to the Clavien-Dindo system[9]; 87.06% of patients in the high ALBI score group (74 out of 85) developed postoperative complications after LT, while only 66.47% of patients in the low ALBI score group (115 out of 173) did. The difference was statistically significant (P < 0.05), which was also reflected in the comprehensive complication index (CCI). In terms of specific complications, a high ALBI score was associated with an increased incidence of biliary complications, intraabdominal bleeding, septicaemia, and AKI (P < 0.05 for all). However, no significant differences were found between the two groups for other complications.
| Complication | Low ALBI grade(n = 173) | High ALBI grade(n = 85) | P-value |
| Total complications (Y/N) | 115/58 | 74/11 | < 0.001 |
| Pneumonia (Y/N) | 51/122 | 33/52 | 0.132 |
| AKI (Y/N) | 79/94 | 57/28 | 0.001 |
| Biliary complication (Y/N) | 11/162 | 14/71 | 0.010 |
| Porta vein thrombosis (Y/N) | 3/170 | 0/85 | 0.546 |
| Rejection (Y/N) | 10/163 | 2/83 | 0.361 |
| Intraabdominal bleeding (Y/N) | 8/165 | 13/72 | 0.003 |
| Coma for 24 h (Y/N) | 1/172 | 4/81 | 0.075 |
| Mechanical ventilation for 72 h (Y/N) | 1/172 | 3/82 | 0.205 |
| Septicemia (Y/N) | 0/173 | 3/82 | 0.013 |
| MOF (Y/N) | 1/172 | 4/81 | 0.075 |
| In-hospital mortality (Y/N) | 3/170 | 4/81 | 0.330 |
| SIRS (Y/N) | 45/128 | 26/59 | 0.439 |
| CCI, median (IQR) | 29.60 (8.70-36.65) | 36.20 (23.40-49.75) | < 0.001 |
| Postoperative hospital stay, median days (IQR) | 17.00 (13.50-24.00) | 19.00 (12.50-25.00) | 0.514 |
The prediction of prognosis is an important part of management in patients with end-stage liver disease. Our current data show that the ALBI score, a simple model incorporating only serum bilirubin and serum albumin levels, performed better than the conventional MELD model in predicting overall survival and postoperative complications after LT. Assessment of liver function is particularly important for patients on the liver-transplant waiting list. Since both serum bilirubin and albumin are part of the commonly used liver function tests, the ALBI score is readily available. In this study, we found that the optimal ALBI cut-off value was -1.48, analysed by the ROC curve to predict survival after LT, which is very close to the cut-off value (-1.39) between ALBI grade 2 and grade 3[6,10]. In fact, by using the cut-off value of the reported ALBI grading system developed for hepatectomy (i.e., -1.39)[6,7,11], we found that patients in the ALBI grade 3 classification had significantly higher mortality and more adverse postoperative outcomes after LT than patients in the ABLI grade 1 or 2 (data not shown) classifications, indicating that the reported ALBI grading system is also relevant in LT. Although many studies have shown that the ALBI grading system is a useful tool to identify patients at risk for adverse outcomes after hepatectomy, as far as we know, the present study is the first to assess the value of the pretransplant ALBI score in predicting outcomes after LT.
Assessment of preoperative liver function is vital to determine liver functional reserve in patients with end stage liver disease. The MELD system was developed in 2002 to prioritize patients waiting for LT[12]. As a numerical scale, MELD was used for adult LT candidates[13-15]. The patient’s urgency for LT within the next three months was determined by personal MELD scores[16]. The MELD scoring system contains two variables for hepatic (dys)function (i.e., total bilirubin and INR) and one variable for renal (dys)function (i.e., creatinine). Although subsequent studies have shown poor outcomes for liver transplant recipients with high MELD scores, its overall capacity to predict posttransplant outcomes is limited[12,16,17].
In the current study, although we found that patients with an MELD score < 10 seemed to have slightly higher survival rates than patients with an MELD score ≥ 10, there were no statistically significant differences in either the univariable or multivariable analyses. These results clearly show that the performance of ALBI is better than MELD in predicting outcomes after LT.
Another model to assess liver function is the C-P system. The C-P grade is determined by five variables, including TBIL, ALB, PT, and degree of ascites and hepatic encephalopathy. The C-P system was developed arbitrarily several decades ago based on clinical observation without proper statistical evidence. Although the C-P system is widely used, there are many limitations for its implementation[18,19]. For instance, the grading of ascites and hepatic encephalopathy is highly subjective[15,18,20]. It is not clear to identify the grade of ascites and hepatic encephalopathy according to guidelines. Some of the parameters, such as serum albumin levels and the extent of ascites, are interrelated. More importantly, the C-P grade failed to show any value in discriminating both survival and complications after LT in our current study.
Of course, there were still some limitations in the study. First, this current study only included population data from one transplant centre; based on the LT data of the single centre, the posttransplant morbidity and mortality were low in the relatively small sample. For example, a relatively small proportion of patients died during follow-up, which may have limited the robustness of the multivariable analysis for adjustment for confounding factors. Second, only patients who received donation after DCD were included in the study; the value of ABLI scores in predicting outcomes of patients who received donation after brain death needs to be further investigated. Third, as the median follow-up time in the current study was only 17.30 mo, we were unable to comment on the effect of pretransplant ALBI scores on longer term outcomes of patients. Additionally, the study aimed to explore the effect of ALBI scores on overall survival, not on liver death related to liver disease (i.e., disease-free survival)[21]. The difficulty of specifically attributing the reason for death after transplantation in the clinic makes no difference in terms of the patients’ outcomes. Lastly, as the nature of this study was retrospective, the results are subject to a selection bias and some residual confounding due to unmeasured or unknown confounders.
In summary, the data reveal that the ALBI score may be better than the MELD score for risk stratification of LT patients. Approximately one-third of our study population was categorized as having a high ALBI score (> -1.48); therefore, the ALBI scoring system is clinically relevant. In addition, the ALBI grading system may be a more readily applicable means to model risk among patients undergoing LT because it relies on fewer variables. The identification of patients who are most likely to benefit from LT remains a remarkable challenge[22].
The albumin-bilirubin (ALBI) score, as a simple assessment of liver function, is objectively calculated by only two variables (albumin and bilirubin). It was proposed as a new method for preoperative risk evaluation to discern patients with the risk of adverse outcomes after hepatectomy. However, its ability to predict outcomes after liver transplantation has not been evaluated. Because of the significant shortage of organs and the increasing number of candidates on the transplant waiting list, there is an urgent need to identify patients who are most likely to benefit from LT.
The main topic of this study was to provide a potential scoring system for the allocation of donor liver resources by investigating the relationship between pretransplant ALBI score and outcomes after liver transplantation.
To retrospectively investigate the value of pretransplant ALBI scores in predicting outcomes after liver transplantation and as a tool for risk-stratifying patients on the liver transplant waiting list.
The research data were obtained from a computerized clinical database from the First Affiliated Hospital of Xi’an Jiaotong University and included 258 consecutive patients who received donation after cardiac death (DCD) and underwent liver transplantation from March 2012 to March 2017. The optimal cut-off value of preoperative ALBI was calculated according to long-term survival status. The performance of the ALBI score in predicting outcomes, including postoperative complications and survival analysis, was measured and evaluated.
This study analysed data from 258 patients. Thirty-five patients died during follow-up [17.30 (interquartile range: 8.90-28.98) mo], with an overall survival rate of 81.0%. The optimal cut-off value of preoperative ALBI scores to predict postoperative survival was -1.48. Patients with an ALBI score > -1.48 had a significantly lower survival rate than those with an ALBI score ≤ -1.48 (73.7% vs 87.6%, P < 0.05), and there were no statistically significant differences in survival rates between patients with a model for end stage liver disease (MELD) score ≥ 10 and < 10 and different Child-Pugh grades. Moreover, a high ALBI score was associated with an increased incidence of biliary complications, intraabdominal bleeding, septicaemia, and acute kidney injury after liver transplantation (P < 0.05 for all). Of course, this study only initially confirmed the predictive value of the ALBI score for liver transplantation outcomes. The predictive value of multi-centre data resources and other donations, except after DCD, need to be further researched and confirmed.
After the ALBI grading system was developed to identify patients at risk for adverse outcomes after hepatectomy, this study hypothesized that this score may also be valuable in evaluating outcomes after liver transplantation. The ALBI score predicted overall survival and postoperative complications after liver transplantation. These data suggest that ALBI may be superior to MELD in risk-stratifying liver transplantation patients. In addition, ALBI may be a more readily applicable tool for modelling risk among patients undergoing liver transplantation because it relies on fewer variables.
The ALBI grading system may be useful in risk-stratifying patients on the liver transplant waiting list. Multi-centre and prospective studies are needed to confirm our findings.
The authors thank all members of the Liver Transplantation Unit for their contributions to this valuable resource.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hilmi I, Hori T S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Xu SL, Zhang YC, Wang GY, Yang Q, Liu B, Zhang J, Li H, Wang GS, Yang Y, Chen GH. Survival analysis of sirolimus-based immunosuppression in liver transplantation in patients with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2016;40:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Klein KB, Stafinski TD, Menon D. Predicting survival after liver transplantation based on pre-transplant MELD score: a systematic review of the literature. PLoS One. 2013;8:e80661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Han S, Kwon JH, Jung SH, Seo JY, Jo YJ, Jang JS, Yeon SM, Jung SH, Ko JS, Gwak MS, Cho D, Son HJ, Kim GS. Perioperative Fresh Red Blood Cell Transfusion May Negatively Affect Recipient Survival After Liver Transplantation. Ann Surg. 2018;267:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Otto G. Liver transplantation: an appraisal of the present situation. Dig Dis. 2013;31:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Dolgin NH. Health Care Rich, Resource Poor: Struggling with the National Shortage of Organs in Liver Transplantation. AMA J Ethics. 2016;18:97-100. [PubMed] |
| 6. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2009] [Article Influence: 200.9] [Reference Citation Analysis (0)] |
| 7. | Andreatos N, Amini N, Gani F, Margonis GA, Sasaki K, Thompson VM, Bentrem DJ, Hall BL, Pitt HA, Wilson A, Pawlik TM. Albumin-Bilirubin Score: Predicting Short-Term Outcomes Including Bile Leak and Post-hepatectomy Liver Failure Following Hepatic Resection. J Gastrointest Surg. 2017;21:238-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J, Peng Y, Li J, Deng H, Guo X. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Turk J Gastroenterol. 2016;27:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8608] [Article Influence: 538.0] [Reference Citation Analysis (0)] |
| 10. | Chan AW, Chong CC, Mo FK, Wong J, Yeo W, Johnson PJ, Yu S, Lai PB, Chan AT, To KF, Chan SL. Applicability of albumin-bilirubin-based Japan integrated staging score in hepatitis B-associated hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1766-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Toyoda H, Lai PB, O'Beirne J, Chong CC, Berhane S, Reeves H, Manas D, Fox RP, Yeo W, Mo F, Chan AW, Tada T, Iñarrairaegui M, Vogel A, Schweitzer N, Chan SL, Sangro B, Kumada T, Johnson PJ. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer. 2016;114:744-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | Rauchfuss F, Zidan A, Scheuerlein H, Dittmar Y, Bauschke A, Settmacher U. Waiting time, not donor-risk-index, is a major determinant for beneficial outcome after liver transplantation in high-MELD patients. Ann Transplant. 2013;18:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Strassburg CP. [Patient selection and indications for liver transplantation]. Chirurg. 2013;84:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1055] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 15. | Huang HC, Lee FY, Huo TI. Major adverse events, pretransplant assessment and outcome prediction. J Gastroenterol Hepatol. 2009;24:1716-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Marroni CP, de Mello Brandão AB, Hennigen AW, Marroni C, Zanotelli ML, Cantisani G, Fuchs SC; Liver Transplantation Group. MELD scores with incorporation of serum sodium and death prediction in cirrhotic patients on the waiting list for liver transplantation: a single center experience in southern Brazil. Clin Transplant. 2012;26:E395-E401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Bernardi M, Gitto S, Biselli M. The MELD score in patients awaiting liver transplant: strengths and weaknesses. J Hepatol. 2011;54:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Ge PL, Du SD, Mao YL. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat Dis Int. 2014;13:361-370. [PubMed] |
| 20. | Rahimi-Dehkordi N, Nourijelyani K, Nasiri-Tousi M, Ghodssi-Ghassemabadi R, Azmoudeh-Ardalan F, Nedjat S. Model for End stage Liver Disease (MELD) and Child-Turcotte-Pugh (CTP) scores: Ability to predict mortality and removal from liver transplantation waiting list due to poor medical conditions. Arch Iran Med. 2014;17:118-121. [PubMed] |
| 21. | Fahrner R, Dondorf F, Ardelt M, Dittmar Y, Settmacher U, Rauchfuß F. Liver transplantation for hepatocellular carcinoma - factors influencing outcome and disease-free survival. World J Gastroenterol. 2015;21:12071-12082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Neuberger J. An update on liver transplantation: A critical review. J Autoimmun. 2016;66:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |