Published online Feb 14, 2018. doi: 10.3748/wjg.v24.i6.725
Peer-review started: October 18, 2017
First decision: November 8, 2017
Revised: November 17, 2017
Accepted: November 28, 2017
Article in press: November 28, 2017
Published online: February 14, 2018
Processing time: 111 Days and 15.3 Hours
To investigate the utility of hepatitis B surface antigen (HBsAg) kinetics in chronic hepatitis B patients during long-term entecavir treatment.
This retrospective study included treatment-naïve chronic hepatitis B patients who received at least 2 years of consecutive entecavir treatment. Patients were followed up at three to six month intervals with liver biochemistry, hepatitis B virus DNA, and abdominal sonography. In hepatitis B e antigen (HBeAg)-positive patients, HBeAg levels were assessed every three to six month until results became negative. Serum HBsAg levels were determined at the baseline, one-year and five-year time points. Liver cirrhosis was diagnosed through liver biopsy, imaging examinations, or clinical findings of portal hypertension. Hepatocellular carcinoma was diagnosed by histological examination or dynamic image studies.
A total of 211 patients were enrolled. The median treatment time was 5.24 (2.00-9.62) years. Multivariate analysis showed that lower baseline HBsAg levels were associated with an earlier virological response, earlier hepatitis B e antigen (HBeAg) seroconversion, and earlier biochemical response in HBeAg-positive patients (cut-off value: 4 log IU/mL) and an earlier virological response in HBeAg-negative non-cirrhotic patients (cut-off value: 2.4 log IU/mL). Although HBsAg levels decreased slowly during long-term entecavir treatment, higher HBsAg decrease rates were found in the first year for HBeAg-positive non-cirrhotic patients, and patients with higher baseline HBsAg levels. More favorable clinical outcomes were not observed by a rapid HBsAg decline per se, but depended on lower baseline HBsAg levels.
Baseline HBsAg can be used to predict treatment responses. HBsAg levels and decrease rates should be considered together according to disease status while interpreting HBsAg changes.
Core tip: Baseline hepatitis B surface antigen (HbsAg) levels could be used to predict virological, serological, and biochemical responses during entecavir treatment. HBeAg-positive non-cirrhotic patients had the highest HBsAg levels at the baseline and throughout entecavir treatment, and had the highest HBsAg decrease rates during the first year of entecavir treatment. HBsAg levels decrease slowly during the treatment. Therefore, HBsAg should be checked at a 1-year interval if hepatitis B virus DNA remains undetectable. A rapid HBsAg decline per se did not achieve better patient outcomes. In the interpretation of HBsAg changes, HBsAg levels and decrease rates should be considered together according to disease status.
- Citation: Lin TC, Chiu YC, Chiu HC, Liu WC, Cheng PN, Chen CY, Chang TT, Wu IC. Clinical utility of hepatitis B surface antigen kinetics in treatment-naïve chronic hepatitis B patients during long-term entecavir therapy. World J Gastroenterol 2018; 24(6): 725-736
- URL: https://www.wjgnet.com/1007-9327/full/v24/i6/725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i6.725
Patients with chronic hepatitis B virus (HBV) infection are at risk of cirrhosis and hepatocellular carcinoma (HCC)[1]. Eradication of chronic HBV infection is difficult because of the presence of covalently closed circular DNA (cccDNA) in infected cells[2]. HBV cccDNA resides in the nucleus of infected cells as an episomal (i.e., nonintegrated) plasmid-like molecule. The paucity of knowledge about cccDNA formation and degradation is a considerable obstacle to the development of anti-chronic HBV infection treatments[2].
Hepatitis B surface antigen (HBsAg) levels have been used to discriminate between different clinical phases[3], predict spontaneous HBsAg seroclearance[4,5], and identify inactive phases in hepatitis B e antigen (HBeAg)-negative patients[6]. HBsAg levels of < 100 IU/mL could predict HBsAg loss in HBeAg seroconverters[7] and identify HBeAg-negative patients with inactive virus[8].
HBsAg levels can also be used to guide pegylated interferon (PegIFN) treatment course. In HBeAg-positive patients with an HBsAg level > 20000 IU/mL after 24 wk of treatment, PegIFN discontinuation is suggested[9]. In HBeAg-negative patients without HBsAg level declines by week 12 and without HBV DNA level declines of > 2 log IU/mL, a sustained response to therapy is considered impossible[10]. PegIFN responders, compared with nonresponders, had greater declines in HBsAg and cccDNA levels. For PegIFN responders, mean HBsAg reduction levels were 2.5 ± 2.3 log IU/mL in HBeAg-positive patients and 2.5 ± 1.3 log IU/mL in HBeAg-negative patients after 48 wk of treatment[11].
HBsAg levels declined much less rapidly during nucleos(t)ide analogue (NA) treatment, compared with PegIFN treatment[12]. The declines in HBsAg levels from the baseline to week 48 during NA treatment were 0.3 to 0.5 log IU/mL in HBeAg-positive patients and -0.1 to 0.1 log IU/mL in HBeAg-negative patients[3]. For most patients, long-term NA treatment renders a consistent but slow reduction (0.084 log IU/year)[13].
For patients receiving NA treatment, HBsAg quantification may help to predict clinical outcomes. HBsAg levels of < 3000 IU/mL at the baseline combined with HBsAg declines of ≥ 75% from the baseline could predict the eventual loss of HBsAg[14]. An HBsAg reduction of > 1 log IU/mL could reflect improved immune control[12,15], and a reduction of ≥ 0.5 log IU/mL after 6 mo of treatment had a high negative predictive value for HBsAg seroclearance[16].
Serum HBsAg is closely related to serum HBV DNA and intrahepatic cccDNA in HBeAg-positive patients, but it is poorly correlated with serum HBV DNA and not correlated with intrahepatic cccDNA in HBeAg-negative patients[11,17]. HBsAg changes after NA treatment were also different between HBeAg-positive and HBeAg-negative patients[3]. Two studies reported that baseline HBsAg levels could help to predict HBsAg decline or loss in HBeAg-negative patients[18,19]. On the contrary, some studies suggested that neither baseline HBsAg nor reduction in HBsAg could predict virological response in HBeAg-negative patients[20,21]. Therefore, these results are rather divergent in HBeAg-negative patients. The aim of the current study was to investigate the role of HBsAg levels in predicting treatment responses and the clinical significance of HBsAg kinetics for different disease statuses during long-term entecavir treatment.
This retrospective study analyzed treatment-naïve chronic hepatitis B patients receiving at least two years of consecutive entecavir treatment at National Cheng Kung University Hospital. The exclusion criteria were (1) prior treatment history with NAs or interferon; (2) coinfection with hepatitis C virus or human immunodeficiency virus infection; (3) end-stage renal disease; (4) systemic chemotherapy due to active cancer; and (5) post-organ transplantation. During the study period, patients received entecavir as the only anti-HBV therapy. Indications for entecavir therapy followed the Asian Pacific Association for the Study of the Liver HBV treatment guideline[22]. Enrolled patients were started on entecavir between December 2007 and January 2015. This study was approved by the Institutional Review Board of National Cheng Kung University Hospital. We analyzed the medical charts and remaining serum samples of these patients in this study. The informed consents of remaining specimen usage were obtained from these patients at the request of the Institutional Review Board of National Cheng Kung University Hospital.
All enrolled patients underwent follow-up liver biochemistry testing, HBV DNA testing, and abdominal sonography at three to six month (twelve to twenty-four week) intervals. In HBeAg-positive patients, HBeAg levels were assessed every three to six month until results became negative. Serum HBsAg quantification was performed at the baseline, one-year (48 wk) time point, and five-year (240 wk) time point after treatment. Liver cirrhosis was diagnosed through liver biopsy, imaging examinations [abdominal sonography, computed tomography (CT), or magnetic resonance imaging (MRI)], or clinical findings of portal hypertension (esophageal or cardiac varices by esophagogastroduodenoscopy). HCC was diagnosed by histological examination (liver biopsy or surgery) or dynamic image studies (CT and MRI).
Virological response to treatment was defined as the point at which serum HBV DNA became undetectable (< 60 IU/mL) during treatment. HBeAg seroclearance was defined as a loss of HBeAg, whereas HBeAg seroconversion was defined as a loss of HBeAg and occurrence of anti-HBe, according to the Asian Pacific Association for the Study of the Liver HBV treatment guideline[22]. Because alanine aminotransferase (ALT) levels usually fluctuated and were affected by multiple factors during treatment, biochemical response was defined as ALT normalization [(≤ upper limit of normal (ULN)] for more than 6 mo during the study period and for the last 6 mo of the study period in patients with elevated baseline ALT levels. The ULN of ALT was 50 U/mL in male patients and 35 U/mL in female patients at National Cheng Kung University Hospital.
Serum HBsAg levels were measured using the Architect HBsAg assay (Abbott, Chicago, IL, United States), with a linear range of 0.05 to 250 IU/mL. Samples with levels higher than 250 IU/mL were retested at a series of dilutions according to the manufacturer’s instructions. Serum HBV DNA levels were determined using the Roche Cobas Amplicor [lower limit of detection (LLD): 60 IU/mL], the Roche Cobas TaqMan 48 analyzer (LLD: 29 IU/mL), the Roche Cobas AmpliPre/Cobas TagMan HBV Test, version 1.0 (LLD: 12 IU/mL), and the Roche Cobas AmpliPre/Cobas TagMan HBV Test, version 2.0 (LLD: 20 IU/mL). Baseline HBV DNA levels of serum samples collected from 22 patients (22/211, 10.4%) between December 2007 and October 2009 were measured by our in-house LightCycler real-time method, which was well correlated with results from the Roche Cobas Amplicor. HBV genotype was determined using melting curve analysis with LightCycler hybridization probes, as described previously[23].
Continuous variables are expressed as mean and standard deviation, except for treatment time, which is expressed as median and range. Categorical variables are expressed as numbers (percentages). Continuous variables were compared using Student’s t test. The distributions of categorical variables were compared using the Chi-square test or Fisher’s exact test when an expected value was less than 5. The cumulative incidence of treatment responses and clinical events with different variables were obtained using the Kaplan-Meier analysis, and the log-rank test was used to test for statistical difference. Multivariate analysis was performed using Cox proportional hazards regression to determine the factors that were independently associated with treatment responses and clinical events. A linear mixed model with a random intercept was used for analysis of longitudinal changes of HBsAg levels. In this model, groups and time points were treated as categorical variables and represented by dummy variables. Statistical analysis was performed using Stata 14.2 (Stata-Corp, Tx, United States). Results were considered statistically significant at P < 0.05.
A total of 211 treatment-naïve chronic hepatitis B patients receiving entecavir monotherapy were enrolled in this study. The median entecavir treatment time was 5.24 (2.00-9.62) years. The mean age was 50.4 ± 11.9 years. Most patients were men (69.7%), HBeAg-negative (70.6%), and non-cirrhotic (68.7%). Compared with HBeAg-negative patients, HBeAg-positive patients had a younger age, higher baseline HBV DNA and HBsAg levels, and lower proportions of liver cirrhosis, HCC diagnosed before or within half a year of entecavir therapy, and genotype B HBV infection (Table 1).
| Characteristics | Total (n = 211) | HBeAg-positive (n = 62) | HBeAg-negative (n = 149) | P value1 |
| Age (yr) | 50.4 ± 11.9 | 43.8 ± 12.2 | 53.2 ± 10.6 | < 0.0001 |
| Male | 147 (69.7) | 39 (63.0) | 108 (72.5) | 0.170 |
| Treatment time (yr) | 5.24 (2.00-9.62) | 4.39 (2.11-9.62) | 5.35 (2.00-9.58) | 0.590 |
| Cirrhosis | 66 (31.3) | 12 (19.4) | 54 (36.2) | 0.016 |
| HCC2 | 32 (15.2) | 5 (8.1) | 27 (18.1) | 0.060 |
| HBV genotype3 | 101:86:2 (53.4%:45.5%:1.1%) | 21:39:01 | 80:47:01 | 0.001 |
| B:C:B + C | (34.4%:63.9%:1.6%) | (62.5%:36.7%:0.8%) | ||
| ALT (× ULN) | 4.12 ± 5.88 | 4.42 ± 6.62 | 3.99 ± 5.56 | 0.630 |
| HBV DNA (log IU/mL) | 5.84 ± 1.70 | 7.24 ± 1.39 | 5.26 ± 1.50 | < 0.0001 |
| HBsAg (log IU/mL) | 3.15 ± 0.80 | 3.80 ± 0.71 | 2.89 ± 0.67 | < 0.0001 |
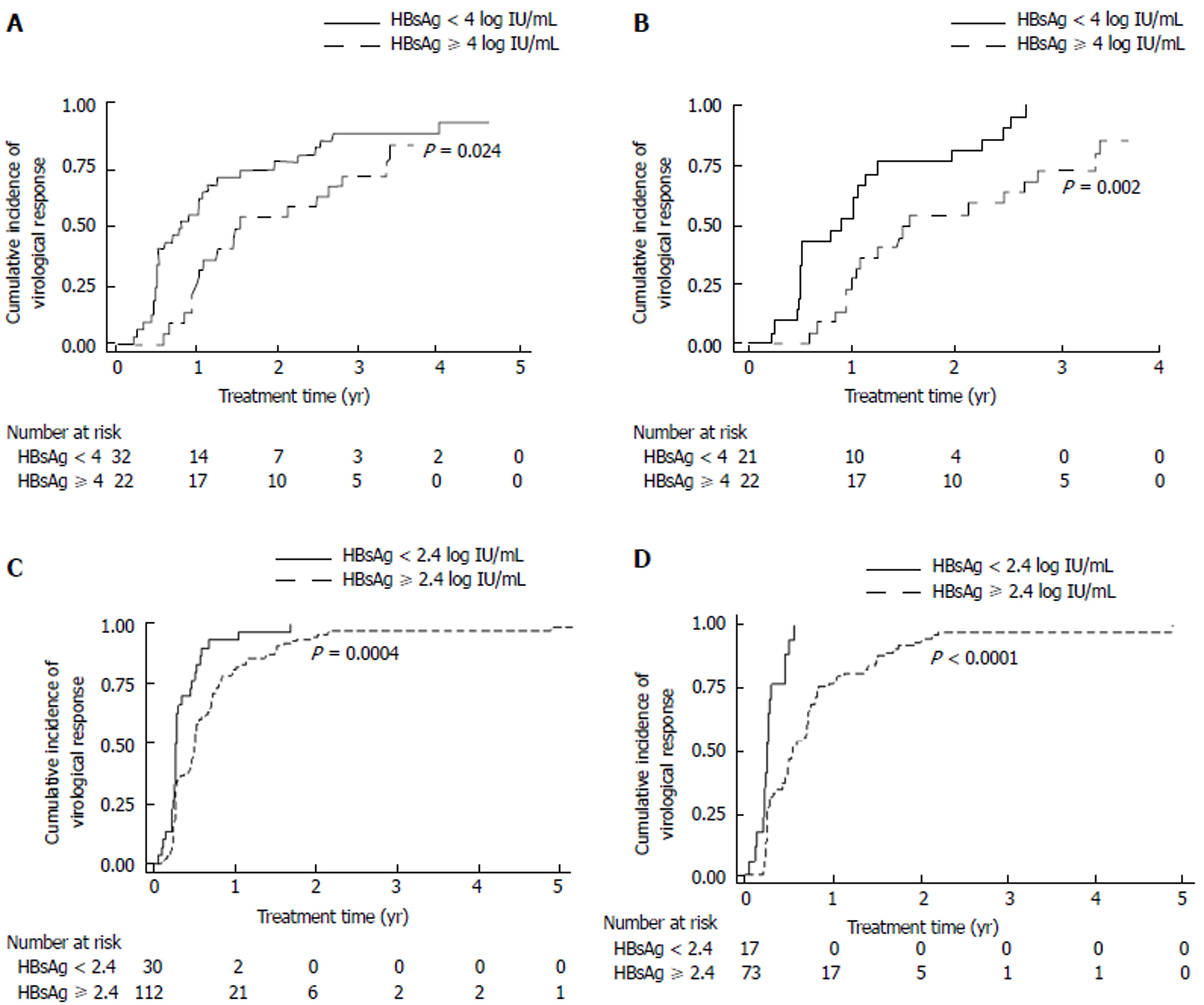
One hundred and ninety-six patients were assessed for virological response to treatment. One hundred and eighty-eight patients (188/196, 95.9%) achieved virological response during treatment. The median time to virological response was 0.50 (0.04-4.88) years. Among HBeAg-positive patients (n = 54), those with a baseline HBsAg level of < 4 log IU/mL had an earlier virological response, compared with those with a baseline HBsAg level of ≥ 4 log IU/mL (P = .024, Figure 1A). Multivariate analysis revealed that the female sex and a baseline HBsAg level of < 4 log IU/mL were independently associated with an earlier virological response [female vs male: hazard ratio (HR): 2.95, 95% confidence interval (CI): 1.33-6.57, P = 0.008; HBsAg < 4 vs ≥ 4 log IU/mL: HR: 4.92, 95%CI: 2.10-11.51, P < 0.001, Table 2]. A subgroup analysis for HBeAg-positive non-cirrhotic patients showed that the female sex, a higher baseline ALT, and a baseline HBsAg level of < 4 log IU/mL were independently associated with an earlier virological response (Figure 1B and Supplemental Table 1A).
| Factors | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex (female vs male) | 1.31 | 0.73-2.36 | 0.37 | 2.95 | 1.33-6.57 | 0.008 |
| Age (yr) | 1.00 | 0.98-1.02 | 0.90 | 0.99 | 0.96-1.01 | 0.390 |
| Cirrhosis (yes vs no) | 0.91 | 0.42-1.96 | 0.81 | 0.94 | 0.32-2.79 | 0.910 |
| HBV genotype (B vs C)1 | 1.14 | 0.62-2.09 | 0.67 | 2.04 | 0.99-4.21 | 0.053 |
| ALT (× ULN) | 1.02 | 0.98-1.06 | 0.25 | 1.04 | 1.00-1.08 | 0.060 |
| HBV DNA (≤ 5 vs > 5 log IU/mL) | 2.72 | 0.96-7.68 | 0.06 | 1.78 | 0.46-6.90 | 0.400 |
| HBsAg (< 4 vs ≥ 4 log IU/mL) | 1.96 | 1.08-3.55 | 0.03 | 4.92 | 2.10-11.51 | < 0.001 |
In HBeAg-negative patients (n = 142), a baseline HBsAg level of < 2.4 log IU/mL predicted virological response in the univariate analysis but not in the multivariate analysis (Figure 1C and Supplemental Table 1B). Therefore, a subgroup analysis for HBeAg-negative non-cirrhotic patients was performed. The results indicated that among HBeAg-negative non-cirrhotic patients (n = 90), those with a baseline HBsAg level of < 2.4 log IU/mL achieved virological response more easily (P < 0.0001, Figure 1D). Multivariate analysis showed that a baseline HBsAg level of < 2.4 log IU/mL was independently associated with an earlier virological response (HR: 3.12, 95%CI: 1.58-6.19, P = 0.001, Table 3). In HBeAg-negative cirrhotic patients (n = 52), baseline HBsAg levels failed to predict virological response.
| Factors | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex (female vs male) | 1.14 | 0.72-1.81 | 0.57 | 1.39 | 0.78-2.47 | 0.27 |
| Age (yr) | 0.99 | 0.97-1.01 | 0.53 | 0.99 | 0.96-1.01 | 0.31 |
| HBV genotype (B vs C)1 | 1.17 | 0.70-1.93 | 0.55 | 1.34 | 0.79-2.30 | 0.28 |
| ALT (× ULN) | 1.00 | 0.96-1.04 | 0.94 | 0.99 | 0.94-1.04 | 0.65 |
| HBV DNA (≤ 4 vs > 4 log IU/mL) | 1.33 | 0.73-2.40 | 0.35 | 0.63 | 0.19-2.07 | 0.45 |
| HBsAg (< 2.4 vs ≥ 2.4 log IU/mL) | 3.95 | 2.19-7.12 | < 0.001 | 3.12 | 1.58-6.19 | 0.001 |
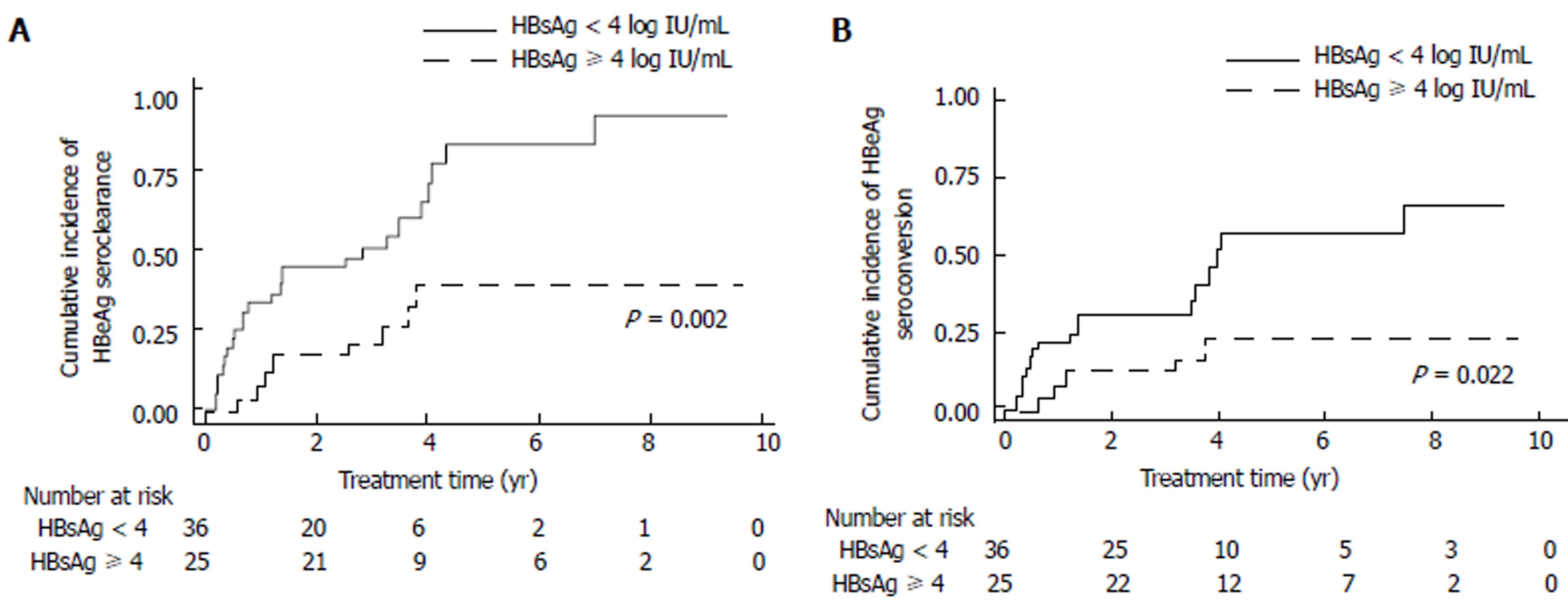
Sixty-one HBeAg-positive patients were assessed for serological response. Thirty-three patients (33/61, 54.1%) achieved HBeAg seroclearance during entecavir treatment. The median time to HBeAg seroclearance was 1.21 (0.19-6.99) years. HBeAg seroclearance occurred more rapidly in patients with a baseline HBsAg level of < 4 log IU/mL, compared with those with a baseline HBsAg level ≥ 4 log IU/mL (P = 0.002, Figure 2A). Statistical significance remained after adjustment (multivariate: HR: 5.74, 95%CI: 2.19-15.00, P < 0.001, Table 4).
| Factors | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex (female vs male) | 0.74 | 0.36-1.54 | 0.43 | 1.14 | 0.49-2.67 | 0.76 |
| Age (yr) | 1.00 | 0.98-1.03 | 0.81 | 0.99 | 0.95-1.02 | 0.42 |
| Cirrhosis (yes no) | 1.42 | 0.64-3.15 | 0.39 | 0.71 | 0.24-2.07 | 0.53 |
| HBV genotype (B vs C)1 | 1.17 | 0.57-2.39 | 0.67 | 2.04 | 0.90-4.62 | 0.09 |
| ALT (× ULN) | 1.02 | 0.96-1.09 | 0.51 | 1.04 | 0.98-1.09 | 0.17 |
| HBV DNA (≤ 5 vs > 5 log IU/mL) | 2.10 | 0.64-6.93 | 0.22 | 3.40 | 0.83-13.87 | 0.09 |
| HBsAg (< 4 vs ≥ 4 log IU/mL) | 3.32 | 1.49-7.43 | 0.003 | 5.74 | 2.19-15.00 | < 0.001 |
HBeAg seroconversion occurred in 22 patients (22/61, 36.1%). The median time to HBeAg seroconversion was 1.21 (0.21-7.49) years. Patients with a baseline HBsAg level of < 4 log IU/mL achieved HBeAg seroconversion more rapidly (P = 0.022, Figure 2B). Multivariate analysis showed that an HBV DNA level of ≤ 5 log IU/mL and HBsAg level of < 4 log IU/mL were independently associated with earlier HBeAg seroconversion (HBV DNA ≤ 5 log IU/mL vs > 5 log IU/mL: HR: 4.15, 95%CI: 1.05-16.44, P = .043; HBsAg < 4 log IU/mL vs ≥ 4 log IU/mL: HR: 5.05, 95%CI: 1.58-16.14, P = 0.006, Table 5).
| Factors | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex (female vs male) | 0.70 | 0.29-1.72 | 0.440 | 1.02 | 0.35-2.97 | 0.970 |
| Age (yr) | 0.99 | 0.95-1.02 | 0.440 | 0.98 | 0.94-1.02 | 0.310 |
| Cirrhosis (yes vs no) | 0.93 | 0.31-2.75 | 0.890 | 0.57 | 0.15-2.16 | 0.410 |
| HBV genotype (B vs C)1 | 0.86 | 0.35-2.13 | 0.750 | 1.49 | 0.52-4.26 | 0.450 |
| ALT (× ULN) | 1.03 | 0.96-1.10 | 0.390 | 1.05 | 0.99-1.11 | 0.120 |
| HBV DNA (≤ 5 vs > 5 log IU/mL) | 3.16 | 0.93-10.76 | 0.070 | 4.15 | 1.05-16.44 | 0.043 |
| HBsAg (< 4 vs ≥ 4 log IU/mL) | 3.05 | 1.12-8.28 | 0.029 | 5.05 | 1.58-16.14 | 0.006 |
One hundred and sixty-eight patients with elevated baseline ALT levels were assessed for biochemical response to treatment. One hundred and thirty-four patients (134/168, 79.8%) achieved biochemical response during entecavir treatment. The median time to biochemical response was 0.26 (0.04-3.09) years. In HBeAg-positive patients with elevated baseline ALT levels (n = 52), an HBsAg level of < 4 log IU/mL was not associated with an earlier biochemical response, as observed in Kaplan-Meier analysis and univariate analysis (Supplemental Figure 1 and Supplemental Table 2). However, multivariate analysis showed that HBV genotype B and an HBsAg level of < 4 log IU/mL were independently associated with more rapid biochemical response (genotype B vs C, HR: 4.59, 95%CI: 1.60-13.15, P = 0.005; HBsAg < 4 log IU/mL vs ≥ 4 log IU/mL, HR: 4.00, 95%CI: 1.41-11.36, P = 0.009, Supplemental Table 2). In HBeAg-negative patients with elevated baseline ALT levels (n = 116), the baseline HBsAg level failed to predict biochemical response, irrespective of cirrhosis status.
New HCC development was defined as HCC diagnosed after half a year of entecavir treatment in patients without a history of HCC. One hundred and seventy-nine patients who had no HCC before and within a half a year of entecavir treatment were assessed for new HCC development. New HCC occurred in 13 patients (13/179, 7.3%). The median time to the development of new HCC was 5.16 (2.11-8.65) years. Liver cirrhosis was associated with increased risk of new HCC (P < 0.001, Supplemental Figure 2). Multivariate analysis revealed that liver cirrhosis was the only independent risk factor for new HCC (HR: 13.02, 95%CI: 2.00-84.99, P = 0.007, Table 6).
| Factors | Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| Sex (female vs male) | 0.42 | 0.09-1.90 | 0.26 | 0.31 | 0.05-1.94 | 0.21 | |
| Age (yr) | 1.08 | 1.03-1.13 | 0.001 | 1.05 | 0.99-1.12 | 0.12 | |
| HBeAg (positive vs negative) | 0.44 | 0.10-1.97 | 0.28 | 0.91 | 0.14-6.00 | 0.92 | |
| Cirrhosis (yes vs no) | 11.32 | 3.11-41.24 | < 0.001 | 13.02 | 2.00-84.99 | 0.007 | |
| HBV genotype (B vs C)1 | 0.58 | 0.18-1.90 | 0.37 | 0.63 | 0.12-3.28 | 0.58 | |
| ALT (× ULN) | 1.02 | 0.95-1.09 | 0.55 | 1.06 | 0.97-1.16 | 0.20 | |
| HBV DNA (log IU/mL) | 0.90 | 0.66-1.22 | 0.48 | 1.06 | 0.59-1.91 | 0.84 | |
| HBsAg (log IU/mL) | 0.80 | 0.41-1.55 | 0.51 | 0.98 | 0.29-3.36 | 0.97 | |
Serum HBsAg levels were determined at the baseline (211 patients; 211/211, 100%), 1-year (175 patients; 175/211, 82.9%), and 5-year time points (68 patients; 68/113, 60.2%) of entecavir treatment.
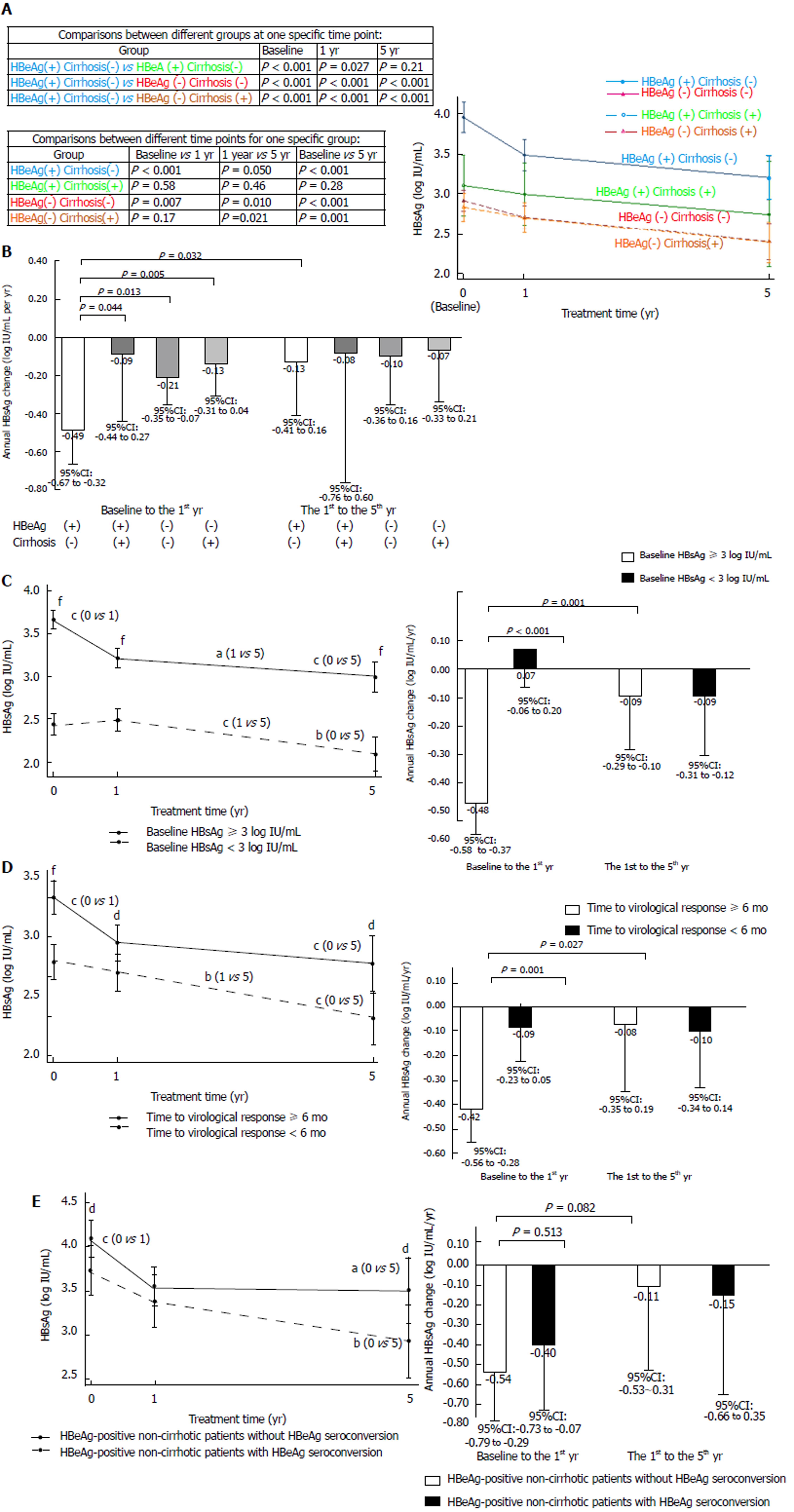
HBsAg levels at different time points, categorized by baseline HBeAg and cirrhosis status, are presented in Figure 3A. The HBeAg-positive non-cirrhotic group had significantly higher HBsAg levels at the baseline, 1-year, and 5-year time points, compared with the other groups, except for the HBeAg-positive cirrhotic group at the 5-year time point, which had a similar trend but did not reach statistical significance. Annual HBsAg changes in different periods, categorized by HBeAg and cirrhosis status, are presented in Figure 3B. The HBsAg decrease in the first year for the HBeAg-positive non-cirrhotic group was higher than that for the other three groups; moreover, the HBsAg decrease in the first year was higher than the decrease observed from the first to the fifth year for the HBeAg-positive non-cirrhotic group.
HBsAg levels at different time points and annual HBsAg changes in different periods, categorized by baseline HBsAg < 3 and ≥ 3 log IU/mL, are presented in Figure 3C. Patients with baseline HBsAg levels ≥3 log IU/mL had higher HBsAg at the baseline, 1-year and 5-year time points than those with baseline HBsAg levels < 3 log IU/mL. The annual HBsAg decrease in the first year for patients with baseline HBsAg levels ≥ 3 log IU/mL was higher than that for patients with baseline HBsAg levels < 3 log IU/mL. Furthermore, the HBsAg decrease in the first year was higher than the decrease from the first to the fifth year for patients with baseline HBsAg levels ≥ 3 log IU/mL.
HBsAg levels at different time points and annual HBsAg changes in different periods, categorized by time to virological response < 6 mo and ≥ 6 mo, are presented in Figure 3D. Patients with a time to virological response of ≥ 6 mo had higher HBsAg levels at the baseline, 1-year and 5-year time points, compared with those with a time to virological response of < 6 mo. The annual HBsAg decrease in the first year for patients with a time to virological response of ≥ 6 mo was higher than for patients with a time to virological response of < 6 mo. Moreover, the HBsAg decrease in the first year was higher than that from the first to the fifth year for patients with a time to virological response of ≥ 6 mo.
HBsAg levels at different time points and annual HBsAg changes in different periods, categorized by HBeAg-positive non-cirrhotic patients with and without HBeAg seroconversion, are presented in Figure 3E. HBeAg-positive non-cirrhotic patients without HBeAg seroconversion had higher baseline and five-year HBsAg levels than those with HBeAg seroconversion. HBeAg-positive non-cirrhotic patients without HBeAg seroconversion had a trend of greater annual HBsAg decrease in the first year than from the first to the fifth year, but these results did not reach statistical significance, which may be due to the limited case number at the 5-year time point.
Our data demonstrate that baseline HBsAg levels can be used to predict virological, serological, and biochemical responses in treatment-naïve chronic hepatitis B patients during entecavir treatment. Furthermore, our study provides a global view of HBsAg kinetics in chronic hepatitis B patients, categorized by baseline HBeAg and cirrhosis status during long-term entecavir treatment. The HBeAg-positive non-cirrhotic group had the highest HBsAg levels at the baseline and throughout entecavir treatment, compared with the other three patient groups. Although HBsAg levels decreased slowly during long-term entecavir treatment, a rapid rate of HBsAg decrease was seen in the first year for HBeAg-positive non-cirrhotic patients.
Previous studies have shown that lower baseline HBsAg levels were also associated with higher chances of HBV DNA suppression[5,20,21,24], HBeAg seroclearance[21,24], and HBsAg seroclearance[25,26]. Because HBeAg-positive patients had higher HBsAg levels than HBeAg-negative patients (Table 1), the finding that the cut-off values of HBsAg for predicting treatment response were different between HBeAg-positive and HBeAg-negative patients is reasonable. In the current study, using an HBsAg cut-off value of 4 log IU/mL indicated that lower baseline HBsAg levels were associated with an earlier virological response, earlier HBeAg seroconversion, and earlier biochemical response in HBeAg-positive patients, and using a cut-off value of 2.4 log IU/mL, lower baseline HBsAg levels were found associated with an earlier virological response in HBeAg-negative non-cirrhotic patients.
HBsAg levels decreased slowly during entecavir treatment in most patients. In HBeAg-positive non-cirrhotic patients, a higher rate of HBsAg decrease was observed in the first year of treatment (Figure 3A and 3B). When patients were categorized according to baseline HBsAg levels, time to virological response, and time to HBeAg seroconversion, higher rates of HBsAg decrease were noted in the first year of treatment for patients with higher baseline HBsAg levels, patients with longer time to virological response, and patients without HBeAg seroconversion (Figure 3C, D, and E). These findings demonstrate that higher rates of HBsAg decrease occurred in the first year of treatment for patients who had higher baseline HBsAg levels. Therefore, rapid rates of HBsAg decline did not necessarily guarantee better clinical outcomes. When interpreting HBsAg changes, both HBsAg levels and decrease rates should be considered according to disease status. These findings are compatible to those of previous studies[27-29]. Because HBsAg levels decrease slowly during entecavir treatment, it could be checked at a 1-year interval if HBV DNA remains undetectable, as mentioned in a recent hepatitis B treatment guideline[1].
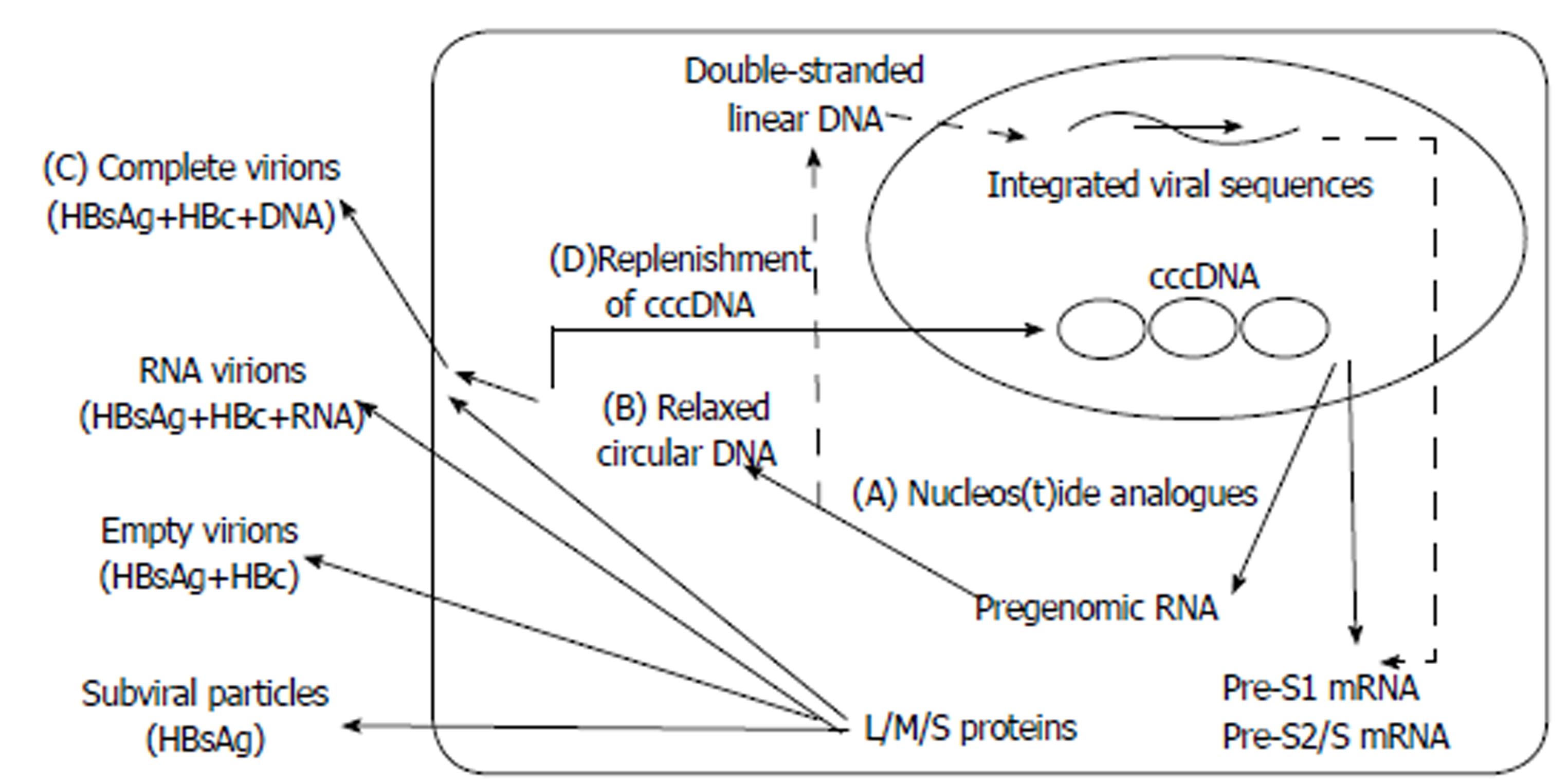
The difference in HBsAg kinetic patterns between HBeAg-positive and HBeAg-negative patients is appealing to consider. This could be related to the dissimilar activity of virus in patients with different HBeAg statuses. HBV cccDNA is the template for pre-S1 mRNA (2.4 kb), pre-S2/S mRNA (2.1 kb), preC mRNA (3.5 kb), pregenomic RNA (3.5 kb), and HBx mRNA (0.7 kb) transcription. Large (L) envelope proteins are translated from the pre-S1 mRNA, whereas middle (M) and small (S) envelope proteins are translated from the pre-S2/S mRNA[12,30]. Serum HBsAg consists of L, M, and S envelope proteins from complete virions (Dane particles), RNA virions, empty virions, and subviral particles (SVP, noninfectious HBsAg particles with spherical or filamentous forms). The amount of SVPs outnumbers complete virions by 1000-fold or greater[12,30-32]. HBsAg is derived not only from cccDNA but also from integrated HBV DNA sequences[12,30,31]. NA inhibits the activity of HBV reverse transcriptase, posing an obstacle to the production of relaxed circular DNA, the packaging and release of complete virions, and the replenishment of cccDNA (Figure 4)[2,30,32]. Notably, these integrated sequences constitute a considerable part of the intrahepatic HBV DNA, and serum HBsAg circulates mainly as defective particles in HBeAg-negative patients[31,33]. This might account for the differences in HBsAg kinetics between HBeAg-positive and HBeAg-negative patients.
There are limitations to this study: it is a retrospective study of a single medical center, thus limiting the diversity of our patient population. In addition, serum samples were unavailable for 39.8% of enrolled patients at the five-year time point, which may have yielded some nonsignificant results for the HBeAg-positive cirrhotic patient group.
In conclusion, this study demonstrated that baseline HBsAg levels could be used to predict virological, serological, and biochemical responses during entecavir treatment. Although HBsAg levels decreased slowly during the treatment, a higher rate of HBsAg decrease was found in the first year of treatment for HBeAg-positive non-cirrhotic patients. Higher rates of HBsAg decrease were observed in the first year for patients with higher baseline HBsAg levels. A rapid HBsAg decline did not necessarily guarantee better outcomes. Clinicians interpreting HBsAg kinetics should consider HBsAg levels and decrease rates together according to a patient’s disease status.
Hepatitis B surface antigen (HBsAg) levels have been studied in the natural course and pegylated interferon treatment course. During nucleos(t)ide analogue (NA) therapy, there are still controversies about using HBsAg to predict treatment responses, especially in HBeAg-negative patients. Besides, HBsAg kinetics and its relationships with outcomes during long-term entecavir therapy have not been fully elucidated.
We hoped to elucidate the utility of HBsAg in the prediction of treatment response in HBeAg-positive and HBeAg-negative patients. Furthermore, we would like to demonstrate the detailed HBsAg kinetics among different disease statuses and their relationships with the treatment outcomes.
We aimed to investigate the utility and kinetics of serum HBsAg in chronic hepatitis B patients during long-term entecavir treatment.
We conducted this retrospective study to analyze the relationships between HBsAg levels and treatment responses in treatment-naïve chronic hepatitis B patients receiving at least two years of consecutive entecavir treatment. Patients were followed up at three to six month intervals with liver biochemistry, hepatitis B virus DNA, and abdominal sonography. Serum HBsAg levels were determined at baseline, one year and five year time points. The cumulative incidence of treatment responses were obtained using the Kaplan-Meier analysis. Multivariate analysis was performed using Cox proportional hazards regression. A linear mixed model with a random intercept was used for analysis of longitudinal changes of HBsAg levels.
We demonstrated that baseline HBsAg levels could be used to predict treatment responses in HBeAg-positive patients with a cut-off value of 4 log IU/mL and in HBeAg-negative non-cirrhotic patients with a cut-off value of 2.4 log IU/mL. Furthermore, our study provides a global view of HBsAg kinetics in chronic hepatitis B patients during long-term entecavir therapy. The HBeAg-positive non-cirrhotic group had the highest HBsAg levels at the baseline and throughout entecavir treatment, as compared with the other three patient groups. Higher rates of HBsAg decrease were observed in the first year for patients with higher baseline HBsAg levels. A rapid HBsAg decline did not necessarily guarantee better outcomes
Baseline HBsAg levels could be used to predict virological, serological, and biochemical responses. In the interpretation of HBsAg changes, HBeAg levels and decrease rates should be considered together according to a patient’s disease status.
HBsAg is a useful biomarker for chronic hepatitis B patients receiving NA therapy. It deserves to be studied in large prospective cohorts with different comorbidities for the future research.
We thank Jia-Jhen Lin and Kai-Ning Shih for their assistance in laboratory work. We are also grateful to Mei-Fang Ke, Su-Erb Lin, Ting-Ting Yang, and Ting-Yin Hou for their assistance in clinical data collection.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lin LJ, Namisaki T S- Editor: Chen K L- Editor: Ma JY E- Editor: Ma YJ
| 1. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2771] [Cited by in F6Publishing: 3218] [Article Influence: 459.7] [Reference Citation Analysis (0)] |
| 2. | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972-1984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 548] [Cited by in F6Publishing: 608] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 3. | Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: a review. Hepatology. 2011;53:2121-2129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS. Determinants of spontaneous surface antigen loss in hepatitis B e antigen-negative patients with a low viral load. Hepatology. 2012;55:68-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Liu J, Yang HI, Lee MH, Batrla-Utermann R, Jen CL, Lu SN, Wang LY, You SL, Hsiao CK, Chen CJ; REVEAL-HBV Study Group. Distinct seromarkers predict different milestones of chronic hepatitis B progression. Hepatology. 2014;60:77-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Liu J, Yang HI, Lee MH, Jen CL, Batrla-Utermann R, Lu SN, Wang LY, You SL, Chen CJ. Serum Levels of Hepatitis B Surface Antigen and DNA Can Predict Inactive Carriers With Low Risk of Disease Progression. Hepatology. 2016;64:381-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Tseng TC, Liu CJ, Su TH, Wang CC, Chen CL, Chen PJ, Chen DS, Kao JH. Serum Hepatitis B Surface Antigen Levels Predict Surface Antigen Loss in Hepatitis B e Antigen Seroconverters. Gastroenterology. 2011;141:517-525.e512. [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Brouwer WP, Chan HL, Brunetto MR, Martinot-Peignoux M, Arends P, Cornberg M, Cherubini B, Thompson AJ, Liaw YF, Marcellin P, Janssen HL, Hansen BE; Good Practice in using HBsAg in Chronic Hepatitis B Study Group (GPs-CHB Study Group). Repeated Measurements of Hepatitis B Surface Antigen Identify Carriers of Inactive HBV During Long-term Follow-up. Clin Gastroenterol Hepatol. 2016;14:1481-1489.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, Liaw YF, Xie Q, Heathcote EJ, Chan HL. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology. 2013;58:872-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 10. | Rijckborst V, Hansen BE, Cakaloglu Y, Ferenci P, Tabak F, Akdogan M, Simon K, Akarca US, Flisiak R, Verhey E. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology. 2010;52:454-461. [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Chuaypen N, Sriprapun M, Praianantathavorn K, Payungporn S, Wisedopas N, Poovorawan Y, Tangkijvanich P. Kinetics of serum HBsAg and intrahepatic cccDNA during pegylated interferon therapy in patients with HBeAg-positive and HBeAg-negative chronic hepatitis B. J Med Virol 2017; 89: 130-138. . [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HLA, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66:398-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 13. | Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 14. | Peng C-Y, Lai H-C, Su W-P, Lin C-H, Chuang P-H, Chen S-H, Chen C-H. Early hepatitis B surface antigen decline predicts treatment response to entecavir in patients with chronic hepatitis B. Sci Rep. 2017;7:42879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wursthorn K, Jung M, Riva A, Goodman ZD, Lopez P, Bao W, Manns MP, Wedemeyer H, Naoumov NV. Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology. 2010;52:1611-1620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | Zhang XX, Li MR, Xi HL, Cao Y, Zhang RW, Zhang Y, Xu XY. Dynamic Characteristics of Serum Hepatitis B Surface Antigen in Chinese Chronic Hepatitis B Patients Receiving 7 Years of Entecavir Therapy. Chin Med J (Engl). 2016;129:929-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Thompson AJV, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, Slavin J, Bowden S, Gane EJ, Abbott W. Serum hepatitis B surface antigen and hepatitis B e antigen titers: Disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933-1944. [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 18. | Striki A, Manolakopoulos S, Deutsch M, Kourikou A, Kontos G, Kranidioti H, Hadziyannis E, Papatheodoridis G. Hepatitis B s antigen kinetics during treatment with nucleos(t)ides analogues in patients with hepatitis B e antigen-negative chronic hepatitis B. Liver Int. 2017;37:1642-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Su TH, Liu CJ, Tseng TC, Liu CH, Yang HC, Chen CL, Chen PJ, Kao JH, Chen DS. Longitudinal change of HBsAg in HBeAg-negative patients with genotype B or C infection. PLoS One. 2013;8:e55916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Lee JM, Ahn SH, Kim HS, Park H, Chang HY, Kim DY, Hwang SG, Rim KS, Chon CY, Han KH. Quantitative hepatitis B surface antigen and hepatitis B e antigen titers in prediction of treatment response to entecavir. Hepatology. 2011;53:1486-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Lee MH, Lee DM, Kim SS, Cheong JY, Cho SW. Correlation of serum hepatitis B surface antigen level with response to entecavir in naïve patients with chronic hepatitis B. J Med Virol. 2011;83:1178-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1652] [Cited by in F6Publishing: 1704] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 23. | Liu W-C, Mizokami M, Buti M, Lindh M, Young K-C, Sun K-T, Chi Y-C, Li H-H, Chang T-T. Simultaneous Quantification and Genotyping of Hepatitis B Virus for Genotypes A to G by Real-Time PCR and Two-Step Melting Curve Analysis. J Clin Microbiol. 2006;44:4491-4497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Cho JY, Sohn W, Sinn DH, Gwak GY, Paik YH, Choi MS, Koh KC, Paik SW, Yoo BC, Lee JH. Long-term real-world entecavir therapy in treatment-naïve hepatitis B patients: base-line hepatitis B virus DNA and hepatitis B surface antigen levels predict virologic response. Korean J Intern Med. 2016;32:636-646. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Fung J, Wong DK, Seto WK, Kopaniszen M, Lai CL, Yuen MF. Hepatitis B surface antigen seroclearance: Relationship to hepatitis B e-antigen seroclearance and hepatitis B e-antigen-negative hepatitis. Am J Gastroenterol. 2014;109:1764-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Seto WK, Wong DK, Fung J, Huang FY, Lai CL, Yuen MF. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy. Hepatology. 2013;58:923-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Seto WK, Liu K, Wong DK, Fung J, Huang FY, Hung IF, Lai CL, Yuen MF. Patterns of hepatitis B surface antigen decline and HBV DNA suppression in Asian treatment-experienced chronic hepatitis B patients after three years of tenofovir treatment. J Hepatol. 2013;59:709-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Seto WK, Lam YF, Fung J, Wong DK, Huang FY, Hung IF, Lai CL, Yuen MF. Changes of HBsAg and HBV DNA levels in Chinese chronic hepatitis B patients after 5 years of entecavir treatment. J Gastroenterol Hepatol. 2014;29:1028-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Wang ML, Chen EQ, Tao CM, Zhou TY, Liao J, Zhang DM, Wang J, Tang H. Pronounced decline of serum HBsAg in chronic hepatitis B patients with long-term effective nucleos(t)ide analogs therapy. Scand J Gastroenterol. 2017;52:1420-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Tong S, Revill P. Overview of hepatitis B viral replication and genetic variability. J Hepatol. 2016;64:S4-S16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 276] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 31. | Janssen HLA. Sonneveld MJ, Brunetto MR. Quantification of serum hepatitis B surface antigen: is it useful for the management of chronic hepatitis B? Gut. 2016;61:641-645. [Cited in This Article: ] |
| 32. | Hu J, Liu K. Complete and Incomplete Hepatitis B Virus Particles: Formation, Function, and Application. Viruses. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 33. | Tripodi G, Larsson SB, Norkrans G, Lindh M. Smaller reduction of hepatitis B virus DNA in liver tissue than in serum in patients losing HBeAg. J Med Virol. 2017;89:1937-1943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |