Published online Mar 21, 2019. doi: 10.3748/wjg.v25.i11.1409
Peer-review started: December 14, 2018
First decision: December 28, 2018
Revised: January 8, 2018
Accepted: January 14, 2019
Article in press: January 14, 2019
Published online: March 21, 2019
Processing time: 96 Days and 12.2 Hours
Life-long removal of gluten from the diet is currently the only way to manage celiac disease (CeD). Until now, no objective test has proven useful to objectively detect ingested gluten in clinical practice. Recently, tests that determine consumption of gluten by assessing excretion of gluten immunogenic peptides (GIP) in stool and urine have been developed. Their utility, in comparison with conventional dietary and analytical follow-up strategies, has not been fully established.
To assess the performance of enzyme-linked immunosorbent assay (ELISA) and point-of-care tests (PoCTs) for GIP excretion in CeD patients on gluten-free diet (GFD).
We conducted an observational, prospective, cross-sectional study in patients following a GFD for at least two years. Using the Gastrointestinal Symptom Rating Scale questionnaire, patients were classified at enrollment as asymptomatic or symptomatic. Gluten consumption was assessed twice by 3-d dietary recall and GIP excretion (by ELISA in stool and PoCTs (commercial kits for stool and urine) in two consecutive samples. These samples and dietary reports were obtained 10 day apart one from the other. Patients were encouraged to follow their usual GFD during the study period.
Forty-four patients were enrolled, of which 19 (43.2%) were symptomatic despite being on a GFD. Overall, 83 sets of stool and/or urine samples were collected. Eleven out of 44 patients (25.0%) had at least one positive GIP test. The occurrence of at least one positive test was 32% in asymptomatic patients compared with 15.8% in symptomatic patients. GIP was concordant with dietary reports in 65.9% of cases (Cohen´s kappa: 0.317). PoCT detected dietary indiscretions. Both ELISA and PoCT in stool were concordant (concomitantly positive or negative) in 67 out of 74 (90.5%) samples. Excretion of GIP was detected in 7 (8.4%) stool and/or urine samples from patients considered to be strictly compliant with the GFD by dietary reports.
GIP detects dietary transgressions in patients on long-term GFD, irrespective of the presence of symptoms. PoCT for GIP detection constitutes a simple home-based method for self-assessment of dietary indiscretions.
Core tip: Excreted gluten immunogenic peptides (GIPs) in stool and urine are specific indicators of gluten consumption in patients with celiac disease.GIP tests detect dietary indiscretions in treated celiac patients, irrespective of the presence of symptoms. GIPs were detected in stool and/or urine samples of patients considered to be strictly compliant with the gluten-free diet according to dietary reports. Point-of-care tests for GIP detection constitute simple home-based methods for self-assessment of dietary indiscretions.
- Citation: Costa AF, Sugai E, Temprano MLP, Niveloni SI, Vázquez H, Moreno ML, Domínguez-Flores MR, Muñoz-Suano A, Smecuol E, Stefanolo JP, González AF, Cebolla-Ramirez A, Mauriño E, Verdú EF, Bai JC. Gluten immunogenic peptide excretion detects dietary transgressions in treated celiac disease patients. World J Gastroenterol 2019; 25(11): 1409-1420
- URL: https://www.wjgnet.com/1007-9327/full/v25/i11/1409.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i11.1409
Life-long removal of gluten from the diet is currently the only way to manage celiac disease (CeD)[1]. In most patients, strict gluten avoidance results in symptomatic, serologic and histological remission. Adherence to the gluten-free diet (GFD) is associated with a reduction and/or normalization of the risk for associated disorders or complications[2]. While the majority of treated patients who are compliant with the GFD are asymptomatic, up to 40% of treated patients remain symptomatic or experience symptom relapse[3]. In this context, the persistence of gluten consumption, or accidental antigen exposure, is considered the main underlying factor[4-6]. Asymptomatic patients on a GFD who have normal serology are not always assessed for adherence to the diet. Furthermore, most guidelines do not reinforce the necessity for monitoring GFD compliance. Until now, there was no objective test to reveal ingested gluten in clinical practice, and evaluations have relied on the presence of symptoms, dietary questionnaires by dietitians and/or serology[7-9].
Quantitative enzyme-linked immunosorbent assays (ELISA) (stool) and quantitative immunocromatography (urine) tests that determine consumption of gluten by assessing the excretion of gluten immunogenic peptides (GIP) have recently been developed[10,11]. These tests are based on the detection of GIP in stool and urine by monoclonal antibodies (anti-33merα-gliadin peptide G12). Previous studies have shown that a positive result constitutes specific evidence of dietary indiscretions. In addition to these, point-of-care tests (PoCT) (both for stool and urine) have been recently developed to simplify their use by patients at home. It is not currently known how these newly developed tests perform compared with laboratory tests for GIP, such as ELISA, traditional dietary assessment and serology. It is also unknown whether PoCT can help in the identification of transgressions in asymptomatic patients on GFD, in whom gluten contamination is not suspected[3].
Thus, our aims were: (1) to assess the performance of ELISA and PoCT for detecting GIP excretion in stool and urine in patients on GFD for more than two years; and (2) to explore the potential association of dietary transgressions with symptoms in CeD patients on long-term GFD.
CD patients (> 18 years old) attending the Celiac Disease Clinic of the “C. Bonorino Udaondo” Gastroenterology Hospital were offered to participate in the study. Inclusion criteria were: (1) a well-established histological and serological diagnosis of CeD; (2) self-reported adherence to the GFD for more than two years; and (3) ability to complete dietary reports, and collect and transport samples to our institution per the protocol. The diagnosis of CeD was based on positive specific serology and concomitant duodenal biopsy showing villous atrophy (Marsh’s 3 damage)[1-4]. Patients not willing to participate, unable to complete dietary diary recall, having concomitant disorders (e.g., type I diabetes, hypothyroidism, etc), type II refractory CeD, or unable to collect and deliver the required samples, were excluded. Serum samples were obtained at the time of enrollment for the determination of CeD-specific antibodies, although levels of serologic tests did not limit patient enrollment.
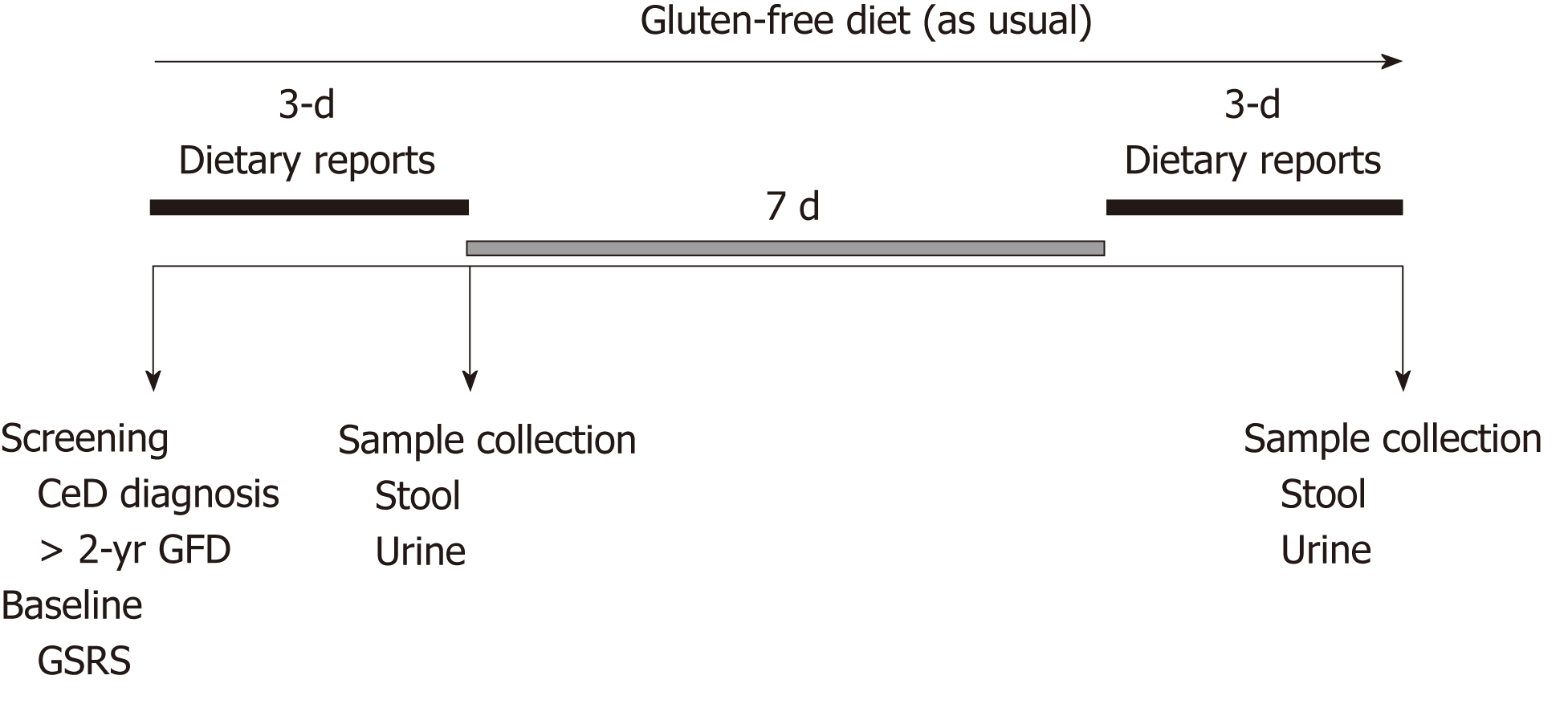
Th study followed an observational, cross-sectional design, and prospectively assessed adult CeD patients on a GFD. Patients fulfilling the inclusion and exclusion criteria were invited to enroll in the study after signing a written informed consent. At baseline, patients were assessed for the presence of GI symptoms by the Gastrointestinal Symptom Rating Scale (GSRS) questionnaire[12]. A patient was classified as symptomatic if reporting ≥3 points for individual syndromes or, ≥2 points in the average score of the five syndromes[13]. Patients were encouraged to follow their usual GFD during the study period (2 wk) (Figure 1).
The first step of the study consisted of a self-written 3-d recall diary on food consumption, as previously described[14]. The following morning, patients delivered a random sample of stool and urine (first morning urine) to the specialized laboratory within 4 h after collection. After a 1-wk clearance period during which patients remained on a GFD to prevent confounding results from potential contaminations prior to the study[5-7], patients completed a second recall diary. Stool and urine samples were collected the consecutive morning, as explained previously[10,11] (Figure 1). The second collection of samples was performed to investigate whether dietary transgressions were isolated or frequent events.
Details on detection limits, lapse of time from consumption to detection, time for clearance of stool and urine, and the quantity of gluten consumption required for detection, are detailed in Table 1.
After collection, blood samples were stored at -20°C until tested. Patients were instructed to collect three sets of 2-4 g of stool from the first morning deposition, and to place them immediately into sealed containers at both time points. Stool samples were transported to the lab within 4 h of collection. Stool samples were kept frozen until GIP quantitative ELISA tests were performed. The urine and stool samples were also used for PoCT detection of GIP, and both determinations were evaluated as soon as they arrived in the lab to prevent any peptide degradation. The manufacturer reported sufficient GIP stability in urine for at least 24 h at room temperature (Cebolla-Ramirez A; personal communication/unpublished).
Testing was performed according to instructions from manufacturers. Briefly, GIP excretion was quantified by a sandwich ELISA kit (iVYLISA GIP-S®, Biomedal S.L.; Sevilla, Spain) designed to detect and quantify GIPs (containing similar epitopes to those found in the immunodominant α-gliadin 33-mer peptide) in samples. Stool samples were incubated for 60 min at 50ºC with gentle agitation in 9 mL of Universal Gluten Extraction Solution (UGES; Biomedal S.L.; Seville, Spain) per gram of stool to release the GIP from the stool matrix. The extracted sample was then added to a plate coated with A1/G12 monoclonal antibodies that specifically detect the epitopes of wheat prolamin (gliadin), rye (secalin) and barley (hordein). Details on analytics and detection limit are shown in Table 1[10].
PoCT in stool and urine samples was performed by lateral flow immunoassays (GlutenDetect®; Biomedal S.L., Spain). First, 2 mL urine samples were mixed with 0.7 mL of the manufacturer solution. If gluten peptides are present, they react with the conjugated antibodies (monoclonal antibodies A1 and G12) previously fixed in the lateral flow strip, producing a red line in the result window. A control antibody-antigen reaction is generated to confirm the correct flow and conditions for antibody binding, which generates a green line to indicate correct test performance. Positive results are revealed by two lines (red and green) and negative results are indicated by a single green line. The limits of quantification in stool and urine are shown in Table 1.
CeD-related serology included: (1) IgA a-tTG (QUANTA Lite TM, h-tTG IgA, INOVA Diagnostic Inc.; San Diego, CA, United States) by ELISA; and (2) IgA deamidated gliadin peptide (IgA DGP) antibodies, both by ELISA. Cut-off for both antibodies was 20 IU/mL. Characteristics of the serologic test have been reported in previous studies[14].
During the 3-d dietary recall, patients were encouraged to be explicit about foods, brands consumed, management strategies and food processing. However, patients were asked to avoid major changes to their usual GFD, and eventual modifications were not determined. A second expert dietitian, blinded to clinical and laboratory results, determined the potential gluten consumption for each dietary report. The degree of adherence was estimated as follows: (1) patients with no evidence of transgression; and (2) patients non-adherent to the diet, which included voluntary or inadvertent transgression with known sources containing gluten, and patients with intake of gluten traces (when hidden gluten or cross contamination was not controlled)[15].
The study was approved by the Institutional Ethical Committee (CEI) and the Local Research Committee (CODEI) from Dr. C. Bonorino Udaondo Gastroenterology Hospital. A written consent was obtained from all patients. Data were analyzed using MedCalc (ver. 11.2.1.0; MedCalc Software; bvba).Comparison between GIP excretion tests and degree of compliance with the GFD was estimated, without considering the existence of a gold standard test. Data are reported as mean and standard deviation or median and range, according to distribution. The proportion (%) of positive or negative tests was established. Results were analyzed using t-tests, Mann-Whitney tests or Fisher exact tests as appropriate, according to data distribution. Concordance between dietary reports and GIP excretion was determined by using the Cohen´s kappa.
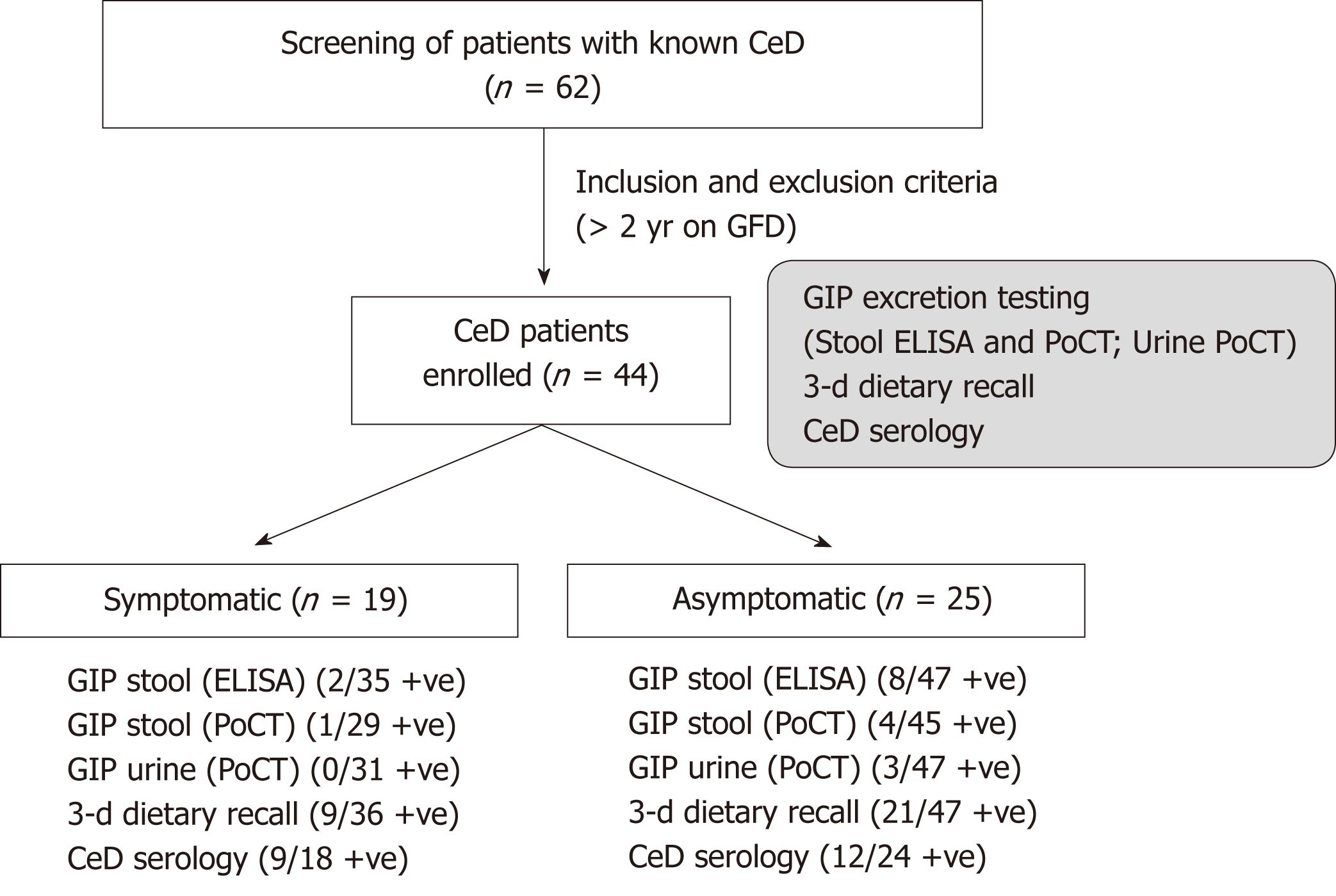
A total of 62 CeD patients were screened and 44 patients were enrolled. Based on the GSRS questionnaire, 19 (43.2%) patients were symptomatic and 25 asymptomatic (Figure 2). Median age was 50 years (range: 25-82). Median time on a GFD was 8 years (range: 2-48). There were no differences in the median time spent on a GFD, age, or median body mass index between patients identified as symptomatic or asymptomatic (pNS) (Table 2). As expected, the symptomatic subpopulation had a significantly higher global GSRS score than asymptomatic patients (P<0.00001) (Table 1). This was also true for individual syndromes (P<0.04 to P< 0.0001) (data not shown).
| Parameter | Asymptomatic | Symptomatic |
| Number of individuals (females) | 25 (21) | 19 (19) |
| Age at enrollment, median (range) | 50 (29-82) | 51 (25-59) |
| Years on a GFD, median (range) | 8 (2-48) | 9 (4-27) |
| Body mass index, median (range) | 25 (19-32) | 25 (19-38) |
| GSRS, median score (range) | 1.16 (0.2-2) | 2.44 (2-3.6)a |
| Serology | ||
| Serum IgA tTG | ||
| Median concentration (range) | 11 (2-100) | 8 (3-200) |
| Number of patients with normal tests (%) | 19/24 (79.2) | 14/18 (77.8) |
| Serum IgA DGP | ||
| Median concentration (range) | 19 (2-78) | 12 (200-5) |
| Number of patients with normal tests (%) | 12/22 (54.5) | 10/18 (55.6) |
| Excretion of GIP (stool and urine) | ||
| N of positive samples (%) | 10/47 (21.3) | 3/36 (8.3) |
| 3-d dietary recall (completed) | (47) | (36) |
| Strict adherence | ||
| Number of patients (%) | 26 (55.3) | 27 (75.0) |
| No adherents | ||
| Number of patients (%) | 21 (44.7) | 9 (25.0) |
Serology was performed on the 42 patients at enrollment (42 for IgA tTG and 40 for IgA DGP). Overall, 21/42 (50.0%) patients had antibody concentrations above the upper limit of normality (ULN) for at least one test. Median serum concentrations (range) for IgA tTG were borderline for the UNL [17.9 AU/mL (2-78) and 21.4(2->100) for IgA DGP]. Nine out of 42 (21.4%) and 18/40 (45.0%) had positive serum concentrations of IgA tTG and IgA DGP, respectively. However, only four had serum antibody concentrations 3X above ULN.In three of them, serum concentrations were concomitantly abnormal for both antibodies. The remaining patients with abnormal serologic values had concentrations below <3X the ULN arbitrary threshold (Tables 3 and 4).
| Patient No. | Serology | First set of determinations | Second set of determinations | |||||||
| IgAtTG AU/mL | IgADGPAU/mL | Dietary report | GIP in stool ELISA μg/g | GIP in stool PoCT | GIP in urine PoCT | Dietary report | GIP in stool ELISA μg/g | GIP in stool PoCT | GIP in urine PoCT | |
| 1 | 12 | 10 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 2 | 11 | 36 | NA | 0.19 | ND | -ve | NA | < 0.16 | ND | -ve |
| 3 | 14 | 13 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 4 | 5 | 7 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 5 | 17 | 22 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | +ve | -ve |
| 6 | 32 | 58 | NA | < 0.16 | -ve | -ve | NA | ND | ND | ND |
| 7 | 16 | 41 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 8 | 6 | 11 | NA | < 0.16 | -ve | +ve | NA | < 0.16 | -ve | -ve |
| 9 | 33 | 18 | NA | < 0.16 | -ve | -ve | NA | < 0.16 | -ve | -ve |
| 10 | 2 | 2 | NA | 0.38 | +ve | -ve | NA | 0.75 | +ve | -ve |
| 11 | 40 | 23 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 12 | 12 | 6 | NA | < 0.16 | -ve | -ve | NA | < 0.16 | -ve | -ve |
| 13 | 8 | 20 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 14 | 8 | 25 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 15 | 9 | ND | NA | < 0.16 | -ve | -ve | NA | < 0.16 | -ve | -ve |
| 16 | 10 | 25 | NA | < 0.16 | -ve | -ve | NA | 0.66 | +ve | +ve |
| 17 | 15 | ND | Strict | 0.26 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 18 | 32 | 16 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 19 | ND | ND | NA | < 0.16 | -ve | -ve | NA | < 0.16 | -ve | -ve |
| 20 | 11 | 21 | Traces | < 0.16 | -ve | -ve | NA | ND | ND | ND |
| 21 | 6 | 13 | Traces | < 0.16 | -ve | -ve | NA | < 0.16 | -ve | -ve |
| 22 | 100 | 78 | ND | 0.66 | -ve | +ve | ND | ND | ND | ND |
| 23 | 8 | 8 | Strict | 0.26 | -ve | -ve | Strict | 0.75 | -ve | -ve |
| 24 | 17 | 22 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 25 | 6 | 6 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| Patient No. | Serology | First set of determinations | Second set of determinations | |||||||
| IgAtTG AU/mL | IgADGPAU/mL | Dietary report | GIP in stool ELISA μg/g | GIP in stool PoCT | GIP in urine PoCT | Dietary report | GIP in stool ELISA μg/g | GIP in stool PoCT | GIP in urine PoCT | |
| 1 | 3 | 5 | Strict | < 0.16 | ND | ND | NA | < 0.16 | -ve | ND |
| 2 | 15 | 9 | NA | < 0.16 | ND | ND | Strict | < 0.16 | ND | ND |
| 3 | 30 | 75 | Strict | 0.38 | ND | ND | Strict | < 0.16 | ND | -ve |
| 4 | 77 | > 200 | NA | < 0.16 | -ve | -ve | NA | < 0.16 | -ve | -ve |
| 5 | 8 | 1 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 6 | 4 | 6 | Strict | < 0.16 | -ve | -ve | NA | < 0.16 | -ve | -ve |
| 7 | 3 | 13 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 8 | 27 | 11 | Strict | ND | ND | -ve | Strict | < 0.16 | -ve | -ve |
| 9 | 3 | 39 | Strict | < 0.16 | ND | -ve | Strict | < 0.16 | -ve | -ve |
| 10 | 5 | 4 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 11 | > 200 | 67 | Strict | < 0.16 | -ve | -ve | Strict | 0.42 | -ve | -ve |
| 12 | 8 | 10 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 13 | 3 | 35 | NA | < 0.16 | -ve | -ve | Strict | < 0.16 | +ve | -ve |
| 14 | ND | ND | NA | < 0.16 | -ve | -ve | NA | < 0.16 | -ve | -ve |
| 15 | 12 | 12 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 16 | 14 | 25 | Strict | < 0.16 | -ve | -ve | ND | ND | ND | ND |
| 17 | 8 | 9 | Strict | < 0.16 | -ve | -ve | ND | ND | ND | ND |
| 18 | 17 | 36 | Strict | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
| 19 | 8 | 24 | NA | < 0.16 | -ve | -ve | Strict | < 0.16 | -ve | -ve |
Forty-four patients returned 83 sets of dietary reports and/or stool and urine samples. Five other patients did not return complete sets and thus were excluded from analysis (Tables 3 and 4). We determined GIP excretion by ELISA in 82 stool samples, and by PoCT in 74 stool samples and in 78 urine samples. Only 73/83 complete sets of samples were returned. Considering both sets of determinations, 11/ 44 patients (25.0%) had at least one positive GIP test, of which 32% were asymptomatic and 15.8% were symptomatic. Ten samples were positive for ELISA in stool, five were positive for PoCT in stool (two of which were the only positive tests) and three were positive for PoCT in urine (one of which was the only positive test). Three samples were positive for both ELISA and PoCT in stool, and one sample was concomitantly positive in the three tests. Stool tests (both ELISA and PoCT) were concordant (concomitantly positive or negative) in 67/74 (90.5%) samples (Table 2). Only two patients who were asymptomatic with negative serology had positive GIP in both sets of samples.
Blinded estimation of the degree of adherence with the GFD using 3-d recall diaries estimated that 53/83 reports (63.9%) showed no evidence of transgressions (strict GFD), while 30/83 (36.1%) reports indicated non-adherence by consumption of foods with potential gluten sources or traces (Tables 3 and 4).
Overall, there was concordance between the estimation of dietary adherence and determinations of GIP excretion in 54/82 (65.9%) samples, while 28/82 (34.1%) cases were discordant (Cohen´s kappa: 0.317). Positive GIP excretion was present in 7/82 (8.4%) cases that were estimated to be strictly adherent by dietary assessment, and in 5/28 (17.9%) that were considered non-adherent by sources or traces. Dietary assessment estimated the consumption of gluten in only 50% of samples that were also positive for GIP.
IgA tTG and IgA DGP were positive in 3/12 and6/12 cases excreting GIP, respectively. Interestingly, three of those cases with high antibody concentrations (above 3X UNL) had positive excretion of GIP in one or more determinations (Tables 3 and 4).
Presence of GIP in stool and/or urine was detected in at least one of two determinations in 9/25(36.0%) asymptomatic patients (Table 3). Although GIP excretion was shown in only 3/19 (15.8%) symptomatic patients (Table 4), the difference did not reach statistical significance (P=0.22). Similarly, no differences were found in subgroup analysis comparing estimation of the degree of adherence with the diet, IgA tTG and IgA DGP antibody mean concentrations, or proportion of patients with normal serology (Table 2). The low number of patients with values above 3X ULN produced no statistically significant results. The median serum concentration for IgA DGP antibodies was very close to the ULN (Table 2).
Strict adherence to the GFD improves or normalizes growth, ameliorates most symptoms, reduces the risk of intestinal and extra-intestinal complications, normalizes immunological reactivity, and heals enteropathy in both pediatric and adult populations[1-4]. Monitoring compliance with the GFD is a key aspect of patient follow-up. Unfortunately, strict adherence is limited by the lack of availability of gluten-free foods, cost, social isolation and frequent cross-contamination[16]. Therefore, caregivers and patients are often confronted with questions regarding the best method for detecting dietary indiscretions. This is the population that requires strict follow-up of the frequency at which these measurements should be performed.
Our first objective compared GIP excretion in urine and feces using laboratory tests, which detect A1 and G12 epitopes similar to those of the gliadin 33-mer peptide[10,11] with the newly developed PoCT. These tests were designed to simplify GIP excretion measurement in stool and/or urine. In 25% of patients who considered themselves compliant with the GFD, we detected GIP excretion in at least one of two different determinations, performed10 d apart. Although there were no statistical differences in terms of the presence or absence of symptoms, the majority of these patients were asymptomatic. A former study showed that the diagnostic sensitivity of the fecal ELISA test was 98.5% and its specificity 100%, with positive and negative predictive values of 100% and 91.7%, respectively[11,17,18]. Furthermore, the specificity for the new PoCT tests have also been previously explored, and were determined to be specific for detecting most of the immunoactivity of gluten peptides in an in vitro research context[19] (Cebolla-Ramirez A, unpublished data). Here we show that these tests can detect gluten contaminations that do not cause symptoms.
In 90.5% of the samples, PoCT and ELISA from stool were concordantly positive or negative. There was lack of concordance in seven pairs of stool samples in which at least one test was positive (ELISA was the only positive test in five stool samples, while PoCT was the only positive fecal test in the remaining two samples). Lack of concordance between stool tests could be related to: intrinsic test factors associated with different extraction quality, the non-homogeneous distribution of GIP in stool, or different sensitivities of the methods in relation to the quantity of gluten ingested, especially in patients subjected to strict protocol procedures. Discordance between tests was more prominent in urine samples compared with detections in stool. GIP in urine was detected in three sets of samples, two of which were in concordance with excretion in stool. The time of gluten intake prior to sample collection (different times to be cleared for detection compared with stool tests) (see Table 1), the degree of urine dilution (related to levels of water consumption), the amount of gluten ingested, and the potential role of deamidation (which decreases antibody reactivity) are all possible reasons for these results. Another explanation is the fact that gluten intake could be below 500 mg/d, since volunteers improve their diets when they are monitored. Interestingly, the urine test has a window of detection ranging from 2-16 or 48 h after gluten intake. Recent unpublished results showed that the performance of PoCT in urine from healthy people consuming regular gluten and CeD patients with strictly controlled GFD had sensitivity, specificity, PPV and NPV of 91%, 99%, 99% and 95%, respectively (Cebolla-Ramirez A, personal communication). Overall, the results suggest that the use of more than one test may enhance the global assessment during patient follow-up.
We also studied the performance of these tests according to symptom presentation. Recent articles suggested that the most common reason for non-responsive CeD is persistent gluten exposure[15,20]. Indeed, a strategy employed in clinical practice with symptomatic patients is the recommendation of a “natural” GFD that, in the majority of cases, resolves symptoms[21]. Although no gold standard is available, the estimation of dietary adherence by dietitians is, in general, quick, simple and mostly recommended[1,3]. However, this method has the setback of being subjective and training-dependent. In this study, food dietary reports were recorded by trained patients and blindly analyzed by an expert nutritionist. We detected a 65.9% concordance between dietary reports and objective evidence of GIP in stool and/or urine samples (Cohen kappa: 0317). Notably, 13.2% of dietary reports that had estimated “strict” GFD adherence showed evidence of gluten consumption, as assessed by GIP excretion in stool and/or urine.
GIP excretion was more prevalent in asymptomatic versus symptomatic patients, although this difference was not statistically significant, which was likely due to the low number of cases enrolled. Interestingly, dietary reports and specific serology were also similar between both subgroups (Tables 2-4). The present study thus confirms the limitations of dietary assessment, since this method failed to detect gluten consumption in 7/13 samples that were positive for GIP excretion. In contrast, dietary reports suggested transgressions in 15 samples in which GIP tests were negative. This could be explained by possible intentional omission in dietary reports by patients. Another possibility is the fact that some foods may content traces of gluten that could not be detected by GIP excretion tools, despite being labeled as gluten-free[22,23], among other factors. Therefore, dietary indiscretions may not explain all cases of persistent symptoms in CeD patients on long-term diets. The consumption of FODMAPs could be a potential explanation for symptom persistence[24]. Additional factors, such as alterations in small bowel microbiota, as previously suggested, may explain some persistent symptomatic cases[25].
Former studies and guidelines have suggested that periodic testing for IgA anti-tTG or IgA anti-DGP is a non-invasive method for monitoring compliance during the initiation of the GFD[1-4]. The decline in serum antibody concentrations is considered a useful indicator of compliance with the diet[26]. However, while highly increased concentrations are strongly associated with continued gluten challenge, serology tests do not identify minor dietary indiscretions[2,3]. In contrast, normal titers are not sensitive enough for ongoing gluten exposure or the persistence of enteropathy[27]. Our study suggests that most patients with abnormal serum antibody values had mildly elevated concentrations. Only in four cases were levels above 3X ULN, which is considered to be the best cut-off to discriminate transgressions. In two of these, there was objective evidence of gluten intake. We confirmed that the most frequently positive test was the IgA DGP antibody, a finding that is in agreement with previous observations[26].
Limitations of this study include the relatively low number of patients, the lack of reliable gold standard testing to monitor real-time adherence with the GFD for comparing with new tests, potential discrepancies between the direct detection of the toxic agent by lab testing and real-time home tests that remain speculative, the subjective essence of dietary reports, and the fact that serology cannot be directly compared with tests that measure real-time consumption. Despite these limitations, our study highlights the difficulties emerging in clinical practice for assessing adherence to the GFD, even in patients who consider themselves compliant.
In conclusion, the study confirms former observations showing that GIP detection in stool and urine are useful adjuvant tools for monitoring adherence to the GFD in real-life conditions of treated CeD patients. It expands this knowledge by showing GIP in stool and urine can also be detected by simple, easy-to-perform PoCT tools. An interesting observation relates to the analysis of asymptomatic patients, in whom it is assumed that dietary adherence is high, and consequently will not be subjected to follow-up. The presence of GIP in the stool and/or urine of patients considered compliant with the diet highlights the limitations of only using dietary estimations. Other factors, such as changes in small intestinal microbiome structure, methods for using these tests, or ways to determine very low gluten consumption, should be explored.
Until now, there was no objective test to reveal objectively ingested gluten in clinical practice. Recently developed stool and urine laboratory tests based on monoclonal antibody technology specifically determine consumption of gluten by assessing the excretion of gluten immunogenic peptides (GIP). These tests were proposed to help in the monitoring of adherence to the gluten-free diet (GFD). More recently, point-of-care tests (PoCT) for stool and urine have been developed that may encourage patient self-monitoring and better compliance with disease management.
Despite recent research, there are at least three unsolved issues regarding the use of objective tests to detect gluten consumption. (1) The utility of GIP excretion tests, in patients with CeD who consider themselves adherent to the GFD, has not been compared with conventional monitoring methods in a real-life-scenario; (2) It is unknown whether consumption of gluten as measured by GIP excretion is different in symptomatic and asymptomatic CeD patients while on GFD; and (3) It is unclear how the new PoCT tests compare with laboratory-performed GIP tests.
We assessed (1) the performance of enzyme-linked immunosorbent assays (ELISA) and point-of-care (PoCTs) GIP excretion tests in patients with CeD on GFD; and (2) its relation to the presence of symptoms.
We conducted an observational, prospective, cross-sectional study in CeD patients on a GFD for at least two years. Patients were categorized as asymptomatic or symptomatic at enrollment, using the Gastrointestinal Symptom Rating Scale questionnaire. Gluten consumption was assessed by 3-d dietary recall and GIP excretion in stool by ELISA, and by PoCTs in stool and urine using commercial kits.
Forty-four of the sixty-two screened CeD patients were enrolled; nineteen (43.2%) were symptomatic despite being on a GFD. Overall, 83 sets of stool and/or urine samples were collected. At least one positive GIP test was detected in 11 out of the total 44 (25.0%) patients,32% of whom were asymptomatic. GIP was concordant with dietary reports in 65.9% of cases (Cohen´s kappa: 0.317). PoCT tests detected dietary indiscretions. Excretion of GIP was detected in 7(8.4%) stool and/or urine samples from patients considered to be strictly compliant with the GFD by dietary reports.
Our study shows that GIP determination in stool and urine detects dietary transgressions in patients on long-term GFD who are unaware of gluten consumption. Our data also suggest that PoCT for GIP detection in stool and urine constitutes simple home-based methods that may aid in self-assessment of dietary indiscretions, especially inadvertent contaminations.GIP excretion is evident in treated patients, irrespective of the presence of symptoms. This observation confirms that patients should be assessed even when they are asymptomatic and/or have negative serology.
The results support the use of specific GIP tests in stool and urine in conjunction with conventional strategies used to determine adherence to the GFD. One potential future research direction includes the use of these new tools to determine patterns of adherence in patients who believe to be adherent to the GFD. PoCT tests might encourage patients to be involved in self-monitoring and, thus, improve adherence to the diet.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Dutta AK, Rostami-Nejad M, Vorobjova T S- Editor:MaRY L- Editor:Filipodia E- Editor: Huang Y
| 1. | Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1272] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 2. | Kelly CP, Bai JC, Liu E, Leffler DA. Advances in diagnosis and management of celiac disease. Gastroenterology. 2015;148:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, Green PH, Hadjivassiliou M, Holdoway A, van Heel DA, Kaukinen K, Leffler DA, Leonard JN, Lundin KE, McGough N, Davidson M, Murray JA, Swift GL, Walker MM, Zingone F, Sanders DS; BSG Coeliac Disease Guidelines Development Group; British Society of Gastroenterology. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 689] [Cited by in RCA: 783] [Article Influence: 71.2] [Reference Citation Analysis (2)] |
| 4. | Bai JC, Ciacci C. World Gastroenterology Organisation Global Guidelines: Celiac Disease February 2017. J Clin Gastroenterol. 2017;51:755-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-76; quiz 677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1157] [Article Influence: 96.4] [Reference Citation Analysis (0)] |
| 7. | Moreno ML, Rodríguez-Herrera A, Sousa C, Comino I. Biomarkers to Monitor Gluten-Free Diet Compliance in Celiac Patients. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 8. | Mahadev S, Murray JA, Wu TT, Chandan VS, Torbenson MS, Kelly CP, Maki M, Green PH, Adelman D, Lebwohl B. Factors associated with villus atrophy in symptomatic coeliac disease patients on a gluten-free diet. Aliment Pharmacol Ther. 2017;45:1084-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Lebwohl B, Murray JA, Rubio-Tapia A, Green PH, Ludvigsson JF. Predictors of persistent villous atrophy in coeliac disease: a population-based study. Aliment Pharmacol Ther. 2014;39:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Comino I. Fernández-Bañares F, Esteve M, Ortigosa L, Castillejo G, Fambuena B, Ribes-Koninckx C, Sierra C, Rodríguez-Herrera A, Salazar JC, Caunedo Á, Marugán-Miguelsanz JM, Garrote JA, Vivas S, Lo Iacono O, Nuñez A, Vaquero L, Vegas AM, Crespo L, Fernández-Salazar L, Arranz E, Jiménez-García VA, Antonio Montes-Cano M, Espín B, Galera A, Valverde J, Girón FJ, Bolonio M, Millán A, Cerezo FM, Guajardo C, Alberto JR, Rosinach M, Segura V, León F, Marinich J, Muñoz-Suano A, Romero-Gómez M, Cebolla Á, Sousa C. Fecal Gluten Peptides Reveal Limitations of Serological Tests and Food Questionnaires for Monitoring Gluten-Free Diet in Celiac Disease Patients. Am J Gastroenterol. 2016;111:1456-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 11. | Moreno ML. Cebolla Á, Muñoz-Suano A, Carrillo-Carrion C, Comino I, Pizarro Á, León F, Rodríguez-Herrera A, Sousa C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut. 2017;66:250-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 12. | Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 1035] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 13. | Nachman F, Mauriño E, Vázquez H, Sfoggia C, Gonzalez A, Gonzalez V, Plancer del Campo M, Smecuol E, Niveloni S, Sugai E, Mazure R, Cabanne A, Bai JC. Quality of life in celiac disease patients: prospective analysis on the importance of clinical severity at diagnosis and the impact of treatment. Dig Liver Dis. 2009;41:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Sugai E, Moreno ML, Hwang HJ, Cabanne A, Crivelli A, Nachman F, Vázquez H, Niveloni S, Argonz J, Mazure R, La Motta G, Caniggia ME, Smecuol E, Chopita N, Gómez JC, Mauriño E, Bai JC. Celiac disease serology in patients with different pretest probabilities: is biopsy avoidable? World J Gastroenterol. 2010;16:3144-3152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Faulkner-Hogg KB, Selby WS, Loblay RH. Dietary analysis in symptomatic patients with coeliac disease on a gluten-free diet: the role of trace amounts of gluten and non-gluten food intolerances. Scand J Gastroenterol. 1999;34:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Sugai E, Vázquez H, Nachman F, Moreno ML, Mazure R, Smecuol E, Niveloni S, Cabanne A, Kogan Z, Gómez JC, Mauriño E, Bai JC. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol. 2006;4:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Leffler D, Kupfer SS, Lebwohl B, Bugin K, Griebel D, Lathrop JT, Lee JJ, Mulberg AE, Papadopoulos E, Tomaino J, Crowe SE. Development of Celiac Disease Therapeutics: Report of the Third Gastroenterology Regulatory Endpoints and Advancement of Therapeutics Workshop. Gastroenterology. 2016;151:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Morón B, Cebolla A, Manyani H, Alvarez-Maqueda M, Megías M, Thomas Mdel C, López MC, Sousa C. Sensitive detection of cereal fractions that are toxic to celiac disease patients by using monoclonal antibodies to a main immunogenic wheat peptide. Am J Clin Nutr. 2008;87:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Moreno Mde L, Cureton PA, Martin ML, Puppa EL, Fasano A. Muñoz-Suano A, López-Casado MÁ, Torres MI, Sousa C, Cebolla Á. Selective capture of most celiac immunogenic peptides from hydrolyzed gluten proteins. Food Chem. 2016;205:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Hollon JR, Cureton PA, Martin ML, Puppa EL, Fasano A. Trace gluten contamination may play a role in mucosal and clinical recovery in a subgroup of diet-adherent non-responsive celiac disease patients. BMC Gastroenterol. 2013;13:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Leonard MM, Cureton P, Fasano A. Indications and Use of the Gluten Contamination Elimination Diet for Patients with Non-Responsive Celiac Disease. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Lee HJ, Anderson Z, Ryu D. Gluten contamination in foods labeled as "gluten free" in the United States. J Food Prot. 2014;77:1830-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Syage JA, Kelly CP, Dickason MA, Ramirez AC, Leon F, Dominguez R, Sealey-Voyksner JA. Determination of gluten consumption in celiac disease patients on a gluten-free diet. Am J Clin Nutr. 2018;107:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Gibson PR, Muir JG, Newnham ED. Other Dietary Confounders: FODMAPS et al. Dig Dis. 2015;33:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Wacklin P, Kaukinen K, Tuovinen E, Collin P, Lindfors K, Partanen J, Mäki M, Mättö J. The duodenal microbiota composition of adult celiac disease patients is associated with the clinical manifestation of the disease. Inflamm Bowel Dis. 2013;19:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Nachman F, Sugai E, Vázquez H, González A, Andrenacci P, Niveloni S, Mazure R, Smecuol E, Moreno ML, Hwang HJ, Sánchez MI, Mauriño E, Bai JC. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur J Gastroenterol Hepatol. 2011;23:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Silvester JA, Kurada S, Szwajcer A, Kelly CP, Leffler DA, Duerksen DR. Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients With Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: a Meta-analysis. Gastroenterology. 2017;153:689-701.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |