Published online Feb 28, 2018. doi: 10.3748/wjg.v24.i8.905
Peer-review started: August 5, 2017
First decision: August 30, 2017
Revised: November 17, 2017
Accepted: December 5, 2017
Article in press: December 5, 2017
Published online: February 28, 2018
Processing time: 207 Days and 11.4 Hours
To determine the frequency and risk factors for colorectal cancer (CRC) development among individuals with resected advanced adenoma (AA)/traditional serrated adenoma (TSA)/advanced sessile serrated adenoma (ASSA).
Data was collected from medical records of 14663 subjects found to have AA, TSA, or ASSA at screening or surveillance colonoscopy. Patients with inflammatory bowel disease or known genetic predisposition for CRC were excluded from the study. Factors associated with CRC developing after endoscopic management of high risk polyps were calculated in 4610 such patients who had at least one surveillance colonoscopy within 10 years following the original polypectomy of the incident advanced polyp.
84/4610 (1.8%) patients developed CRC at the polypectomy site within a median of 4.2 years (mean 4.89 years), and 1.2% (54/4610) developed CRC in a region distinct from the AA/TSA/ASSA resection site within a median of 5.1 years (mean 6.67 years). Approximately, 30% (25/84) of patients who developed CRC at the AA/TSA/ASSA site and 27.8% (15/54) of patients who developed CRC at another site had colonoscopy at recommended surveillance intervals. Increasing age; polyp size; male sex; right-sided location; high degree of dysplasia; higher number of polyps resected; and piecemeal removal were associated with an increased risk for CRC development at the same site as the index polyp. Increasing age; right-sided location; higher number of polyps resected and sessile endoscopic appearance of the index AA/TSA/ASSA were significantly associated with an increased risk for CRC development at a different site.
Recognition that CRC may develop following AA/TSA/ASSA removal is one step toward improving our practice efficiency and preventing a portion of CRC related morbidity and mortality.
Core tip: Screening colonoscopy reduces colorectal cancer morbidity and mortality risks through detection and treatment of precursor lesions. However, screening colonoscopy has a 3.5% false negative rate for detection of colorectal cancer (CRC) resulting in 17% of patients who had undergone colon screening within 3 years being diagnosed with CRC. We report that 3% of patients with advanced polyps in a surveillance program developed interval CRC. Recognition that CRC could develop following advanced polyp removal despite adherence to guidelines is one step toward improving our practice efficiency and preventing a portion of CRC related morbidity and mortality.
- Citation: Mouchli MA, Ouk L, Scheitel MR, Chaudhry AP, Felmlee-Devine D, Grill DE, Rashtak S, Wang P, Wang J, Chaudhry R, Smyrk TC, Oberg AL, Druliner BR, Boardman LA. Colonoscopy surveillance for high risk polyps does not always prevent colorectal cancer. World J Gastroenterol 2018; 24(8): 905-916
- URL: https://www.wjgnet.com/1007-9327/full/v24/i8/905.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i8.905
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States[1-4]. Colonoscopy with removal of premalignant lesions has contributed to a recent decline in CRC incidence and the number of deaths from this disease; nevertheless 5%-9% of patients diagnosed with CRC have undergone screening colonoscopy within the 3 years prior to detection of cancer[5]. Than et al[3] reported that colonoscopy has a 3.5% false negative rate for detection of CRC since 17% of patients with newly diagnosed CRC had been investigated with bowel-specific investigations within the previous 3 years. Winawer et al[6] reported that 6% of patients with advanced adenomas (AA) are missed by colonoscopy. The development of CRC despite colonoscopy may reflect missed superficial depressed lesions (cancer or high risk adenoma), incompletely resected adenomas[7], de novo cancer[8], or delayed diagnosis because of failed biopsy detection[9,10].
Adenomatous polyps are the most common neoplastic finding at colonoscopy[11]. These neoplastic polyps have malignant potential and are classified histologically as villous, tubulovillous, or tubular adenomas[12]. The malignant potential of these polyps correlates with type, size, and degree of dysplasia of the polyp. Advanced adenomas (AA) are those which are larger than 10 mm, have tubulovillous or villous architecture, or have high grade dysplasia[13].
The term “serrated adenoma” was introduced by Longacre et al[14] to describe polyps with dysplastic (adenomatous) cytology and serrated crypt architecture. Later, Torlakovic et al[15] coined the term sessile serrated adenoma to describe a different lesion, one with serrated crypts and characteristic architectural changes but usually no cytologic dysplasia. In order to avoid (or at least minimize) confusion, the Longacre lesion was renamed “traditional serrated adenoma.” Despite the shared terminology, SSA and TSA are not necessarily related lesions[16]. After a few more terminology modifications, the current World Health Organization classification for serrated polyps is: hyperplastic polyp, sessile serrated polyp (SSP) without dysplasia; sessile serrated adenoma (SSA) with cytological dysplasia, and traditional serrated adenoma[17]. The risk of developing CRC from a serrated lesion correlates with larger size (> 10 mm), presence of dysplasia and higher number of synchronous polyps.
Surveillance is recommended by the United States Preventive Services Task Force (USPSTF) 3 years after removal of AA, TSA, or advanced SSA[11] while the European guidelines recommend surveillance at 1 years for high risk polyps (≥ 20 mm) but three years for intermediate risk polyps(10 mm to < 20 mm)[18]. Despite frequent colonoscopy, CRC has been shown to develop at an incidence rate of 1.2/1000[19]. Though several large studies have illustrated the rates of post colonoscopy CRC to be low[20], we were particularly interested in how often CRC develops in the highest risk patients, namely those who have AA, TSA, or advanced SSA.
In this IRB-approved nested case cohort study (IRB 622-00), we reviewed the colonoscopy database and pathology reports for patients who were seen at Mayo Clinic, Rochester, Minnesota for colonoscopy related to any indication and found to have high-risk AA (villous architecture; high grade dysplasia and/or size > 10 mm), TSA, or Advanced SSA (any dysplasia and/or size > 10 mm), then identified 4160 patients who had at least one surveillance exam following the index polypectomy for their AA/TSA/ASSA. Surveillance exams were performed only for follow up and were not done in response to clinical symptoms. Colonoscopy reports prior to the incident advanced polyp lesion were not available in the electronic medical record on most patients and thus were not included in this study.
We included all patients ≥ 18 years of age diagnosed with either AA between January 1990 to December 2010 or ASSA/TSA between January 2000 to December 2010. Patients were followed through August 2016. Patients with a diagnosis of a polyposis syndrome, inflammatory bowel disease, or a known genetic predisposition for CRC were excluded from the study. We identified all patients from this cohort who had developed CRC (n = 84) and then randomly selected 252 patients who had an AA, TSA, or ASSA at index colonoscopy but who had not developed CRC. Clinical and pathological features of high-risk polyps (i.e., size, histology, site, and degree of dysplasia, time of index polypectomy), number and timing of surveillance colonoscopies and post polypectomy CRC (i.e., size, site, grade and stage) were collected via chart abstraction for this cohort of patients. Subjects who had not developed post-polypectomy CRC were randomly selected from a pool of 10 patients matched to the post-polypectomy CRC group based on polyp histology and size (< or ≥ 20 mm), degree of dysplasia and decade that the index polyp was removed. ASSA was classified as being at higher risk for malignant transformation if the polyp was > 10 mm, had dysplasia or higher number of synchronous polyps (≥ 3 polyps in small polyps measuring < 10 mm or ≥ 2 large polyps measuring > 10 mm)[17].
Post-polypectomy CRC was classified as same site cancer if the cancer arose in the region of the colon in which the high risk polyp had been removed. Since our surveillance intervals and time from index AA/TSA/ASSA to cancer development extended beyond three years in some cases, we did not use the term interval cancer[21], but rather post-polypectomy cancer. We acknowledge that it is impossible to know if the development of CRC in the same region as the high risk polyp that had prompted surveillance, we would anticipate that this high risk polyp would be the most likely source for the cancer.
Though these cases spanned from 1990 to 2010 for the AA and from 2000 to 2010 for the TSA and ASSA, we applied the most current USPSTF guidelines to all of these cases in order to assess the ability of these recommendations for polyp management of these high risk patients[11]. We similarly assessed using the European surveillance guidelines distinguishing intermediate versus high risk AA/TSA/ASSA based on polyp size. A polyp was classified as persistent if polyp clearance was not achieved on any of the surveillance procedures and as recurrent if the polyp had been successfully treated, not detected on at least one subsequent colonoscopy but recurred at the tattooed site of the original AA/TSA/ASSA.
The data are reported as mean (± SD), median (interquartile range, IQR), ranges, and categorical variables by counts and percentages as appropriate. We included only cancers occurring at least one year after polypectomy to minimize the risk of detection bias and misclassification. Patients with a past history of CRC diagnosed were included in our study. Estimates of the rate of cancer for the entire cohort were determined by using the Kaplan-Meier survival curve with log-rank test. To identify risk factors associated with development of cancer, we performed univariate time-to-event analysis with Cox proportional regression models that accounted for the case-cohort design by using case weights to account for the sampling frame and robust estimates of variance[22-24]. Variables with P < 0.05 on univariate analysis were included in a multivariate Cox proportional hazard analysis to identify independent risk factors associated with malignancy. Finally, penalized regression models were run using Lasso regression, with 10-fold cross validation, to provide robust estimates of the model coefficients, which should provide better predictions when used with external data[25]. All statistical analyses were conducted using JMP version 10 for Windows (SAS Institute Inc., Cary, NC, United States), SAS (version 9) or R (version 3.2.3).
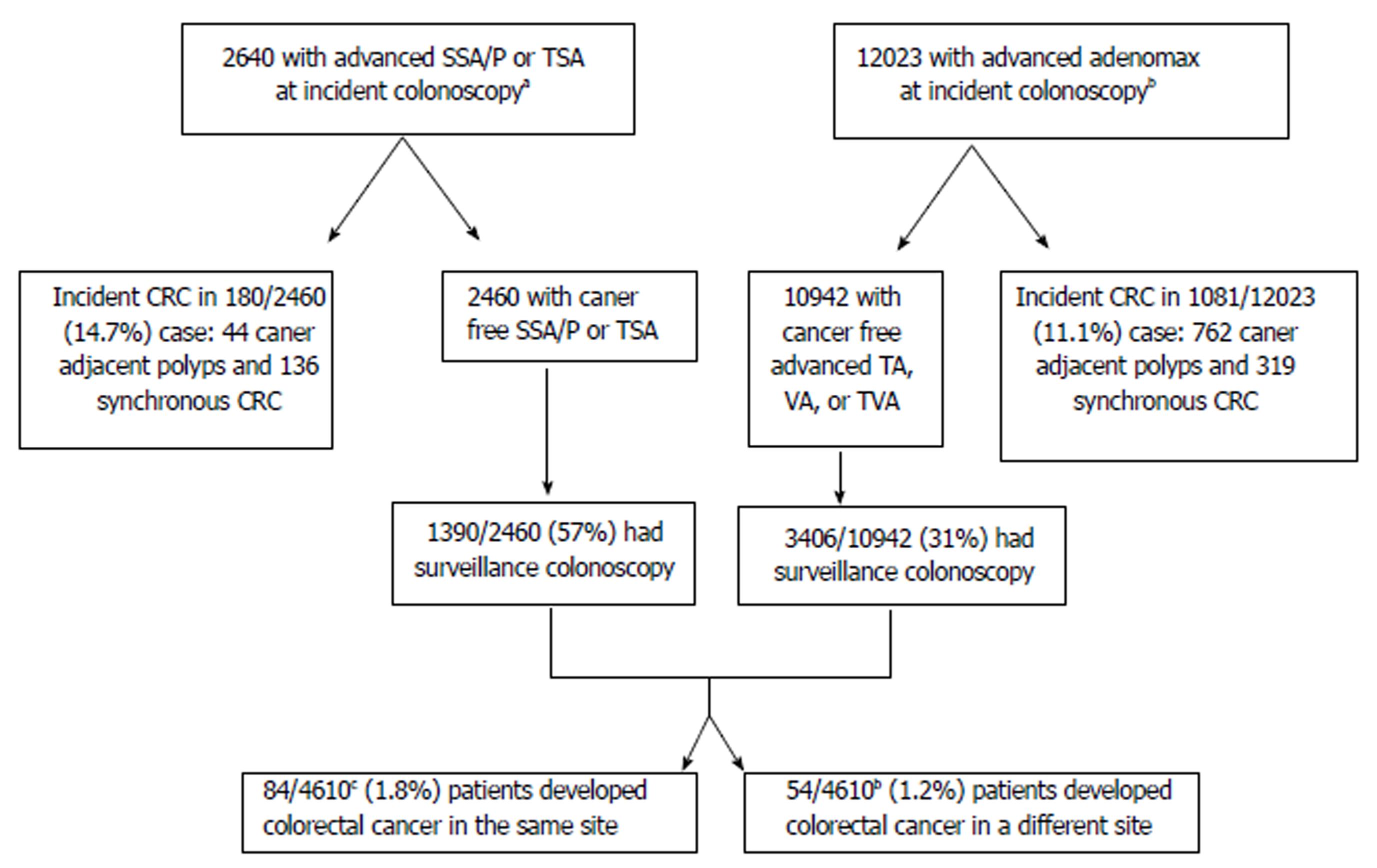
AA/TSA/ASSA were detected in 14633 patients at incident colonoscopy. Of those, 1261 were excluded since they were found to have incident CRC at the time of AA/TSA/ASSA detection. After excluding patients who did not undergo a surveillance colonoscopy after this index polypectomy, 4610 patients were evaluated. Thirty- one of the 1390 (1.67%) of the TSA and ASSA were found to have subsequent CRC, and 107/3406 (3.14%) of the AA patients developed subsequent CRC (P = 0.11) (Figure 1).
Sixty-three patients with history of AA (41 villous, 22 tubular), two with TSA and 19 with ASSA (15 without dysplasia and 4 with dysplasia) who developed CRC at the same site as the index polyp were identified. These 84 patients were compared to a randomly selected cohort of 252 of the AA/TSA/ASSA patients who did not develop post-polypectomy CRC. Patients who developed CRC at the index polypectomy site were significantly older (47.6% vs 33.7%, P = 0.02); had larger index polyps (15.5% vs 7.1%, P = 0.02); had an increased number of synchronous polyps at time of polypectomy (16.7% vs 8.3%, P = 0.03) and were more likely to have AA/TSA/ASSA in the right colon (75% vs 43%; P < 0.01) than the patients who did not develop post-polypectomy CRC. Patients with smaller polyps (> 10 mm and < 20 mm) that would be categorized by EU guidelines were less likely to develop post-polypectomy CRC (P = 0.03). Other findings are shown in Table 1.
| Characteristics | Developed CRC (n = 84) | No CRC (n = 252) | P value1 |
| Demographics | |||
| Male sex | 56 (66.7) | 138 (54.8) | 0.06 |
| Age, yr (≥ 70) | 40 (47.6) | 85 (33.7) | 0.02 |
| Time interval from first treatment for advanced adenoma till cancer or last surveillance colonoscopy in years (median, IQR) | 4.24 (1.51-7.23) | 6.0 (4.05-9.35) | < 0.01 |
| Number of colonoscopies between first polypectomy and cancer (mean ± SD) | 1.65 ± 2.30 | 1.47 ± 1.6 | 0.83 |
| Adenoma size (1-2 cm) | 31 (42.5) | 146 (58.2) | 0.03 |
| Adenoma size (> 2 cm) | 20 (27.4) | 27 (10.8) | 0.0007 |
| Flat/sessile | 76 (91.6) | 161 (66.8) | < 0.01 |
| Degree of dysplasia | |||
| High grade | 28 (33.3) | 66 (26.2) | 0.18 |
| Low grade/no dysplasia | 56 (66.7) | 186 (73.8) | 0.18 |
| Number of attempts to remove the polyp (mean ± SD) | 2.05 ± 1.62 | 1.26 ± 0.60 | < 0.01 |
| Number of polyps resected (> 3 polyps) | 14 (16.7) | 21 (8.3) | 0.03 |
| Polypectomy device used | |||
| Hot snare | 19 (24.1) | 41 (16.3) | 0.11 |
| Cold snare | 5 (6.3) | 26 (10.3) | 0.11 |
| Snare NOS | 60 (75.9) | 185 (73.4) | 0.11 |
| Additional treatments | |||
| Piecemeal removal | 21 (27.6) | 30 (13.1) | < 0.01 |
| Mucosal lift | 13 (17.1) | 23 (10.0) | 0.10 |
| Polyp location | |||
| Right colon | 63 (75.0) | 110 (43.7) | < 0.01 |
| Left colon | 8 (9.52) | 106 (42.1) | < 0.01 |
| Rectum | 13 (15.5) | 36 (14.3) | < 0.01 |
CRC developed over a median follow-up of 2.31 years (IQR: 0.58-4.27). The most common site of post- polypectomy CRC was the cecum (33.3%) followed by the ascending colon (20.2%). Mean tumor size was 3.25 ± 2.0 cm. The proportion of patients with stage 1-2 and stage 3-4 were 38.1% and 53.6%, respectively. Altogether, 6 patients (7.1%) were diagnosed with metastatic disease.
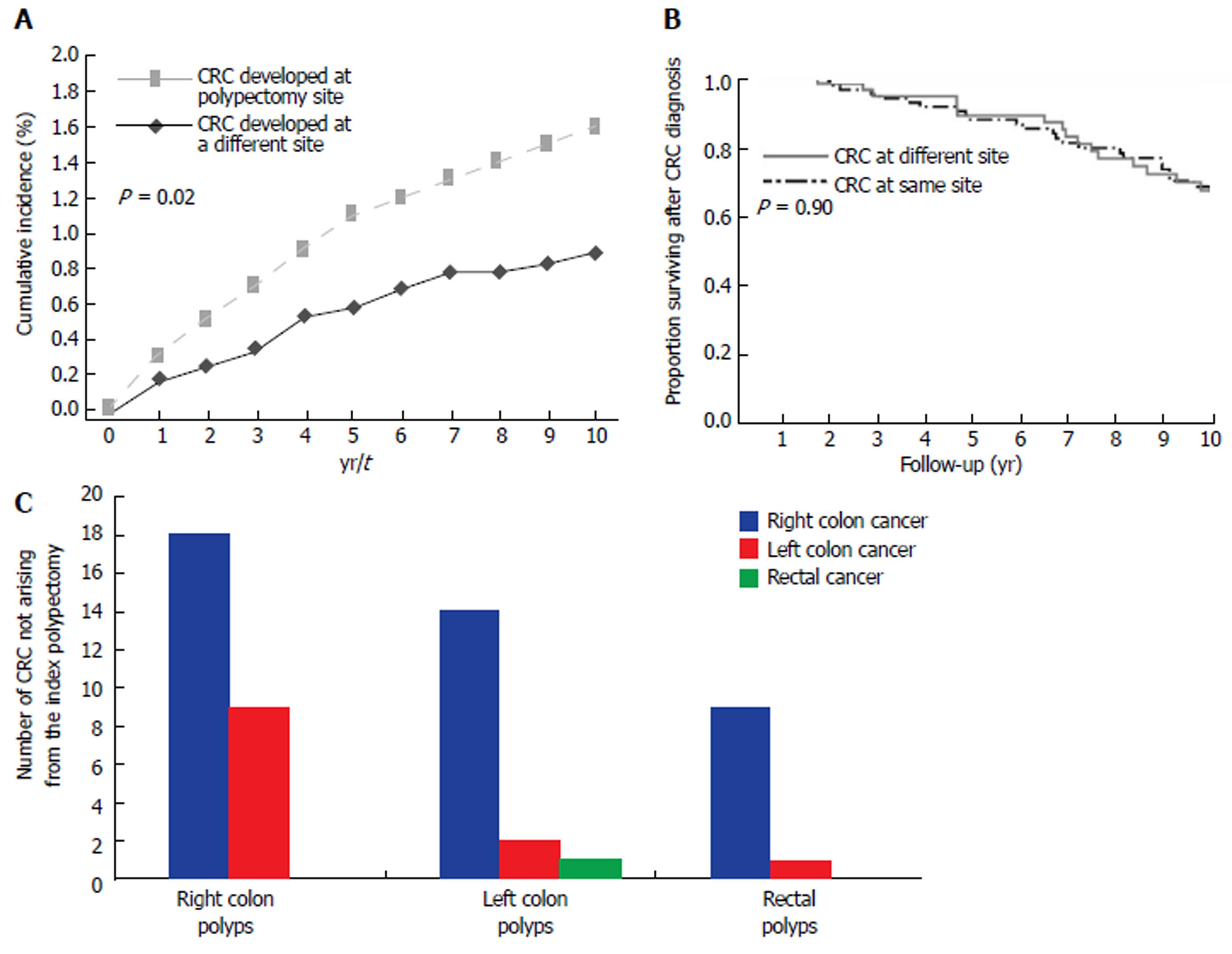
The most common causes associated with post-polypectomy CRC development were non-adherence to recommended surveillance interval (27.4%), incomplete resection of high risk polyp (25.0%), and unknown causes (30%) (Supplementary table 1). Notably, the median time from the index polypectomy to post-polypectomy cancer development ranged from 0.7 years for patients with persistent or recurrent polyps at the index polypectomy site to 3.5 years for patient who developed CRC but had at least one negative surveillance colonoscopy done after the index polypectomy. Patients who had their surveillance colonoscopy later than recommended or who were recommended by their healthcare providers to have follow up of their index AA/TSA/ASSA later than guideline recommendations developed CRC at a median of 6 years after treatment for the index AA/TSA/ASSA (Supplementary table 1). The 1-, 5-, and 10- year cumulative incidences of cancer were 0.3%, 1.1%, and 1.6%, respectively (Figure 2A).
By multivariate analysis, patient age ≥ 70 at time of polypectomy (HR = 2.31, 95%CI: 1.04-5.12, P = 0.04), male sex (HR = 2.87, 95%CI: 1.14-6.81, P = 0.03), polyp size ≥ 20 mm (HR = 3.70, 95%CI: 1.07-12.77, P = 0.04); degree of dysplasia (High vs Low) (HR = 2.59, 95%CI: 1.09-6.18, P = 0.03), higher number of polyps resected (HR = 5.94, 95%CI: 1.98-17.79, P < 0.01), and piecemeal compared to en bloc resection (HR = 5.42, 95%CI: 1.82-16.20, P = 0.01) were all significant factors associated with CRC development. Left vs right colon AA/TSA/ASSA location was associated with decreased risk (HR = 0.09, 95%CI: 0.03-0.29, P < 0.01) (Table 2).
| Risk factors | Univariate analysis, HR (95%CI) | P value | Multivariate analysis, HR (95%CI) | P value |
| Male sex (M:F) | 1.74 (1.01-2.98) | 0.04 | 2.87 ( 1.14-6.81) | 0.03 |
| Age at polypectomy (≥ 70) | 2.51 (1.47-4.27) | < 0.01 | 2.31 (1.04-5.12) | 0.04 |
| Polyp size (≥ 20 mm) | 2.60 (1.17-5.78) | 0.02 | 3.70 (1.07-12.77) | 0.04 |
| Degree of Dysplasia (High:Low) | 1.90 (1.06-3.41) | 0.03 | 2.59 (1.09-6.18) | 0.03 |
| Polyp location (Left colon: Right colon) | 0.10 (0.04-0.22) | < 0.01 | 0.09 (0.03-0.29) | < 0.01 |
| Polyp location (Rectum: Right colon) | 0.47 (0.22-1.02) | 0.06 | - | - |
| Number of polyps resected at polypectomy (> 3 polyps) | 2.40 (1.10-5.24) | 0.03 | 5.94 (1.98-17.79) | < 0.01 |
| Polypectomy device used (Hot snare: Cold snare) | 1.24 (0.49-3.75) | 0.46 | - | - |
| Polyp shape (Flat: Pedunculated) | 6.15 (2.67-14.15) | < 0.01 | 2.79 (0.94-15.23) | 0.06 |
| Piecemeal removal (Yes:No) | 2.80 (1.44-5.46) | < 0.01 | 5.42 (1.82-16.20) | 0.01 |
| Injection-assisted endoscopic mucosal resection (EMR) (Yes:No) | 2.15 (0.98-4.68) | 0.06 | - | - |
Forty-four patients with history of AA (27 villous, 17 tubular); three with TSA and seven with ASSA (four with dysplasia) later developed CRC at a site distinct from that of the incident AA/TSA/ASSA. One hundred and sixty-two patients who underwent polypectomy for AA/TSA/ASSA but did not later develop CRC (Table 3) were randomly selected to be the comparison group matched to the control group based on polyp histology and degree of dysplasia.
| Characteristics | Patients with post-polypectomy CRC (n = 54) | Patients who did not develop CRC (n = 162) | P value1 |
| Demographics | |||
| Male sex | 27 (50.0) | 88 (54.3) | 0.58 |
| Age ≥ 70 | 26 (48.2) | 52 (32.1) | 0.04 |
| Time interval from first treatment for advanced adenoma till cancer or last surveillance colonoscopy in years (median, IQR) | 5.11 (2.67-10.37) | 6.8 (4.0-10.26) | 0.08 |
| Number of colonoscopies between first polypectomy and cancer (mean ± SD) | 1.24 ± 1.58 | 1.65±1.68 | 0.03 |
| Adenoma size (10-20 mm) | 25 (48.1) | 106 (65.8) | 0.03 |
| Adenoma size (≥ 20 mm) | 9 (17.3) | 20 (12.4) | 0.51 |
| Flat/sessile | 47 (92.2) | 91 (58.71) | < 0.01 |
| Degree of dysplasia | |||
| High grade | 17 (31.5) | 51(31.5) | - |
| Low grade/no dysplasia | 37 (68.5) | 111 (68.5) | - |
| Number of attempts to remove the polyp (mean ± SD) | 1.35 ± 0.68 | 1.33 ± 0.68 | < 0.01 |
| Number of polyps resected at polypectomy (> 3 polyps) | 11 (20.4) | 15 (9.5%) | 0.04 |
| Polypectomy Device used | |||
| Hot snare | 12 (22.2) | 26(16.05) | 0.63 |
| Cold snare | 4 (7.4) | 12(7.0) | 0.63 |
| Snare NOS | 38 (70.5) | 127 (76.5) | 0.63 |
| Additional treatments | |||
| Piecemeal removal | 9 (16.7) | 21(13.0) | 0.5 |
| Injection-assisted EMR | 3 (5.6) | 16 (10.0) | 0.33 |
| Polyp location | |||
| Right colon | 31 (57.4) | 40 (24.7) | < 0.01 |
| Left colon | 13 (24.1) | 89 (54.9) | < 0.01 |
| Rectum | 10 (18.5) | 33 (20.4) | < 0.01 |
For the 54 patients who developed CRC at a site distinct from the index polypectomy, the most common sites of the index polyp were the rectum (18.5%) and the transverse colon (18.5%) followed by the ascending colon (16.7%). Forty-eight% of these patients were ≥ 70, while 32.1% of patients who did not develop CRC were ≥ 70 years old (P = 0.04). Fifty percent of these patients with post-polypectomy CRC and 54% of those in the comparison group were male. CRC developed over a median follow-up of 2.64 years (1.0-6.33). Mean tumor size was 4.48 ± 4.81 cm. The most common site of CRC was the transverse colon (22.2%) followed by the cecum (18.5%), the ascending colon (18.5%), and the hepatic flexure (18.5%). The proportion of patients with stage 1-2 and stage 3-4 were 31.5% and 57.4%, respectively. In 10 cases, the patients (18.5%) were diagnosed with metastatic disease. Details of patient characteristics are shown in Table 3.
The most common causes associated with post-polypectomy CRC development at another site were non-adherence to recommended USPSTF surveillance intervals (31.5%), followed by unknown causes (27.8%), and incomplete colonoscopy (26.0%) (Supplementary table 2). The 1-, 5-, and 10- year cumulative incidences of cancer were 0.17%, 0.56%, and 0.87%, respectively (Figure 2A). The median survival after CRC development was not significantly different for patients who developed CRC at the same site as compared to those who developed CRC at a different site (15.2 years vs 12.7 years, P = 0.90)( Figure 2B).
Right-sided post-polypectomy CRC were more common than left-sided CRC (P < 0.01) (Figure 2C). By multivariate analysis, patient age ≥ 70 years at time of polypectomy (HR = 3.02, 95%CI: 1.23-7.41, P = 0.02); polyp shape (sessile vs pedunculated) (HR = 3.92, 95%CI: 1.10-14.04, P = 0.04) and number of polyps resected (HR = 4.05, 95%CI: 1.38-11.90, P = 0.01) were significant factors associated with CRC development at another site. Polyp location (left vs right) (HR = 0.23, 95%CI: 0.08-0.63, P < 0.01) was associated with decreased risk (Table 4).
| Risk factors | Univariate analysis, HR (95%CI) | P value | Multivariate analysis, HR (95%CI) | P value |
| Male sex (M:F) | 0.79 (0.40-1.57) | 0.50 | - | - |
| Age at polypectomy (≥ 70) | 3.68 (1.82-7.48) | < 0.01 | 3.02 (1.23-7.41) | 0.02 |
| Polyp size (≥ 20 mm) | 1.93 (0.77-4.83) | 0.16 | - | - |
| Degree of dysplasia (High: Low) | 1.41 (0.65-3.08) | 0.39 | - | - |
| Polyp location (Left colon: Right) | 0.15 (0.07-0.34) | < 0.01 | 0.23 (0.08-0.63) | < 0.01 |
| Polyp location (Rectum:Right colon) | 0.22 (0.07-0.68) | < 0.01 | 0.23 (0.05-1.00) | 0.05 |
| Number of polyps resected at polypectomy (> 3 polyps) | 2.94 (1.14-7.54) | 0.03 | 4.05 (1.38-11.90) | 0.01 |
| Polypectomy device used (Hot snare:Cold snare) | 0.64 (0.22-2.34) | 0.24 | - | - |
| Polyp shape (Flat/sessile: Pedunculated) | 8.0 (2.62-24.05) | < 0.01 | 3.92 (1.10-14.04) | 0.04 |
| Piecemeal removal (Yes:No) | 1.68 (0.67-4.22) | 0.27 | - | - |
| Injection-assisted EMR (Yes:No) | 0.70 (0.19-2.60) | 0.59 | - | - |
This study showed that there is a persistent risk for post-polypectomy CRC despite surveillance colonoscopy for those polyps known to have the highest risk for malignant transformation. Even under watchful, directed colonoscopic surveillance and management of those polyps with the highest risk, 1.8% of patients developed post polypectomy CRC at the index polyp site and 1.2% developed CRC at a site distinct from the index AA/TSA/ASSA.
Villous and tubular adenomas were the most commonly observed histologies. ASSA/TSA were less common, possibly due to limited recognition of the serrated-cancer pathway during the time frame in this study, but which has improved within the last decade. One-third of patients developed CRC at the polypectomy site despite following appropriate surveillance intervals. This could be secondary to high endoscopic miss rate or rapidly-progressing cancer development. We found that increasing age at the time of polypectomy, number of polyps, polyp size, location, degree of dysplasia, and piecemeal resection were associated with increased CRC risk.
CRC developed at the index AA/TSA/ASSA polypectomy site in 1.8% (84/4610) of patients despite apparent initial complete resection of the high risk polyp. In 25% of these cases in which CRC developed at the index polypectomy site, the polyp had been found on surveillance colonoscopy either to have persisted or recurred, and subsequently progressed to cancer. It is possible that some polyps were missed, since colonoscopy has a failure rate of 6%-12% in detecting adenomas > 10 mm[26,27]. Alternatively, this could be explained by rapid progression from adenoma to CRC or by de novo CRC formation[28]. In spite of at least one surveillance colonoscopy in which there was no endoscopic evidence of recurrence of the index polyp, CRC was identified at the index polypectomy site on subsequent colonoscopy in nearly one third of those who developed same site post polypectomy CRC.
Polypectomy techniques have been implicated as one potential risk factor for post polypectomy CRC. Endoscopic piecemeal mucosal resection has been reported to be associated with 12.2%-55% rate for recurrence at the polypectomy site[29-33], which is known to be suboptimal for full resection of flat polyps in part because of the difficulty to completely identify, and thus include with the resection, the tissue bordering the polyp. Walsh et al studied 65 patients with large flat polyps treated with piecemeal resection with electrocautery snare. Nearly 14% of the polyps recurred after at least one negative intervening examination, and CRC developed in 17% of the patients after complete resection of the large polyp[30]. In another study, the rate of recurrence after endoscopic mucosal resection (EMR) with mucosal lift was observed in 7% of patients with flat polyps[34]. In our study, CRC occurred in 21 (27.6%) and 13(17.1%) of the patients who received piecemeal resection and en bloc EMR with mucosal lift, respectively. Piecemeal snare excision but not EMR with mucosal lift was an independent risk factor for post polypectomy CRC in this study; but further prospective studies are needed to examine the prognostic utility of EMR with CRC development.
In our study, poor adherence to current surveillance guidelines appeared to contribute to 8.3% of the cases of post-colonoscopy CRC. A previous study showed that delayed surveillance interval was associated with the development of CRC in almost 2% of patients post polypectomy for advanced adenoma[35]. Risk factors for pent surveillance was associated with a colonoscopist’s having finished colonoscopy training prior to 1990; presently being in training; practicing in a non-academic setting, and performing a low life time number of colonoscopies[36].
In our study, we confirmed the finding by Robertson et al[37] that patients who are older at the time of polypectomy for AA are more likely to develop post-polypectomy CRC. Toll et al reported that CRC developed in 7% of patients with large polyps with high grade dysplasia over an average of 7 mo[38]. We also found that patients with right-sided AA/TSA/ASSA are more likely than those left-sided high risk polyps to develop same site post-polypectomy CRC, possibly due to the fact that flat polyps are more likely to arise in the right-side of the colon and are more easily missed[39]. Our study, like other studies, have implicated large (≥ 20 mm ) polyps as particularly high risk and in need of a follow-up colonoscopy relatively soon after initial resection because the residual polyp could persist or recur with subsequent progression to CRC after polypectomy[40]. In this group of patients with persistent or recurrent high risk polyps, markers that predict whether a polyp needs to be removed with a colon resection to prevent CRC have yet to be identified. The clinical or molecular clues that distinguish the three quarters of patients with recurrent AA/TSA/ASSA who are able to be successfully treated with colonoscopic therapy from the ¼ of patients in whom the recurrent polyps will progress to cancer need to be expanded beyond the current features that declare a polyp as “high risk”.
Our study highlights the risk of missing additional adenomas or cancers at a surveillance colonoscopy for follow up of an index AA/TSA/ASSA. Recognition that post-polypectomy CRC can happen at a site distinct from the index polypectomy even in individuals undergoing more intensive surveillance may be leveraged to improve the success rates of surveillance colonoscopy. It is possible that by expanding the proceduralist’s attention beyond evaluation of the target lesion - in addition to utilizing each opportunity at the surveillance colonoscopy to perform a thorough examination of the entire colon - may decrease the unanticipated and undesired outcome of CRC developing in spite of repeated surveillance. Adenoma miss rates during colonoscopic surveillance have been reported to range from 6% to 27%[41]. Bressler et al[2] reported that the rates for new/missed colon cancer which developed within 6-36 mo after colonoscopy were approximately 6.0% in the right colon; 5.5% in the transverse colon; 2.0% in the descending colon; and 2.3% in the rectosigmoid colon[2]. Positive screening tests such as Cologuard™ could improve colonoscopy performance. Johnson et al found that endoscopists who were aware of the Cologuard™ results spent more time and found more hemorrhagic and precancerous polyps than blinded endoscopists[41]. Features other than colonoscopy adenoma detection and polypectomy skills may contribute to these post-polypectomy CRCs at either the index site or in other areas of the colon.
Our study has several limitations. In addition to the retrospective nature of our study, we were not able to obtain data on all patients who did not develop CRC due to the large size of this cohort. To obtain reliable data would have necessitated manual review of over 4000 patient medical records not available in the electronic medical record to confirm the colonoscopy and pathology data for surveillance colonoscopies done both at Mayo and at other healthcare centers. Therefore, the relatively small number of post-polypectomy CRC cases was compared to a randomly selected portion of patients who did not develop post polypectomy CRC, rather than to the entire cancer free cohort. Another limitation is the relatively low number of TSA or ASSA patients compared to those with AA. We did not account for other confounding factors associated with higher lifetime risks and mortality from CRC such as the patient’s BMI, smoking exposure, exercise, use of aspirin or NSAIDs, prior colonoscopy exams, or the adenoma detection rate of the performing colonoscopist[42].
This study shows that the applicability of current evidence-based surveillance guidelines to some patients with AA/TSA/ASSA is limited. There is insufficient data to provide explicit guidance for the follow up of polyps removed using specific treatments such as piecemeal endoscopic resection[11]. Current surveillance guidelines do not incorporate the impact of multiple high-risk features such as the risk of a large AA/TSA/ASSA being more recalcitrant or at higher risk for progressing to cancer if present in the right versus left side of the colon or the age of the patient. Current USPSTF guidelines recommend 3-year surveillance interval following polypectomy of adenoma with high-grade dysplasia but does not account for other features[11,43]. European guidelines stratify intermediate risk polyps as having a lower risk than EU guideline high risk polyps ≥ 20 mm, and recommend surveillance at 1 year for high risk polyps. Our findings that post-polypectomy CRC was significantly associated with high, but not intermediate risk polyps as classified by EU guidelines supports the need for a one year surveillance colonoscopy for these larger polyps currently not addressed in the USPSTF recommendations. Developing a risk score to optimize risk stratifications of patients with AA/TSA/ASSA might result in better discrimination between low- and high-risk patients. A recent study developed a scoring system based on older age, male sex, adenoma number, size ≥ 10 mm, villous histology, and proximal location at index colonoscopy; which were found to be independent predictors for detecting AA/TSA/ASSA, but not cancer, at surveillance endoscopy[44]. Having additional tools to risk stratify polyps will assist with making recommendations for surveillance, could identify tissue or molecular features that might be used to improve visualization of polyps, and stratify the risks that a polyp might recur or progress to cancer.
To our knowledge, this is the first study to determine risk factors for incident CRC at the same site or at another site in the colon following polypectomy of advanced lesions. Current guidelines are still limited in detecting such patients. Our study supports Atkin et al’s[45] study who recently reported that the incidence of CRC in patients was higher in patients with suboptimal quality colonoscopy, proximal polyps, large or high-grade polyps at baseline. Patients with increasing age and a history of large, multiple, highly dysplastic, right-sided, and difficult to remove adenomas requiring piecemeal resection are a high-risk population for the development of CRC at the same site. Increasing age and the presence of flat and/or right-sided adenomas increased the risk of CRC at another site. A diagnosis of CRC soon after complete colonoscopy may imply the need for shortened surveillance intervals. Understanding risk factors for subsequent CRC development and developing molecular markers predictive of progression to cancer are important for individualizing surveillance recommendations following adenoma removal since colonoscopy is not 100% sensitive tool in the identification or prevention of CRC in this population. In order to better stratify a polyp’s risk for recurrence and subsequent CRC will require further research to identify molecular or other features to guide more individualized polyp management.
Screening colonoscopy has a 3.5% false negative rate for detection of colorectal cancer (CRC) resulting in 17% of patients who had undergone colon screening within 3 years being diagnosed with CRC. However, no large studies have assessed the frequency and risk factors for CRC development among individuals following advanced adenoma (AA)/traditional serrated adenoma (TSA)/advanced sessile serrated adenoma (ASSA) removal. Recognition of this group at high-risk for interval CRC is one step toward preventing morbidity and mortality associated with CRC development.
Recognition that CRC could develop following AA/TSA/ASSA removal despite adherence to guidelines is one step toward improving our practice efficiency and preventing a portion of CRC related morbidity and mortality. Understanding risk factors and developing molecular markers that predict progression may become important in order to individualize surveillance recommendations and recognize those AA/TSA/ASSA patients at high-risk for interval CRC.
To report the frequency of interval CRC development following high-risk polypectomy at the polypectomy site and another site distinct from polypectomy site and to identify risk factors associated with development of cancer. Realizing these objective is critical for future research since current evidence-based surveillance guidelines are limited in predicting CRC risk in these patients.
We reviewed medical records of all adult patients ( ≥ 18 years of age) who underwent colonoscopy (between January 1990 to December 2010 ) and were found to have high-risk polyps ( either AA between January 1990 to December 2010 or ASSA/TSA between January 2000 to December 2010 ) to identify 4160 patients who had at least one follow-up surveillance colonoscopy following polypectomy. We excluded patients with IBD, polyposis syndromes or other genetic syndromes predisposing for CRC. Patients with a past history of CRC were not excluded from our study. From this cohort, we identified 84 patients who had developed CRC and matched to 252 patients who had not developed CRC based on polyp histology and size (< or ≥ 20 mm), degree of dysplasia and decade that the index polyp was removed. Data abstracted included clinical and pathological features of high-risk polyps, number and timing of surveillance colonoscopies and post polypectomy CRC.
The data are reported as mean (± SD), median (interquartile range, IQR), ranges, and categorical variables by counts and percentages as appropriate. Estimates of the rate of cancer for the entire cohort were determined by using the Kaplan-Meier survival curve with log-rank test. We performed univariate time-to-event analysis with Cox proportional regression models to identify risk factors associated with development of cancer. Variables with P < 0.05 on univariate analysis were included in a multivariate Cox proportional hazard analysis to identify independent risk factors associated with malignancy. Finally, penalized regression models were run using Lasso regression, with 10-fold cross validation, to provide robust estimates of the model coefficients, which should provide better predictions when used with external data. All statistical analyses were conducted using JMP version 10 for Windows (SAS Institute Inc., Cary, NC, United States), SAS (version 9) or R (version 3.2.3).
Despite colonoscopic surveillance and management of high-risk polyps, 1.8% of patients developed post polypectomy CRC at or the index polyp site and 1.2% developed CRC at a site distinct from the index AA/TSA/ASSA. About one-third of patients developed CRC at the polypectomy site despite following appropriate surveillance intervals. Increasing age at the time of polypectomy, number of polyps, polyp size, location, degree of dysplasia, and piecemeal resection were associated with increased CRC risk. Current surveillance guidelines are not sufficient since it does not take into account the impact of multiple high-risk features of high-risk polyps for CRC development. This study also highlights the risk of missing additional adenomas or cancers at a surveillance colonoscopy for follow up of an index AA/TSA/ASSA. Resection technique (Piecemeal snare excision) was an independent risk factor for post polypectomy CRC in this study; but further prospective studies are needed to examine the prognostic utility of EMR with CRC development.
1.8% of patients developed post polypectomy CRC at the index polyp site and 1.2% developed CRC at a site distinct from the index AA/TSA/ASSA despite surveillance colonoscopy. Surveillance colonoscopy for high-risk polyp does not always prevent CRC cancer development. Current surveillance guidelines are not sufficient in predicting CRC risk in some patients. Incorporate the impact of multiple high-risk features of resected polyps in surveillance guidelines. Interval CRC develops after high-risk polyp resection despite being in a surveillance program. We compared patients who had developed interval CRC after high-risk polyp resection at same site and different site and matched to patients who had not developed CRC to identify risk factors associated with CRC development. Patients with increasing age and a history of large, multiple, highly dysplastic, right-sided, and difficult to remove adenomas requiring piecemeal resection are a high-risk population for the development of CRC at the same site. Increasing age and the presence of flat and/or right-sided adenomas increased the risk of CRC at another site. Colonoscopy is not 100% sensitive tool in the identification or prevention of CRC. Shortened surveillance intervals may be needed post-polypectomy in some patients with multiple high-risk features.
Interval CRC cancer rate after high-risk polyp resection is low yet CRC does develop in spite of post-polypectomy surveillance. We require further research to identify molecular or other features to guide more individualized polyp management. Study molecular features of patients who developed CRC at the polypectomy site despite following appropriate surveillance intervals
We wish to thank our work study collaborators Paige Diedrick and Alexander Leehan for their assistance in reviewing clinical records.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Augustin G, Chiu CT, Lakatos PL, Perez-Cuadrado-Robles E, Sali L, Zorzi M S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ
| 1. | Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 428] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 2. | Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 449] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 3. | Than M, Witherspoon J, Shami J, Patil P, Saklani A. Diagnostic miss rate for colorectal cancer: an audit. Ann Gastroenterol. 2015;28:94-98. [PubMed] |
| 4. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2912] [Article Influence: 364.0] [Reference Citation Analysis (3)] |
| 5. | Welch HG, Robertson DJ. Colorectal Cancer on the Decline--Why Screening Can’t Explain It All. N Engl J Med. 2016;374:1605-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1501] [Cited by in RCA: 1437] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 7. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 554] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 8. | Umetani N, Sasaki S, Masaki T, Watanabe T, Matsuda K, Muto T. Involvement of APC and K-ras mutation in non-polypoid colorectal tumorigenesis. Br J Cancer. 2000;82:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, Cross AJ, Zauber AG, Church TR, Lance P. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 338] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 10. | Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1445] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 12. | Colucci PM, Yale SH, Rall CJ. Colorectal polyps. Clin Med Res. 2003;1:261-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 2000;95:3053-3063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 418] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 431] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 16. | Wiland HO 4th, Shadrach B, Allende D, Carver P, Goldblum JR, Liu X, Patil DT, Rybicki LA, Pai RK. Morphologic and molecular characterization of traditional serrated adenomas of the distal colon and rectum. Am J Surg Pathol. 2014;38:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-29; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 830] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 18. | Steele RJ, Pox C, Kuipers EJ, Minoli G, Lambert R; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Management of lesions detected in colorectal cancer screening. Endoscopy. 2012;44 Suppl 3:SE140-SE150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Leung K, Pinsky P, Laiyemo AO, Lanza E, Schatzkin A, Schoen RE. Ongoing colorectal cancer risk despite surveillance colonoscopy: the Polyp Prevention Trial Continued Follow-up Study. Gastrointest Endosc. 2010;71:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 691] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 21. | Sanduleanu S, le Clercq CM, Dekker E, Meijer GA, Rabeneck L, Rutter MD, Valori R, Young GP, Schoen RE; Expert Working Group on ‘Right-sided lesions and interval cancers’, Colorectal Cancer Screening Committee, World Endoscopy Organization. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64:1257-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 22. | Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 371] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | Binder S. Hazards of low-level lead exposure recognized. Am J Public Health. 1992;82:1043-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Prentice RL. On the design of synthetic case-control studies. Biometrics. 1986;42:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Simon N, Friedman J, Hastie T, Tibshirani R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. J Stat Softw. 2011;39:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1357] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 26. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1049] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 27. | Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1495] [Cited by in RCA: 1284] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 28. | Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, Axon AT. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 410] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Seo GJ, Sohn DK, Han KS, Hong CW, Kim BC, Park JW, Choi HS, Chang HJ, Oh JH. Recurrence after endoscopic piecemeal mucosal resection for large sessile colorectal polyps. World J Gastroenterol. 2010;16:2806-2811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Walsh RM, Ackroyd FW, Shellito PC. Endoscopic resection of large sessile colorectal polyps. Gastrointest Endosc. 1992;38:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Zlatanic J, Waye JD, Kim PS, Baiocco PJ, Gleim GW. Large sessile colonic adenomas: use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc. 1999;49:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Brooker JC, Saunders BP, Shah SG, Williams CB. Endoscopic resection of large sessile colonic polyps by specialist and non-specialist endoscopists. Br J Surg. 2002;89:1020-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Khashab M, Eid E, Rusche M, Rex DK. Incidence and predictors of “late” recurrences after endoscopic piecemeal resection of large sessile adenomas. Gastrointest Endosc. 2009;70:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Ferrara F, Luigiano C, Ghersi S, Fabbri C, Bassi M, Landi P, Polifemo AM, Billi P, Cennamo V, Consolo P. Efficacy, safety and outcomes of ‘inject and cut’ endoscopic mucosal resection for large sessile and flat colorectal polyps. Digestion. 2010;82:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | van Heijningen EM, Lansdorp-Vogelaar I, Steyerberg EW, Goede SL, Dekker E, Lesterhuis W, ter Borg F, Vecht J, Spoelstra P, Engels L. Adherence to surveillance guidelines after removal of colorectal adenomas: a large, community-based study. Gut. 2015;64:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Iskandar H, Yan Y, Elwing J, Early D, Colditz GA, Wang JS. Predictors of Poor Adherence of US Gastroenterologists with Colonoscopy Screening and Surveillance Guidelines. Dig Dis Sci. 2015;60:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Robertson DJ, Greenberg ER, Beach M, Sandler RS, Ahnen D, Haile RW, Burke CA, Snover DC, Bresalier RS, McKeown-Eyssen G. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 38. | Toll AD, Fabius D, Hyslop T, Pequignot E, DiMarino AJ, Infantolino A, Palazzo JP. Prognostic significance of high-grade dysplasia in colorectal adenomas. Colorectal Dis. 2011;13:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Nawa T, Kato J, Kawamoto H, Okada H, Yamamoto H, Kohno H, Endo H, Shiratori Y. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol. 2008;23:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 40. | Nivatvongs S, Snover DC, Fang DT. Piecemeal snare excision of large sessile colon and rectal polyps: is it adequate? Gastrointest Endosc. 1984;30:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Ahn SB, Han DS, Bae JH, Byun TJ, Kim JP, Eun CS. The Miss Rate for Colorectal Adenoma Determined by Quality-Adjusted, Back-to-Back Colonoscopies. Gut Liver. 2012;6:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 42. | Meester RG, Doubeni CA, Lansdorp-Vogelaar I, Jensen CD, van der Meulen MP, Levin TR, Quinn VP, Schottinger JE, Zauber AG, Corley DA. Variation in Adenoma Detection Rate and the Lifetime Benefits and Cost of Colorectal Cancer Screening: A Microsimulation Model. JAMA. 2015;313:2349-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, Zauber AG, Jiang R, Ahnen DJ, Bond JH. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 44. | van Heijningen EM, Lansdorp-Vogelaar I, van Hees F, Kuipers EJ, Biermann K, de Koning HJ, van Ballegooijen M, Steyerberg EW; SAP Study Group. Developing a score chart to improve risk stratification of patients with colorectal adenoma. Endoscopy. 2016;48:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Atkin W, Wooldrage K, Brenner A, Martin J, Shah U, Perera S, Lucas F, Brown JP, Kralj-Hans I, Greliak P. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18:823-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |