Published online Mar 21, 2019. doi: 10.3748/wjg.v25.i11.1398
Peer-review started: December 17, 2018
First decision: January 6, 2019
Revised: January 22, 2019
Accepted: January 26, 2019
Article in press: January 26, 2019
Published online: March 21, 2019
Processing time: 95 Days and 17.6 Hours
Liver cirrhosis is a major risk factor for hepatocellular carcinoma (HCC) development in chronic hepatitis B (CHB). Serum Mac-2 binding protein glycosylation isomer (M2BPGi) is a novel serological marker for fibrosis. The role of M2BPGi in prediction of HCC is unknown.
To examine the role of serum M2BPGi in predicting HCC development in hepatitis B e antigen (HBeAg)-negative patients.
Treatment-naive CHB patients with documented spontaneous HBeAg seroconversion were recruited. Serum M2BPGi was measured at baseline (within 3 years from HBeAg seroconversion), at 5 years and 10 years after HBeAg seroconversion and expressed as cut-off index (COI). Multivariate cox regression was performed to identify predictors for HCC development. ROC analysis was used to determine the cut-off value of M2BPGi.
Among 207 patients (57% male, median age at HBeAg seroconversion 40 years old) with median follow-up of 13.1 (11.8-15.5) years, the cumulative incidence of HCC at 15 years was 7%. Median M2BPGi levels were significantly higher in patients with HCC compared to those without HCC (baseline: 1.39 COI vs 0.38 COI, P < 0.001; 5-year: 1.45 COI vs 0.47 COI, P < 0.001; 10-year: 1.20 COI vs 0.55 COI, P = 0.001). Multivariate analysis revealed age at HBeAg seroconversion [odds ratio (OR) = 1.196, 95% confidence interval (CI): 1.034-1.382, P = 0.016] and baseline M2BPGi (OR = 4.666, 95%CI: 1.296-16.802, P = 0.018) were significant factors predictive of HCC. Using a cut-off value of 0.68 COI, baseline M2BPGi yielded AUROC of 0.883 with 91.7% sensitivity and 80.8% specificity.
High serum M2BPGi within 3 years after HBeAg seroconversion was a strong predictor for subsequent HCC development in treatment-naive HBeAg-negative CHB patients.
Core tip: Serum Mac-2 binding protein glycosylation isomer (M2BPGi) is a novel marker to assess severity of liver disease. The aim of this study was to assess its role in prediction of incident hepatocellular carcinoma (HCC) in patients with chronic hepatitis B who were prospectively followed-up for a median duration of 13.1 years. High serum M2BPGi increased HCC risk by 4-5 folds. If M2BPGi is below the threshold (0.68 cut-off index), there is > 99% chance that the patient will not develop liver cancer in the subsequent 15 years.
- Citation: Mak LY, To WP, Wong DKH, Fung J, Liu F, Seto WK, Lai CL, Yuen MF. Serum Mac-2 binding protein glycosylation isomer level predicts hepatocellular carcinoma development in E-negative chronic hepatitis B patients. World J Gastroenterol 2019; 25(11): 1398-1408
- URL: https://www.wjgnet.com/1007-9327/full/v25/i11/1398.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i11.1398
As of year 2015, chronic hepatitis B virus infection (CHB) affects 257 million persons worldwide[1]. It accounts for 50%-80% of all cases of hepatocellular carcinoma (HCC) globally[2,3], which is the 5th most common cancer worldwide and still demonstrates rising incidence[1,4]. Up to 15%-40% of hepatitis B virus (HBV) carriers will develop cirrhosis and/or HCC in their lifetime[5,6]. Identifying CHB patients with high risk for HCC is essential for implementation of preventive measures, including initiation of antiviral therapy and regular surveillance for HCC.
Known risk factors for HCC in CHB patients include both host factors [e.g., older age, male gender, genetic polymorphism, family history, presence of cirrhosis, co-existing chronic liver diseases, co-infection with hepatitis C virus (HCV) or human immunodeficiency virus (HIV)] and viral factors (e.g., high HBV DNA levels, core promoter mutations)[7-12]. Risk stratification models for prediction of HCC risk in CHB patients have been developed and validated, namely the REACH-B, GAG-HCC and CU-HCC[13-15]. Common variables among these prediction scores include age, gender, HBV DNA, cirrhosis, liver biochemistries. Among these factors, diagnosis of cirrhosis is particularly prone to inter-observer variability due to the nature of qualitative assessment by imaging. Improving diagnostic accuracy of cirrhosis without histological assessment could be achieved by non-invasive means. Quantitative assessment with imaging-based (e.g., liver stiffness measurement) or serum-based tests is increasingly used in estimating the severity of liver fibrosis for risk stratification.
Serum Mac-2 binding protein glycosylation isomer (M2BPGi), also known as Wisteria floribunda agglutinin positive Mac-2 binding protein (WFA+-M2BP), is a novel glycan-based marker for assessment of liver fibrosis[16]. The sugar chain structure of M2BP changes correspondingly to progression of hepatic fibrosis. WFA, a lectin used to recognize the altered glycan parts of M2BP, is detected by a lectin-antibody sandwich immunoassay and liver fibrosis can thus be quantified. Serum M2BPGi has recently been shown to be a reliable marker for diagnosing advanced liver fibrosis and cirrhosis in various liver diseases[17-19]. The predictive value of M2BPGi for risk of HCC development has also been demonstrated in CHB patients[20-23]. However, these studies were mostly retrospective with a relatively short duration of follow-up of ≤ 5 years. In addition, the patient population was heterogeneous, including both treatment-naive and treatment-experienced patients. In the only study evaluating M2BPGi in treatment-naive patients, serum M2BPGi higher than a pre-defined cut-off [≥ 2.0 cut-off index (COI)] was associated with higher risk of HCC[22]. However, the serum M2BPGi levels in CHB patients may differ between populations with different ethnicity and disease stage and the cut-off values should be properly defined for each. Moreover, as the majority of HCC in CHB is diagnosed in Hepatitis B e antigen (HBeAg) negative patients[24], the predictive value of M2BPGi in HBeAg negative patients should be investigated in a well-defined cohort. Therefore, the aim of this longitudinal study was to examine the relationship between serum M2BPGi and the development of HCC in treatment-naive HBeAg-negative patients, and to define an optimal cut-off value for prediction of subsequent HCC development.
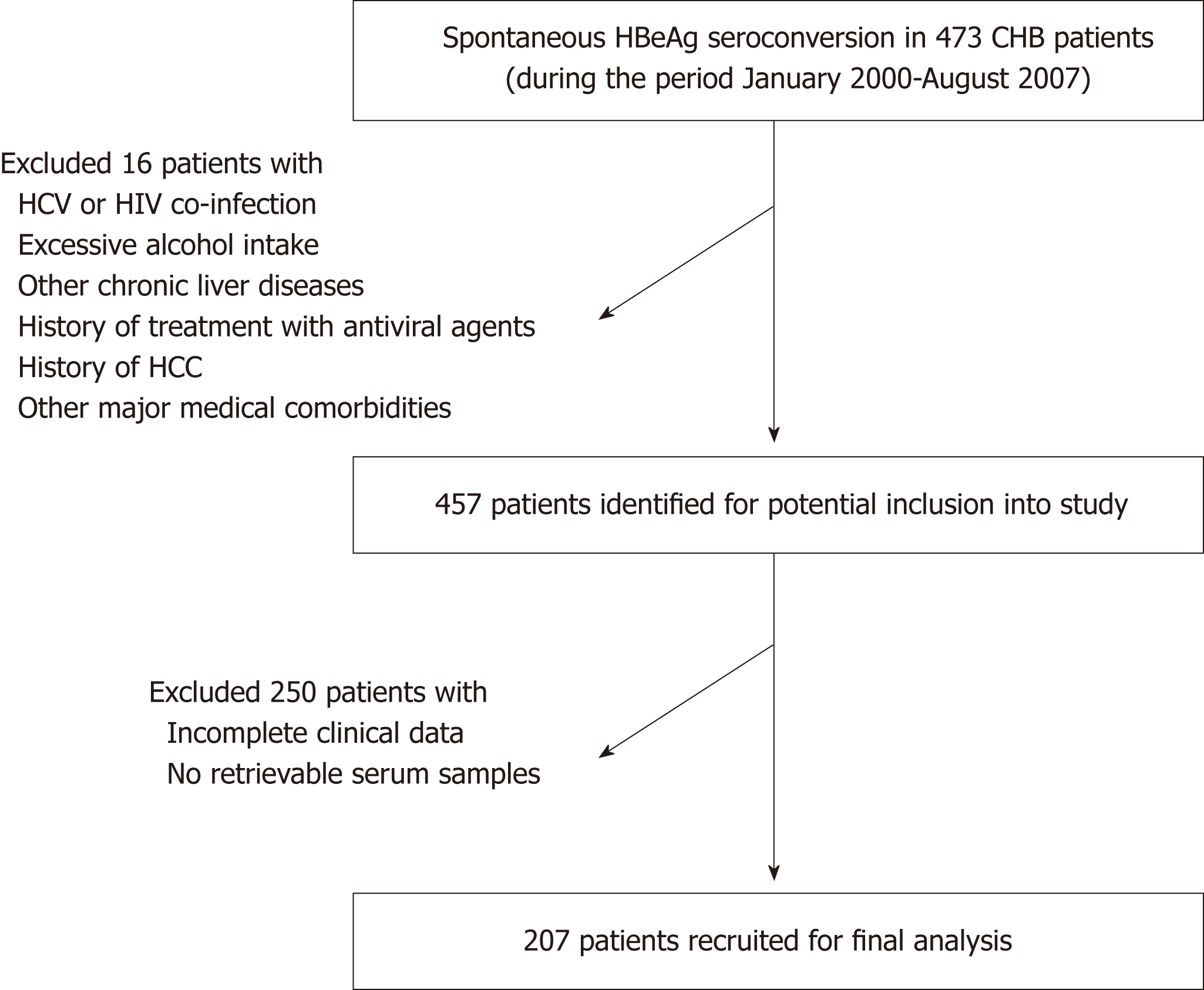
The present study recruited CHB patients who were aged ≥ 18 years old and were managed in the Liver Clinics in Queen Mary Hospital, Hong Kong. All recruited patients had persistent positivity for serum HBV surface antigen (HBsAg) ≥ 6 mo, and were positive for HBeAg on presentation with subsequent documented spontaneous HBeAg seroconversion between year 2000-2007. Patients were excluded for the following conditions: co-infection with chronic HCV, HIV, excessive alcohol intake (≥ 30 g/d for male, ≥ 20 g/d for female), other chronic liver diseases (e.g., primary biliary cholangitis, autoimmune hepatitis, Wilson’s disease), history of treatment with antiviral agents, history of HCC, or other major medical comorbidities, e.g., heart failure and pulmonary disease. Patients with incomplete clinical data or without retrievable serum samples were also excluded. A total of 207 patients were recruited for this study. All patients were treatment-naïve at recruitment. Subsequent antiviral therapy consisting of nucleos(t)ide analogues were initiated for either hepatitis flare [defined as alanine aminotransferase (ALT) > 2 times the upper limit of normal with serum HBV DNA > 2000 IU/mL] or development of liver-related complications (cirrhosis or HCC)[25]. This study population was also examined for the role of hepatitis B core-related antigen (HBcrAg) on the HCC development in a previous study[26]. The study protocol was approved by the Institutional Review Board/ Ethics Committee of the University of Hong Kong and the Hong Kong West Cluster of Hospital Authority. Figure 1 depicts the patient disposition.
All recruited patients had regular clinic visits every 6 months or more frequently if clinically indicated. For the purpose of this study, the date of HBeAg seroconversion was defined as the start of follow-up. The end of follow-up was defined as the date of diagnosis of HCC, or the date of last clinic visit for patients without HCC development. Clinical parameters including age at presentation, age at HBeAg seroconversion and gender were documented. Blood tests including liver biochemistry and alpha feto-protein (AFP) were measured at the time of HBeAg seroconversion and subsequent visits. Serum HBV DNA levels were measured at baseline by Cobas Taqman (Roche Diagnostics, Branchburg, NJ, United States) with lower limit of detection of 20 IU/mL.
Serum M2BPGi was measured using a chemiluminescent enzyme-linked immunoassay by two-step sandwich method and the details of the test were reported elsewhere[18]. Briefly, serum M2BPGi was measured by HISCL M2BPGi reagent kit (Sysmex, Hyogo, Japan) on an automatic immunoanalyzer HISCL-800 (Sysmex, Hyogo, Japan). Serum M2BPGi level was expressed as COI. Serum M2BPGi level was measured at 3 time points: at baseline (defined as within 3 years after HBeAg seroconversion), at 5 years and at 10 years after HBeAg seroconversion.
Regular 6 monthly ultrasonography of the hepatobiliary system was advised to all patients and AFP levels were measured regularly at the interval of 3-6 mo. For those with abnormal AFP levels and/or abnormal ultrasound findings, contrast-enhanced imaging, either computerized tomography or magnetic resonance imaging would be arranged. HCC was diagnosed by the typical features of arterial phase hyper-enhancement and porto-venous washout of contrast, with or without histological proof. Cirrhosis was diagnosed in the presence of small nodular liver, splenomegaly or ascites.
Liver stiffness measurement (LSM) was performed using Fibroscan (Echosens®, Paris, France). Liver stiffness (LS) was expressed as the median value of ≥ 10 successful acquisitions in units of kilopascals (kPa). LSM was only considered reliable with a success rate of ≥ 60%, combined with an interquartile range of < 30%. The operator received prior formal training from Echosens® and had performed at least 500 transient elastography procedures. As the Fibroscan machine was only available in our centre since year 2006, LSM was performed at year 5 from HBeAg seroconversion. Advanced fibrosis was defined as LS ≥ 9 kPa according to the European Association for Study of Liver, Asociación Latinoamericana para el Estudio del Hígado clinical guidelines[27].
Continuous variables were expressed as median [interquartile range (IQR), as specified]. Mann-Whitney U test was used for comparison of median between 2 groups. Categorical variables, expressed as proportions, were compared using Chi-square test and Fisher’s Exact test when appropriate. Pearson’s correlations were performed to evaluate the relationship between LS and serum M2BPGi. To determine whether factors were independently associated with subsequent HCC development, variables with a P < 0.05 in univariate analyses were entered into multivariate analysis performed by binary logistic regression, with odds ratio (OR) and 95% confidence interval (CI) calculated. To evaluate the diagnostic performance of serum M2BPGi in predicting subsequent HCC development, receiver-operating characteristic (ROC) curve analysis was carried out. Diagnostic accuracy was expressed as the specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), and area under the ROC curve (AUROC). The optimal cut-off values were obtained by maximizing the Youden’s index. Kaplan-Meier survival analysis was used to compare the incidence of HCC using the derived M2BPGi cut-off value. A two-tailed P value of < 0.05 was considered statistically significant. All statistical analysis was performed using Statistical package for Social Sciences (SPSS) version 20.0 (SPSS Inc, Chicago, IL, United States). The statistical review of the study was performed by a biomedical statistician.
The baseline characteristics of 207 enrolled patients were shown in Table 1. One-hundred and eighteen (57%) were male. The median age at HBeAg seroconversion was 40 (IQR: 34-45) years old. The median follow-up duration was 13.1 (IQR: 11.8-15.5) years. The median HBV DNA was 4.0 (IQR: 3-5.4) log IU/mL and the median ALT was 32 (IQR: 21-50) U/L. Seven patients (3.6%) had HBsAg seroclearance after a median duration of 6.4 years and none of them developed HCC. Subsequent antiviral therapy was initiated in 102 (49.3%) patients after a median duration of 5.5 years due to hepatitic flare or diagnosis of liver-related complications including cirrhosis and HCC. The details of the antiviral therapy are listed in Supplementary Table 1.
| Characteristics | Value |
| Gender (male, %) | 118 (57%) |
| Age of HBeAg seroconversion (yr) | 40 (34-45)1 |
| Follow-up duration (yr) | 13.1 (11.8-15.5)1 |
| Liver biochemistry | |
| Bilirubin (μmoL/L) | 12 (9-16)1 |
| ALP (U/L) | 69 (57-82)1 |
| AST (U/L) | 29 (23-37)1 |
| ALT (U/L) | 32 (21-50)1 |
| GGT (U/L) | 23 (16-36)1 |
| Albumin (g/dL) | 43 (41-45)1 |
| Globulin | 34 (31-36)1 |
| AFP (ng/mL) | 4 (3-7)1 |
| HBV DNA (log IU/mL) | 4 (3-5.4)1 |
| Baseline M2BPGi | 0.42 (0.27-0.68)1 |
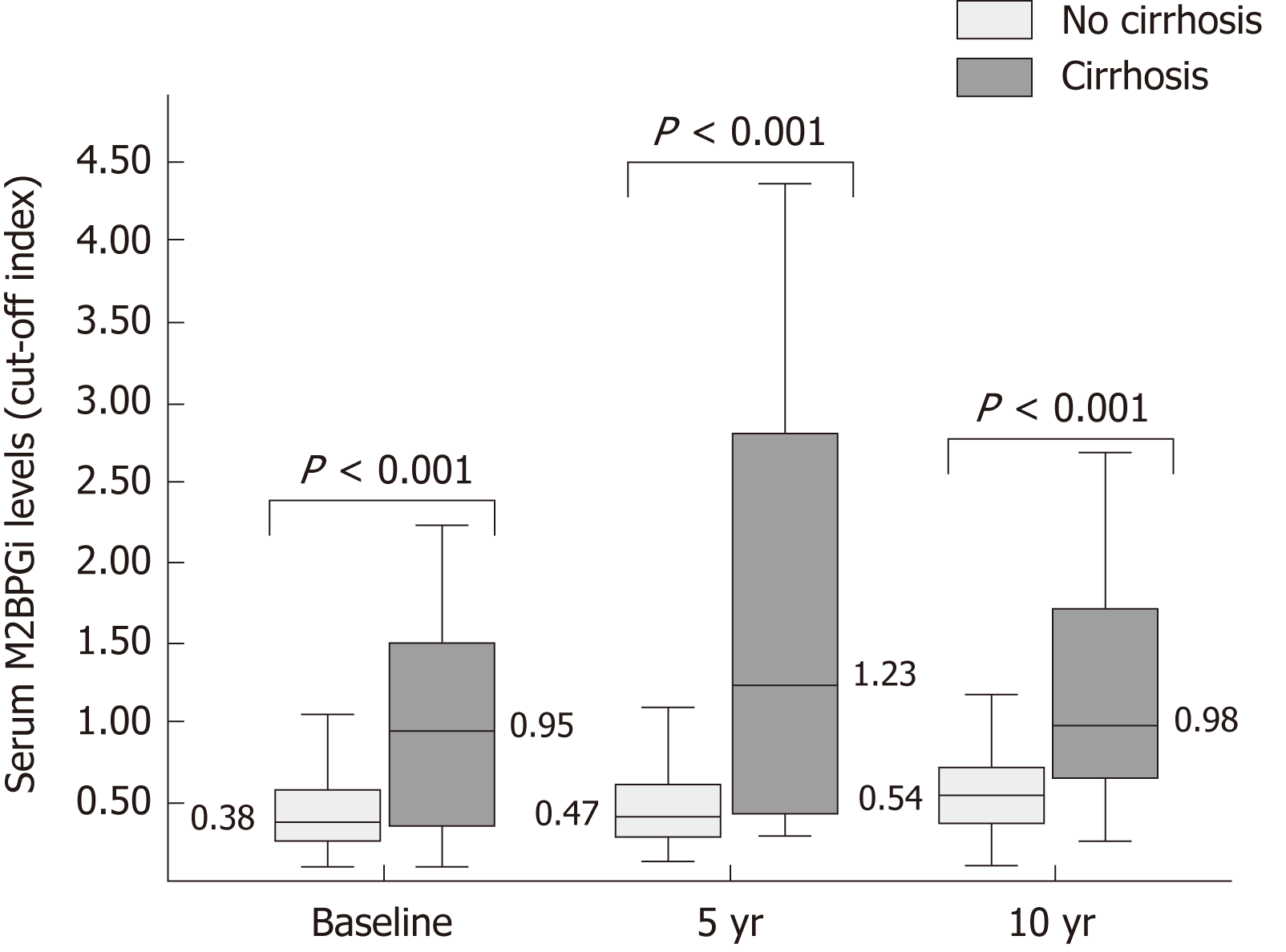
The median serum M2BPGi at baseline, 5-year and 10-year was 0.42 (IQR: 0.27-0.68), 0.5 (IQR: 0.29-0.68) and 0.56 (0.38-0.76) COI, respectively. Cirrhosis was present in 35 (16.9%) patients at the end of follow-up. Compared to non-cirrhotic group, the median serum M2BPGi levels in the cirrhotic group were significantly higher at all 3 time points (0.38 COI vs 0.95 COI, 0.47 COI vs 1.23 COI, 0.54 COI vs 0.98 COI, respectively, P < 0.001 for all time points) (Figure 2). Among 167 patients with 5-year LSM, the median LS was 6.5 (5-8.8) kPa. Advanced fibrosis was present in 39 (23.4%) patients. The median LS at 5-year was significantly higher in the cirrhotic group than the non-cirrhotic group (14.4 kPa vs 6.1 kPa, P < 0.001). Serum M2BPGi at baseline and 5-year demonstrated linear correlations with LSM at 5-year (r = 0.232, P = 0.009 and r = 0.563, P < 0.001, respectively). Baseline serum M2BPGi level was significantly higher in patients with subsequent LSM at 5-year showing advanced fibrosis compared to those without (0.61 COI vs 0.37 COI, P = 0.008).
Since almost half patients were eventually started on antiviral therapy, it would be impractical to exclude these patients from subsequent analysis. Previous report stated that antiviral therapy would lead to decline in serum M2BPGi level and reduction in histological fibrosis[18]. To address this issue, we performed additional analysis to compare the serum M2BPGi levels at 5-year and 10-year between those who were subsequently initiated with antiviral therapy. The median serum M2BPGi levels in patients requiring treatment compared to patients not requiring treatment were 0.50 (IQR: 0.37-0.71) COI vs 0.50 (IQR: 0.28-0.64) COI and 0.66 (IQR: 0.43-0.95) COI vs 0.52 (IQR: 0.34-0.62) COI at 5-year (P = 0.167) and 10-year (P < 0.001), respectively.
Among 207 patients, HCC developed in 14 patients (6.8%) at a median of 4.7 (IQR: 2.7-8.9) years at median age of 59.1 years. The 5-year, 10-year and 15-year cumulative incidences of HCC were 3.9%, 5.9% and 7%, respectively.
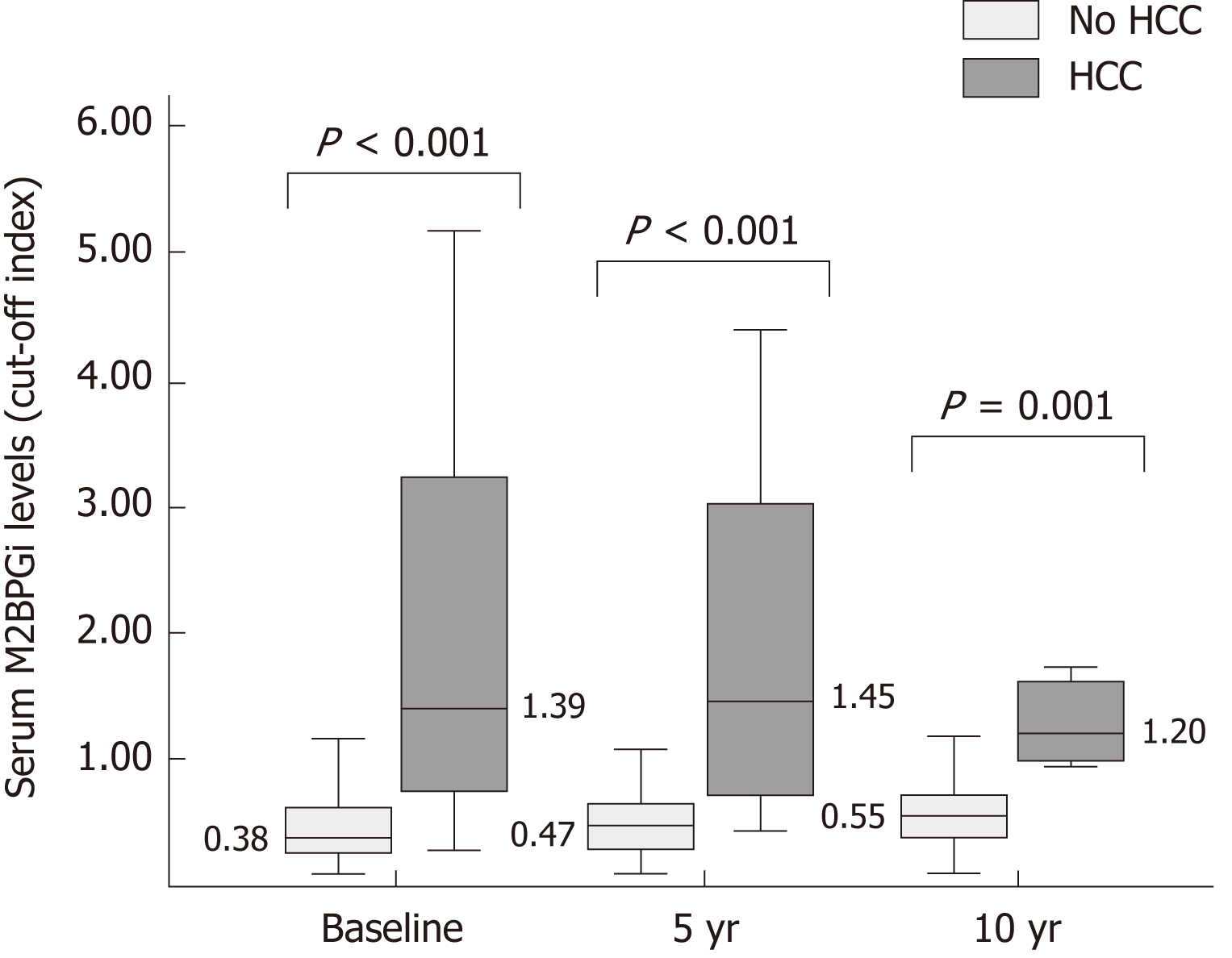
Compared to patients without HCC development, HCC patients were older at HBeAg seroconversion (median age: 40 years old vs 52 years old, P < 0.001). HCC patients had higher baseline serum aspartate aminotransferase (AST, 44 U/L vs 24 U/L, P = 0.002), alanine aminotransferase (ALT, 61 U/L vs 27 U/L, P = 0.007), and gamma glutamyl transferase (GGT, 59 U/L vs 22 U/L, P = 0.003). The proportion of patients with cirrhosis was higher in the HCC group compared to non-HCC group (92.8% vs 11.4%, P < 0.001). The median serum M2BPGi at baseline, 5-year and 10-year were significantly higher in HCC group compared to non-HCC group (1.39 COI vs 0.38 COI, 1.45 COI vs 0.47 COI and 1.20 COI vs 0.55 COI, respectively; P < 0.001, P < 0.001 and P = 0.001, respectively) (Figure 3). The 5-year LS was significant higher in HCC group compared to non-HCC group (12.6 kPa vs 6.4 kPa, P = 0.028) (Table 2).
| Univariate analysis | Multivariate analysis | |||||
| HCC (n = 14) | No HCC (n = 193) | P value | OR | 95%CI | P value | |
| Gender (being male, %) | 10 (71.4%) | 108 (56%) | 0.403 | |||
| Age of HBeAg seroconversion (yr) | 53 | 39 | < 0.001 | 1.196 | 1.034-1.382 | 0.016 |
| Bilirubin (μmoL/L) | 11 | 10 | 0.797 | |||
| ALP (U/L) | 74 | 58 | 0.177 | |||
| AST (U/L) | 44 | 24 | 0.002 | 1.042 | 0.906-1.199 | 0.564 |
| ALT (U/L) | 61 | 27 | 0.007 | 1.030 | 0.969-1.094 | 0.347 |
| GGT (U/L) | 59 | 22 | 0.003 | 0.981 | 0.932-1.033 | 0.471 |
| Albumin (g/L) | 43 | 44 | 0.427 | |||
| Globulin | 36 | 34 | 0.157 | |||
| AFP (ng/mL) | 4 | 3 | 0.587 | |||
| HBV DNA (log IU/mL) | 4.6 | 4.0 | 0.446 | |||
| Baseline M2BPGi (COI) | 1.39 | 0.38 | < 0.001 | 4.666 | 1.296-16.802 | 0.018 |
| 5-year M2BPGi (COI) | 1.45 | 0.47 | < 0.001 | |||
| 10-year M2BPGi (COI) | 1.2 | 0.55 | 0.001 | |||
| 5-year LS (kPa) | 12.6 | 6.4 | 0.028 | |||
| Cirrhosis (%) | 13 (92.8%) | 22 (11.4%) | < 0.001 | 7.142 | 0.270-188.693 | 0.239 |
| Treatment after HBeAg seroconversion | 10 (71.4%) | 92 (47.7%) | 0.102 | |||
| HBsAg seroclearance (%) | 0 (0%) | 7 (3.6%) | 0.608 | |||
As more than half of HCC cases in this cohort developed between baseline and 5-year, multivariate analysis was only performed on baseline variables for association with subsequent HCC development. Multivariate analysis showed that age at HBeAg seroconversion (OR = 1.196, 95%CI: 1.034-1.382, P = 0.016) and baseline serum M2BPGi (OR = 4.666, 95%CI: 1.296-16.802, P = 0.018) were independent factors associated with HCC development (Table 2).
The AUROC of baseline serum M2BPGi for predicting subsequent HCC development was 0.883 (95%CI: 0.771-0.995, P < 0001). The optimal cut-off value was 0.685 COI, which predicted HCC development with 91.7% sensitivity and 80.8% specificity. The PPV and NPV were 25.8% and 99.3%, respectively (Supplementary Figure 1).
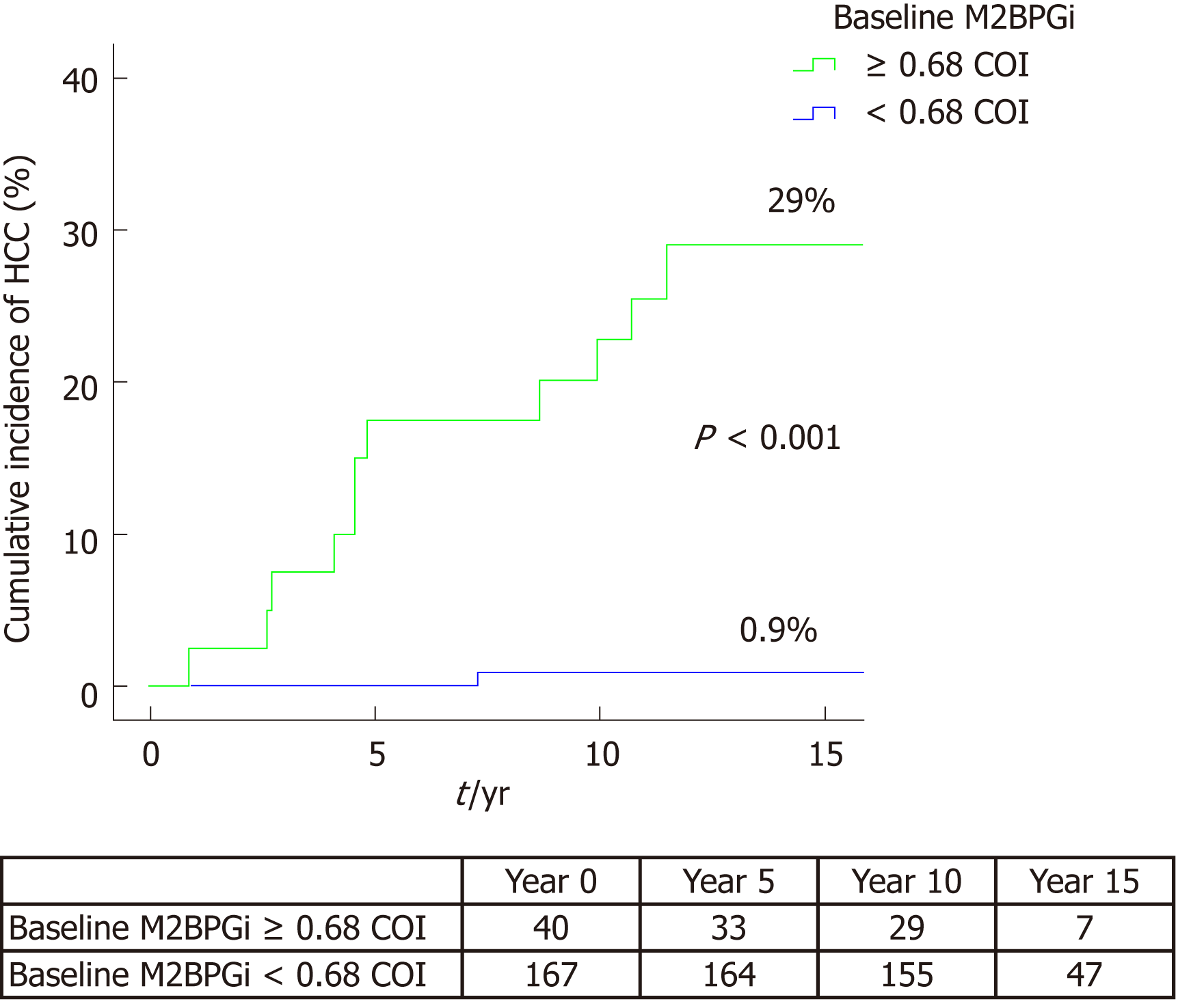
The cumulative incidence of HCC at 15-year of follow-up was significantly higher in patients with baseline M2BPGi ≥ 0.68 COI compared to < 0.68 COI (29% vs 0.9%, respectively, P < 0.001) (Figure 4).
The present longitudinal study investigated a homogenous CHB patient population with a well-defined time point of HBeAg seroconversion, with a median follow-up length of 13.1 years representing a relatively long duration of follow up in the existing literature regarding risk prediction for HCC development in such a population. HCC development was shown to be highly predictable by baseline serum M2BPGi (OR = 4.666). Serum M2BPGi provides an objective quantitative assessment of liver fibrosis, rather than qualitative sonographic features. In comparison, sonographic diagnosis of cirrhosis was not a significant factor for HCC development upon multivariate analysis (P = 0.239), highlighting the limitations of qualitative assessment. Although 5-year LSM and 5-year M2BPGi were not included in the multivariate analysis, they showed good linear correlation with each other (r = 0.563, P < 0.001). Baseline M2BPGi also dictated advanced liver fibrosis at subsequent 5-year LSM (P = 0.008). It would be of great interest for future studies to determine whether a combined M2BPGi and fibroscan measurement at baseline would further enhance the predictability for HCC development.
The cut-off value of baseline serum M2BPGi was derived by established statistical methods with an excellent performance (AUROC = 0.883). Using a cut-off value of 0.68 COI, the sensitivity and specificity was > 90% and > 80%, respectively, with very high NPV (99.3%). The low PPV (25.8%) was mainly related to the relatively low incidence of HCC in this HBeAg negative cohort and is not unexpected from their inactive disease profile. In view of such, serum M2BPGi would be useful to exclude patients with low risk of HCC. As LSM has been incorporated into HCC prediction scores[28], combining other markers for liver fibrosis, like M2BPGi, into risk prediction models should also be explored.
Apart from baseline M2BPGi, older age at HBeAg seroconversion (OR = 1.196) was another independent factor associated with the development of HCC. This is consistent with previous reports that the risk of HCC was higher if spontaneous HBeAg seroconversion occurred at an older age[29,30]. As HBeAg seroconversion marks the transition from immune active phase to residual low replicative phase of CHB[31,32]. patients with late HBeAg seroconversion might experience more cumulative liver insult from more active viral replication during the longer HBeAg positive immune active phase compared to patients who had HBeAg seroconversion at a younger age.
In this study, a few known risk factors were not statistically significant variables for subsequent HCC development. The patients in this study were all treatment-naive at the time of recruitment, implying a relatively inactive disease profile. Expectantly, both groups (HCC and non-HCC) had relatively low viral load at the time of HBeAg seroconversion (HBV DNA 4.6 and 4.0 log IU/mL, respectively). Similarly, serum albumin in both groups were in the normal range (43 and 44 g/L, respectively), indicating preserved liver synthetic function in this selected population. The lack of statistical significance for male gender in this study might be related to the small sample size and low number of patients with HCC. A higher proportion of patients who subsequently developed HCC were initiated with antiviral therapy compared to patients who did not develop HCC (71.4% vs 47.7% for non-HCC, P = 0.102). Although statistical significance was not reached, those that did not need antiviral therapy may intrinsically have a better disease profile with more favourable virological and biochemical characteristics[30], which accounted for an apparently lower risk of HCC in those who do not require antiviral therapy.
Antiviral treatment cannot be withheld if treatment indications were reached, including not only hepatitic flare but also development of cirrhosis or HCC. Therefore, 49.3% of patients in this study eventually received treatment at a median duration of 5.5 years. We showed that subsequent antiviral therapy did not signficantly change the serum M2BPGi levels at 5-year, probably related to the short duration of treatment. Paradoxically, the median M2BPGi level was significantly higher at 10-year in those requiring antiviral treatment compared to those who did not (0.66 vs 0.52, P < 0.001). This reflects the intrinsically more advanced liver disease in those requiring antiviral treatment. As the timing of antiviral therapy initiation was heterogenous (IQR: 2.7-8.6 years after recruitment), it is difficult to assess the effect of antiviral therapy on fibrosis regression, taking into consideration the differences in the severity of liver disease accumulated at the timing of therapy. Therefore, the differences in serum M2BPGi levels between HCC and non-HCC group at 5-year and 10-year could not be explained by antiviral therapy alone.
There are a few limitations in the present study. Firstly, platelet counts were not available, precluding the use of other serum-based indices for fibrosis assessment (e.g., FIB-4, APRI). Secondly, viral factors including HBV genotype and specific mutations - known risk factors for HCC development - were not included in the analysis. Thirdly, due to the low number of HCC cases at 5-year (n = 8) and 10-year (n = 3), 5-year and 10-year serum M2BPGi as well as 5-year LS were not included in multivariate analysis. Larger scale studies including more patients would be needed to evaluate the role of longitudinal assessment of these markers in HCC risk prediction.
In summary, serum M2BPGi accurately predicted subsequent HCC development in treatment-naive HBeAg-negative CHB patients across a long-term follow-up duration. The derived cut-off value of serum M2BPGi would be a valuable tool for risk stratification regarding HCC risk prediction.
Hepatocellular carcinoma (HCC) is the most dreadful complication of chronic hepatitis B infection (CHB). Recent research showed that serum Mac-2 binding protein glycosylation isomer (M2BPGi) is a novel biomarker for liver fibrosis and cirrhosis, and preliminary studies reported its potential role in predicting risk of HCC in both untreated and treated patients.
The current literature has limited data on the role of serum M2BPGi in predicting risk of HCC in patients with hepatitis B e antigen (HBeAg) seroconversion (HBeAg-negative disease), and studies with long-term follow-up are lacking.
We would like to know if serum M2BPGi can predict subsequent HCC development in untreated CHB patients who underwent HBeAg seroconversion.
This is a retrospective study by a tertiary center in Hong Kong. Treatment-naive patients with documented spontaneous HBeAg seroconversion were recruited. Serum M2BPGi was measured at baseline, at 5-years and 10-years from HBeAg-seroconversion. We investigated the relationship between serum M2BPGi levels and subsequent HCC development.
The cumulative HCC incidence at 15 years was 7% among 207 recruited patients (median follow-up of 13.1 years). Serum M2BPGi was significantly higher in patients with HCC compared to those without HCC at all 3 time points (all P < 0.01). Baseline serum M2BPGi was significantly associated with HCC development (odds ratio of 4.666, P = 0.018). The area under the receivor operating characteristics curve for baseline M2BPGI was 0.883, with sensitivity and specificity of 91.7% and 80.8%, respectively, when the derived cut-off value of 0.68 cut-off index was used to predict HCC development.
High serum M2BPGi level at HBeAg seroconversion was a strong predictor for subsequent HCC development in CHB patients.
The derived cut-off value of serum M2BPGi would be a valuable tool for risk stratification regarding HCC risk prediction. Further validation studies are warranted.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee CL, Namisaki T S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
| 1. | Lam YF, Seto WK, Wong D, Cheung KS, Fung J, Mak LY, Yuen J, Chong CK, Lai CL, Yuen MF. Seven-Year Treatment Outcome of Entecavir in a Real-World Cohort: Effects on Clinical Parameters, HBsAg and HBcrAg Levels. Clin Transl Gastroenterol. 2017;8:e125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Yuen MF, Hou JL, Chutaputti A; Asia Pacific Working Party on Prevention of Hepatocellular Carcinoma. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol. 2009;24:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 353] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1946] [Cited by in RCA: 1973] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 4. | Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 357] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 5. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] |
| 6. | Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 337] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Yuen MF, Yuan HJ, Wong DK, Yuen JC, Wong WM, Chan AO, Wong BC, Lai KC, Lai CL. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Yuen MF, Tanaka Y, Shinkai N, Poon RT, But DY, Fong DY, Fung J, Wong DK, Yuen JC, Mizokami M, Lai CL. Risk for hepatocellular carcinoma with respect to hepatitis B virus genotypes B/C, specific mutations of enhancer II/core promoter/precore regions and HBV DNA levels. Gut. 2008;57:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Varbobitis I, Papatheodoridis GV. The assessment of hepatocellular carcinoma risk in patients with chronic hepatitis B under antiviral therapy. Clin Mol Hepatol. 2016;22:319-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH; REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2363] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 11. | Zhang H, Zhai Y, Hu Z, Wu C, Qian J, Jia W, Ma F, Huang W, Yu L, Yue W, Wang Z, Li P, Zhang Y, Liang R, Wei Z, Cui Y, Xie W, Cai M, Yu X, Yuan Y, Xia X, Zhang X, Yang H, Qiu W, Yang J, Gong F, Chen M, Shen H, Lin D, Zeng YX, He F, Zhou G. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat Genet. 2010;42:755-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 13. | Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK; REACH-B Working Group. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 519] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 14. | Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, But DY, Chan AO, Wong BC, Mizokami M, Lai CL. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 487] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 15. | Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, Lui YY, Chan AT, Sung JJ, Yeo W, Chan HL, Mok TS. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28:1660-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 16. | Kuno A, Sato T, Shimazaki H, Unno S, Saitou K, Kiyohara K, Sogabe M, Tsuruno C, Takahama Y, Ikehara Y, Narimatsu H. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin-assisted glycan profiling. Proteomics Clin Appl. 2013;7:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M, Narimatsu H. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Mak LY, Wong DK, Cheung KS, Seto WK, Lai CL, Yuen MF. Role of serum M2BPGi levels on diagnosing significant liver fibrosis and cirrhosis in treated patients with chronic hepatitis B virus infection. Clin Transl Gastroenterol. 2018;9:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, Gotoh M, Narimatsu H, Korenaga M, Mizokami M, Nishie A, Aishima S, Maehara Y. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J Gastroenterol. 2015;50:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 20. | Kim SU, Heo JY, Kim BK, Park JY, Kim DY, Han KH, Ahn SH, Kim HS. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts the risk of HBV-related liver cancer development. Liver Int. 2017;37:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Cheung KS, Seto WK, Wong DK, Mak LY, Lai CL, Yuen MF. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts liver cancer development in chronic hepatitis B patients under antiviral treatment. Oncotarget. 2017;8:47507-47517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Liu J, Hu HH, Lee MH, Korenaga M, Jen CL, Batrla-Utermann R, Lu SN, Wang LY, Mizokami M, Chen CJ, Yang HI. Serum Levels of M2BPGi as Short-Term Predictors of Hepatocellular Carcinoma in Untreated Chronic Hepatitis B Patients. Sci Rep. 2017;7:14352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Heo JY, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Park YN, Ahn SS, Han KH, Kim HS. Use of Wisteria Floribunda Agglutinin-Positive Human Mac-2 Binding Protein in Assessing Risk of Hepatocellular Carcinoma Due to Hepatitis B Virus. Medicine (Baltimore). 2016;95:e3328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Yuen MF, Lai CL. Natural history of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2000;15 Suppl:E20-E24. [PubMed] |
| 25. | European Association for the Study of the Liver, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3790] [Article Influence: 473.8] [Reference Citation Analysis (1)] |
| 26. | To WP, Wong DK, Mak LY, Cheung KS, Yue Y, Fung J, Lai CL, Yuen MF. Relationship between hepatitis B core-related antigen and chronic hepatitis B outcome in HBeAg negative patients: a 10-year longitudinal study. J Hepatol. 2018;68 Suppl 1:S478. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1330] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 28. | Wong GL, Chan HL, Wong CK, Leung C, Chan CY, Ho PP, Chung VC, Chan ZC, Tse YK, Chim AM, Lau TK, Wong VW. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol. 2014;60:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 29. | Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology. 2010;51:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Fung J, Cheung KS, Wong DK, Mak LY, To WP, Seto WK, Lai CL, Yuen MF. Long-term outcomes and predictive scores for hepatocellular carcinoma and hepatitis B surface antigen seroclearance after hepatitis B e-antigen seroclearance. Hepatology. 2018;68:462-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 32. | Yuen MF, Lai CL. Treatment of chronic hepatitis B. Lancet Infect Dis. 2001;1:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |