Published online Feb 14, 2018. doi: 10.3748/wjg.v24.i6.706
Peer-review started: December 1, 2017
First decision: December 13, 2017
Revised: December 19, 2017
Accepted: December 27, 2017
Article in press: December 27, 2017
Published online: February 14, 2018
Processing time: 67 Days and 1.8 Hours
To investigate the modulatory effect of recombinant-expressed vasoactive intestinal peptide (VIP) analogue (rVIPa) on trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats.
Forty-eight rats were randomized into six groups: normal control group (Control), model control group (TNBS), ethanol treatment group (ETOH), and VIP treatment groups with different dosage (rVIPa1nmol, rVIPa2nmol, rVIPa4nmol). Diarrhea and bloody stool were observed. Colonic damage was evaluated histologically. The levels of tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), myeloperoxidase (MPO) and endotoxin in colonic tissue and serum were determined by enzyme-linked immunosorbent assay (ELISA). The expression of occludin, ZO-1, Toll-like receptor 4 (TLR4), and nuclear factor-kappa B p65 (NF-κB p65), IκBα, and p-IκBα were detected by Western blot.
Administration with 2 nmol rVIPa prevented TNBS-induced necrosis, hyperemia, swelling, inflammation, etc., pathologic changes observed in the inner surface of colon in experimental rats. Moreover, rVIPa significantly decreased colonic TNF-α level (P < 0.001), MPO activity (P < 0.001) and serum endotoxin level (P < 0.01), and remarkably increased colonic IL-10 content (P < 0.001) in rats with TNBS-induced colitis. Furthermore, compared to the TNBS-induced colitis group, 2 nmol rVIPa treatment up-regulated the levels of occludin (P < 0.05) and ZO-1 (P < 0.05), NF-κB p65 (P < 0.01) and IκBα (P < 0.001), and down-regulated the levels of TLR4.
rVIPa ameliorates TNBS-induced colonic injury and inflammation and effectively protected the intestinal mucosal barrier function in rats. The mechanism may be related to TLR4/NF-κB-mediated signaling pathway. rVIPa could be used as a new alternative therapy for intestinal inflammatory disorders.
Core tip: Vasoactive intestinal peptide (VIP) is a neuropeptide with potent anti-inflammatory activities. Recombinant VIP analogue (rVIPa with amino acid sequence “HSKAVFTKNYTRLRKQMAVKKYLNSILN”) with higher antimicrobial activity and stability than natural peptide was produced by an effective and low-cost production method. The current study first indicated that rVIPa could alleviated TNBS-induced colitis via TLR4/NF-κB-mediated signaling pathway. In summary, these results suggest a protective role and anti-inflammatory effect of rVIPa in inflammatory bowel disease and indicate that rVIPa has therapeutic potential for intestinal inflammatory disorders. The study contributes to identify and produce novel anti-inflammatory agents from human innate host defense mechanisms in the process of biological evolution.
- Citation: Xu CL, Guo Y, Qiao L, Ma L, Cheng YY. Recombinant expressed vasoactive intestinal peptide analogue ameliorates TNBS-induced colitis in rats. World J Gastroenterol 2018; 24(6): 706-715
- URL: https://www.wjgnet.com/1007-9327/full/v24/i6/706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i6.706
Crohn’s disease (CD) belonging to a type of inflammatory bowel disease (IBD), is a group of chronic and relapsing intestinal inflammatory disorders[1]. A proportion of patients with CD given the currently available medical therapies remains incurable[2]. CD causes transmural inflammation of the gastrointestinal wall. Furthermore, prolonged inflammation of the intestinal tract affects the patients’ quality of life and increases the risk of colorectal cancer development[3]. Rapid increase of multidrug-resistant pathogenic microorganisms makes it urgent to research and develop promising and alternative anti-inflammatory molecules. Endogenous bioactive peptides with remarkably structural and functional diversity represent an emerging and potential anti-inflammatory agents[3,4].
Vasoactive intestinal peptide (VIP) is considered a paradigm as an endogenous neuroendocrine-immune mediator with therapeutic potential for a variety of inflammatory disorders such as IBD[5-7]. Previous studies indicated that VIP exerts a lines of important modulatory effects in intestinal pathophysiology, including CD[8-10]. VIP can inhibit the neurodegeneration induced by the loss of neurons, which may be mediated by glial cells through the production of neurotrophic factors and the inhibition of proinflammatory mediators[11]. VIP is also recognized to belong to host defense peptides[12,13]. Furthermore, VIP is a small and linear peptide. VIP as an endogenous hormone, can exhibit efficient adjustment effect for a low dose. These advantages suggest that VIP possesses a promising potential for developing as a novel and safe anti-inflammatory agent.
However, due to several shortcomings such as instability, low efficient and expensive production methods[14,15], VIP has not obtained widely application at present. Therefore, in order to promote the development and application of VIP, choosing the VIP analogue with high stability and anti-inflammatory activity and establishing an effective, low-cost production strategy are very urgent. Previous research indicate that molecular modification of VIP enhances its antimicrobial activity and stability compared with the nature VIP[16]. For the effective and low-cost production method, recently, different recombinant expression strategies have been conducted to produce unique antimicrobial peptides such as fusion partner-mediated Escherichia coli expression system[17-19].
In our previous study, we obtained a kind of VIP analogue with an enhanced antimicrobial potency, no-hemolysis, and high stability through the rational design and scanning, and established an effective and low-cost genetic engineering production technology. However, it remains to be elucidated whether molecular modification and recombinant expression affect its natural anti-inflammatory activities or not. Therefore, the current study was conducted to investigate its anti-inflammatory activity, and the possible mechanism of VIP analogue produced by recombinant expression after rational design. The study contributes to better understanding of colitis and leads to advancement in novel treatment design.
rVIPa with amino acid sequence “HSKAVFTKNYTRLRKQMAVKKYLNSILN” was prepared in our laboratory based on the recombinant expression and molecular modification of natural VIP. Moreover, its quality and purity were identified by high-performance liquid chromatography (HPLC) and electrospray ionization mass spectrometry (ESI-MS). The 2, 4, 6-trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma Chemical Co. (St.Louis, MO, United States). ELISA kits for tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), and myeloperoxidase (MPO) were purchased from R&D Systems (Minneapolis, MN, United States). Primary antibodies against ZO-1, occludin, TLR4, p-IκBα, IκBα, NF-κB p65 and β-actin, and peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, United States). Total superoxide dismutase (T-SOD) activity assay kit was purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). Bicinchoninic acid (BCA) protein assay kit was from Solarbio Life Sciences Co. (Beijing, China).
This experiment was approved by the Institutional Animal Care and Use Committee of the Northwestern Polytechnical University (permit Number: 2016014) and was conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals. Forty-eight adult male Sprague-Dawley (SD) rats (200 ± 20 g) were purchased from the Experimental Animal Center of Xi’an Jiaotong University. During the whole experimental period, rats were maintained in the Animal Experimental Center of Northwestern Polytechnical University at room temperature of 25 °C, relative humidity of 50% and a 12 h light and dark cycle.
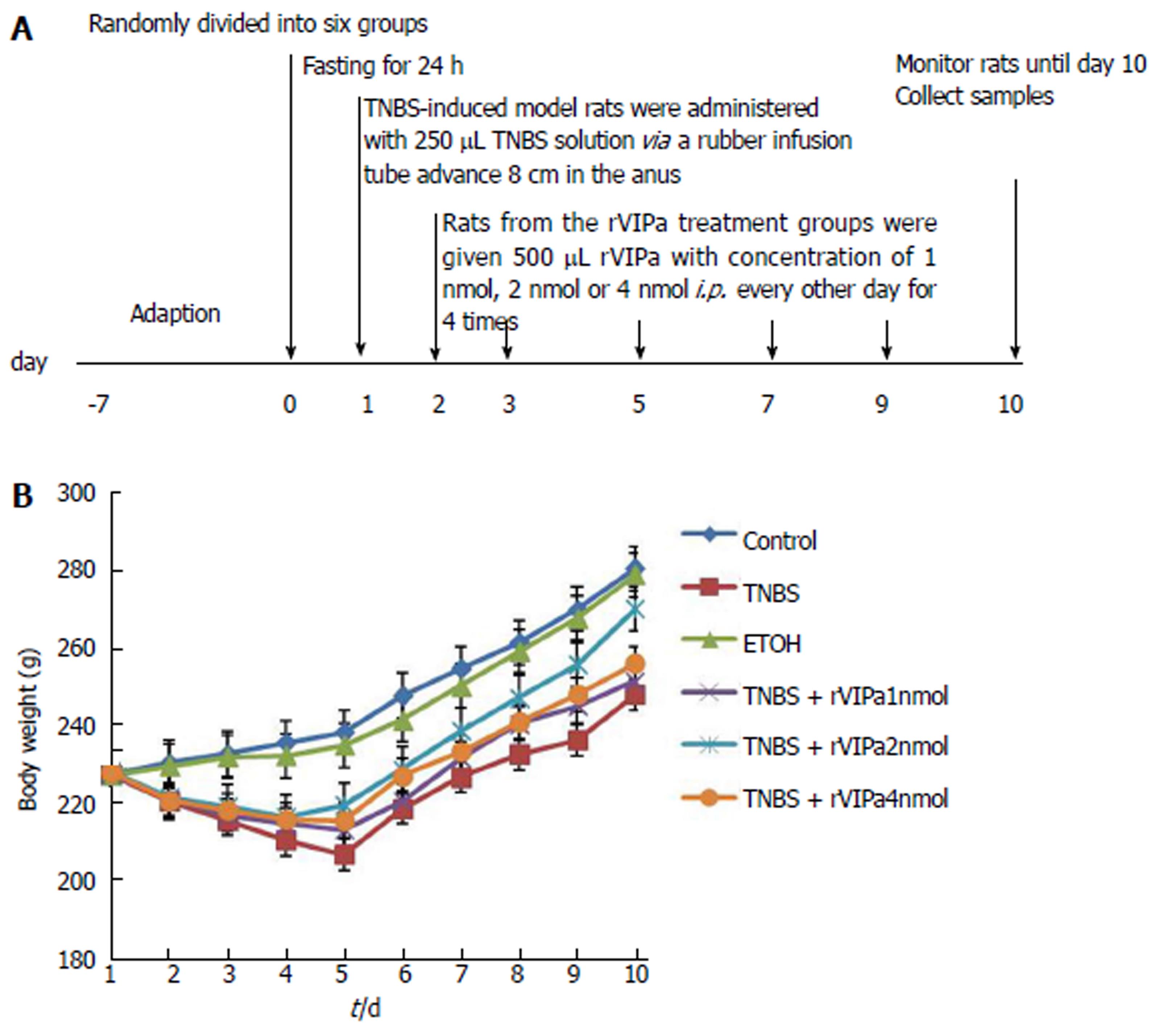
In the experiment, healthy male SD rats were assigned randomly to six groups: Normal control group saline and phosphate buffer solution (PBS), model group TNBS and PBS, positive control group (50% ETOH and PBS), and rVIPa groups (TNBS and rVIPa of 1 nmol, 2 nmol, and 4 nmol), with eight rats in each group. Colon colitis was induced rectally by TNBS, as in the following description[20]. First, TNBS was dissolved in 50% ethanol while all experimental rats fasted for 24 h. Then, SD rats from the TNBS-induced model groups were anesthetized and administered with 20 mg (in 250 μL of 50% ethanol) of TNBS via a 15-cm (diameter 2.0 mm) length of rubber infusion tube advanced 8 cm in the anus. The rats were seized by the tail for duration of 30s to ensure uniform contact with colonic mucosa. Following the same protocol, positive control animals were given 250 μL 50% ethanol. Controls were given the same volume of normal saline. Rats from the rVIPa treatment groups were given 500 μL rVIPa with concentration of 1 nmol, 2 nmol, or 4 nmol intraperitoneally (i.p.) every other day after TNBS induction of colitis until the end. Non-rVIPa-treated rats were administered the same volume of PBS. The period of animal experiment lasted 9 d. The animals’ weight, diarrhea, and rectal bleeding were observed and recorded daily.
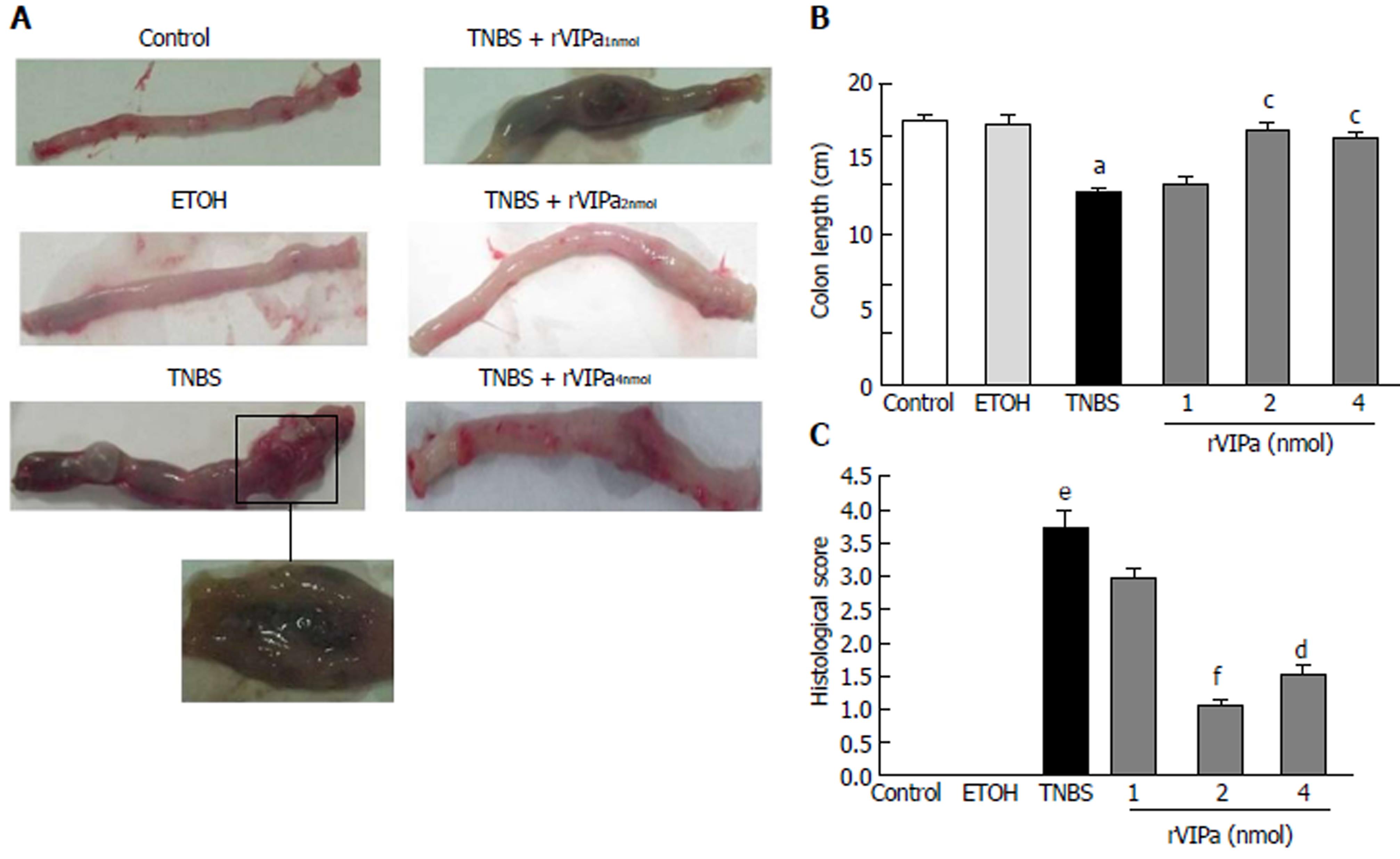
The rats were anesthetized with ether and the abdomen was opened. The entire colon was immediately removed and the total length was measured. Then the colon was split longitudinally, and gently washed with normal saline. Histologicals assessment of colonic damage was according to the following scoring criteria: 0, no ulcer and no inflammation; 1, no ulcer and local hyperemia; 2, ulcer and no inflammation; 3, ulceration and inflammation at only one site; 4, ulceration and inflammation at two or more sites; 5, area of ulceration extending more than 0.5 cm.
After the experimental rats were anesthetized with isoflurane, blood samples were collected by cardiac puncture and used for preparing serum according to general method. Then, 20 mg colon tissue was homogenized in 200 μL ice-cold RIPA lysate with 2 μL PMSF with a tissue homogenizer. The supernatant of homogenates was collected by centrifuging with 10000 g at 4 °C for 5 min. All samples of serum and supernatant of colon homogenates were stored at -80 °C until further analysis. MPO and SOD activities were determined by corresponding assay kits. The absorbance was measured by using a microplate reader (Synergy2, BioTek Instruments, Inc., Winooski, VT, United States) at 450 nm.
TNF-α, IL-10, and endotoxin level in serum and colon tissue were measured by specific rat ELISA assay kits according to the manufacturer’s instructions, respectively.
Supernatant of colon was prepared according to the following method. Protein concentration in the supernatant was measured by BCA kit. Approximately 40 μg protein from each sample was loaded to the 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane. After being blocked for 2 h in Tris-buffered saline/Tween 20 (TBST, 2 mmol/L Tris-HCl, pH 7.6, 13.7 mmol/L NaCl, and 0.1% Tween 20) containing 5% skimmed milk, membrane were incubated with primary antibodies TLR4 (1:500), ZO-1 (1:1000), occludin (1:1000), p-IκBα (1:1000), NF-κB p65 (1:1000), IκBα (1:1000), and β-actin (1:500) at 4 °C overnight. Subsequently, the membrane was incubated with the secondary antibody. The immunoreactive protein bands were visualized using a Clarity Western ECL substrate kit (BioRad, CA, United States) with HRP-conjugated secondary antibody for film-based imaging. Denditometry analysis of protein bands from scanned-ray films were performed using Scion Image software (Scion Corp., Frederick, MD, United States). The values were normalized against the intensity of β-actin.
Data were analyzed by one-way analysis of variance (ANOVA) and by using Student’s t test (SPSS 19.0, Chicago, IL, United States). All data are shown as mean ± standard error of mean (SEM), and differences were considered to be significant at P < 0.05.
The experimental scheme of induction of colitis and administration of rVIPa is shown in Figure 1A. During the whole period, the activity, diet, stool traits, and body weight of each group of rats were observed. TNBS-treated-alone colitis rats exhibited bradykinesia, reduced food intake, profound and sustained body weight loss, and diarrhea. Moreover, compared to the normal control and vehicle-treated rats, the feces from TNBS-treat-colitis rats contained blood and mucus. Conversely, moderate concentration of rVIPa (2 nmol) significantly ameliorated the TNBS-induced physiopathological effects and improved the TNBS-induced body weight loss in contrast with the untreated group or other concentrations of rVIPa-treated groups (as shown in Figure 1B).
As shown in Figure 2A, histological examination demonstrated a regular morphology on normal rats. Compared to the normal control or ethanol-treated controls, the macroscopic assessment of colon tissue exhibited superficial bleeding and erosion, necrosis, hyperemia, and inflammation in the mucosa. Treatment with 2 nmol or 4 nmol rVIPa effectively relieved the pathological symptoms and prevented the hyperemia and inflammation in the experimental rats’colon (Figure 2B). Moreover, 2 nmol rVIPa inhibited TNBS-induced decrease of colon length (Figure 2C).
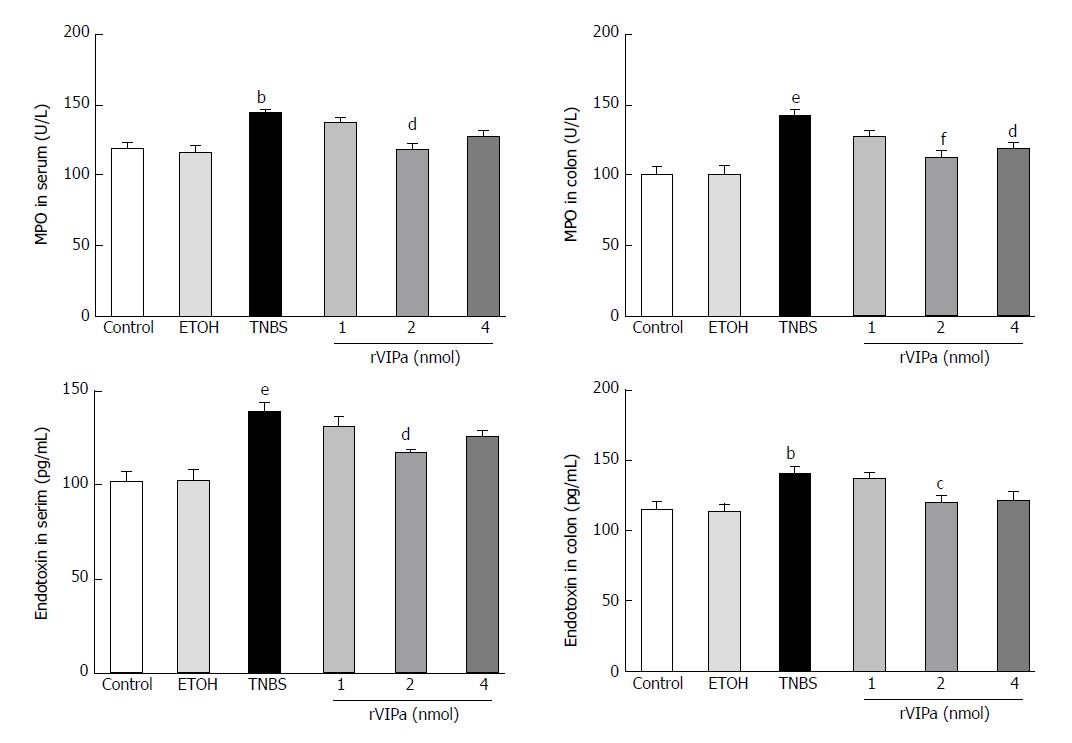
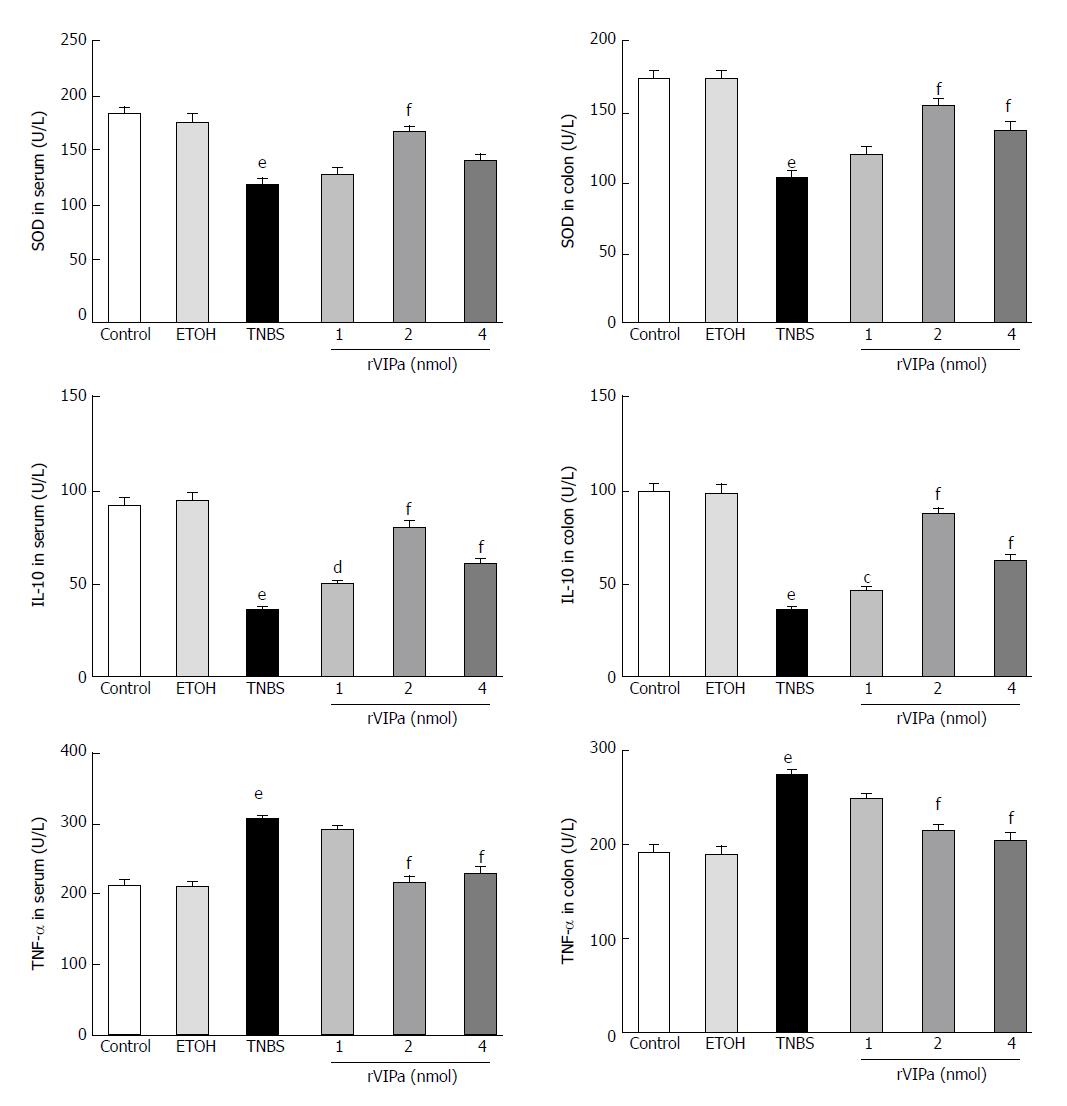
As shown in Figure 3, the current results showed that compared with the normal control and ethanol-treated control rats, colon TNBS treatment significantly increased the activity of MPO. However, 2 nmol rVIPa treatment significantly inhibited the TNBS-induced increase of MPO activity in colon tissue. At the same time, we examined the T-SOD activity in serum and colon tissue. The results showed that compared with the normal control and ethanol-treated rats, administration with TNBS significantly reduced the T-SOD activity in serum and colon tissue. In contrast, 2 nmol rVIPa treatment obviously relieved the TNBS-induced increase of the T-SOD activity in serum and colon tissue.
As shown in Figure 4, the levels of TNF-α and endotoxin in the serum and colon of TNBS-treated group were apparently higher than those in the normal control and vehicle groups. However, the level of anti-inflammatory factor IL-10 in the serum and colon of TNBS-treated model rats was significantly lower than that in the normal control and vehicle groups. Conversely, compared with the TNBS-treated group, 2 nmol and 4 nmol rVIPa treatment reduced the level of TNF-α and endotoxin, and increased the level of IL-10 in the serum and colon.
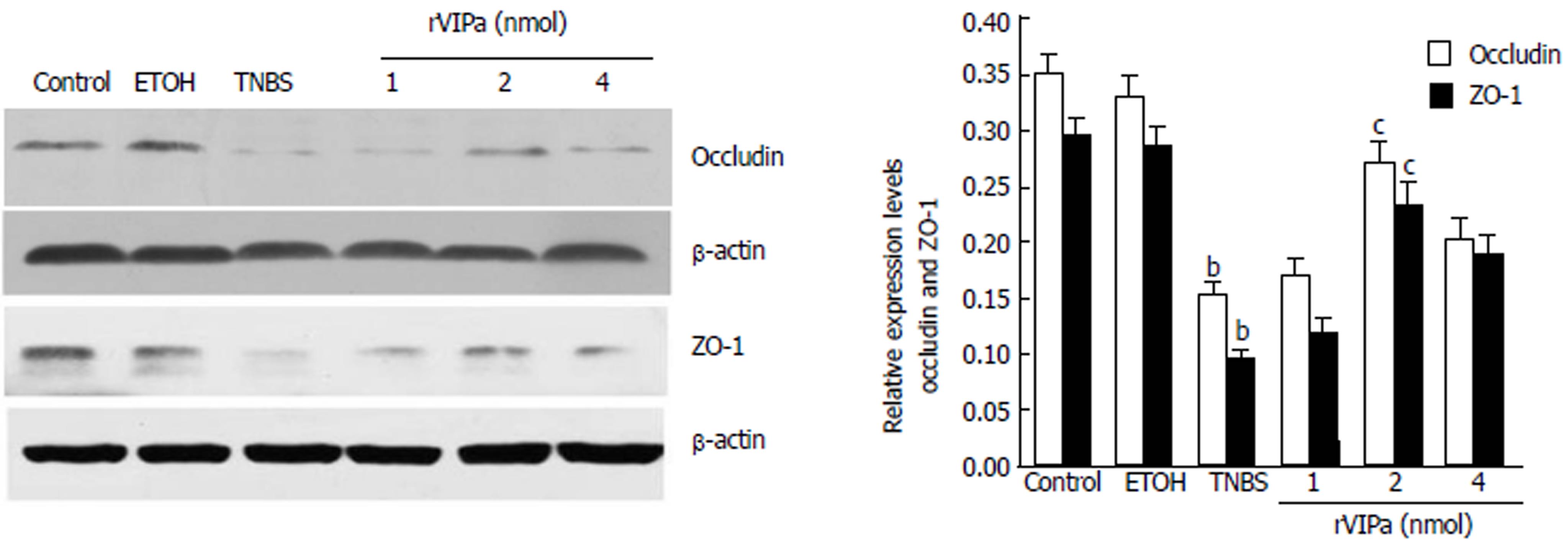
The results of intestinal tight junction (TJ) protein-occludin and ZO-1 expression levels determined by Western blot analysis were shown in Figure 5 and demonstrated that compared with the normal control rats, occludin and ZO-1 expression were decreased in the TNBS-treated rats. Conversely, 2 nnol rVIPa inhibited the above effect. However, relatively low and high concentrations of rVIPa did not exhibit protective effects on the intestinal barrier function damage.
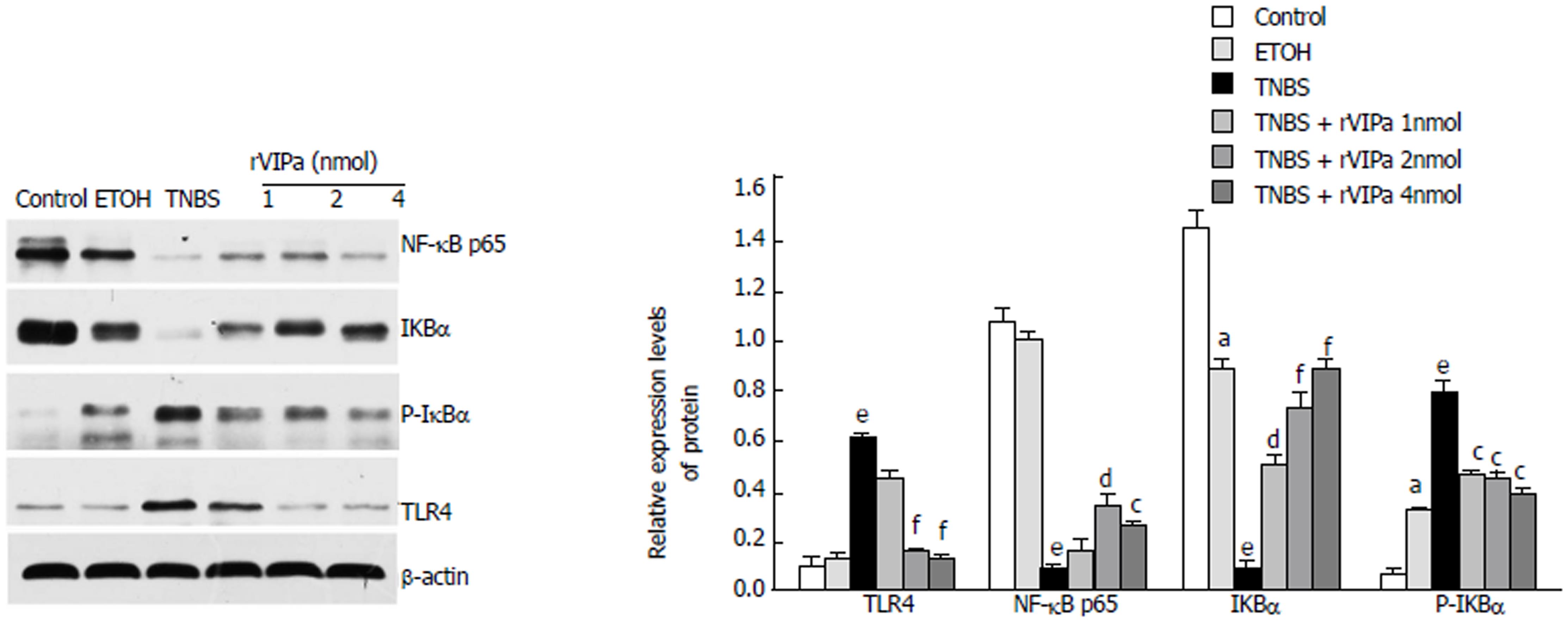
As shown in Figure 6, compared with the control rats, the protein level of TLR4 in TNBS-induced colitis was increased. However, 2 nmol rVIPa treatment significantly down-regulated the level of TLR4 during the TNBS-induced colitis. NF-κB is an important regulator of inflammation-related gene expression. Moreover, compared with the normal control, TNBS treatment significantly down-regulated NF-κB p65 and IκBα expression levels, and up-regulated the expression level of p-IκBα in colon tissue. NF-κB p65 and IκBα expression levels in the rVIPa treatment groups were higher than those in the model group, which suggested that rVIPs may exhibit anti-inflammatory efficiency via TLRs/ NF-κB signaling pathway.
IBD mainly includes CD and ulcerative colitis (UC), which affect quality of life for patients, and even give rise to the risk of enteric cancers. The lack of effective prevention and therapeutic measures are largely due to the pathogenesis of IBD, which is poorly understood. VIP is a neuroimmunopeptide concerned with homeostasis and health. Considerable lines of data suggested that VIP may be a potential candidate which can be developed to treat inflammatory diseases including CD[5-7]. Moreover, the VIP-receptor plays a key role in the modulatory effect of VIP on IBD[5].
As a neuroendocrine mediator, VIP possesses a wide range of biological functions. However, some disadvantages limit its practical application. Therefore, in our previous research, we designed a VIP analogue, via amino residue substitution (Asp3→Lys3, Asp8→Lys8), with safety, favorable stability and improved antimicrobial activities, and established a green, efficient and low-cost production technology via E. coli-recombinant expression system[21]. However, it remains to be elucidated whether the molecular modification and recombinant generation affect its anti-inflammatory activity or not. TNBS-induced colitis shows the similar pathological features to the human CD[22,23]. Therefore, an experimental acute CD model usually established by intrarectal administration of TNBS in mice or rats. In the current study, we investigated the role of rVIPa in TNBS-induced colitis. The results indicated that 2 nmol rVIPa effectively relieved body weight loss, pathological symptoms and prevented hyperemia and inflammation in the colon tissue in the experimental rat model of colitis. This study first verified the the beneficial effect of rVIPa on colitis via in vivo experiment. Notably, high concentration of rVIPa exhibited reduced anti-inflammatory effects. Therefore, as for rVIPa derived from an endogenous neurotransmitter belonging to the hormone family, dose is very crucial. In the treatment of IBD, the benefical effect of rVIPa on the damaged colon may be associated with its anti-inflammatory, antibacterial and immune-modulatory activities. In the TNBS-induced colitis rats, increase of TNF-α and endotoxin level and MPO activity, and decrease of IL-10 level and T-SOD activity were observed in the serum and colon tissue. Conversely, treatment with proper dose of rVIPa significantly suppressed the UC development and inflammatory response. VIP restores immune tolerance by down-regulation of the inflammatory response and by induction of regulatory T cells[15]. VIP protects against bacterial pathogen EPEC-induced epithelial barrier disruption and colitis, which may be associated with the inhibition of PKCε activation by VIP[24]. Previous research indicate that exogenous VIP alleviates symptoms of histopathology of TNBS-induced colitis, which may be related to the decrease of the inflammatory and T helper cell type 1 (Th1)-driven autoimmune components[25]. Impaired goblet cell development and obviously intestinal barrier dysfunction were observed in VIP knockout (KO) (VIP KO) mice[26]. Moreover, VIPKO enhanced the susceptibility of mice to DNBS and DSS-induced colitis. However, VIP-treated VIPKO mice restores the phenotype and effectively protects against DSS-colitis[7]. However, other research suggests that deficient in VIP reduces the pathology caused by TNBS-induced colitis in mice[27]. The role of VIP in colitis need further investigate.
Intestinal epithelial cells, as the important component of intestinal physical barrier, play an key role in resisting harmful substances such as pathogenic microorganism and toxin, etc., and maintaining the intestinal homeostasis[28,29]. The intestinal epithelium barrier can be easily disrupted during gut inflammation such as UC or CD. Recent evidence suggests that it is the crucial pathological feature of IBD that colonic epithelial barrier dysfunction heightens bacterial translocation, and followed by stimulating inflammatory response in the colonic mucosa[30,31]. Moreover, impaired intestinal epithelium permeability contributes to ongoing bowel symptoms in patients with IBD and mucosal healing[32]. In the current study, we found that treatment with rVIPa increased the expression of occludin. Future studies may further reveal the key roles of the intestinal barrier function in the pathophysiology of IBD, which will contribute to explore alternative and targeted therapy methods[33].
Inflammatory molecules s have been considered as elementary inducing factors involved in the disruption of the intestinal TJs, and gut permeability and bacterial translocation[34]. TNF-α is recognized as one of the important proinflammatory cytokines. Treatment with rVIPa reduced the production of systemic and mucosal inflammatory factors and rescued the T colon injuries caused by TNBS and improved the expression of TJsi. TNF-α triggers the increase of intestinal epithelial permeability by disrupting TJs which is related to the MLCK up-regulation[35]. Moreover, NF-κB is involved in positively regulating MLCK as the positive upstream regulator[35]. Activation of NF-κB plays an important role in the intestinal inflammation[36], which is usually recognized as a central “switch” in the key initial steps of inflammation response. The present study also confirmed the idea. Compared with that of normal rats, up-regulation of NF-κB p65 and p-IκBα, and down-regulation of IκBα in inflammatory colonic tissue. Administration of rVIPa decreased the expression of NF-κB p65 and inhibited the degradation of IκBα.
VIP regulates the immune response via TLRs signaling pathway. VIP modulating TLR has been tested in the mucosal immune system using TNBS-induced colitis[37]. VIP exerts an important role in regulating the balance of proinflammatory and anti-inflammatory factors not only in the colon but also in mesenteric lymph nodes. TLRs mediates the expression regulation of inflammation-related genes in a cascade style[38]. The current study shows that rVIPa treatment inhibites TNBS-induced the up-regulation of TLR4, which suggests that rVIPa could regulate the inflammatory response by modulating TLR expression. The VIP/pituitary adenylate cyclase-activating peptide (PACAP) system is considered a potential candidate applicated to anti-inflamatory therapy as a neuroendocrine-immune mediator. Most data validate the therapeutic effect through the modulation of TLR2 and 4[39]. The protective effect of rVIPa is mediated by the down-regulation of inflammatory cytokines, which may be involved in TLRs/ NF-κB /VIP system.
In summary, the study indicated that the VIP analogue produced by recombinant expression significantly inhibited the progression of colitis, exhibiting a protective effect on the colon, which may be related to the inhibition of proinflammatory cytokines, up-regulated TJ expression, and promotion of anti-inflammatory cytokines via TLRs/NF-κB signaling pathway. By modifying the structure of VIP, its anti-inflammatory activity could be further improved. This study provides a simple, low-cost strategy for identifying and producing a novel anti-inflammatory agent from human innate host defense mechanisms in the process of biological evolution.
Crohn’s disease (CD) is a type of inflammatory bowel disease (IBD) Prolonged inflammation of the intestinal tract affects the patients’ quality of life and increases the risk of colorectal cancer development. Endogenous antimicrobial and immunoregulatory peptides represent an emerging category of therapeutic agents, are gaining considerable interest in the scientific community. VIP is a neuropeptide with potent anti-inflammatory activities. Recombinant expressed VIP analogue with higher antimicrobial activity and stability than natural peptide was produced by an effective and low-cost production method. The results indicated that rVIPa alleviated TNBS-induced colitis via TLR4/NF-κB-mediated signaling pathway. rVIPa could be used as a new alternative therapy for intestinal inflammatory disorders. The study contributes to advancement in novel treatment design.
At present, a proportion of patients with Crohn’s disease could not obtain high efficacy of the available medical therapies. Therefore, it is urgent to develop new anti-inflammatory agents with high efficacy, safety and low cost. The natural biological peptides and their analogues will provide important and significant resources for the development of molecules that can block inflammatory pathways.
rVIPa with high antimicrobial activity and stability was produced by an effective and low-cost biotechnology. The current study was conducted to investigate the modulatory effect of rVIPa on colon in rats with TNBS-induced colitis. The study contributes to the development of a kind of new and novel therapeutic agent from endogenous bioactive peptides for IBD.
The current study investigated the anti-inflammatory activity, and the possible mechanism of rVIPa through establishing acute colitis model in rats administrated of TNBS intrarectally. In addition to the body weight change, histological assessment, MPO and endotoxin analyzed by ELISA, and tight junction proteins levels analyzed by Western Blot, TLR4/NF-κB-mediated signaling pathway were also investigated.
The current study first find that VIP analogue produced by recombinant expression significantly ameliorates the colon injury and inflammation caused by TNBS in rats, and exhibits a protective effect on colitis. Moreover, administration with rVIPa inhibited proinflammatory cytokines, up-regulated tight junction proteins expression, and promotion of anti-inflammatory cytokines via TLRs/NF-κB signaling pathway.
rVIPa alleviated TNBS-induced inflammation and effectively protected the intestinal mucosal barrier function in rats, which may be related to TLR4/NF-κB-mediated signaling pathway. These results suggested that rVIPa could be explored to a new alternative therapy for intestinal inflammatory disorders.
By rational design and molecular modification, the antimicrobial and anti-inflammatory activity, and stability for endogenous bioactive peptides could be further improved. This study provides a simple, low-cost strategy for identifying and producing a novel anti-inflammatory agent from human innate host defense mechanisms in the process of biological evolution.
We are grateful to Xiang-Jin Qiao, Yao Meng, and Kai Chen for their technical help.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Daniel F, Sergi CM, Tantau A S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ
| 1. | Luo X, Yu Z, Deng C, Zhang J, Ren G, Sun A, Mani S, Wang Z, Dou W. Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci Rep. 2017;7:16374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Jauregui-Amezaga A, Somers M, De Schepper H, Macken E. Next generation of biologics for the treatment of Crohn’s disease: an evidence-based review on ustekinumab. Clin Exp Gastroenterol. 2017;10:293-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, Fischetti VA, Foster S, Gilmore BF, Hancock RE, Harper D. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect Dis. 2016;16:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 576] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 4. | Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front Cell Infect Microbiol. 2016;6:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 900] [Cited by in RCA: 1216] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 5. | Abad C, Gomariz R, Waschek J, Leceta J, Martinez C, Juarranz Y, Arranz A. VIP in inflammatory bowel disease: state of the art. Endocr Metab Immune Disord Drug Targets. 2012;12:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Jönsson M, Norrgård O, Forsgren S. Epithelial expression of vasoactive intestinal peptide in ulcerative colitis: down-regulation in markedly inflamed colon. Dig Dis Sci. 2012;57:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Wu X, Conlin VS, Morampudi V, Ryz NR, Nasser Y, Bhinder G, Bergstrom KS, Yu HB, Waterhouse CC, Buchan AM. Vasoactive intestinal polypeptide promotes intestinal barrier homeostasis and protection against colitis in mice. PLoS One. 2015;10:e0125225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Abad C, Cheung-Lau G, Coûté-Monvoisin AC, Waschek JA. Vasoactive intestinal peptide-deficient mice exhibit reduced pathology in trinitrobenzene sulfonic acid-induced colitis. Neuroimmunomodulation. 2015;22:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Del Valle-Pinero AY, Sherwin LB, Anderson EM, Caudle RM, Henderson WA. Altered vasoactive intestinal peptides expression in irritable bowel syndrome patients and rats with trinitrobenzene sulfonic acid-induced colitis. World J Gastroenterol. 2015;21:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Vu JP, Million M, Larauche M, Luong L, Norris J, Waschek JA, Pothoulakis C, Pisegna JR, Germano PM. Inhibition of vasoactive intestinal polypeptide (VIP) induces resistance to dextran sodium sulfate (DSS)-induced colitis in mice. J Mol Neurosci. 2014;52:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Deng G, Jin L. The effects of vasoactive intestinal peptide in neurodegenerative disorders. Neurol Res. 2017;39:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Augustyniak D, Nowak J, Lundy FT. Direct and indirect antimicrobial activities of neuropeptides and their therapeutic potential. Curr Protein Pept Sci. 2012;13:723-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Jiang W, Wang H, Li YS, Luo W. Role of vasoactive intestinal peptide in osteoarthritis. J Biomed Sci. 2016;23:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Yamaguchi Y, Ouchi Y. Antimicrobial peptide defensin: identification of novel isoforms and the characterization of their physiological roles and their significance in the pathogenesis of diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:152-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Pozo D, Gonzalez-Rey E, Chorny A, Anderson P, Varela N, Delgado M. Tuning immune tolerance with vasoactive intestinal peptide: a new therapeutic approach for immune disorders. Peptides. 2007;28:1833-1846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Campos-Salinas J, Cavazzuti A, O’Valle F, Forte-Lago I, Caro M, Beverley SM, Delgado M, Gonzalez-Rey E. Therapeutic efficacy of stable analogues of vasoactive intestinal peptide against pathogens. J Biol Chem. 2014;289:14583-14599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Aleinein RA, Hamoud R, Schäfer H, Wink M. Molecular cloning and expression of ranalexin, a bioactive antimicrobial peptide from Rana catesbeiana in Escherichia coli and assessments of its biological activities. Appl Microbiol Biotechnol. 2013;97:3535-3543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Zorko M, Jerala R. Production of recombinant antimicrobial peptides in bacteria. Methods Mol Biol. 2010;618:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | LaVallie ER, DiBlasio EA, Kovacic S, Grant KL, Schendel PF, McCoy JM. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology. 1993;11:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 511] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Zuo D, Liu X, Shou Z, Fan H, Tang Q, Duan X, Cao D, Zou Z, Zhang L. Study on the interactions between transplanted bone marrow-derived mesenchymal stem cells and regulatory T cells for the treatment of experimental colitis. Int J Mol Med. 2013;32:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Xu C, Guo Y, Qiao X, Shang X, Niu W, Jin M. Design, Recombinant Fusion Expression and Biological Evaluation of Vasoactive Intestinal Peptide Analogue as Novel Antimicrobial Agent. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 623] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 23. | Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 981] [Cited by in RCA: 1041] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 24. | Morampudi V, Conlin VS, Dalwadi U, Wu X, Marshall KC, Nguyen C, Vallance BA, Jacobson K. Vasoactive intestinal peptide prevents PKCε-induced intestinal epithelial barrier disruption during EPEC infection. Am J Physiol Gastrointest Liver Physiol. 2015;308:G389-G402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology. 2003;124:961-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Coskun M, Troelsen JT, Nielsen OH. The role of CDX2 in intestinal homeostasis and inflammation. Biochim Biophys Acta. 2011;1812:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Abad C, Cheung-Lau G, Coûté-Monvoisin AC, Waschek JA. Vasoactive intestinal peptide-deficient mice exhibit reduced pathology in trinitrobenzene sulfonic acid-induced colitis. Neuroimmunomodulation. 2015;22:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 785] [Article Influence: 98.1] [Reference Citation Analysis (0)] |
| 29. | Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 471] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 30. | Capaldo CT, Powell DN, Kalman D. Layered defense: how mucus and tight junctions seal the intestinal barrier. J Mol Med (Berl). 2017;95:927-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 31. | Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol. 2017;4:33-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 448] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 32. | Chang J, Leong RW, Wasinger VC, Ip M, Yang M, Phan TG. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients With Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology. 2017;153:723-731.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 33. | Okamoto R, Watanabe M. Functional relevance of intestinal epithelial cells in inflammatory bowel disease. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Ribeiro AB, Giusti H, Souza APT, Franci CR, Saia RS. Dexamethasone Prevents Lipopolysaccharide-Induced Epithelial Barrier Dysfunction in Rat Ileum. Shock. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | He F, Peng J, Deng XL, Yang LF, Camara AD, Omran A, Wang GL, Wu LW, Zhang CL, Yin F. Mechanisms of tumor necrosis factor-alpha-induced leaks in intestine epithelial barrier. Cytokine. 2012;59:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1530] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 37. | Gomariz RP, Arranz A, Abad C, Torroba M, Martinez C, Rosignoli F, Garcia-Gómez M, Leceta J, Juarranz Y. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukoc Biol. 2005;78:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2272] [Cited by in RCA: 2239] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 39. | Arranz A, Abad C, Juarranz Y, Leceta J, Martinez C, Gomariz RP. Vasoactive intestinal peptide as a healing mediator in Crohn’s disease. Neuroimmunomodulation. 2008;15:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |