Published online Feb 21, 2018. doi: 10.3748/wjg.v24.i7.775
Peer-review started: December 6, 2017
First decision: December 12, 2017
Revised: December 13, 2017
Accepted: December 19, 2017
Article in press: December 19, 2017
Published online: February 21, 2018
Processing time: 64 Days and 15.3 Hours
To investigate by immunostaining glucose transporter expression in human colorectal mucosa in controls and patients with inflammatory bowel disease (IBD).
Colorectal samples were obtained from patients undergoing lower endoscopic colonoscopy or recto-sigmoidoscopy. Patients diagnosed with ulcerative colitis (n = 18) or Crohn’s disease (n = 10) and scheduled for diagnostic colonoscopy were enrolled. Patients who underwent colonoscopy for prevention screening of colorectal cancer or were followed-up after polypectomy or had a history of lower gastrointestinal symptoms were designated as the control group (CTRL, n = 16). Inflammatory status of the mucosa at the sampling site was evaluated histologically and/or endoscopically. A total of 147 biopsies of colorectal mucosa were collected and processed for immunohistochemistry analysis. The expression of GLUT2, SGLT1, and GLUT5 glucose transporters was investigated using immunoperoxidase labeling. To compare immunoreactivity of GLUT5 and LYVE-1, which is a marker for lymphatic vessel endothelium, double-labeled confocal microscopy was used.
Immunohistochemical analysis revealed that GLUT2, SGLT1, and GLUT5 were expressed only in short epithelial portions of the large intestinal mucosa. No important differences were observed in glucose transporter expression between the samples obtained from the different portions of the colorectal tract and between the different patient groups. Unexpectedly, GLUT5 expression was also identified in vessels, mainly concentrated in specific areas where the vessels were clustered. Immunostaining with LYVE-1 and GLUT5 antibodies revealed that GLUT5-immunoreactive (-IR) clusters of vessels were concentrated in areas internal to those that were LYVE-1 positive. GLUT5 and LYVE-1 did not appear to be colocalized but rather showed a close topographical relationship on the endothelium. Based on their LYVE-1 expression, GLUT5-IR vessels were identified as lymphatic. Both inflamed and non-inflamed mucosal colorectal tissue biopsies from the IBD and CTRL patients showed GLUT5-IR clusters of lymphatic vessels.
Glucose transporter immunoreactivity is present in colorectal mucosa in controls and IBD patients. GLUT5 expression is also associated with lymphatic vessels. This novel finding aids in the characterization of lymphatic vasculature in IBD patients.
Core tip: Our study demonstrates that GLUT2, SGLT1, and GLUT5 glucose transporters are expressed in the colorectal mucosa in controls and patients with inflammatory bowel disease (IBD). In addition, it provides first evidence that GLUT5 expression is associated with lymphatic vessels in controls and IBD patients. GLUT5-immunoreactive vessels were isolated or clustered in specific areas. We interpreted the presence of clusters as a pattern related to proliferative zones. As GLUT5 is the main fructose transporter, fructose may have a role in the atypical aggregation of lymphatic vessels. This novel finding yields further insight into the characterization of lymphatic vasculature, whose dysfunction is a long-recognized feature in humans with IBD.
- Citation: Merigo F, Brandolese A, Facchin S, Missaggia S, Bernardi P, Boschi F, D’Incà R, Savarino EV, Sbarbati A, Sturniolo GC. Glucose transporter expression in the human colon. World J Gastroenterol 2018; 24(7): 775-793
- URL: https://www.wjgnet.com/1007-9327/full/v24/i7/775.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i7.775
Facilitative monosaccharide transport across the cell membrane is mediated by the GLUT family of membrane proteins encoded by the solute carrier transporter 2 (SLC2) gene. The GLUT protein family comprises 14 isoforms that differ by expression pattern and by affinity for sugars, polyols, and other carbon compounds[1]. The GLUT5 and GLUT2 (also known as SLC2A5 and SLC2A2, respectively) isoforms are predominantly but not exclusively distributed in the intestine.
GLUT5 is a sugar transporter that facilitates fructose uptake. In mice, GLUT5 mRNA is abundantly detected by in situ hybridization and PCR in the small intestine but not in the large intestine. It is expressed in the small intestine and other organs such as the brain, testes, skeletal muscle, adipocytes, and kidneys[2,3]. In enterocytes, it is located on the apical membrane. In the small intestine of rats, GLUT5 expression may be increased by exposure to fructose (or its metabolites), steroids, and thyroid hormones depending on age and weaning stage[4-7]. GLUT5 expression differs according to insulin-resistance in adipocytes and duodenal enterocytes[8,9]. It is also implicated in the development of metabolic diseases such as fructose-induced hypertension and non-alcoholic fatty liver disease[10].
GLUT2 expression is widely present throughout the digestive system[11], greatest in the small intestine and least in the terminal ileum. Molecular biology studies indicate that it is not expressed in the large intestine in mice[12]. In humans, GLUT2 expression has been observed in enterocytes and enteroendocrine L-cells. In enterocytes, it is mainly located in the basolateral membrane where it facilitates the passage of glucose towards the interstitial space and bloodstream. In mice, it has been observed in the apical region of enterocytes after a sugar-rich meal or when insulin-resistance develops[13-16]. GLUT2 is also expressed in enteroendocrine L-cells of the distal small intestine and large intestine in humans, and it is responsible for the secretion of glucagon-like peptide 1 (GLP-1)[17-19]. Because of the gradient of glucose concentration between the cytosol and the intestinal lumen, glucose uptake by enterocytes depends mostly on SGLT1, which is a Na+/glucose co-transporter of the human family SLC5[20]. SGLT1 is located on the apical membrane of enterocytes[21]. In mice, SGLT1 expression was found to be intensely present from the duodenum to the end of the ileum where its levels tend to decrease, as demonstrated by in situ hybridization and PCR techniques. In the large intestine, SGLT1 expression remains controversial because SGLT1 mRNA in the proximal colon has been detected by in situ hybridization but not by PCR[12]. SGLT1 is also known to be implicated in glucose-mediated GLP1 exocytosis through depolarization of the L-cell membrane[22].
Further implications of these transporters other than sugar uptake and metabolism have been proposed for inflammation, malignancy, and gut microbiota regulation. Research has shown that GLUT5 activity and expression are regulated under normal conditions but they are altered in pathological conditions[7]. GLUT5 is over-expressed in malignant but not in normal human tissues, including colonic and hepatic tissues, and it can be considered an important diagnostic or therapeutic target in tumors relying on fructose for proliferation such as breast cancer and pancreatic cancer[23-25]. GLUT5 mRNA levels in non-inflamed small intestine of rats with iodacetamide-induced colitis were observed to be lower than in controls, suggesting a possible link between this fructose transporter and inflammatory cytokines. On the other hand, fructose absorption in rabbit was found to be inversely proportional to TNF-alpha expression and bacterial lipopolysaccharide (LPS) exposure[26,27]. In mice models, GLUT5 deficiency has been reported to be associated with severe malabsorption due to dilation of the cecum and colon[28].
GLUT2 has a high affinity for glucosamine, an amino-monosaccharide with anti-inflammatory activity against sodium dextran sulfate-induced colitis, which is a model for inflammatory bowel disease (IBD) in rats[29]. Moreover, it has also been reported to have a role in modulating gut microbiota: after intestinal-specific deletion of GLUT2 in mice, an increase in commensal bacteria was observed in association with an improvement in inflammatory status[19].
SGLT1 (like GLUT2) mRNA and protein levels were found to be significantly increased in intestinal biopsies of diabetic human subjects[9]. Some mechanisms of cellular survival linked to SGLT1 have been demonstrated in Caco-2 cells models when exposed to bacterial LPS[30]. Furthermore, SGLT1 expression in these models was found to be inversely proportional to TNF-alpha expression, implicating its potential involvement in colonic inflammatory diseases[31]. In addition, in vivo studies on mice have shown that oral glucose and 3-O-methyl-d-glucose (a non-metabolizable glucose analogue ) administration promotes survival in endotoxic shock induced by LPS[32]. These studies suggest a protective role for SGLT1 in inflammatory processes or sepsis, possibly by maintaining intestinal barrier integrity. SGLT1 acts through the inhibition of intestinal epithelial cell activation mediated by phosphorylation of the signaling molecule Nf-KB, a protein complex involved in the gene regulation of several cellular responses, which is a known mechanism in the epithelium of patients with Crohn’s disease (CD) and ulcerative colitis (UC)[33-35].
These findings raise expectations for future discoveries; however, most studies to date on sugar transporters in the gastrointestinal tract have been performed using molecular biology in murine or in vitro models. Since topographical variations in glucose transporters have been detected mainly by biochemical rather than standard immunohistochemical analysis also in humans, the localization and distribution of glucose transporters in the large intestinal mucosa of the colon have not yet been clarified.
For this reason, the aim of this study was to investigate by immunostaining at light and confocal microscopy the expression of the major intestinal glucose transporters (GLUT5, GLUT2, SGLT1) in human colonic mucosa in control subjects and subjects with IBD.
The study protocol was approved by the Ethics Committee of Padua University Hospital (study No. 2813P). Written, informed consent was obtained from all participants prior to inclusion in the study. The study was prospectively conducted from December 2013 to November 2015.
Patients diagnosed with UC or CD and scheduled for diagnostic colonoscopy were enrolled. Patients who underwent colonoscopy for prevention screening of colorectal cancer or were followed-up after polypectomy or lower gastrointestinal symptoms were designated as the control group (CTRL). Inclusion criteria are presented in Table 1.
| Patient group | Periodic Follow-up (n) | New stadiation of IBD (n) | Abdominal pain (n) | Chronic diarrhea (n) | Colorectal cancer screening (n) | Stipsis (n) | Hemorrhoids (n) |
| UC | 16 | 2 | - | - | - | - | - |
| CD | 2 | 8 | - | - | - | - | - |
| CTRL | 31 | - | 5 | 4 | 2 | 1 | 1 |
The UC patient group (n = 18) included 8 men and 10 women (mean age 47 years, range 30-66; average body-mass index (BMI, weight in kg divided by height in m squared) 24 kg/m2, range 18-32.9 kg/m2.
The CD patient group (n = 10) included 5 men and 5 women (mean age 37 years, range 19-53; average BMI 23.6 kg/m2, range19.6-26.1 kg/m2.
The CTRL patient group (n = 16) included 6 men and 10 women (mean age 56 years, range 27-72, average BMI 24.85 kg/m2, range 18.1-31.1 kg/m2. All CTRL patients were evaluated based on histological and/or endoscopic examination of intestinal biopsies; none was affected by UC or CD.
Patients were classified by their BMI into one of three groups: normal weight for BMI < 24.9 kg/m2, overweight for 25.0-29.9, and obese for > 30 kg/m2.
Colorectal samples were obtained from patients undergoing lower endoscopic colonoscopy or recto-sigmoidoscopy (2 in the CTRL group). Biopsies of portions of the colonic tract were taken for diagnostic purposes according to the endoscopist‘s judgment, and for immunohistochemistry (IHC). The biopsies were collected from adjacent sites to compare the level of inflammation in independent samples. In 14 of the 44 patients who underwent a complete colonoscopy, biopsies were obtained of all 6 portions of the colon-rectum (cecum, ascending colon, transverse, descending, sigmoid colon, rectum). In the remaining 30 patients, biopsies were obtained only from the endoscopically examined portions.
A total of 147 biopsies of colonic mucosa were collected for IHC analysis. Inflammatory status of the mucosa at the sampling site was evaluated endoscopically in all biopsies and histologically in 127 out of 147 biopsies by an experienced pathologist who evaluated the mononuclear and polymorphonuclear cell infiltration of the mucosal layer. For the endoscopic findings, inflammatory status was graded according to the Mayo endoscopic score in the UC patients (Mayo score of 0 indicates normal colonic mucosa, > 0 evidence of macroscopic active inflammation), and according to the Rutgeerts score in previously resected CD (3 out of 10) patients (Rutgeerts score of 0-1 indicates normal ileocolic anastomosis, and > 1 macroscopic relapse of CD). For the non-resected CD and CTRL patients, inflammatory mucosal status was graded as documented in the endoscopic report. Based on histological and endoscopic grading, their status towards inflammation and classification as inflamed or non-inflamed was determined. When the endoscopic grade differed from the histological grade, the final biopsy status was decided on the basis of the pathologist’s assessment. The complete list of all biopsies analyzed by IHC, classified according to inflammation status, is shown in Table 2.
| Patient group | Biopsy status | Large | Intestine | ||||
| Proximal | Tract | Distal | Tract | ||||
| Cecum colon (n) | Ascending colon (n) | Transverse colon (n) | Descending colon (n) | Sigmoid colon (n) | Rectum (n) | ||
| UC | Inflamed | 2 | 6 | 4 | 6 | 7 | 7 |
| Non-Inflamed | 7 | 9 | 5 | 6 | 3 | 3 | |
| CD | Inflamed | 0 | 4 | 3 | 4 | 4 | 4 |
| Non-Inflamed | 1 | 1 | 0 | 1 | 2 | 1 | |
| CTRL | Inflamed | 2 | 4 | 3 | 2 | 4 | 4 |
| Non-Inflamed | 7 | 5 | 6 | 6 | 7 | 7 | |
After sampling, specimens were fixed in 40 g/L formaldehyde and processed by embedding in paraffin using standard methods. Sections were cut to 7 μm thickness, mounted on polylysine-coated microscope slides, and processed for immunoperoxidase and double immunofluorescence labeling. Primary and secondary antibodies are listed in Table 3.
| Antibody | Host | Dilution | Source |
| Anti-GLUT2 | Goat | 1:200 | cat#ab111117, abcam, Cambridge, United Kingdom |
| Anti-GLUT5 | Rabbit | 1:400 | cat#ab36057, abcam |
| Anti-SGLT1 | Rabbit | 1:200 | cat#ab14686, abcam |
| Anti-LYVE-1 | Rabbit | 1:500 | cat#Bs-1311R, Bioss,Woburn, MA, United States |
| Anti-VEGF | Rabbit | 1:200 | cat#RB-222, Thermo Scientific, Fremont, CA, United States |
| Polyclonal anti-rabbit IgG/biotinylated | Swine | 1:400 | cat#E0353, Dako, Milan, Italy |
| Polyclonal anti-goat IgG/biotinylated | Rabbit | 1:400 | cat#E0466, Dako |
| CyTM3-Fab fragment anti-rabbit IgG | Goat | 1:100 | cat#111-167, Jackson Lab., Baltimore, MD, United States |
| FITC-fab fragment anti-rabbit IgG | Goat | 1:100 | cat#111-097, Jackson Lab. |
| Unconjugated fab fragment anti-rabbit IgG | Goat | 1:30 | cat#111-007, Jackson Lab. |
Sections were deparaffinized in xylene, rehydrated through passages in alcohol with decreasing concentrations to water, and then microwaved at 800 W for three five-minute cycles in 0.01 mol/L citrate buffer (pH 6) to optimize antigen retrieval. Endogenous peroxidase activity was blocked by incubating the slides in 30 mL/L H2O2 in methanol for 30 min. After rinsing in PBS, the slides were first incubated with blocking solution (3 mL/L Triton X-100, 10 g/L bovine serum albumin, and 10 mL/L normal swine or rabbit serum); the solution was used to dilute the antibodies. Subsequently, the sections were incubated with primary antibodies overnight at 4 °C and then reacted with biotinylated secondary antibody for 1 h at room temperature. The immunoreaction was detected using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, United States) and then visualized with 3.3’-diaminobenzidine tetrahydrocloride (Dako, Milan, Italy) for 5-10 min. Finally, the sections were mounted in Entellan (Merck, Milan, Italy).
Sections processed as above, but without the primary antibody, were used as negative control. Control sections for SGLT1 were also prepared by preabsorbing the primary antibodies with the corresponding peptide (5 μg/mL of antibody; SGLT1 peptide, cat#ab99447, abcam). Sections of human small intestine were used as positive controls.
Sections were examined with an Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with a digital camera (DKY-F58 CCD JVC, Yokohama, Japan). Digital images were analyzed and processed with Image-ProPlus 7.0 software (Media Cybernetics, Silver Spring, MD, United States). Images were composed with Adobe Photoshop software ver. 6.0 (Adobe Systems, Mountain View, CA, United States) to regulate contrast and brightness.
Double immunofluorescent staining was used to compare immunolabeling of GLUT5 with that of lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), a marker for lymphatic vessel endothelium in humans and rodents, and with that vascular endothelial growth factor (VEGF), a marker of vascular endothelial cells.
The double-label assay was carried out sequentially using a method that relied on the use of secondary monovalent Fab fragments because all primary antibodies are raised in the same species[36,37]. Paraffin sections (processed as described above) were used for double staining, which was performed as described in Merigo et al[38]. After completing the staining protocol, the sections were immersed 1 g/L Sudan Black B (Merck) in 70% ethanol for 20 min at room temperature to reduce tissue autofluorescence. After rinsing, the sections were mounted with fluorescent mounting medium (DAKO) and observed with a Zeiss LSM 510 confocal microscope equipped with argon (488 nm) and helium/neon (543 nm) excitation beams. Sequential acquisition, i.e., one color at a time, was utilized on double-labeled tissues to avoid side-band excitation of the inappropriate fluorophore. All images for publication were composed using Adobe Photoshop software, adjusting only brightness and contrast.
Control sections were prepared using one of the following methods: (1) replacing the second primary antibody with normal rabbit serum; or (2) exchanging the fluorophores of the secondary antibodies; or (3) omitting the primary antibody; or (4) changing the sequence of secondary antibody application.
Sections of colonic mucosa, previously immunostained for GLUT5 antibody, were reused for scanning electron microscopy (SEM) analysis. After removal of the coverslip, the samples were brought to absolute alcohol, processed by critical point drying (CPD 030, Balzers, Vaduz, Liechtenstein), mounted on stubs with colloidal silver, sputtered with gold by means of an MED 010 coater (Balzers), and examined under an FEI XL30 scanning electron-microscope (FEI Company, Eindhoven, NL).
The results of immunohistochemical analysis of samples from the cecum, ascending and transverse colon were grouped together as data of the proximal tract; the results of samples from the descending, sigmoid colon, and rectum were grouped as data of the distal portion of the large intestine.
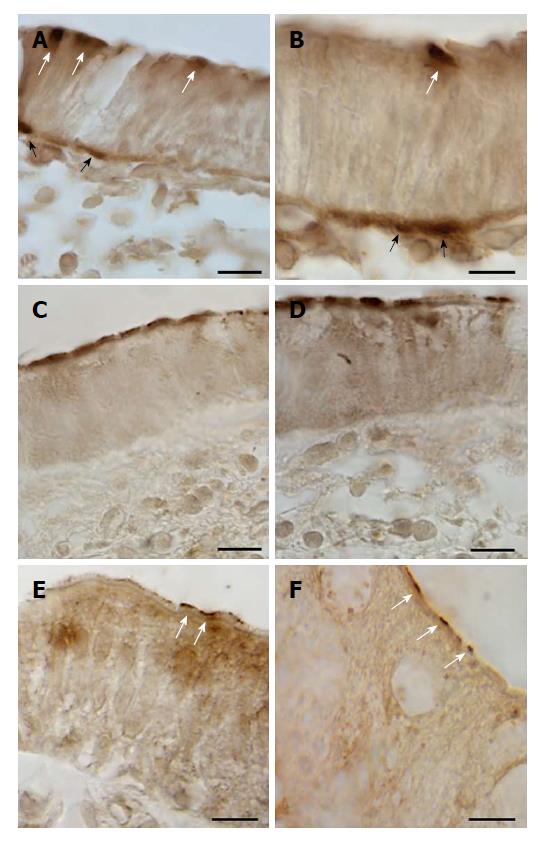
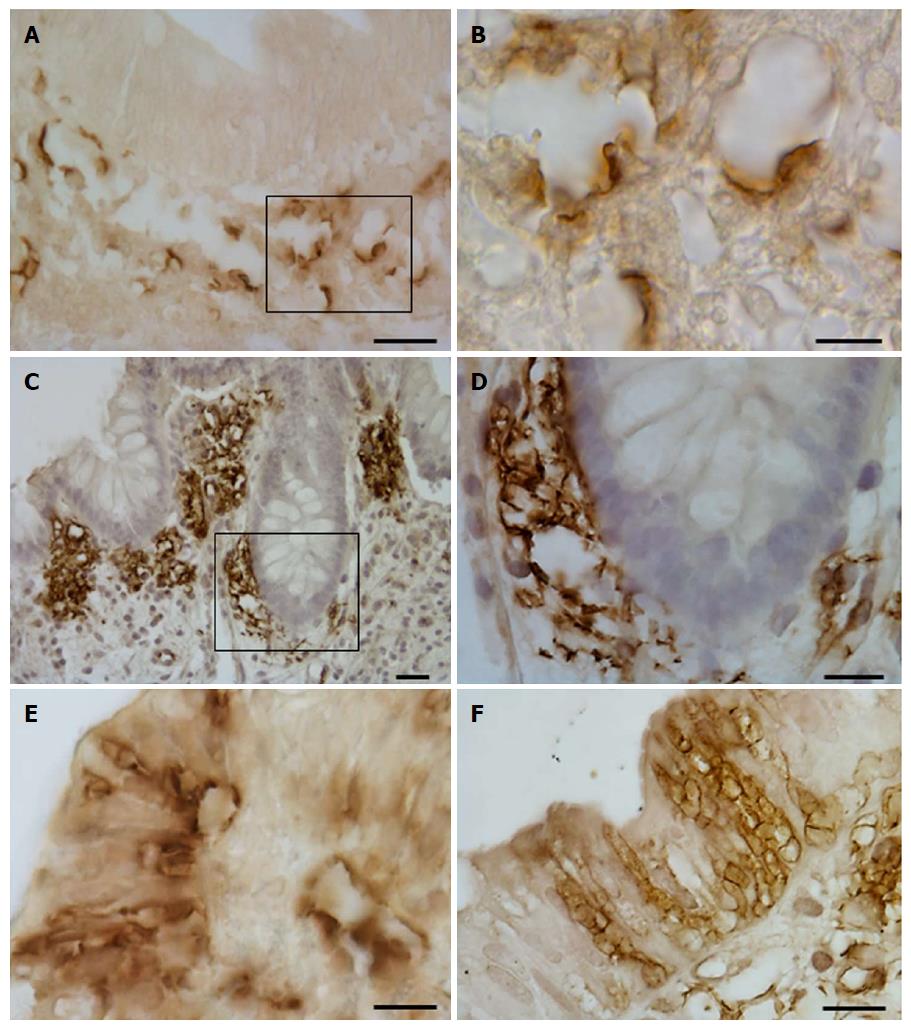
Immunostaining for GLUT2 was detected on the apical cell pole and/or basolateral membrane of epithelial intestinal cells. Only small portions of the epithelium were positive for GLUT2 (Figure 1A-D). A few samples in each group of patients were immunostained for GLUT2 in both the proximal and the distal tract (Table 4).
| Patient group | Large | Intestine | ||||||
| Biopsy status | Proximal | Tract | Biopsy status | Distal | Tract | |||
| GLUT2, % | SGLT1, % | GLUT5, % | GLUT2, % | SGLT1, % | GLUT5, % | |||
| UC | Inflamed (n = 12) | 8 | 9 | 17 | Inflamed (n = 20) | 0 | 15 | 15 |
| Non-inflamed (n = 21) | 5 | 5 | 33 | Non-inflamed (n = 12) | 8 | 8 | 17 | |
| CD | Inflamed (n = 7) | 0 | 0 | 0 | Inflamed (n = 12) | 8 | 8 | 0 |
| Non-inflamed (n = 2) | 0 | 0 | 0 | Non-inflamed (n = 4) | 0 | 0 | 0 | |
| CTRL | Inflamed (n = 9) | 11 | 0 | 33 | Inflamed (n = 10) | 30 | 10 | 0 |
| Non-inflamed (n = 18) | 17 | 0 | 17 | Non-inflamed (n = 20) | 20 | 1 | 15 | |
Immunoreactivity for SGLT1 was concentrated mainly on the apical pole of epithelial cells, which were often observed in small clusters of 2 or 3 (Figure 1E and F). SGLT1 expression was observed in the samples from the distal tract; no substantial differences between the three patient groups were noted (Table 4).
Two distinct expression patterns of GLUT5 immunoreactivity were observed throughout the portions of the large intestine. GLUT5 expression was found on the apical membrane of epithelial cells, most likely representing labeling of the brush border membrane (Figure 2A-L). Only short portions of the epithelium showed positive epithelial cells. This staining was observed from the cecum to the rectum in both the UC and CTRL groups, but without significant differences between them (Table 4).
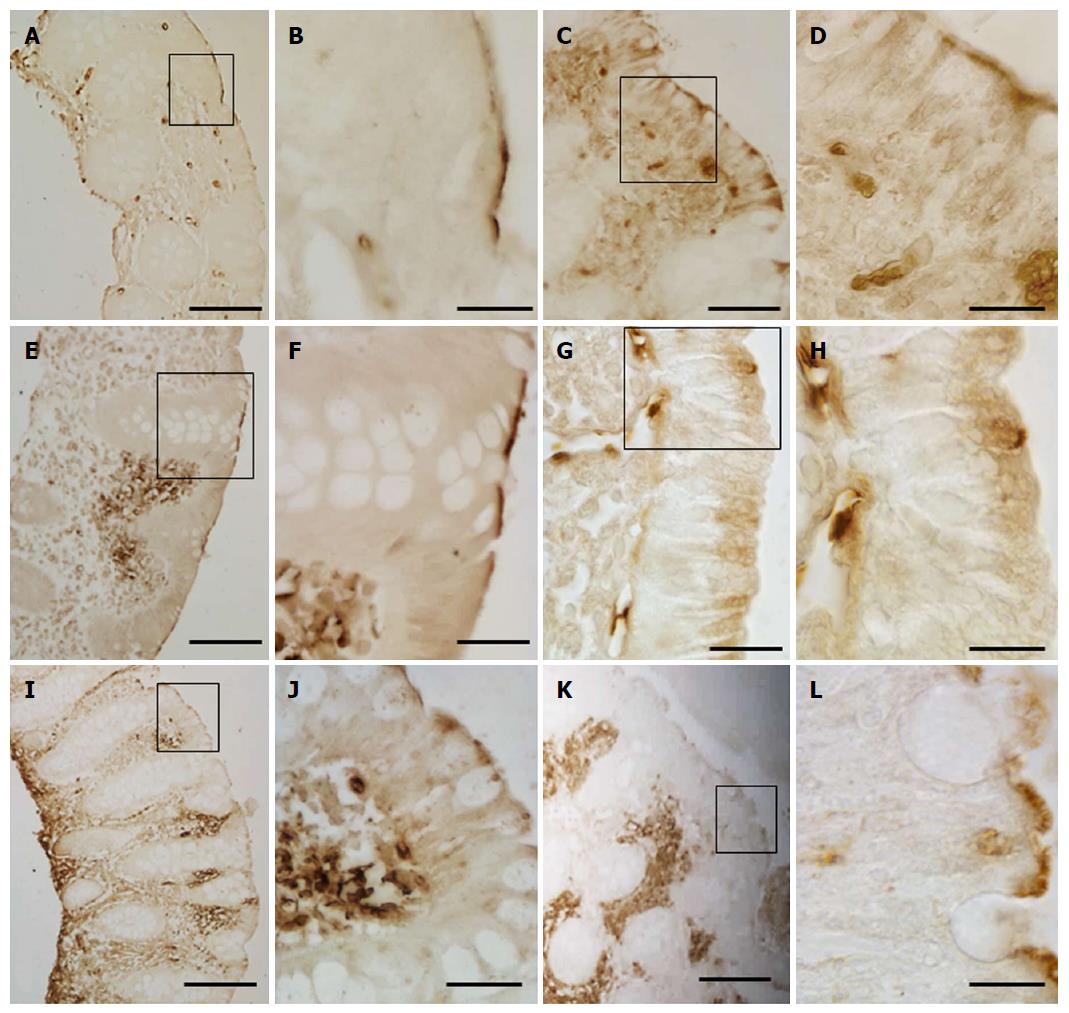
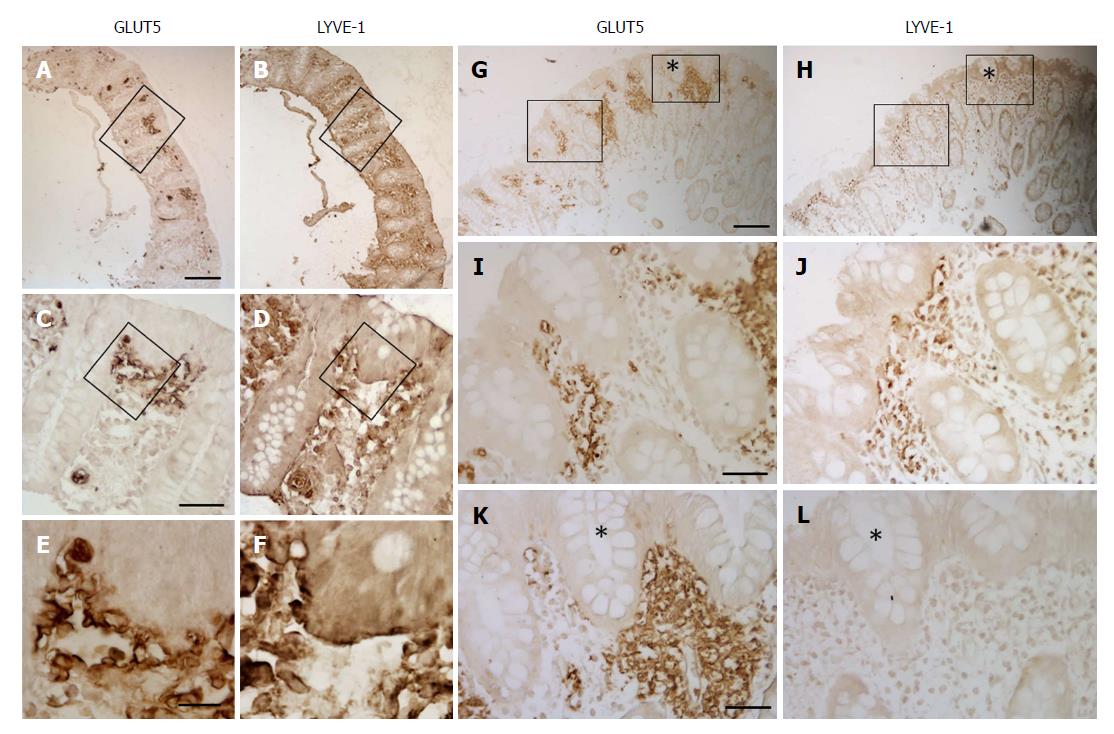
GLUT5 staining was also seen in vascular structures, where it displayed a heterogeneous pattern of expression and distribution. GLUT5 immunoreactivity was observed in the endothelial cells of small vessels, with well-defined endothelium, and slit-like or rounded luminal spaces. The vessels were scattered separately just below the epithelium or around the glands in the intestinal mucosa (Figure 3A-C). This pattern was defined as normal GLUT5 expression in vessels because no samples lacking this characteristic were observed.
In some samples, however, this immunoreactivity pattern was detected in small, numerous vessels which, though remaining individually distinct, tended to aggregate. They were mainly concentrated below the epithelium or around the glands (Figure 3D-I).
No specific labeling was seen in the control sections when IHC was performed without the primary antibody (Figure 3J).
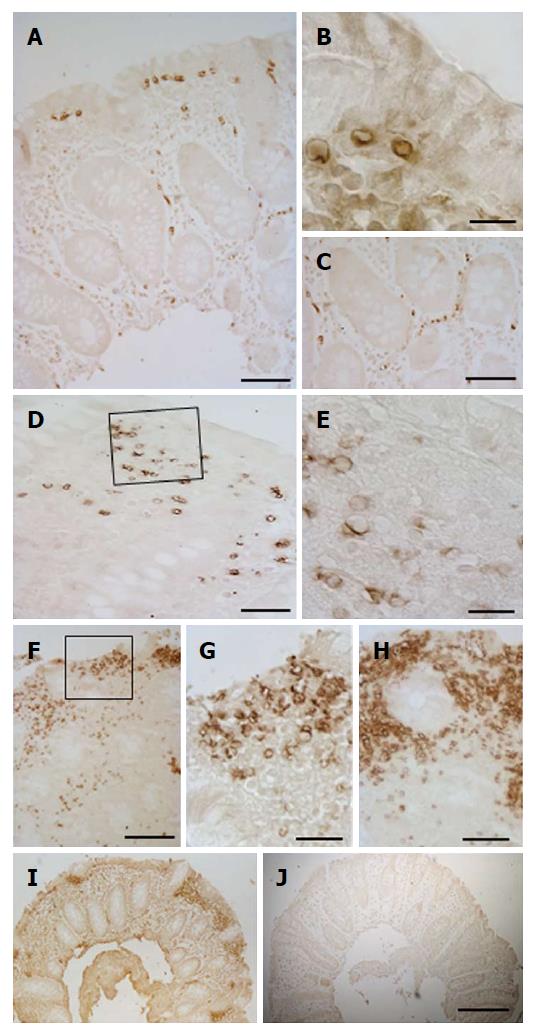
Aggregation of GLUT5-immunoreactive (-IR) vessels was much more evident in other samples, where GLUT5 immunostaining was observed to be mainly concentrated in specific areas formed by clusters of GLUT5-IR vessels scattered throughout the intestinal tract (Figure 4A-F). The clusters varied in number (number ranged from 1 to 12 per section) and size (min area 262 μm2; max area 10695 μm2) and were predominantly localized beneath the epithelium (Figure 5A and B) or around the glands (Figure 5C and D). They consisted of many non-rounded vessels which appeared to be strongly dilated, with only short portions lining the lumen covered by endothelial cells. The flat endothelial cells were stained for GLUT5 (Figure 5B). The epithelium overlying these aggregates often appeared damaged by the presence of vacuolar structures (Figure 5E and F). The epithelium was missing in some cases.
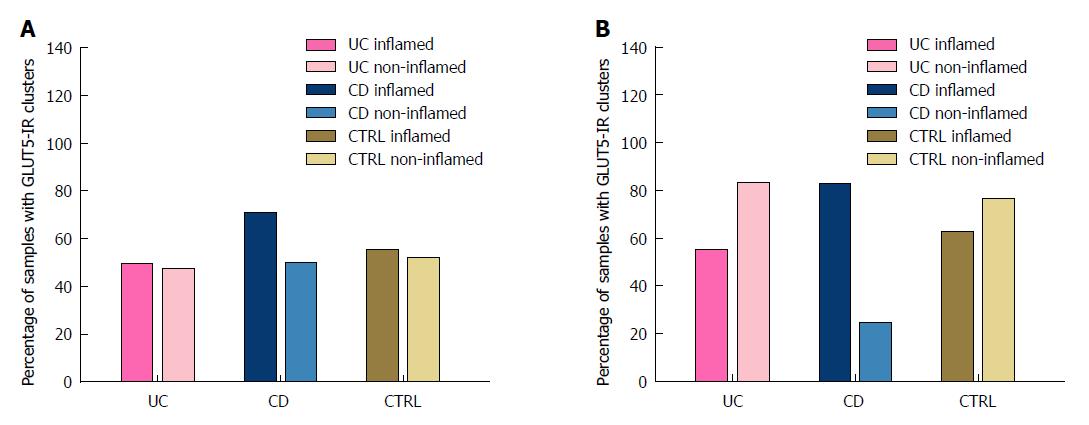
GLUT5-IR clusters were observed throughout the entire tract of the large intestine in the samples of both inflamed and non-inflamed mucosal tissue from the UC, CD, and CTRL patients. The percentage of samples with GLUT5-IR clusters is shown in Table 5.
| Patient group | Large | Intestine | ||
| Proximal | Tract | Distal | Tract | |
| Biopsy status | GLUT5, % | Biopsy status | GLUT5, % | |
| UC | Inflamed (n = 12) | 50.0 | Inflamed (n = 20) | 55.0 |
| Non-Inflamed (n = 21) | 47.6 | Non-Inflamed (n = 12) | 83.3 | |
| CD | Inflamed (n = 7) | 71.0 | Inflamed (n = 12) | 83.0 |
| Non-Inflamed (n = 2) | 50.0 | Non-Inflamed (n = 4) | 25.0 | |
| CTRL | Inflamed (n = 9) | 55.5 | Inflamed (n = 10) | 60.0 |
| Non-Inflamed (n = 18) | 44.4 | Non-Inflamed (n = 20) | 85.0 | |
In the UC patients, the expression of GLUT5-IR clusters was higher in the samples from the distal than from the proximal tract, especially in the samples of non-inflamed tissue (83.3% vs 47.6%). The percentage of inflamed and non-inflamed samples was similar in the proximal tract (50% vs 47.6%), whereas in the distal tract the percentage of non-inflamed samples was higher (83.3% vs 55%).
In the CD patients, the percentage of GLUT5-IR clusters was higher in inflamed samples obtained from the distal than from the proximal tract (83% vs 71%), whereas the percentage was higher in non-inflamed samples from the proximal tract than from the distal tract (50% vs 25%). However, it was always higher in the inflamed than in the non-inflamed samples from both the proximal (70% vs 50%) and the distal tract (83% vs 25%).
In the CTRL group, the percentage of GLUT5-IR clusters in both the inflamed (60% vs 55.5%) and non-inflamed tissue samples (85% vs 44.4%) was higher in those obtained from the distal tract than from the proximal tract. The percentage was similar for the inflamed samples and non-inflamed samples from the proximal tract (55.5% vs 44.4%) and higher in the non-inflamed as compared to the inflamed tissue samples from the distal tract (85% vs 60%). Figure 6 reports the percentages of samples with GLUT5-IR clusters as bar graphs, for both the proximal (Panel A) and the distal (Panel B) tract.
The expression of GLUT5-IR clusters was also evaluated in a sample group obtained by classifying the patients according to their BMI (Table 6). In the UC and CTRL patients, the expression of GLUT5-IR clusters was higher in the inflamed samples of patients with normal weight as compared with non-inflamed samples from the proximal tract; whereas in the distal tract the percentage of GLUT5-IR clusters was always higher in the non-inflamed samples as compared to the inflamed samples from all three groups. The percentage was higher in the inflamed than in the non-inflamed tissue samples from both the proximal and distal tract in the normal weight and overweight CD patients.
| Patient group | Biopsy status | Large | Intestine | ||||
| Proximal | Tract | Distal | Tract | ||||
| Patient | BMI | Patient | BMI | ||||
| Normal weight, % | Over-weight, % | Obese, % | Normal weight, % | Over-weight, % | Obese, % | ||
| UC | Inflamed | 71 | 20 | 0 | 70 | 43 | 33 |
| Non-inflamed | 57 | 25 | 50 | 87 | 100 | 0 | |
| CD | Inflamed | 80 | 50 | 0 | 87 | 75 | 0 |
| Non-inflamed | 100 | 0 | 0 | 50 | 0 | 0 | |
| CTRL | Inflamed | 80 | 25 | 0 | 67 | 50 | 0 |
| Non-inflamed | 22 | 50 | 100 | 82 | 80 | 100 | |
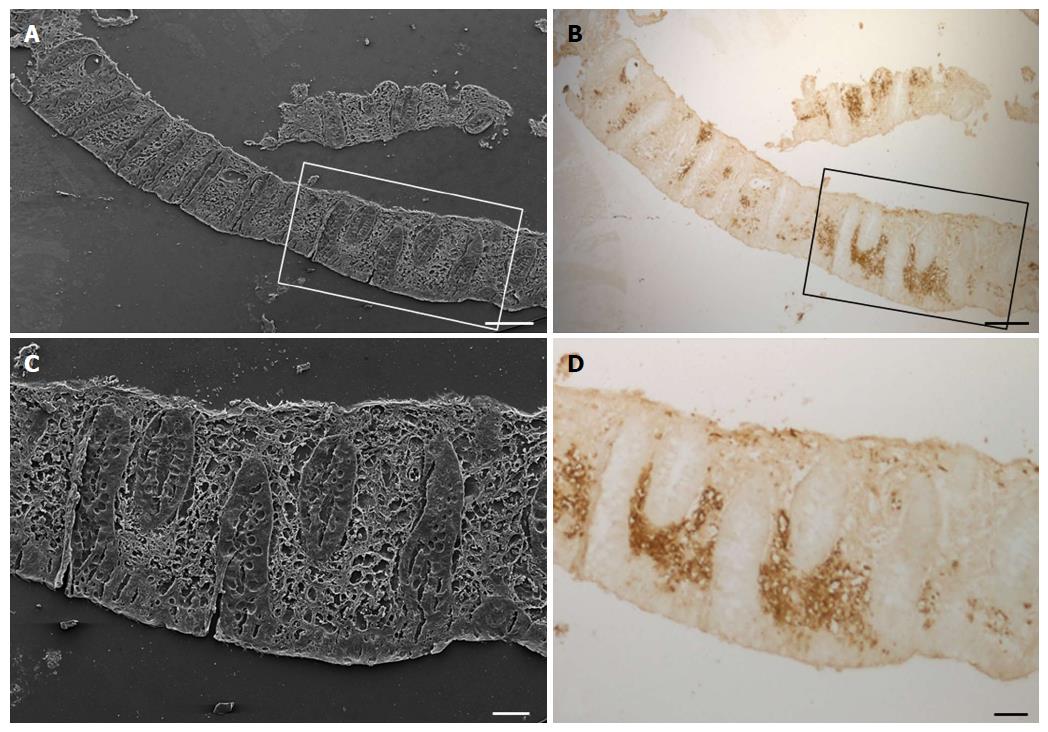
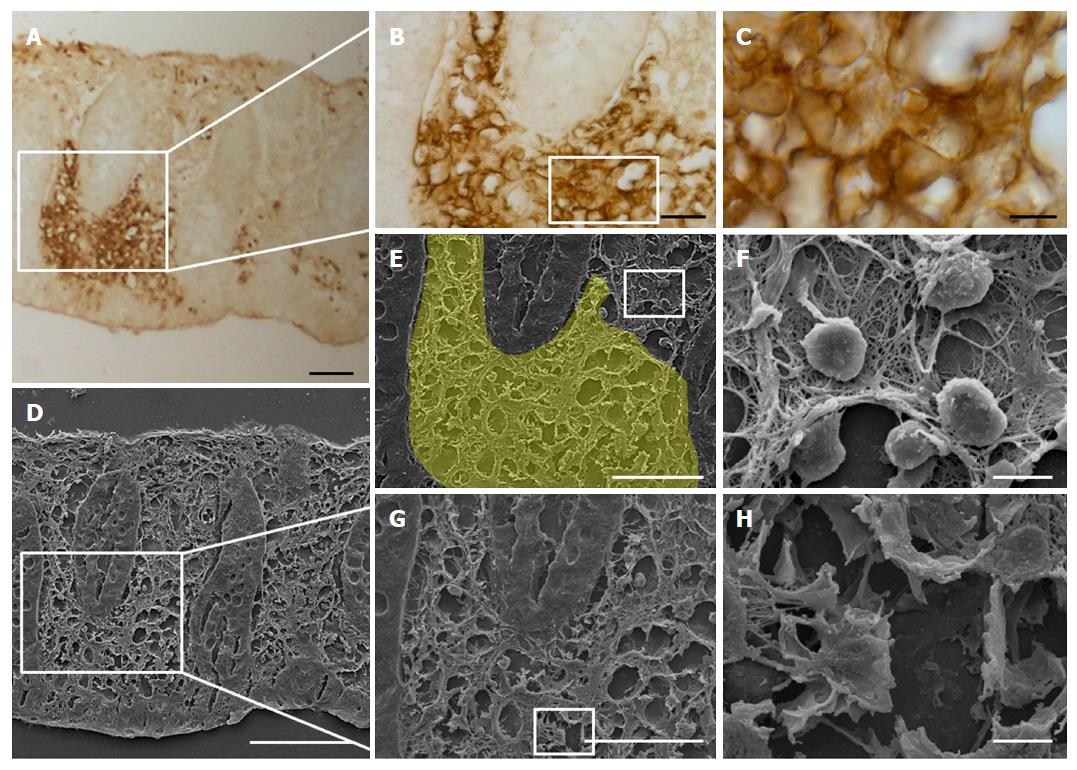
The GLUT5 positive sections were also observed at SEM for a more detailed evaluation of the labeled areas. Comparison between the SEM (Figure 7A and C) and the light microscope (Figure 7B and D) images showed that the area with GLUT5 immunoreactivity (Figure 8A-E) was characterized by large spaces and numerous dilated vessels, probably lymphatic because of the absence of red blood cells inside the lumen (Figure 8G and H). The vessels had a non-continuous endothelium, as observed at light microscopy. In the non-labeled areas, there were vessels containing cells inside the lumen that, judging by their rounded appearance, were probably lymphocytes or plasma cells (Figure 8F).
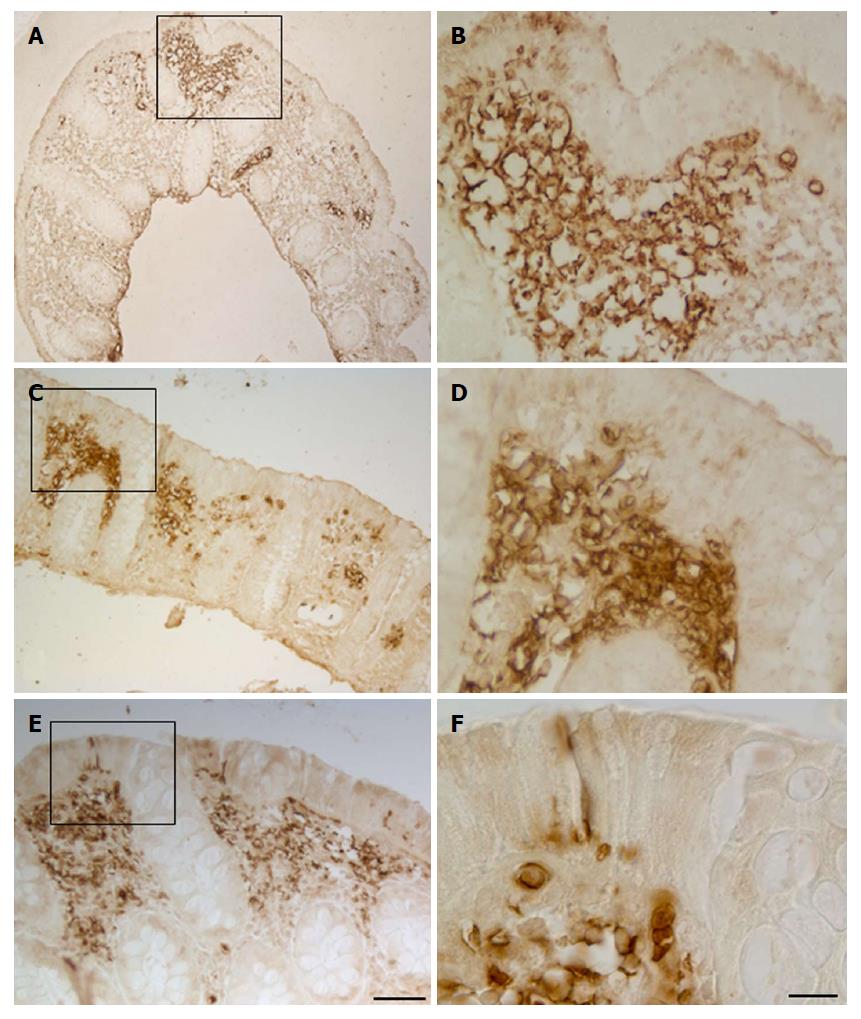
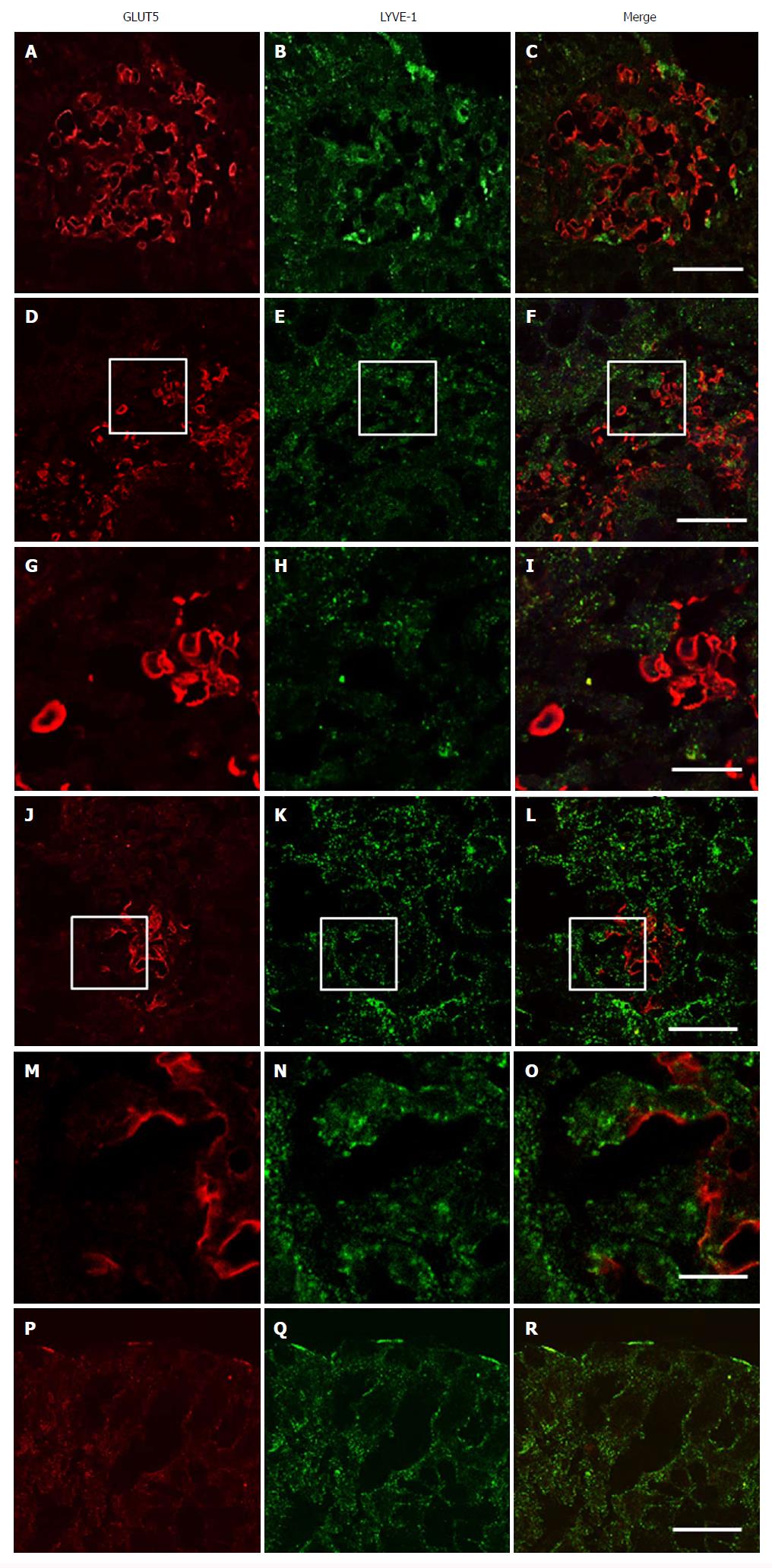
GLUT5 expression was compared with expression of LYVE-1 and VEGF by light immunohistochemistry in contiguous sections and by laser-scanning confocal microscopy in double staining. Light immunohistochemistry revealed the presence of GLUT5 immunoreactivity in areas that were also LYVE-1 positive (Figure 9A, B, G and H). GLUT5 positivity was limited to areas usually located under the epithelium (Figure 9C and I), whereas LYVE-1 staining was distributed in large areas of the lamina propria (Figure 9D and J). The GLUT5 expression pattern was generally much more marked than LYVE-1 labeling, which was formed by short spots (Figure 9E and F). However, some corresponding areas in contiguous sections showed GLUT5 immunoreactivity (Figure 9G and K) but lacked LYVE-1 staining (Figure 9H and L).
Double immunofluorescence microscopy was consistent with light-microscopic evaluation. GLUT5-IR clusters were found distributed inside LYVE-1 positive areas and formed by numerous vessels, most of which were irregularly shaped and with ill-defined lumina (Figure 10A-F and J-L). GLUT5 and LYVE-1 expression were never colocalized on the morphologically undefined wall but rather were expressed in close proximity to some areas of the endothelium (Figure 10M-O). Some vessels showed only GLUT5 expression (Figure 10G-I).
In addition to staining the lymphatic vessels, LYVE-1 also labeled the brush border membrane of some epithelial cells which was observed in both immunohistochemistry (Figure 9D) and double-immunofluorescent experiments, in the latter also seen colocalized with GLUT5 expression (Figure 10P-R).
To further discriminate between lymphatic vessels and blood vessels, expression of GLUT5 and VEGF antibodies was compared in contiguous sections using light immunohistochemistry and immunofluorescence. Both analyses revealed that VEGF expression was absent in GLUT5-IR clusters, confirming that GLUT5-immunoreactivity is selective for lymphatic vessels (data not shown).
In control sections, no specific double-labeling was seen when the second antibody was replaced with normal rabbit serum.
Here we show that GLUT2, SGLT1, and GLUT5 glucose transporters are expressed in the epithelial cells of tissue samples of the large intestine, and that they are mainly located in the brush border membrane from IBD and control patients. Also in the colonic mucosa their location reflects the canonical expression of the small intestine and confirms the tissue-specific expression of GLUT isoforms also in humans. However, unlike the small intestine, their expression is present only in short epithelial portions, involving a limited number of cells. We observed no important differences in glucose transporter expression between the samples obtained from the proximal and distal tracts and between the different patient groups. Nevertheless, we consider this finding important as it provides the first evidence for the distribution of glucose transporter immunoreactivity throughout the epithelial tract of the human large intestine. Because of the limited expression of the glucose transporters, we were unable to compare their expression across the three patient groups. However, we believe that our data support previous findings. In humans, GLUT5 expression has been observed in normal colonic cells; expression of both GLUT2 and GLUT5 has been reported in colon carcinoma and expression of GLUT5 with higher levels of staining than in normal colon cells[23]. GLUT2 and GLUT5 have been found to be overexpressed in many other types of tumors. Since both transporters are involved in fructose uptake, it was suggested that tumor cells utilize fructose as an energy substrate[23].
On the other hand, while excessive, chronic consumption of the GLUT5 and GLUT2 substrate fructose has been associated with numerous diseases and syndromes, including hypertension, obesity, diabetes, hyperinsulinemia, and non-alcoholic fatty liver disease, the role of fructose transporters in causing or contributing to the diseases is still unclear[10].
In addition, we also identified GLUT5 expression in vessels, mainly concentrated in specific areas where the vessels were clustered. This staining was detected in the samples from IBD and control patients. Furthermore, immunohistochemical and SEM evaluation revealed that the vessels in the GLUT5-IR clusters had a reticular architecture, indistinct and dilated lumen, and were labeled on the endothelium that appeared to be fragmented. Large spaces between the vessels were present. Immunostaining with LYVE-1, a specific marker of lymphatic endothelium, and GLUT5 antibodies in contiguous sections revealed that GLUT5-IR clusters of vessels were concentrated in areas that were well-circumscribed but internal to those that were LYVE-1 positive. This finding was also confirmed in double staining studies. GLUT5 and LYVE-1 did not appear to be colocalized but rather showed a close topographical relationship on the endothelium lining the lumen. Based on their LYVE-1 expression, GLUT5-IR vessels were unambiguously identified as lymphatic. We interpreted the presence of clusters as an impaired pattern of lymphatic capillary networks, probably related to proliferative zones, and consisting of vessels with morphological characteristics resembling those of immature lymphatics. They differed markedly from the vessels that we classified as “normal”, which were characterized by a well-defined lumen lined by endothelial cells, a rounded appearance, and a non-aggregated distribution.
To the best of our knowledge, this is the first study to provide evidence that GLUT5 expression is associated with lymphatic vessels in controls and UC and CD patients. This novel finding yields further insight into the characterization of lymphatic vasculature, whose dysfunction is a long-recognized feature in humans with IBD.
There is considerable evidence for the proliferative expansion of lymphatic vessels during the course of inflammatory disease[39-41]. Alteration and remodeling of the lymphatic system, besides blood vessel angiogenesis, are implicated in IBD[42-44]. Increased lymphatic vessels density is a well-established feature in biopsy samples from CD and UC patients and is correlated with disease severity[42,44-47]. Similarly, a reduced density of lymphatic vessels was demonstrated to be associated with CD recurrence[46].
Moreover, studies have reported that lymphatic vessel dysfunction contributes to perpetuating intestinal inflammation in both UC and CD patients and that the intestinal lymphatic system can profoundly influence gut immune homeostasis[48,49]. However, it is still unclear whether expansion of the lymphatic network in IBD is a defensive mechanism or contributes to worsening the condition. It can be interpreted as a protective/adaptive, local response linked to the necessity to reduce fluid accumulation and to remove infiltrated immune cells, thus limiting further tissue injury. But lymphatic vessels also undergo morphological and functional changes in inflammatory conditions that impair their ability to drain fluid[50].
Lymphangiectasia is typically observed in the colonic mucosa of CD and UC patients[44,45], and impaired intestinal lymphatic transport function was found to play a role in the early-stages and development of IBD[51,52]. The relevance of the lymphatic transport function in IBD was also demonstrated in an experimental murine model that showed that acute colitis was aggravated by compromised lymphatic function; in contrast, immune cell clearance, fluid transport, and course of the disease were improved by induction of lymphangiogenesis[53,54].
While it is beyond doubt that increased lymphatic vessel density is closely linked to inflammatory disease, whether this process in IBD is an event that precedes inflammation or is its direct consequence remains to be elucidated.
Here we demonstrated a relevant presence of GLUT5-IR clusters of lymphatic vessels in both inflamed and non-inflamed tissue samples of large intestine from IBD and CTRL patients. The intestinal distal tract was, by far, the area most affected by their presence, especially in the UC and CTRL groups. This finding is consistent with the abundance of lymphatic tissue in the most distal regions of the intestine[50].
To our surprise, we found more non-inflamed than inflamed tissue samples expressing GLUT5-IR clusters in the distal tract of the UC patients and controls: these percentages were similar across all three patient groups when classified by BMI. Differently, in the CD patients, similar results were found for the proximal and the distal intestinal tract, and the number of inflamed samples with GLUT5-IR clusters was higher than non-inflamed tissue samples, also in relation to the BMI of patients. Our data indicate that GLUT5-IR clusters are present in both UC patients and controls, and that their presence does not appear to be conditioned by the level of inflammation of the mucosal area where the clusters are found. The opposite finding in the CD group may indicate a particular aspect of this condition. Since the amount of tissue analyzed in the CD patients was limited, it may not be completely representative of the whole group, however.
The few studies that have investigated the relationship between lymphoproliferation and inflammation of the area where it occurs have shown that lymphatic proliferation and inflammation do not occur in close association. Several explanations for this have been proposed. Increased lymphatic vessel density has been documented as a persistent feature in samples from IBD patients, in which it was also observed in fibrotic specimens from end-stage disease characterized by low inflammation[42]. Similarly, the increased lymphatic vessel density detected in both the inflamed and non-inflamed tissue samples from the UC and CD patients has been interpreted as a process that occurs before the disease becomes clinically active, and therefore can manifest itself independent of inflammation[46]. No obvious correlation between increased lymphatic vessel density and inflammation was demonstrated in the colonic mucosa of the UC patients, where it has been predicted that structural changes in the mucosa, including “muscularis mucosa expansion, infiltration by muscle fibers, and filiform epithelial changes” are an essential prerequisite to justify lymphatic proliferation[45].
Both these interpretations may also be consistent with our data: the first is especially valid for the controls where the presence of vessel clusters can be a subclinical aspect, at times not necessarily related to a particular pathology. The second can be valid for IBD: we sometimes observed vacuolar structures in the epithelium over the areas where lymphatic vessel clusters were present and altered epithelial cell integrity. Recent studies have shown that epithelial barrier dysfunction in IBD can contribute to disease progression by activating immunoregulatory processes, probably following increased exposure of the mucosa to the luminal microbiota or their products[55-58]. Although the underlying mechanism is unclear, defects in intestinal barrier function are now attributed a direct or indirect role in the onset or progression of IBD. We observed morphological epithelial damage limited to certain areas of the epithelium in some samples and only at the light microscope, in which the real damage may be underestimated.
Other mechanisms/factors have been investigated to explain the stimulation of lymphatic vessels in IBD, given that it may occur via different processes in UC and CD. Lymphatic system expansion during inflammation in peripheral tissues progresses through the expansion and proliferation of an existing vascular network or sprouting of new vessels (lymphangiogenesis)[40,59]. However, the expression of lymphangiogenic factors (e.g., VEGF-C, VEGF-D) and cytokines (e.g., TNF and IL-1b), which have been found to be increased in inflammatory conditions, were decreased in the inflamed and non-inflamed colonic tissues of the IBD patients[46,60].
LYVE-1 has also been demonstrated to be selectively expressed in inflammation. In cultured primary lymphatic endothelial cells, LYVE-1 uptake and degradation and inhibition of its gene expression were seen to occur under the stimulus of pro-inflammatory cytokines, leading to a subsequent reversible loss of the surface expression of LYVE-1[61]. Subsequently, quantification of LYVE-1 by RT-PCR in human colonic mucosa revealed a lower mRNA level in both the non-inflamed and inflamed samples from the UC patients than in the controls, but no differences in LYVE-1 mRNA levels were seen in the CD patients[46]. In the UC patients, the reduction was more evident in the non-inflamed than the inflamed samples.
In this study, double-immunofluorescence staining revealed that GLUT5-IR clusters were present in zones with LYVE -1 positivity, however, the appearance of the vessels inside the clusters was very heterogeneous, as was their phenotypic expression: GLUT5-positive but LYVE-1 negative or GLUT5-negative but LYVE-1 positive vessels were often identified, whereas the simultaneous expression of LYVE-1 and GLUT5 was limited to restricted endothelial areas of some clustered vessels. In the cases where LYVE-1 was missing, we saw the expression of GLUT5, which appeared in close continuity with LYVE-1, without ever being colocalized with it. These data strongly suggest a reduced expression of LYVE-1, which may depend on the different degrees of vessel differentiation within the clusters. There is considerable evidence that immature vessels lack LYVE-1 expression, particularly intratumoral lymphatic vessels, that differ morphologically from lymphatic vessels found within normal tissue[62,63]. The reason for this difference is unclear; however, it has been also demonstrated that altered expression of lymphatic endothelial markers such as LYVE-1 may compromise lymphatic transport function[50].
Collectively, our results regarding GLUT5 expression in IBD patients are consistent with previous data obtained for lymphatic proliferation in IBD. The presence of GLUT5 alone does not imply that it is involved in lymphatic proliferation because it was found to be expressed in both normal and clustered lymphatic vessels. However, its expression indicates that GLUT5 may play a role in controlling the formation of lymphatic vessels. It is plausible that aberrant lymphatic growth in clusters may result from the effect of GLUT5 on cellular mechanisms underlying the differentiation of new lymphatic vessels. It may also be that fructose, GLUT5 being its main transporter, may be the energy substrate that endothelial cells use for lymphatic expansion.
In this regard, we speculate that fructose present in the lumen may have some effect on the proliferation of these vessels. We found that a percentage of samples expressed GLUT5 in the brush border membrane of epithelial cells from both the UC and CTRL patients. This finding merits further investigation because it could be a clear example of crosstalk between the cells projecting into the intestinal lumen and underlying environments.
In conclusion, our study provides evidence that GLUT2, SGLT1, and GLUT5 glucose transporters are expressed in the epithelial cells of the mucosa of the large intestine of IBD and control patients. Furthermore, both inflamed and non-inflamed mucosal colorectal tissue biopsies from the IBD and control patients showed GLUT5-IR lymphatic vessel clusters. This finding aids in the characterization of lymphatic vessels in IBD. As GLUT5 is the main fructose transporter in the human intestine, it is conceivable that fructose is a metabolic substrate that has a role in the atypical aggregation of lymphatic vessels.
Quantification of lymphatic vessel density in the gastrointestinal tract, particularly in cancer and chronic inflammatory disease, holds interest because it can be predictive of the early stages of disease. In this regard, GLUT5 expression on endothelial lympho-vascular cells may have implications for routine use in the histopathological evaluation of lymphangiogenesis, also in combination with LYVE-1, a marker of lymphatic endothelium that can be down-modulated under inflammatory conditions.
A future area of focus is the precise function of GLUT5 expression in lymphatic vessels and the factors that interfere with the development or maintenance of IBD.
Glucose transporter expression is present throughout the digestive system, greatest in the small intestine and least in the terminal ileum. Most studies to date on glucose transporters in the gastrointestinal tract have been performed using biochemical rather than standard immunohistochemical analysis also in humans.
The localization and distribution of glucose transporters in the intestinal mucosa of the human colon has not yet been clarified despite evidences that support the role of glucose transporters in broad areas of pure glucose absorption and metabolism, such as inflammation, malignancy, and gut microbiota regulation.
The aim of this study was to investigate by immunostaining at light and confocal microscopy the expression of the major intestinal glucose transporters (GLUT2, SGLT1, GLUT5) in human colonic mucosa in control subjects and subjects with IBD.
Patients diagnosed with ulcerative colitis or Crohn’s disease and scheduled for diagnostic colonoscopy were enrolled. Patients who underwent colonoscopy for prevention screening of colorectal cancer or were followed-up after polypectomy or lower gastrointestinal symptoms were designated as the control group. Colorectal samples were obtained from patients undergoing lower endoscopic colonoscopy or recto-sigmoidoscopy. Biopsies of portions of the colonic tract (cecum, ascending colon, transverse, descending, sigmoid colon, rectum) were taken for diagnostic purposes according to the endoscopist’s judgment and for immunohistochemistry. Inflammatory status of the mucosa at the sampling site was evaluated endoscopically and histologically.
Samples were fixed in formaldehyde and embedded in paraffin. The expression of GLUT2, SGLT1, and GLUT5 glucose transporters was investigated using immunoperoxidase labeling. Immunoreactivity of GLUT5 was compared with that of LYVE-1, which is a marker for lymphatic vessel endothelium, using double-labeled confocal microscopy.
GLUT2, SGLT1, and GLUT5 glucose transporter immunostaining was found in short epithelial portions of the large intestine from IBD and control patients. No difference in glucose transporter expression was observed between the samples obtained from the proximal and distal tracts and between the different patient groups. GLUT5 immunostaining was also detected in vessels, which were mainly concentrated in specific areas. In double fluorescent-labeled sections with GLUT5 and LYVE-1, GLUT5-immunoreactive clusters of vessels were concentrated in areas internal to those that were LYVE-1 positive. The GLUT5 and LYVE-1 labeling patterns were never colocalized but rather showed a close topographical relationship on the endothelium lining the lumen. Based on their LYVE-1 expression, GLUT5 immunoreactive vessels were identified as lymphatic and observed both in inflamed and non-inflamed mucosal colorectal tissue biopsies from the IBD and CTRL patients. This novel finding yields further insight into the characterization of lymphatic vasculature, whose dysfunction is a long-recognized feature in humans with IBD.
This study provides evidence that GLUT2, SGLT1, and GLUT5 glucose transporters are expressed in the colorectal mucosa in controls and IBD patients. Furthermore, it provides first evidence that GLUT5 expression is associated with lymphatic vessels in controls and IBD patients. Its expression indicates that GLUT5 may play a role in controlling the formation of lymphatic vessels. GLUT5 expression on endothelial lymphovascular cells may have implications for routine use in the histopathological evaluation of lymphangiogenesis, also in combination with LYVE-1, a marker of lymphatic endothelium that can be down-modulated under inflammatory conditions.
A future area of focus is the precise function of GLUT5 expression in lymphatic vessels and the factors that interfere with the development or maintenance of IBD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Huerta-Franco MR, Maric I, Nakase H, Yu LCH S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1153] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 2. | Kayano T, Burant CF, Fukumoto H, Gould GW, Fan YS, Eddy RL, Byers MG, Shows TB, Seino S, Bell GI. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990;265:13276-13282. [PubMed] |
| 3. | Blakemore SJ, Aledo JC, James J, Campbell FC, Lucocq JM, Hundal HS. The GLUT5 hexose transporter is also localized to the basolateral membrane of the human jejunum. Biochem J. 1995;309:7-12. [PubMed] |
| 4. | Douard V, Cui XL, Soteropoulos P, Ferraris RP. Dexamethasone sensitizes the neonatal intestine to fructose induction of intestinal fructose transporter (Slc2A5) function. Endocrinology. 2008;149:409-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Matosin-Matekalo M, Mesonero JE, Laroche TJ, Lacasa M, Brot-Laroche E. Glucose and thyroid hormone co-regulate the expression of the intestinal fructose transporter GLUT5. Biochem J. 1999;339:233-239. [PubMed] |
| 6. | David ES, Cingari DS, Ferraris RP. Dietary induction of intestinal fructose absorption in weaning rats. Pediatr Res. 1995;37:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab. 2008;295:E227-E237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 329] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | Litherland GJ, Hajduch E, Gould GW, Hundal HS. Fructose transport and metabolism in adipose tissue of Zucker rats: diminished GLUT5 activity during obesity and insulin resistance. Mol Cell Biochem. 2004;261:23-33. [PubMed] |
| 9. | Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241-G248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 232] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Douard V, Ferraris RP. The role of fructose transporters in diseases linked to excessive fructose intake. J Physiol. 2013;591:401-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Thorens B, Cheng ZQ, Brown D, Lodish HF. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990;259:C279-C285. [PubMed] |
| 12. | Yoshikawa T, Inoue R, Matsumoto M, Yajima T, Ushida K, Iwanaga T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem Cell Biol. 2011;135:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 14. | Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, Citadelle D, Kellett GL, Thorens B, Leturque A, Brot-Laroche E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol. 2003;552:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350 Pt 1:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Ait-Omar A, Monteiro-Sepulveda M, Poitou C, Le Gall M, Cotillard A, Gilet J, Garbin K, Houllier A, Château D, Lacombe A. GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes. 2011;60:2598-2607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 612] [Cited by in RCA: 576] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 18. | Depoortere I. Taste receptors in the gut tune the release of peptides in response to nutrients. Peptides. 2015;66:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Schmitt CC, Aranias T, Viel T, Chateau D, Le Gall M, Waligora-Dupriet AJ, Melchior C, Rouxel O, Kapel N, Gourcerol G. Intestinal invalidation of the glucose transporter GLUT2 delays tissue distribution of glucose and reveals an unexpected role in gut homeostasis. Mol Metab. 2016;6:61-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004;447:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature. 1987;330:379-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 762] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 22. | Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 556] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 23. | Godoy A, Ulloa V, Rodríguez F, Reinicke K, Yañez AJ, García Mde L, Medina RA, Carrasco M, Barberis S, Castro T. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol. 2006;207:614-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Wuest M, Trayner BJ, Grant TN, Jans HS, Mercer JR, Murray D, West FG, McEwan AJ, Wuest F, Cheeseman CI. Radiopharmacological evaluation of 6-deoxy-6-[18F]fluoro-D-fructose as a radiotracer for PET imaging of GLUT5 in breast cancer. Nucl Med Biol. 2011;38:461-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Liu H, Huang D, McArthur DL, Boros LG, Nissen N, Heaney AP. Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Res. 2010;70:6368-6376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | García-Herrera J, Marca MC, Brot-Laroche E, Guillén N, Acin S, Navarro MA, Osada J, Rodríguez-Yoldi MJ. Protein kinases, TNF-{alpha}, and proteasome contribute in the inhibition of fructose intestinal transport by sepsis in vivo. Am J Physiol Gastrointest Liver Physiol. 2008;294:G155-G164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | García-Herrera J, Abad B, Rodríguez-Yoldi MJ. Effect of lipopolysaccharide on D-fructose transport across rabbit jejunum. Inflamm Res. 2003;52:177-184. [PubMed] |
| 28. | Barone S, Fussell SL, Singh AK, Lucas F, Xu J, Kim C, Wu X, Yu Y, Amlal H, Seidler U. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem. 2009;284:5056-5066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Uldry M, Ibberson M, Hosokawa M, Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002;524:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Yu LC, Flynn AN, Turner JR, Buret AG. SGLT-1-mediated glucose uptake protects intestinal epithelial cells against LPS-induced apoptosis and barrier defects: a novel cellular rescue mechanism? FASEB J. 2005;19:1822-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Barrenetxe J, Sánchez O, Barber A, Gascón S, Rodríguez-Yoldi MJ, Lostao MP. TNFα regulates sugar transporters in the human intestinal epithelial cell line Caco-2. Cytokine. 2013;64:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Palazzo M, Gariboldi S, Zanobbio L, Selleri S, Dusio GF, Mauro V, Rossini A, Balsari A, Rumio C. Sodium-dependent glucose transporter-1 as a novel immunological player in the intestinal mucosa. J Immunol. 2008;181:3126-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Yomogida S, Kojima Y, Tsutsumi-Ishii Y, Hua J, Sakamoto K, Nagaoka I. Glucosamine, a naturally occurring amino monosaccharide, suppresses dextran sulfate sodium-induced colitis in rats. Int J Mol Med. 2008;22:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Reed KL, Fruin AB, Gower AC, Gonzales KD, Stucchi AF, Andry CD, O’Brien M, Becker JM. NF-kappaB activation precedes increases in mRNA encoding neurokinin-1 receptor, proinflammatory cytokines, and adhesion molecules in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2005;50:2366-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Visekruna A, Joeris T, Seidel D, Kroesen A, Loddenkemper C, Zeitz M, Kaufmann SH, Schmidt-Ullrich R, Steinhoff U. Proteasome-mediated degradation of IkappaBalpha and processing of p105 in Crohn disease and ulcerative colitis. J Clin Invest. 2006;116:3195-3203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Lewis Carl SA, Gillete-Ferguson I, Ferguson DG. An indirect immunofluorescence procedure for staining the same cryosection with two mouse monoclonal primary antibodies. J Histochem Cytochem. 1993;41:1273-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Negoescu A, Labat-Moleur F, Lorimier P, Lamarcq L, Guillermet C, Chambaz E, Brambilla E. F(ab) secondary antibodies: a general method for double immunolabeling with primary antisera from the same species. Efficiency control by chemiluminescence. J Histochem Cytochem. 1994;42:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. Glucose transporters are expressed in taste receptor cells. J Anat. 2011;219:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 925] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 41. | Kerjaschki D. The lymphatic vasculature revisited. J Clin Invest. 2014;124:874-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Geleff S, Schoppmann SF, Oberhuber G. Increase in podoplanin-expressing intestinal lymphatic vessels in inflammatory bowel disease. Virchows Arch. 2003;442:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807-4812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 196] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 44. | Pedica F, Ligorio C, Tonelli P, Bartolini S, Baccarini P. Lymphangiogenesis in Crohn’s disease: an immunohistochemical study using monoclonal antibody D2-40. Virchows Arch. 2008;452:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Kaiserling E, Kröber S, Geleff S. Lymphatic vessels in the colonic mucosa in ulcerative colitis. Lymphology. 2003;36:52-61. [PubMed] |
| 46. | Rahier JF, De Beauce S, Dubuquoy L, Erdual E, Colombel JF, Jouret-Mourin A, Geboes K, Desreumaux P. Increased lymphatic vessel density and lymphangiogenesis in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Fogt F, Pascha TL, Zhang PJ, Gausas RE, Rahemtulla A, Zimmerman RL. Proliferation of D2-40-expressing intestinal lymphatic vessels in the lamina propria in inflammatory bowel disease. Int J Mol Med. 2004;13:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Vranova M, Halin C. Lymphatic Vessels in Inflammation. J Clin Cell Immunol. 2014;5:250. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Becker F, Yi P, Al-Kofahi M, Ganta VC, Morris J, Alexander JS. Lymphatic dysregulation in intestinal inflammation: new insights into inflammatory bowel disease pathomechanisms. Lymphology. 2014;47:3-27. [PubMed] |
| 50. | Alexander JS, Chaitanya GV, Grisham MB, Boktor M. Emerging roles of lymphatics in inflammatory bowel disease. Ann N Y Acad Sci. 2010;1207 Suppl 1:E75-E85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Von Der Weid PY, Rehal S. Lymphatic pump function in the inflamed gut. Ann N Y Acad Sci. 2010;1207 Suppl 1:E69-E74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Linares PM, Gisbert JP. Role of growth factors in the development of lymphangiogenesis driven by inflammatory bowel disease: a review. Inflamm Bowel Dis. 2011;17:1814-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | D’Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest. 2014;124:3863-3878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 54. | Becker F, Potepalov S, Shehzahdi R, Bernas M, Witte M, Abreo F, Traylor J, Orr WA, Tsunoda I, Alexander JS. Downregulation of FoxC2 Increased Susceptibility to Experimental Colitis: Influence of Lymphatic Drainage Function? Inflamm Bowel Dis. 2015;21:1282-1296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 56. | Berry D, Reinisch W. Intestinal microbiota: a source of novel biomarkers in inflammatory bowel diseases? Best Pract Res Clin Gastroenterol. 2013;27:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | Klag T, Stange EF, Wehkamp J. Defective antibacterial barrier in inflammatory bowel disease. Dig Dis. 2013;31:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Lanis JM, Kao DJ, Alexeev EE, Colgan SP. Tissue metabolism and the inflammatory bowel diseases. J Mol Med (Berl). 2017;95:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Wirzenius M, Tammela T, Uutela M, He Y, Odorisio T, Zambruno G, Nagy JA, Dvorak HF, Ylä-Herttuala S, Shibuya M. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J Exp Med. 2007;204:1431-1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 60. | Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, Pepper MS, Zawieja DC, Ran S. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood. 2010;115:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 61. | Johnson LA, Prevo R, Clasper S, Jackson DG. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. J Biol Chem. 2007;282:33671-33680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315-1320. [PubMed] |
| 63. | Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 683] [Article Influence: 29.7] [Reference Citation Analysis (0)] |