Published online Feb 21, 2018. doi: 10.3748/wjg.v24.i7.810
Peer-review started: November 7, 2017
First decision: November 30, 2017
Revised: December 11, 2017
Accepted: December 19, 2017
Article in press: December 19, 2017
Published online: February 21, 2018
Processing time: 95 Days and 5.6 Hours
To demonstrate the feasibility of cryopreservation of peripheral blood mononuclear cells (PBMCs) for prognostic circulating tumor cell (CTC) detection in gastroesophageal cancer.
Using 7.5 mL blood samples collected in EDTA tubes from patients with gastroesopheagal adenocarcinoma, CTCs were isolated by epithelial cell adhesion molecule based immunomagnetic capture using the IsoFlux platform. Paired specimens taken during the same blood draw (n = 15) were used to compare number of CTCs isolated from fresh and cryopreserved PBMCs. Blood samples were processed within 24 h to recover the PBMC fraction, with PBMCs used for fresh analysis immediately processed for CTC isolation. Cryopreservation of PBMCs lasted from 2 wk to 25.2 mo (median 14.6 mo). CTCs isolated from pre-treatment cryopreserved PBMCs (n = 43) were examined for associations with clinicopathological variables and survival outcomes.
While there was a significant trend to a decrease in CTC numbers associated with cryopreserved specimens (mean number of CTCs 34.4 vs 51.5, P = 0.04), this was predominately in samples with a total CTC count of > 50, with low CTC count samples less affected (P = 0.06). There was no significant association between the duration of cryopreservation and number of CTCs. In cryopreserved PBMCs from patient samples prior to treatment, a high CTC count (> 17) was associated with poorer overall survival (OS) (n = 43, HR = 4.4, 95%CI: 1.7-11.7, P = 0.0013). In multivariate analysis, after controlling for sex, age, stage, ECOG performance status, and primary tumor location, a high CTC count remained significantly associated with a poorer OS (HR = 3.7, 95%CI: 1.2-12.4, P = 0.03).
PBMC cryopreservation for delayed CTC isolation is a valid strategy to assist with sample collection, transporting and processing.
Core tip: This study demonstrates a novel and robust protocol for the cryopreservation and thawing of patient blood samples, demonstrating reliable circulating tumor cell isolation and characterisation after the long term storage of patient samples. Using the largest patient cohort reported to date, we validated our method by confirming the independent prognostic association of circulating tumor cell (CTC) enumeration from cryopreserved peripheral blood mononuclear cells. Cryopreservation may assist with the wider incorporation of CTC collection and analysis in biobanking, retrospective studies, and large international clinical trials, by facilitating specimen storage, bulk transporting, and batch processing.
- Citation: Brungs D, Lynch D, Luk AW, Minaei E, Ranson M, Aghmesheh M, Vine KL, Carolan M, Jaber M, de Souza P, Becker TM. Cryopreservation for delayed circulating tumor cell isolation is a valid strategy for prognostic association of circulating tumor cells in gastroesophageal cancer. World J Gastroenterol 2018; 24(7): 810-818
- URL: https://www.wjgnet.com/1007-9327/full/v24/i7/810.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i7.810
Circulating tumor cell (CTC) analysis continues to be a rapidly developing field in oncology, offering a promising tool to both prognosticate and guide managements for patients[1]. Despite recent advancements in the field, one persisting challenge to the widespread adoption of CTC analysis for translational clinical trials or routine clinical care is the limited time frame considered best for blood processing and CTC isolation. Usually fresh blood is processed for CTCs within 24 h after blood draw, requiring prompt transfer to specialised centres for CTC isolation and analysis, which offers significant logistical challenges[2]. To overcome this issue, some studies use blood collection tubes that contain fixatives. Fixation of blood samples can allow CTC processing delayed by several days which has proven very useful for some CTC analyses[3,4]. However, fixatives may interfere with down-stream molecular analyses that require isolation of nucleic acids[5]. An alternative is the use of cryopreservation protocols for peripheral blood mononuclear cells (PBMCs) to allow delayed CTC isolation from these cells followed by CTC analysis. Cryopreservation should overcome fixation related analysis limitations and allow far more flexible time frames for batched CTC processing. However, a defined, robust cryopreservation protocol that is proven to enable analysis of the same or at least a relevant proportion of CTCs to that found in fresh samples, needs to be adopted and confirmation is needed whether cryopreserved CTCs can still predict disease outcome.
The advantage of cryopreservation of PBMCs is that it requires only minimal local processing, possible in most diagnostic settings, as well as feasible cryostorage and frozen transport of PBMC samples.
While there are a large number of approaches used to isolate and identify circulating tumor cells (recently reviewed by van der Toom et al[6]), the best established and most widely used is the CellSearchTM system (Menarini-Silicon Biosystems), which uses positive immunomagnetic isolation of epithelial cell adhesion molecule (EpCAM, an epithelial cell marker) expressing cells followed by cytokeratin (CK), CD45, and DAPI staining[2]. The CTCs are then identified with automated immunofluorescence microscopy, defined by an EpCAM/CK/DAPI positive and CD45 negative phenotype. Cell Search CTC counts have shown to be prognostic in large patient series in a variety of cancers[7-9], including gastroesophageal cancer[10-12], but the instrument offers limited sensitivity in resectable gastroesophageal cancer, with CTCs detected in less than 15% of patients[10,13]. The IsoFlux system (Fluxion) uses a similar definition of CTCs to CellSearch (EpCAM/CK/DAPI positive, CD45 negative phenotype), but has shown a greater sensitivity for CTC detection[14-16]. This platform uses EpCAM targeted immunomagnetic isolation of CTCs within a microfluidic setting, improving isolation of CTCs with lower EpCAM expression, minimising leukocyte contamination, and allowing downstream applications including staining, enumeration, or sequencing, as shown for fresh blood samples[16].
Here, we use a viable method of PBMC cryopreservation that allows subsequent isolation and immunocytochemical analysis of CTCs. We demonstrate the feasibility of PBMC cryopreservation for delayed CTC isolation using paired cryopreserved and freshly processed blood samples drawn at the same time from patients with gastroesophageal adenocarcinoma. Importantly, we also provide data confirming that cryopreserved CTCs remain clinically applicable as a circulating prognostic marker for overall survival (OS).
Blood samples were collected from patients with histologically confirmed distal oesophageal, gastroesophageal junction, or gastric adenocarcinomas treated at Wollongong Hospital, Australia. Blood samples were collected in 7.5 mL EDTA Vacutainer tubes (Sarstedt AG & Co.) and maintained at room temperature until processing.
In the initial cohort (Cohort 1) to confirm the feasibility of cryopreservation, 15 patients with gastroesophageal carcinomas had 2 specimens taken during the one blood draw, one processed within 24 h (“fresh” specimen), and one cryopreserved with delayed CTC isolation and analysis (“cryopreserved” specimen). Pre-treatment blood samples were cryopreserved from a second, larger cohort of patients for correlation with clinical outcomes (Cohort 2). The study was approved by South Western Sydney Local Health District Human Research Ethics Committee (Project Number 15/072). A written informed consent was obtained from each participant before sample collection.
Blood samples were processed within 24 h to recover the PBMC fraction using 50 mL SepMate tubes and Lymphoprep according to manufacturer’s instructions (Stemcell Technologies, Vancouver, BC, Canada).
PBMCs used for fresh analysis were resuspended in Isoflux Binding Buffer and immediately processed for CTC isolation (see below).
PBMCs for cryopreservation were well resuspended in 1 mL of diluted plasma (the supernatant of the PBMC preparation from the matching patient) with the addition of 7.5% final DMSO, and stored at -80 °C until further processing. Cryopreserved samples were thawed according to the protocol from Fluxion Biosciences, San Francisco, California, United States[17]. In brief, warmed (37 °C) thawing buffer, consisting of RPMI 1640 with 10% Fetal Bovine Serum (FBS, Bovogen Biologicals, Australia) and 50 Unit/mL Benzonase (Sigma-Aldrich, Germany), was added to thawed samples, washed once in thawing buffer, and resuspended in IsoFlux Binding Buffer with 5% FBS.
As per the Fluxion protocol, immunomagnetic beads preconjugated with anti-EpCAM antibodies (CTC Enrichment Kit; Fluxion Biosciences Inc) were added to PBMCs suspended in IsoFlux Binding Buffer, and incubated for 90 min at 4 °C with passive mixing on a rotator. Samples were then loaded into the sample well of the microfluidic cartridge and underwent immunomagnetic isolation of CTCs with the IsoFlux using the standard protocol (Fluxion Biosciences Inc).
Recovered CTCs were blocked with a final concentration of 1.2 μg/μL mouse IgG in binding buffer (Jackson ImmunoResearch, Baltimore, PA, United States) for 30 min, washed and fixed in fixing solution (Fluxion Biosciences Inc). The CTCs were then blocked in 10% FBS in binding buffer for 15 min, then underwent immunofluorence staining for anti-CD45 antibody conjugated to Alexa Fluor 647 (Biolegend, Clone HI30). The CTCs were also stained for urokinase plasminogen activator receptor (uPAR, CD87), a key receptor in the plasminogen activator system and clinically relevant biomarker in primary gastroesophageal cancer[18], using anti-uPAR antibody conjugated to AF594 (ThermoFischer, Clone R4). After permeabilization with 0.1% Triton X-100, cells were probed with anti-cytokeratin antibody conjugated to FITC (Sigma-Aldrich, Clone PCK-26). CTCs were finally stained with Hoechst and mounted using Isoflux mounting media to 24-well glass bottom plates (MoBioTec, Goettingen, Germany) for imaging.
Imaging was performed with an inverted epifluorescence microscope (Leica DMi8, Leica Microsystems Pty Ltd) using the Leica Application Suite. Cells were considered CTCs if they were CK positive, CD45 negative, nucleated and morphologically intact. The proportion of uPAR positive CTCs was recorded.
The CTC recovery from matched cryopreserved and fresh samples were compared with the paired t-test. Correlation between cryopreservation time and CTC number was described with a Pearson correlation coefficient, and the Fisher exact test and t-test were used to compare the status of CTCs with categorical clinicopathologic factors.
For survival analyses, in the absence of established cut-offs for prognostic CTC numbers, the median CTC count (17) was used as the discriminator between high and low CTC counts. Survival analyses are conducted using Kaplan-Meier methods, with median survival reported. Unadjusted and multivariable Cox proportional hazards regression analyses were used to estimate the association between CTC counts and survival, and to calculate corresponding hazard ratios (HRs) and 95% confidence intervals (CIs). The following variables were included in the multivariate model: age, sex, ECOG, TNM stage, primary tumor location, and CTC count. All statistical analyses were performed using SAS 9.2 software (SAS Institute, Inc., Cary, NC, United States).
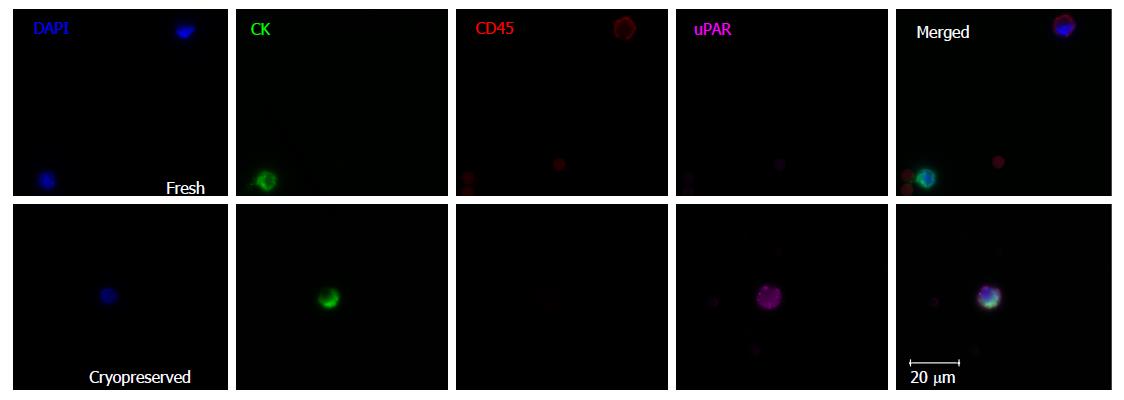
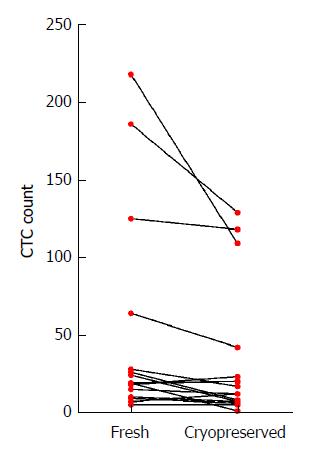
Matching parallel blood samples, collected from 15 gastroesophageal cancer patients (10 patients had blood taken prior to treatment, 5 patients were already on treatment), that had either been cryopreserved before CTC processing or were processed fresh, were compared. Cryopreservation of PBMCs lasted from 2 wk to 25.2 mo (median 14.6 mo). There was no significant correlation between cryopreservation time and CTC number (Pearson r -0.25, P = 0.09). CTCs isolated from cryopreserved samples appeared morphologically similar to fresh samples (Figure 1). There was a significant difference between CTC numbers isolated from the cryopreserved samples compared to fresh samples (mean number of CTCs 34.4 cryopreserved vs 51.5 fresh, P = 0.04, Figure 2), however this difference was predominately attributable to a larger fall in CTC numbers in samples with very high CTC counts (> 50 CTCs in the fresh specimen). There was no significant difference in CTC count between cryopreserved and fresh samples for specimens with CTC count less than 50 (n = 11 patients, mean number of CTCs 10.7 vs 16.3, P = 0.06). Thus CTC loss by cryopreservation in patient samples with low CTC counts appears relatively minor (mean proportion of CTCs lost in cryopreserved samples = 23.95%).
A larger cohort of 43 gastroesophageal cancer patients (cohort 2) was analyzed to validate whether detectable CTC counts post cryopreservation correlated to disease outcomes. All patient samples were taken prior to treatment commencement and had undergone cryopreservation before CTC isolation. Cohort 2 included the 10 treatment naive patients from cohort 1. Patient characteristics of cohort 2 are summarised in Table 1. Twenty-four patients had resectable disease (Stage II or III). Post CTC evaluation, 11 of these patients received neoadjuvant chemoradiotherapy prior to resection (CROSS regimen), 3 received perioperative chemotherapy (MAGIC regimen), and 10 had surgery alone. Nineteen patients had metastatic disease (stage IV). Most of these patients received chemotherapy (7 patients: platinum and capecitabine doublet, 3 patients: anthracycline, capecitabine, and platinum triplet, 1 patient: irinotecan or paclitaxel monotherapy), immunotherapy (2 patients), and 6 patients received no active systemic treatments.
| CTC count | |||
| All patients | Low (CTC ≤ 17) | High (CTC > 17) | |
| n = 43 | n = 23 | n = 20 | |
| Age | |||
| Mean (range) | 64 (39-89) | 65 (39-89) | 64 (48-83) |
| Sex | |||
| Male | 32 (74.4) | 15 (65.2) | 20 (85.0) |
| Female | 11 (25.6) | 8 (34.8) | 3 (15.0) |
| ECOG | |||
| 0-1 | 36 (83.7) | 22 (95.6) | 14 (70.0) |
| 2-4 | 7 (16.3) | 1 (4.3) | 6 (30.0) |
| Primary tumor location | |||
| Distal oesophageal | 12 (27.9) | 8 (34.8) | 4 (20.0) |
| Gastroesophageal junction | 14 (32.6) | 4 (17.4) | 10 (50.0) |
| Gastric | 17 (37.5) | 11 (47.8) | 6 (30.0) |
| Stage | |||
| II | 18 (41.9) | 13 (56.5) | 5 (25.0) |
| III | 6 (14.0) | 4 (17.4) | 2 (10.0) |
| IV | 19 (44.2) | 6 (26.1) | 13 (65.0) |
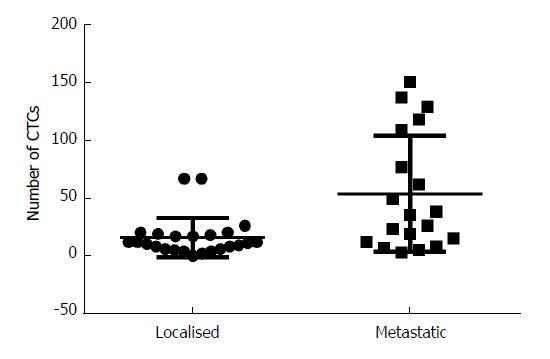
CTCs were detected in 42/43 patients (95.5%), with a median CTC of 17 (interquartile range 8-38). Patients with metastatic disease had a higher number of CTCs than those with resectable disease (Figure 3, mean CTC count 53.8 vs 15.8, P = 0.0013).
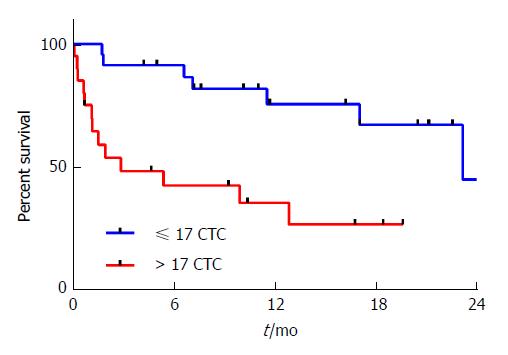
Currently there are no established cut-offs for prognostic CTC numbers detected using the IsoFlux in gastroesophageal adenocarcinoma. Therefore we opted to divide our patients by their CTC counts, above versus equal or lower than the median CTC count, to test for any correlation with clinical outcomes. Patients with a high CTC count (> 17) had a poorer OS than those with a lower CTC count (≤ 17) (Figure 4, median OS 2.8 mo vs 23.2 mo, HR = 4.4, 95%CI: 1.7-11.7, P = 0.0013). In multivariate analysis, after controlling for sex, age, stage, ECOG performance status, and primary tumor location, a high CTC count remained an independent prognostic factor associated with poor OS (Table 2, HR = 3.7, 95%CI: 1.2-12.4, P = 0.03). This association was stronger when the analysis was restricted to patients with metastatic disease (n = 19, HR = 5.5, 95%CI: 1.2-25.5, P = 0.01), but not observed in patients with resectable disease (n = 24, P = 0.39), although a high CTC count (> 17) was associated with a non-significant trend to shorter recurrence free survival in these patients (HR = 3.1, 95%CI: 0.8-12.6, P = 0.09).
| Univariate | Multivariate | |||
| Factor | HR (95%CI) | P value | HR (95%CI) | P value |
| CTC count (high vs low) | 4.4 (1.7-11.7) | 0.001 | 3.7 (1.2-12.4) | 0.03 |
| Age (≥ 65 vs <65 yr old) | 0.7 (0.3-1.8) | 0.46 | 1.0 (0.9-1.1) | 0.76 |
| ECOG (2-4 vs 0-1) | 7.2 (2.2-23.7) | 0.0002 | 2.3 (0.5-10.1) | 0.14 |
| Sex (male vs female) | 1.2 (0.4-3.8) | 0.7 | 0.7 (0.2-2.1) | 0.49 |
| Stage (IV vs II-III) | 10.0 (3.3-30.8) | < 0.0001 | 9.9 (2.9-33.8) | 0.0003 |
| Primary tumor location (gastric vs oesophageal/GOJ) | 0.3 (0.1-1.01) | 0.05 | 0.4 (0.2-1.6) | 0.22 |
Most patients had some uPAR positive CTCs (40/43, 93.0%), however the proportion of uPAR positive CTCs was similar between patients with localised and metastatic disease (mean proportion uPAR positive CTCs 48.8% vs 47.7% respectively, P = 0.89), and there was no association with survival outcomes (Supplementary Figure 1, median OS 17.0 mo vs 12.8 mo, P = 0.6).
In this study we report the reliable isolation, immunocytochemical identification, and enumeration of gastroesophageal cancer CTCs from cryopreserved PBMCs using the IsoFlux platform. The included cohort is the largest reported study analysing cryopreservation of patient PBMCs for CTC detection. Our data confirms that CTCs isolated from cryopreserved samples remain an independent prognostic factor associated with OS.
The timely processing of patient samples for CTC isolation, usually is recommended within 24 h for most isolation methods[19], presenting significant logistical challenges for researchers and prohibits inclusion of patients from remote areas into clinical trials that would rely on CTCs as outcome measures. This is mainly because current methods of CTC analysis require significant expertise, instrumentation, time and laboratory resources, usually performed in specialised research centres. Protocols using isolation of CTCs from cryopreserved specimens would require some basic processing and cryopreservation at the site of blood draw, but offer many advantages, including the ability to biobank patient samples for prolonged periods of time before central processing. This would be a huge benefit for larger scale clinical trials as it would allow inclusion of geographically separated sites.
Previous work has shown that the immunochemical properties of CK, EpCAM and CD45, central to the isolation and identification of CTCs, are not affected by cryopreservation and thawing[20,21]. In agreement, our results demonstrate a similar morphological and immunofluorescent profile between cryopreserved and fresh CTCs and leukocytes, suggesting current techniques are suitable for cryopreserved samples. This approach is further supported by other work showing close concordance in genetic alterations seen on paired fresh and frozen CTCs[21].
Our results also show that enumeration of CTCs isolated from cryopreserved PMBCs is a valid prognostic biomarker in gastroesophageal cancer. Patients with metastatic disease had a significantly higher number of CTCs than those with resectable disease (mean CTC count 53.8 vs 15.8, P = 0.0013). Moreover, patients with a high CTC count (> 17) had a much poorer OS than those with a lower CTC count (≤ 17) (HR = 4.4, P = 0.0013). High CTC count remained significant in the multivariate analysis as an independent predictor of poorer OS (HR = 3.7, P = 0.03), after controlling for age, ECOG, sex, stage and primary tumour location, particularly when analysis was restricted to patients with metastatic disease only (HR = 5.5, P = 0.01). These results are concordant with other studies which confirm CTC enumeration as an important prognostic factor in gastroesophageal cancer[10-12].
Given our previous findings that the uPA system is a clinically relevant biomarker in primary gastroesophageal cancer[18], we undertook and successfully probed for uPAR expression in CTCs derived from cryopreserved and fresh samples. We previously have shown that higher expression of uPA, uPAR and PAI-1 in the primary tumour is associated with higher risk disease and poorer prognosis. However, in this study, there was no correlation between CTC uPAR expression with disease parameters. This suggests that the selection of epithelial (EpCAM-positive) CTCs might have affected any correlation of uPAR with patient outcome, as CTCs that present mesenchymal phenotypes, such as uPAR expressing cells, can escape standard methods of isolation reliant on epithelial markers[22]. Indeed Vishnoi et al[23]. has previously reported the isolation of subsets of EpCAM-negative, uPAR and integrin β1 positive breast cancer CTCs, which further supports the concept of CTC heterogeneity[23]. Ultimately, we have successfully stained for a novel biomarker, uPAR, which further supports our cryopreservation method as a valid CTC isolation approach.
One important concern with cryopreservation is the potential for loss of CTCs due to cell loss during freezing, storage, or thawing. In a study by Nejlund et al[20], tumor cell recovery from cryopreserved spiked tumor cells in normal controls was variable, with up to a 40% tumor cell loss. However in clinical samples using matched fresh and cryopreserved specimens from the same patient, there was no consistent loss of CTCs, with the variation in CTC enumeration similar to those seen in paired fresh samples in other studies[2,20]. Friedlander et al[21] found that cryopreservation of PBMCs had no significant effect on the cell recovery from patients with metastatic prostate cancer. We noted a small loss of CTCs associated with cryopreservation, however this was predominately in samples with large numbers of CTCs (> 50), where loss of some CTCs is more acceptable than samples with low CTC counts. We noted samples with high numbers of CTCs were more prone to cell clumping despite benzonase. This is normally due to the release of viscous DNA from cell lysis on thawing, leading to aggregates which prevent accurate CTC counting. We speculate that the higher disease burden in these patients, coupled with a corresponding systemic inflammatory response, lead to poorer cell integrity within the PBMCs of high CTC-count samples. Some loss of CTCs in these samples will have little impact for prognostic and down-stream biomarker analysis purposes. There was no significant loss of CTCs in samples were the total CTC count was ≤ 50 (P = 0.06).
Similar to previously published work, we found that the duration of cryopreservation was not correlated with number of isolated CTCs[20]. Moreover, we were able to isolate CTCs from specimens stored at -80 °C for over two years, suggesting cryopreservation is a suitable approach for long term projects that involve biobanking of patient samples.
Even when using cryopreservation prior to CTC isolation, we found higher numbers of CTCs (median CTC count 17) and a higher number of patient samples with CTCs (98%) compared to other studies using EpCAM based CTC capture in gastroesophageal cancer[10-12,24]. The correlation of CTC numbers with disease progression implies that the CTCs we identified are indeed disease related. Increased CTC counts are consistent with the higher reported sensitivity of the IsoFlux system compared to other platforms, particularly in isolating CTCs with a lower expression of EpCAM[14-16]. Our results confirm, in the largest cohort of patients reported to date, that a high CTC count (> 17) in cryopreserved specimen was an independent prognostic factor associated with poorer OS (HR = 3.7). As expected from the minimal CTC loss during cryopreservation, these data indicate that indeed our method is suitable for delayed and centralised CTC analysis which could help recruiting patients for major clinical trials. In this setting it would be advantageous compared to fixation of blood which allows CTC processing delayed by only several days rather than long term biobanking. We are currently testing if cryopreservation is also able to overcome limitations associated with using fixative for molecular down-stream analysis of CTCs that involves nucleic acid extraction[4,5].
In conclusion, we have tested a robust PBMC cryopreservation protocol that allows successful CTC isolation even 2 years post freezing. Cryopreservation of CTCs is feasible, with a small loss of tumor cells predominantly in samples with a high CTC load. Enumeration of CTCs from cryopreserved samples remained a clinically important prognostic biomarker. Cryopreservation may assist with the wider incorporation of CTC collection and analysis in biobanking, retrospective studies, and large international clinical trials, by facilitating specimen storage, bulk transporting, and batch processing. It may also help to develop diagnostic settings that can service even remote patients with diagnostic CTC data potentially relevant for their disease management.
A persisting challenge to the field of circulating tumor cell (CTC) research is the requirement for prompt analysis of samples at specialised centres. This has presented significant logistical challenges to researchers, compounded by the significant expertise, time and laboratory resources required for CTC analysis.
Current methods to overcome this issue, such as fixation of blood samples, extend the time for CTC processing for several days, but may interfere with downstream molecular analyses.
Cryopreservation of patient samples permits the wider incorporation of CTC collection and analysis in biobanking, retrospective studies, and large international clinical trials, by facilitating specimen storage, bulk transporting, and batch processing. However, up to now, there has been little research in how cryopreservation affects CTC recovery, and whether cryopreservation retains predictive value of CTCs.
The primary objective of our study was to investigate the feasibility and reliability of delayed CTC isolation from cryopreserved peripheral blood mononuclear cells (PBMCs) layer. This was determined by percentage of CTC loss during cryopreservation and thawing, and clinical validity of CTC enumeration from cryopreserved samples.
CTCs were isolated from 7.5 mL blood samples collected from patients with gastroesophageal adenocarcinoma using EpCAM based immunomagnetic capture with the IsoFlux platform. CTC loss with cryopreservation was determined by comparing CTC enumeration from matched cryopreserved and freshly processed blood samples collected during the same blood draw. CTCs isolated from pre-treatment cryopreserved PBMCs were examined for association with clinicopathological variables and survival outcomes.
We found a minor loss of tumor cells in matched cryopreserved and freshly processed samples, mostly in samples with high CTC counts. A high CTC count isolated from cryopreserved PBMCs remained a statistically significant independent prognostic factor in gastroesophageal cancer.
Our study demonstrates a feasible and robust protocol facilitating CTC isolation from cryopreserved PBMCs even after 2 years post freezing. Our results have immediate applicability in the design and conduct of translational studies, as it facilitates incorporation of CTC analysis in large international trials and biobanking projects.
There is an increasing variety of techniques used for CTC isolation described in the literature. While the current work confirms the reliability of CTC isolation from cryopreserved samples using immunomagnetic separation, further work needs to be undertaken to confirm its suitability for other isolation approaches.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dumitrascu DL S- Editor: Chen K L- Editor: A E- Editor: Huang Y
| 1. | Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1012] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 2. | Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-6904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1954] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 3. | Qin J, Alt JR, Hunsley BA, Williams TL, Fernando MR. Stabilization of circulating tumor cells in blood using a collection device with a preservative reagent. Cancer Cell Int. 2014;14:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Yee SS, Lieberman DB, Blanchard T, Rader J, Zhao J, Troxel AB, DeSloover D, Fox AJ, Daber RD, Kakrecha B. A novel approach for next-generation sequencing of circulating tumor cells. Mol Genet Genomic Med. 2016;4:395-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Luk AWS, Ma Y, Ding PN, Young FP, Chua W, Balakrishnar B, Dransfield DT, Souza P, Becker TM. CTC-mRNA (AR-V7) Analysis from Blood Samples-Impact of Blood Collection Tube and Storage Time. Int J Mol Sci. 2017;18:pii: E1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | van der Toom EE, Verdone JE, Gorin MA, Pienta KJ. Technical challenges in the isolation and analysis of circulating tumor cells. Oncotarget. 2016;7:62754-62766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Bidard FC, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 626] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 8. | Lim SH, Becker TM, Chua W, Caixeiro NJ, Ng WL, Kienzle N, Tognela A, Lumba S, Rasko JE, de Souza P. Circulating tumour cells and circulating free nucleic acid as prognostic and predictive biomarkers in colorectal cancer. Cancer Lett. 2014;346:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Miyamoto DT, Sequist LV, Lee RJ. Circulating tumour cells-monitoring treatment response in prostate cancer. Nat Rev Clin Oncol. 2014;11:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Uenosono Y, Arigami T, Kozono T, Yanagita S, Hagihara T, Haraguchi N, Matsushita D, Hirata M, Arima H, Funasako Y. Clinical significance of circulating tumor cells in peripheral blood from patients with gastric cancer. Cancer. 2013;119:3984-3991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Reeh M, Effenberger KE, Koenig AM, Riethdorf S, Eichstädt D, Vettorazzi E, Uzunoglu FG, Vashist YK, Izbicki JR, Pantel K. Circulating Tumor Cells as a Biomarker for Preoperative Prognostic Staging in Patients With Esophageal Cancer. Ann Surg. 2015;261:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Lee SJ, Lee J, Kim ST, Park SH, Park JO, Park YS, Lim HY, Kang WK. Circulating tumor cells are predictive of poor response to chemotherapy in metastatic gastric cancer. Int J Biol Markers. 2015;30:e382-e386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa Y, Suda K, Ando T, Kumagai K, Irino T, Yoshikawa T, Matsuda S. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol. 2008;15:3092-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Alva A, Friedlander T, Clark M, Huebner T, Daignault S, Hussain M, Lee C, Hafez K, Hollenbeck B, Weizer A. Circulating Tumor Cells as Potential Biomarkers in Bladder Cancer. J Urol. 2015;194:790-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Sánchez-Lorencio MI, Ramirez P, Saenz L, Martínez Sánchez MV, De La Orden V, Mediero-Valeros B, Veganzones-De-Castro S, Baroja-Mazo A, Revilla Nuin B, Gonzalez MR. Comparison of Two Types of Liquid Biopsies in Patients With Hepatocellular Carcinoma Awaiting Orthotopic Liver Transplantation. Transplant Proc. 2015;47:2639-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Harb W, Fan A, Tran T, Danila DC, Keys D, Schwartz M, Ionescu-Zanetti C. Mutational Analysis of Circulating Tumor Cells Using a Novel Microfluidic Collection Device and qPCR Assay. Transl Oncol. 2013;6:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Cryopreservation of CTC samples for biobanking and sample storage. Available from: https://liquidbiopsy.fluxionbio.com/application-notes. |
| 18. | Brungs D, Chen J, Aghmesheh M, Vine KL, Becker TM, Carolan MG, Ranson M. The urokinase plasminogen activation system in gastroesophageal cancer: A systematic review and meta-analysis. Oncotarget. 2017;8:23099-23109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Fehm T, Solomayer EF, Meng S, Tucker T, Lane N, Wang J, Gebauer G. Methods for isolating circulating epithelial cells and criteria for their classification as carcinoma cells. Cytotherapy. 2005;7:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Nejlund S, Smith J, Kraan J, Stender H, Van MN, Langkjer ST, Nielsen MT, Sölétormos G, Hillig T. Cryopreservation of Circulating Tumor Cells for Enumeration and Characterization. Biopreserv Biobank. 2016;14:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Friedlander TW, Ngo VT, Dong H, Premasekharan G, Weinberg V, Doty S, Zhao Q, Gilbert EG, Ryan CJ, Chen WT. Detection and characterization of invasive circulating tumor cells derived from men with metastatic castration-resistant prostate cancer. Int J Cancer. 2014;134:2284-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Königsberg R, Obermayr E, Bises G, Pfeiler G, Gneist M, Wrba F, de Santis M, Zeillinger R, Hudec M, Dittrich C. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 2011;50:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 23. | Vishnoi M, Peddibhotla S, Yin W, T Scamardo A, George GC, Hong DS, Marchetti D. The isolation and characterization of CTC subsets related to breast cancer dormancy. Sci Rep. 2015;5:17533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Matsusaka S, Chìn K, Ogura M, Suenaga M, Shinozaki E, Mishima Y, Terui Y, Mizunuma N, Hatake K. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci. 2010;101:1067-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |