Published online Feb 28, 2018. doi: 10.3748/wjg.v24.i8.941
Peer-review started: December 25, 2017
First decision: January 4, 2018
Revised: January 15, 2018
Accepted: January 18, 2018
Article in press: January 18, 2018
Published online: February 28, 2018
Processing time: 64 Days and 23.6 Hours
To observe gene polymorphisms of TPMT and NUDT15, and compare their predictive value for azathioprine (AZA)-induced leukopenia in inflammatory bowel disease (IBD).
This study enrolled 219 patients diagnosed with IBD in Xiangya Hospital, Central South University, Changsha, China from February 2016 to November 2017. Peripheral blood of all patients was collected to detect their genotypes of TPMT and NUDT15 by pyrosequencing at the Department of Clinical Pharmacology, Hunan Key Laboratory of Pharmacogenetics, Xiangya Hospital. Eighty patients were treated with AZA according to the disease condition. During the first month, patients who received AZA underwent routine blood tests and liver function tests once a week. The endpoint of the study was leukopenia induced by AZA. By analyzing patient characteristics, genotypes and leukopenia induced by drug use, we found the risk factors associated with AZA-induced leukopenia.
There were 219 patients with IBD (160 men and 59 women), including 39 who were confirmed with ulcerative colitis (UC), 176 with Crohn’s disease (CD) and 4 with undetermined IBD (UIBD). There were 44 patients (20.1%) with mutant genotype of NUDT15 (C/T); among them, 16 received AZA, and 8 (50%) developed leukopenia. There were 175 patients (79.7%) with wild genotype of NUDT15 (C/C); among them, 64 received AZA, and 11 (17.2%) developed leukopenia. A significant difference was found between NUDT15 C/T and its wild-type C/C (P = 0.004). There were only 3 patients with TPMT mutant genotype of A/G (1.4%) who participated in the research, and 1 of them was treated with AZA and developed leukopenia. The remaining 216 patients (98.6%) were found to bear the wild genotype of TPMT (A/A); among them, 79 patients received AZA, and 18 (22.8%) developed leukopenia, and there was no significant difference from those with A/G (P = 0.071). The frequency of TPMT mutation was 1.4%, and NUDT15 mutation rate was significantly higher and reached 20.1% (P = 0.000). Therefore, NUDT15 gene polymorphism was obviously a better biomarker than TPMT gene polymorphism in the prediction of AZA-induced leukopenia.
Mutation rate of NUDT15 in Chinese IBD patients is higher than that of TPMT. NUDT15 polymorphism is a better predictor for AZA-induced leukopenia than TPMT polymorphism.
Core tip: Azathioprine (AZA) plays an important role in the remission maintenance therapy of inflammatory bowel disease (IBD). However, serious drug adverse reactions, especially leukopenia, limit its clinical application. European consensus has identified TPMT polymorphism as a predictor of AZA-induced leukopenia. However, the predictive value of TPMT is controversial in Asians with low mutation rate and effectiveness. NUDT15 polymorphism has recently been found to be strongly associated with AZA-induced leukopenia in Asians but there are few data in Chinese populations. This study aimed to observe TPMT and NUDT15 polymorphisms and compare their predictive value for AZA-induced leukopenia in Chinese IBD patients.
- Citation: Wang HH, He Y, Wang HX, Liao CL, Peng Y, Tao LJ, Zhang W, Yang HX. Comparison of TPMT and NUDT15 polymorphisms in Chinese patients with inflammatory bowel disease. World J Gastroenterol 2018; 24(8): 941-948
- URL: https://www.wjgnet.com/1007-9327/full/v24/i8/941.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i8.941
Inflammatory bowel disease (IBD) is a chronic nonspecific intestinal inflammatory disease[1]. The pathogenesis of IBD is still unknown but is possibly related to genetic and environmental factors, as well as intestinal immune dysfunction. IBD mainly includes ulcerative colitis (UC), Crohn’s disease (CD) and unclassified IBD (UIBD). During the 21st century, IBD has become a global disease with accelerating incidence in the newly industrialized countries, including Asia, and has caused a huge social and economic burden. An epidemiological study among the ACCESS countries showed an incidence of IBD in China of 3.3 cases per 100000 population, with an increasing trend[2].
The main goal of IBD treatment is to induce and maintain remission and prevent complications[3]. Treatment strategies of IBD include 5-aminosalicylate compounds, steroids, immune modulators and biological agents. Patients with IBD need long-term treatment to maintain remission due to its lifelong relapsing-remitting tendency. Immune modulators, especially azathioprine (AZA), have played an important role in maintaining remission. However, the adverse reactions of AZA, especially leukopenia, seriously limit its clinical application[4]. Research on the metabolic enzymes and adverse reactions of AZA has become popular and controversial. What is the value of these enzymes? How do they influence adverse reactions of AZA?
Thiopurine S-methyltransferase (TPMT), an enzyme of AZA metabolism, has been reported as a risk predictor for AZA-induced leukopenia in Caucasians. The United States Food and Drug Administration has proposed that TPMT status should be assessed before AZA therapy[5]. Researchers[6] have pointed out that AZA should not be used if TPMT activity is deficient because of a high risk of AZA-induced leukopenia. AZA should be used at a lower dose to reduce adverse reactions if TPMT activity is intermediate. However, AZA-induced leukopenia still occurs in patients with normal activity of TPMT. Moreover, although the mutation rate of TPMT is lower in Asians (1%-3%) than that in Caucasians (about 10%), the incidence of AZA-induced leukopenia in Asians (about 35.4%) is higher than that in Caucasians (about 5%)[7]. Thus, the predictive value of TPMT polymorphism for AZA-induced leukopenia is uncertain, especially among Asian populations.
Recent studies have revealed that NUDT15 polymorphism is a better predictor for drug adverse reactions with a higher mutation rate in Asians than TPMT[8]. However, little research has been done on the correlation between NUDT15 polymorphism and AZA-induced leukopenia in Chinese populations. Therefore, our study aimed to observe the gene polymorphism frequency distributions of TPMT and NUDT15, and compare their value in predicting AZA-induced leukopenia in patients with IBD to help optimize AZA therapy for IBD.
We enrolled 219 patients with IBD (160 men and 59 women), including 39 cases of UC, 176 of CD and 4 of UIBD. All of the patients visited Xiangya Hospital, Central South University (Changsha, China) from February 2016 to November 2017. Study inclusion criteria were: (1) diagnosis and disease activity based on the Consensus on Diagnosis and Treatment of Inflammatory Bowel Disease (2012, Guangzhou)[9]; (2) age 16-80 years; and (3) written informed consent obtained before examination. Exclusion criteria were: (1) severe cardiac, hepatic and/or renal insufficiency; (2) mucous biopsy diagnosed as carcinoma or lymphoma; (3) active tuberculosis; (4) active viral hepatitis with an obvious increase in transaminase (> 2 times the upper limit of normal), and/or hepatitis B e antigen(+) with hepatitis B virus DNA > 105 copies/mL or hepatitis B e antibody(+) with hepatitis B virus DNA > 104 copies/mL; (5) comorbidity such as bacterial or viral infection that had not been controlled effectively; and (6) acute abdominal disease, or other surgical indications (such as complicated fistula and intestinal obstruction caused by fibrosis).
Peripheral blood samples (2-3 mL) were obtained from enrolled patients and total genomic DNA was extracted from peripheral leukocytes. The variants in the NUDT15 gene of R139C (c.415C>T, rs116855232) and the TPMT variants of TPMT*3C (p.Tyr240Cys, c.719A>G, rs1142345) were genotyped at the Department of Clinical Pharmacology, Hunan Key Laboratory of Pharmacogenetics, Xiangya Hospital, Central South University. Genotyping was performed using pyrosequencing and the results were validated by Sanger sequencing. Pyrosequencing and Sanger sequencing primers were designed with PyroMark Assay Design software 2.0 (Qiagen, Hilden, Germany) and PrimerQuest Tool (IDT, San Jose, CA, United States), respectively. The sequences of the forward, reverse and sequencing primers for rs116855232 were 5’-GTGGGTTCCTTGGGAAGAACTA-3’, 5’-ATCCCACCAGATGGTTCAGATCTT-3’ and 5’-GCTTTTCTGGGGACTG-3’, respectively. The sequences of the forward, reverse and sequencing primers for rs1142345 were 5’-TGGGGAATTGACTGTCTTTTTGA-3’, 5’-TCCATTACATTTTCAGGCTTTAGC-3’ and 5’-GACTGTCTTTTTGAAAAGTT-3’, respectively. Conditions for polymerase chain reaction were 35 cycles of 30 s at 95 °C for denaturation, 30 s at 57 °C for annealing and 30 s at 72 °C for extension.
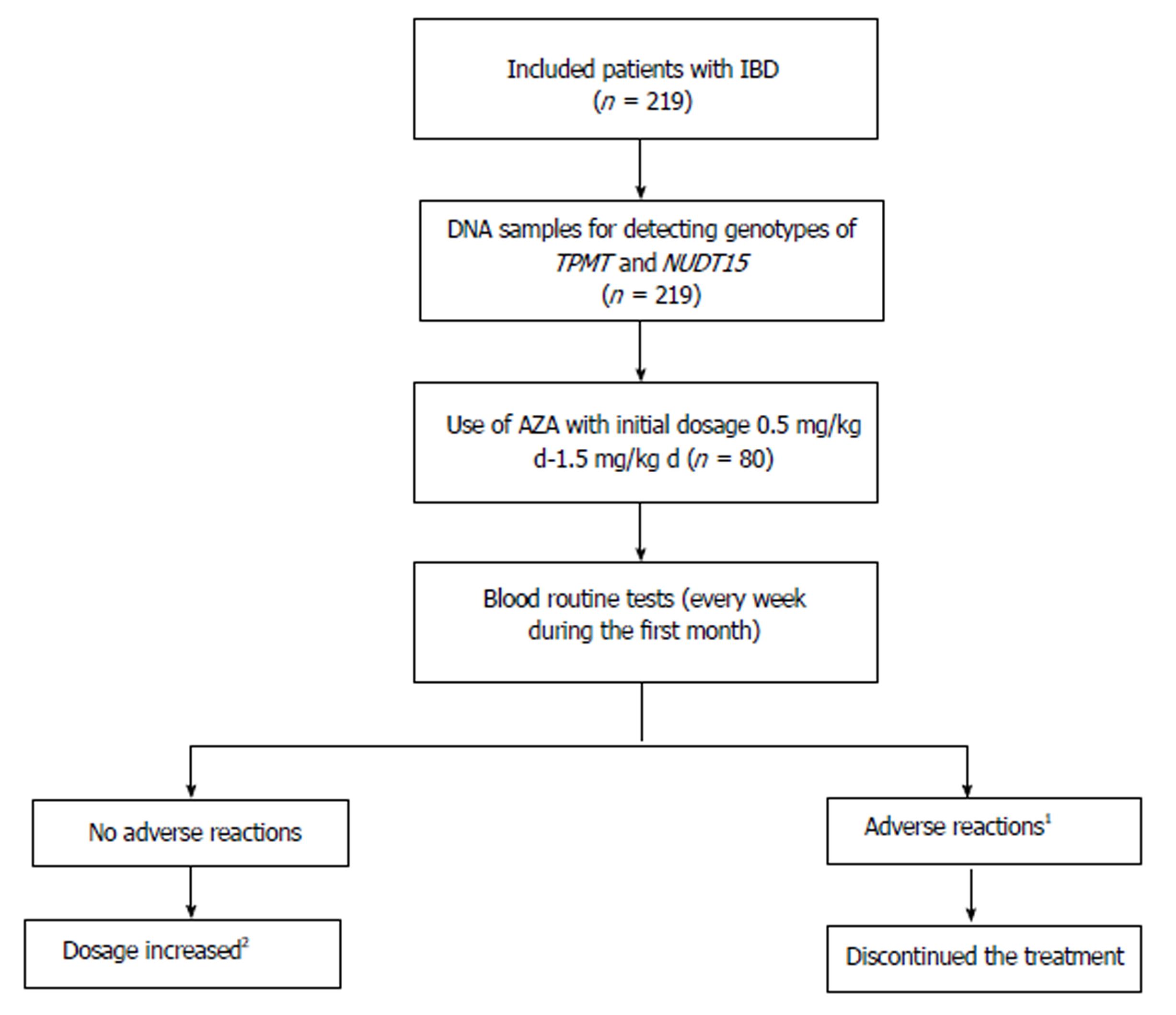
Initial dose of AZA was 0.5-1.5 mg/kg daily, based on the Consensus on Diagnosis and Treatment of Inflammatory Bowel Disease (2012, Guangzhou)[9]. Patients who used AZA underwent routine blood tests every week during the first month, then every 2 wk for 2 mo, followed by every month, as well as liver function tests every month. If patients had no adverse reactions, then AZA dose was increased by 0.5 mg/kg daily every month to 1.0-2.0 mg/kg/d. If AZA was effective without adverse reactions, patients should take it for life. If patients developed leukopenia (white blood cell count < 3.5 × 109/L or neutrophils < 1.5 × 109/L) and/or other severe adverse reactions, such as leukopenia, hepatoxicity, severe gastrointestinal adverse reactions and/or severe hair loss, the treatment was discontinued. The workflow of the study is shown in Figure 1.
The study was approved by the ethics committee of the hospital, and written informed consent was obtained before examination.
Data such as baseline characteristics including age, sex, disease stage, initial dose and duration of AZA were compared using the χ2 or Student’s t test. Combined use of other drugs and the frequency of leukopenia for each genotype were compared by analysis of χ2 tests. Logistic regression analyses were also performed to identify the associations of leukopenia with each genotype and other factors in multivariate analyses. To study the implication of different genes (NUDT15 and TPMT) in AZA-mediate leukopenia, we calculated relative risk (RR), etiological fraction (EF; if RR > 1) and preventive fraction (PF; if RR < 1). P < 0.05 was considered significant. All analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, United States).
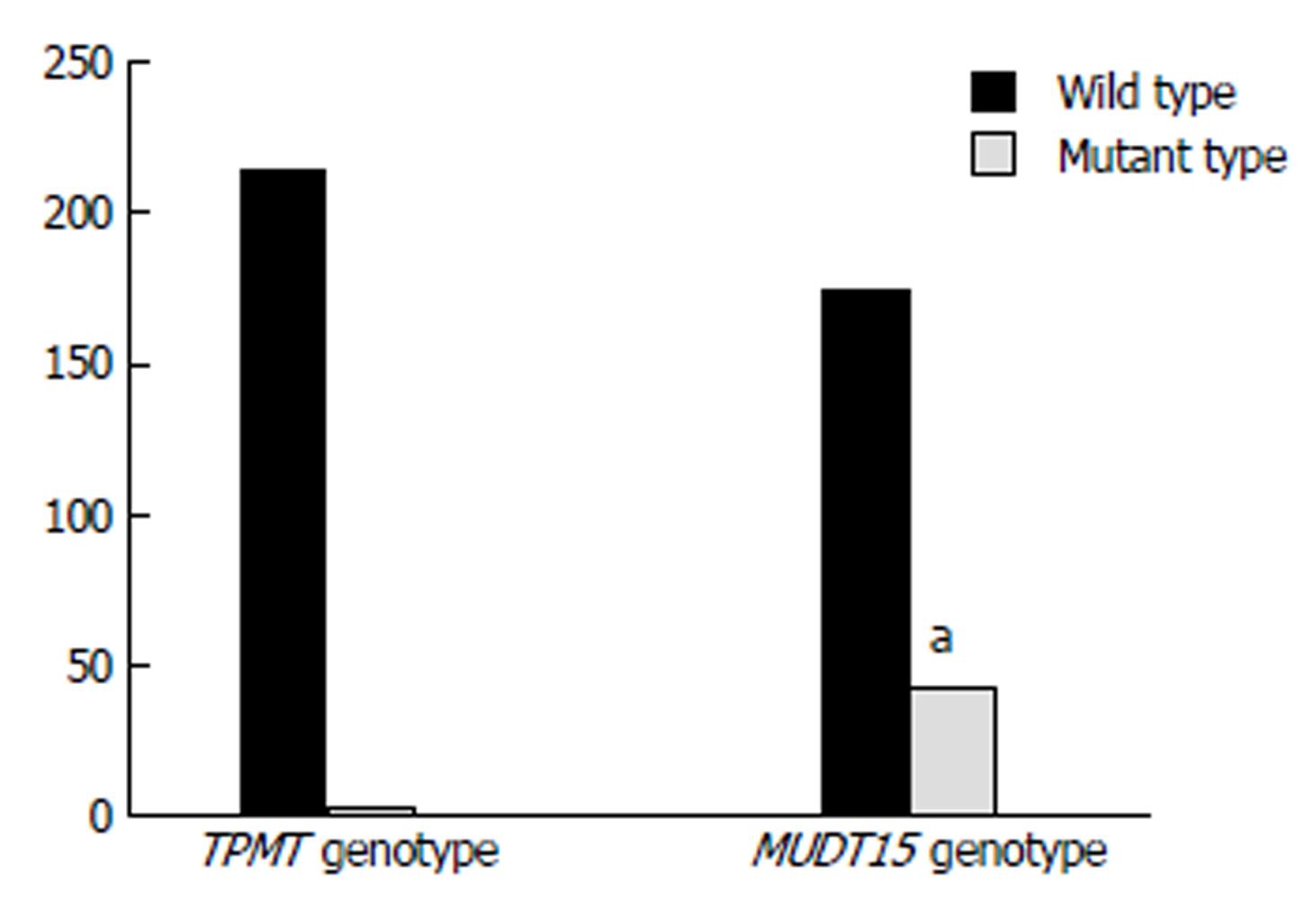
A total of 219 patients (UC 39, CD 176 and UIBD 4) participated in this study and 80 of them received AZA therapy. All patients were checked for their genotypes of NUDT15 R139C and TPMT *3C. The baseline characteristics of the patients are summarized in Table 1. More men than women received AZA treatment. Mutation rate of TPMT (1.4%) was significantly lower than that of NUDT15 (20.1%) (P = 0.000), which was in accordance with other studies (Figure 2).
| UC | CD | UIBD | Total | |
| No. of patients (n) | 39 (17.8) | 176 (80.4) | 4 (1.8) | 219 |
| Age in yr | 42.74 ± 13.60 | 30.51 ± 11.84 | 45.25 ± 16.62 | 33.44 ± 13.08 |
| Sex, M/F | 27/12 | 132/44 | 1/3 | 161/59 |
| TPMT genotype | ||||
| A/A | 38 (97.4) | 174 (98.9) | 4 (100) | 216 (98.6) |
| A/Ga | 1 (2.6) | 2 (1.1) | 0 | 3 (1.4) |
| NUDT15 genotype | ||||
| C/C | 31 (79.5) | 140 (79.5) | 4 (100) | 175 (79.9) |
| C/T | 8 (20.5) | 36 (20.5) | 0 | 44 (20.1) |
| AZA therapy | 3 (7.7) | 77 (43.8) | 0 | 80 (36.5) |
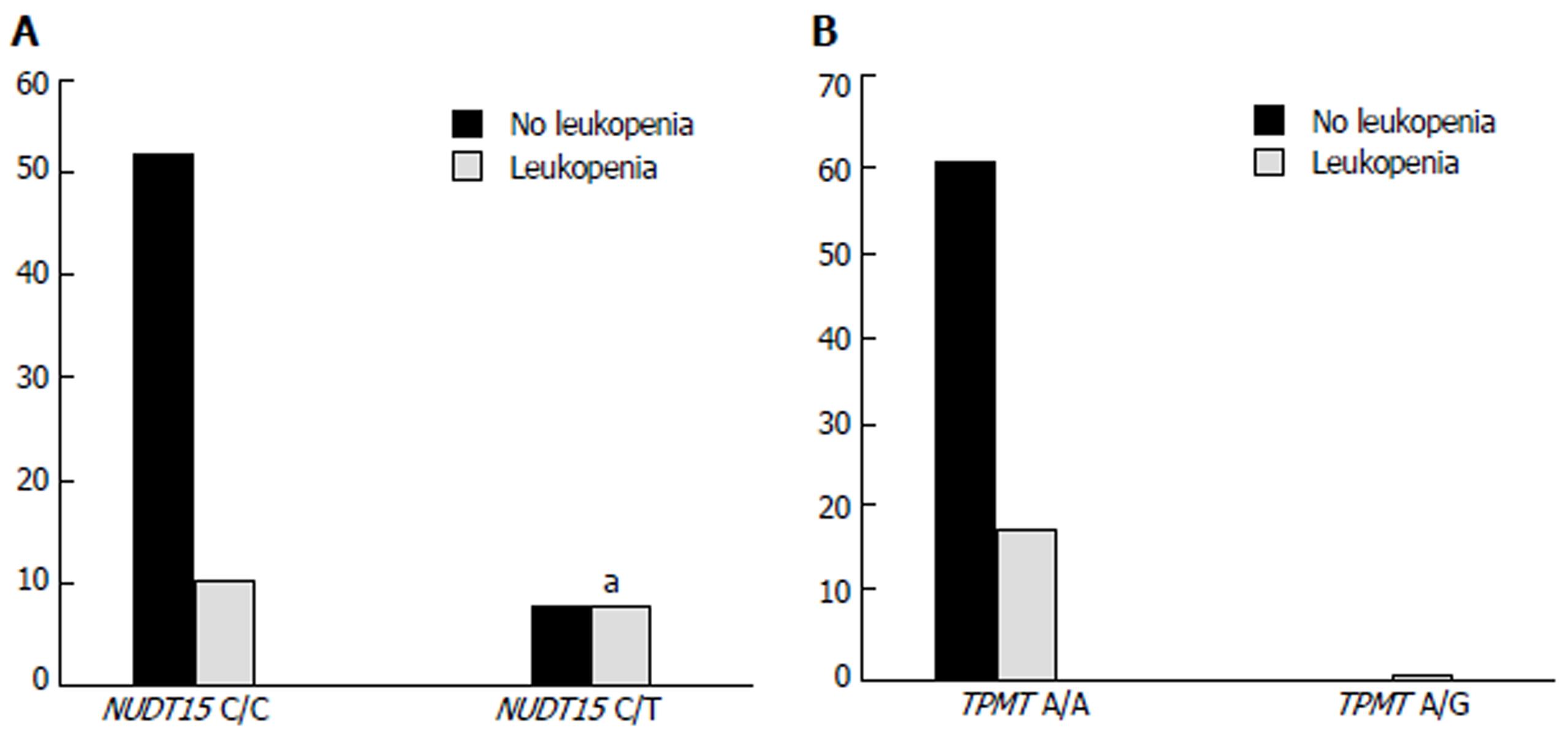
Patients characteristics, AZA initial dose, AZA duration, disease stage, adverse events and genotype of NUDT15 are summarized in Table 2. Sixty-four patients with NUDT15 C/C (80.0%) and 16 with C/T (20.0%) received AZA therapy. There were no significant differences in the age, sex, disease stage, initial dose of AZA, duration of AZA and combined treatment between these two groups. Eight of 16 patients with NUDT15 C/T developed leukopenia after receiving AZA therapy, while only 11/64 (11.7%) patients with NUDT15 C/C had leukopenia; there was a significant difference between the two groups (P = 0.006) (Figure 3A). Thus, patients with NUDT15 C/T had a higher risk of AZA-induced leukopenia than those with NUDT15 C/C.
| Genotype of NUDT15 | C/C | C/T | Total | P value |
| No. of patients (gene frequencies) | 64 (90.0) | 16 (10.0) | 80 | - |
| Age in yr | 33.69 ± 11.31 | 31.13 ± 12.40 | 33.18 ± 11.50 | 0.429 |
| Sex, M/F | 48/16 | 11/5 | 59/21 | 0.611 |
| Disease stages | ||||
| Remission | 6 (9.4) | 3 (18.8) | 9 (11.3) | 0.253 |
| Mild activity | 10 (15.6) | 2 (12.5) | 12 (15.0) | |
| Moderate activity | 35 (54.7) | 5 (31.3) | 40 (50.0) | |
| Severe activity | 13 (20.3) | 6 (37.5) | 19 (23.8) | |
| Initial dosage of AZA in mg/kg daily | 1.044 ± 0.318 | 1.241 ± 0.252 | 1.082 ± 0.441 | 0.596 |
| Duration of AZA in mo | 8.1 ± 11.19 | 9.3 ± 9.95 | 8.4 ± 10.90 | 0.686 |
| Combined with corticosteroids | 28 (43.8) | 5 (31.3) | 33 (41.3) | 0.071 |
| Combined with IFX | 14 (21.9) | 7 (43.8) | 21 (26.3) | |
| Combined with IFX and corticosteroid | 0 | 1 (6.3) | 1 (1.3) | |
| Leukopenia | 11 (17.2) | 8 (50.0)a | 19 (23.8) | 0.006 |
We also analyzed TPMT gene polymorphism. Most patients (98.75%) were homozygous for the wild allele of TPMT (A/A) and 18 (22.8%) developed leukopenia after using AZA, while only 1 patient showed heterozygous TPMT (A/G) and then developed leukopenia, with no significant differences between the TPMT polymorphism and incidence of leukopenia (P = 0.071) (Figure 3B). Based on these results, we suggest that TPMT polymorphism does not predict AZA-induced leukopenia efficiently.
In the logistic regression analysis with age, sex, combined drug use, disease stage, TPMT risk allele counts and NUDT15 risk allele counts, we found that NUDT15 polymorphism was significantly associated with AZA-induced leukopenia (P = 0.004, OR = 7.663, 95%CI: 1.893-31.023, RR = 2.909, EF = 0.276). In addition, we found that the incidence of AZA-induced leukopenia negatively correlated with corticosteroid usage and female sex (Table 3). Fewer women than men developed leukopenia after receiving AZA (P = 0.039, odds ratio (OR) = 0.146, 95% confidence interval (CI): 0.023-0.909, RR = 0.527, PF = 0.124). Combination with glucocorticoids reduced the risk of AZA-induced leukopenia (P = 0.023, OR = 0.201, 95%CI: 0.050-0.798, RR = 0.437, PF = 0.253). We found that age, disease stage, duration of AZA and combination with other drugs were not significantly associated with AZA-induced leukopenia, except for corticosteroids.
| Factor | P value | OR (95%CI) | RR | EF/PF |
| Age | 0.714 | - | - | - |
| Sex, female | 0.039 | 0.146 (0.023-0.909) | 0.527 | 0.124 |
| Disease stages | 0.509 | - | - | |
| Duration of AZA | 0.438 | - | - | |
| Combined with corticosteroids | 0.023 | 0.201 (0.050-0.798) | 0.437 | 0.253 |
| Combined with IFX | 0.339 | - | - | - |
| Combined with IFX and corticosteroid | 1.000 | - | - | - |
| TPMT | 1.000 | - | - | - |
| NUDT15 | 0.004 | 7.663 (1.893-31.023) | 2.909 | 0.276 |
AZA is an antimetabolic immunosupressor that plays a key role in remission maintenance treatment of IBD. It can be used in patients who are dependent on corticosteroids or in whom corticosteroids are ineffective, as well as in those with fistulas or operations[10]. AZA is metabolized by multiple enzymes through three main pathways[11]. (1) Steps depending on hypoxanthine phosphoribosyl transferase, inosine monophosphate dehydrogenase and GMP synthetase and eventually transferred into 6-thioguanosine triphosphate (6-TGTP). 6-TGTP is an effective form of AZA that is incorporated into double-strand DNA to inhibit its synthesis and trigger apoptosis, as well as causing bone marrow toxicity[12]; (2) Oxidization by xanthine oxidase and conversion into 6-thiouric acid, an inactive product of AZA; and (3) Catalysis by TPMT and conversion into 6-methylmercaptopurine. These three pathways are mutually competitive.
Although AZA is cost-effective, adverse reactions such as leukopenia may lead to severe and life-threatening infections that result in treatment discontinuation. A study by Kakuta et al[13] showed that 34 (25.2%) of 135 Japanese patients with IBD developed leukopenia after receiving AZA therapy. Yang et al[8] found that 346 (35.4%) of 978 Korean patients with CD developed AZA-induced leukopenia. Consistent with these results, we found that 19 (23.8%) of 80 Chinese patients with IBD discontinued AZA treatment due to leukopenia. So, the incidence of AZA-induced leukopenia is high in Asians and should not be ignored. Research on AZA has found that TPMT polymorphism is significantly associated with AZA-induced leukopenia, and > 40 different variant TPMT alleles (TPMT*2-*41) have been studied[14,15]. Among them, TPMT*3C is the most popular variant in Asians, but the gene frequency is low (1%-3%)[16]. Gazouli et al[17] have found no association between TPMT polymorphisms and the occurrence of AZA-related adverse events. In our study, the variation of TPMT (1.4%) was rare and not significantly associated with AZA-induced leukopenia in Chinese patients with IBD, which was consistent with previous reports. Thus, the predictive value of TPMT polymorphism is controversial in Asians.
NUDT15 also known as MTH2, is a member of the Nudix hydrolytic enzyme family, which degrades 8-o-dGTP into 8-o-dGMP and prevents DNA from mismatch. Carter et al[18] have revealed that NUDT15 has a strong effect on dGTP, 6-TGTP and dUTP. It hydrolyzes 6-TGTP into 6-TGMP, decreases the level of AZA active metabolites and prevents AZA-induced leukopenia. Yang et al[8] have reported that NUDT15 polymorphism is strongly associated with thiopurine-induced early leukopenia in Koreans. Zhu et al[19] have found in Chinese patients that NUDT15 has a high mutation rate (22.5%) that is strongly associated with the incidence of AZA-induced leukopenia. A study of Singapore patients with IBD[20] has also revealed that patients with low and intermediate NUDT15 activity have significantly higher risks of developing AZA-induced leukopenia. We verified the same conclusion that the incidence of an aberrant NUDT15 was 10.0% significantly higher than that of TPMT (P = 0.000) and NUDT15 could be an important pharmacogenetic marker for predicting AZA-induced leukopenia in a Chinese cohort (P = 0.004, OR = 7.663, 95%CI: 1.893-31.023, RR = 2.909, EF = 0.276).
This is a prospective study that has a lot of clinical value. Our results were significant to optimize AZA therapy for Chinese patients with IBD and helpful to provide more data in Chinese populations and to further multicenter large-scale research. Moreover, we are the first to describe that combined use of corticosteroids is a negative factor for developing AZA-induced leukopenia. Patients who received corticosteroids along with AZA treatment, or those with corticosteroid-induced remission and maintenance by AZA had a lower risk of developing leukopenia (P = 0.023, OR = 0.201, 95%CI: 0.050-0.798, RR = 0.437, PF = 0.253). This may be explained by the function of corticosteroids[22] in enhancing bone marrow hematopoiesis and promoting neutrophil release and increasing the number of circulating neutrophils, as well as reducing the infiltration and consumption of neutrophils in the inflammatory regions.
In contrast to the study by Kakuta et al[13], which showed that sex was not associated with AZA-induced leukopenia, we found that women were less likely to develop leukopenia after receiving AZA than men (P = 0.039, OR = 0.146, 95%CI: 0.023-0.909, RR = 0.527, PF = 0.124). The exact mechanism was unknown. We supposed that might be related to sex differences in pharmacokinetics. More specifically, females may have a lower apparent volume of distributions than males because of the solubility of the drug so that AZA might be removed more quickly in females, which reduces the risk of AZA-induced leukopenia. However, since more men received AZA treatment than women in our study, further study is needed to confirm the relationship between sex and AZA-induced leukopenia.
Our study had a few limitations, including that no patients with NUDT15(T/T) participated in our study. Recent studies have detected additional variants of NUDT15, except R139C, including Arg139His, Val18Ile and p.Val18_Val19insGlyVal, and defined six haplotype (*1 to *6) combinations of these variants[21], but we have not investigated these in the present study. So, further multicenter studies with larger sample size are warranted in this area.
In conclusion, the mutation rate of NUDT15 in Chinese patients with IBD is significantly higher than that of TPMT. NUDT15 polymorphism is a better predictor for AZA-induced leukopenia than TPMT polymorphism. Combined use of corticosteroids is a potential way to reduce the risk of AZA-induced leukopenia. Males have a higher risk of developing AZA-induced leukopenia and need to be more closely monitored than females. Further research is necessary to verify this relationship and determine the precise mechanism.
Azathioprine (AZA) plays a key role in remission maintenance therapy of inflammatory bowel disease (IBD), although it causes serious adverse reactions, including leukopenia. TPMT polymorphism is a predictor of AZA-induced leukopenia in Caucasians. However, the predictive value of TPMT is controversial in Asians. NUDT15 polymorphism is a more effective predictor of AZA-induced leukopenia in Asians, but there are few data in Chinese populations.
The purpose of this study was to observe TPMT and NUDT15 polymorphisms and compare their values in predicting AZA-induced leukopenia in Chinese IBD patients.
To find a more valuable predictor of AZA-induced leukopenia in Chinese patients with IBD, improve our ability to manage these patients more safely, and optimize AZA therapy.
A total of 219 patients diagnosed with IBD in Xiangya Hospital, Central South University were enrolled. Peripheral blood of all patients was collected to detect their genotypes of TPMT and NUDT15 by pyrosequencing at the Department of Clinical Pharmacology, Hunan Key Laboratory of Pharmacogenetics, Xiangya Hospital. Eighty patients were treated with AZA according to the disease condition. Patients who received AZA underwent routine blood tests and liver function tests once a week. Data analysis was performed by χ2 test, Student’s t test or logistic regression analyses using SPSS version 18.
We enrolled 219 patients with IBD (160 men and 59 women). There were 44 patients (20.1%) with mutant genotype of NUDT15 (C/T); among them, 16 received AZA, and 8 (50%) developed leukopenia. There were 175 patients (79.7%) with wild genotype of NUDT15 (C/C); among them, 64 received AZA, and 11 (17.2%) developed leukopenia. A significant difference was found between NUDT15 C/T and its wild-type C/C (P = 0.004). There were only 3 patients with TPMT mutant genotype of A/G (1.4%) and 1 was treated with AZA and then developed leukopenia. The remaining 216 patients (98.6%) were found to bear the wild genotype of TPMT (A/A); among them, 79 received AZA, and 18 (22.8%) developed leukopenia. There was no significant difference from those with A/Gs (P = 0.071). The frequency of TPMT mutation was 1.4%, and NUDT15 mutation rate was significantly higher and reached 20.1% (P = 0.000). Therefore, NUDT15 gene polymorphism was obviously a better biomarker than TPMT gene polymorphism for prediction of AZA-induced leukopenia. Moreover, combined use of corticosteroids reduced the risk of AZA-induced leukopenia (P = 0.023, odds ratio (OR) = 0.201, 95% confidence interval (CI): 0.050-0.798, relative risk (RR) = 0.437, preventive fraction (PF) = 0.253) and fewer women than men developed leukopenia after receiving AZA (P = 0.039, OR = 0.146, 95%CI: 0.023-0.909, RR = 0.527, PF = 0.124).
In Chinese patients with IBD, the mutation rate of NUDT15 is significantly higher than that of TPMT. NUDT15 polymorphism is more strongly associated with AZA-induced leukopenia than TPMT is. Detecting NUDT15 genotype before AZA treatment is more effective at predicting the risk of developing leukopenia than detecting TPMT genotype. Combined use of corticosteroids is a potential way to reduce the risk of leukopenia. Males have a higher risk of developing AZA-induced leukopenia and need to be more closely monitored than females. Further research is necessary to verify this relationship and determine the precise mechanism.
According to our study, NUDT15 polymorphism may predict AZA-induced leukopenia more effectively than TPMT does. Female sex and corticosteroid usage were negatively associated with developing AZA-induced leukopenia. This might be helpful for AZA-induced leukopenia prevention and to optimize AZA therapy for IBD. While the definite relationship was obscure, more research about how they affect AZA metabolism should be carried out in the future. To learn more about the interaction between them, multicenter studies with larger samples and functional genomics technology may be carried out in future research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gazouli M, Link A, Trifan A S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma YJ
| 1. | Lakatos PL. Prevalence, predictors, and clinical consequences of medical adherence in IBD: how to improve it? World J Gastroenterol. 2009;15:4234-4239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MN, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJ, Chan FK; Asia-Pacific Crohn’s and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 3. | Bernstein CN, Eliakim A, Fedail S, Fried M, Gearry R, Goh KL, Hamid S, Khan AG, Khalif I, Ng SC. World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease: Update August 2015. J Clin Gastroenterol. 2016;50:803-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Bär F, Sina C, Fellermann K. Thiopurines in inflammatory bowel disease revisited. World J Gastroenterol. 2013;19:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Lichtenstein GR, Sbreu MT, Cohen R, Tremaine W. [American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease]. Rev Gastroenterol Mex. 2006;71:351-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 344] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | Gazouli M, Pachoula I, Panayotou I, Chouliaras G, Anagnou NP, Chroussos G, Roma E. Thiopurine methyltransferase genotype and thiopurine S-methyltransferase activity in Greek children with inflammatory bowel disease. Ann Gastroenterol. 2012;25:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Lee KM, Kim YS, Seo GS, Kim TO, Yang SK; IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of Thiopurines in Inflammatory Bowel Disease: A Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015;13:193-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, Haritunians T, Ye BD, Kim KJ, Park SH. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 422] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 9. | Hu PJ, Qian JM, Wu KC, Rang ZH. Consensus on Diagnosis and Management of Inflammatory Bowel Disease (2012·Guang Zhou). Neike Lilun Yu Shijian. 2013;1:62-75. [DOI] [Full Text] |
| 10. | Meijer B, Mulder CJ, van Bodegraven AA, de Boer NK. How I treat my inflammatory bowel disease-patients with thiopurines? World J Gastrointest Pharmacol Ther. 2016;7:524-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 11. | Fangbin Z, Xiang G, Liang D, Hui L, Xueding W, Baili C, Huichang B, Yinglian X, Peng C, Lizi Z. Prospective Evaluation of Pharmacogenomics and Metabolite Measurements upon Azathioprine Therapy in Inflammatory Bowel Disease: An Observational Study (Baltimore). Medicine. 2016;95:e3326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, Lin TN, Hoshitsuki K, Nersting J, Kihira K. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 13. | Kakuta Y, Naito T, Onodera M, Kuroha M, Kimura T, Shiga H, Endo K, Negoro K, Kinouchi Y, Shimosegawa T. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016;16:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Appell ML, Berg J, Duley J, Evans WE, Kennedy MA, Lennard L, Marinaki T, McLeod HL, Relling MV, Schaeffeler E. Nomenclature for alleles of the thiopurine methyltransferase gene. Pharmacogenet Genomics. 2013;23:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Iu YPH, Helander S, Kahlin AZ, Cheng CW, Shek CC, Leung MH, Wallner B, Mårtensson LG, Appell ML. One amino acid makes a difference-Characterization of a new TPMT allele and the influence of SAM on TPMT stability. Sci Rep. 2017;7:46428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Takatsu N, Matsui T, Murakami Y, Ishihara H, Hisabe T, Nagahama T, Maki S, Beppu T, Takaki Y, Hirai F. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2009;24:1258-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Gazouli M, Pachoula I, Panayotou I, Mantzaris G, Syriopoulou VP, Goutas N, Vlachodimitropoulos D, Anagnou NP, Roma-Giannikou E. Thiopurine S-methyltransferase genotype and the use of thiopurines in paediatric inflammatory bowel disease Greek patients. J Clin Pharm Ther. 2010;35:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Carter M, Jemth AS, Hagenkort A, Page BD, Gustafsson R, Griese JJ, Gad H, Valerie NC, Desroses M, Boström J. Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2. Nat Commun. 2015;6:7871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Zhu X, Wang XD, Chao K, Zhi M, Zheng H, Ruan HL, Xin S, Ding N, Hu PJ, Huang M. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn’s disease. Aliment Pharmacol Ther. 2016;44:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Sutiman N, Chen S, Ling KL, Chuah SW, Leong WF, Nadiger V, Tjai M, Choon Kong CS, Schwender BJ, Chan W. Predictive role of NUDT15 variants on thiopurine-induced myelotoxicity in Asian inflammatory bowel disease patients. Pharmacogenomics. 2018;19:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Chao K, Wang X, Cao Q, Qian J, Wu K, Zhu X, Yang H, Liang J, Lin L, Huang Z. Combined Detection of NUDT15 Variants Could Highly Predict Thiopurine-induced Leukopenia in Chinese Patients with Inflammatory Bowel Disease: A Multicenter Analysis. Inflamm Bowel Dis. 2017;23:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Le Jeunne C. [Pharmacology of glucocorticoids]. Presse Med. 2012;41:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |