Published online Feb 7, 2018. doi: 10.3748/wjg.v24.i5.583
Peer-review started: November 15, 2017
First decision: December 3, 2017
Revised: December 10, 2017
Accepted: December 26, 2017
Article in press: December 26, 2017
Published online: February 7, 2018
Processing time: 76 Days and 17.9 Hours
To assess the effect of enteral nutrition (EN) supplemented with glutamine on recovery after ileal pouch-anal anastomosis (IPAA) in rats, to provide an experimental basis for nutritional support in patients with ulcerative colitis (UC) after IPAA.
Male Sprague-Dawley (SD) rats were randomly divided into three groups (n = 8) after IPAA operation using a microsurgical technique. From the third postoperative day, rats in the control group, EN group, and immune nutrition (IN) group were fed standard rat chow, short peptide EN, and short peptide EN combined with glutamine ad libitum, respectively. The rats’ general condition was observed throughout the study. Serum levels of total protein (TP), albumin (ALB), prealbumin (PA), and transferrin (TF) were detected on the 30th postoperative day, using an automatic biochemical analyzer. The ileal pouch mucosa was stained with hematoxylin and eosin (HE), and occludin protein levels were detected by immunohistochemistry.
The body weight of rats in the EN group (359.20 ± 10.06 g) was significantly higher than that in the control group (344.00 ± 9.66 g) (P < 0.05) and lower than that in the IN group (373.60 ± 9.86 g) (P < 0.05) on the 30th postoperative day. The levels of serum TP, ALB, PA, and TF in the EN group were significantly higher than those in the control group (P < 0.01 for all) and lower than those in the IN group (P < 0.05 for all). Histopathological score (EN: 0.80 ± 0.37; IN: 0.60 ± 0.40; control group: 2.29 ± 0.18) and expression level of occludin protein (EN: 0.182 ± 0.054; IN: 0.188 ± 0.048; control group: 0.127 ± 0.032) were significantly lower in the control group compared with the EN and IN groups (P < 0.05 for all), but there were no significant differences between the latter two groups (P > 0.05 for all).
EN combined with glutamine may effectively improve nutritional status after IPAA. Our results suggest a benefit of glutamine supplementation in EN for UC patients undergoing IPAA, although human studies are required to confirm this finding.
Core tip: We assessed the effect of enteral nutrition (EN) supplemented with glutamine on recovery after ileal pouch-anal anastomosis (IPAA) in rats, to provide an experimental basis for nutritional support in patients with ulcerative colitis after IPAA. Male Sprague-Dawley rats underwent IPAA and were then fed standard rat chow, short peptide EN, or short peptide EN combined with glutamine from postoperative day 3. The rats’ general condition was observed throughout the study, and serum levels of total protein, albumin, prealbumin, and transferrin were measured on the 30th postoperative day. The ileal pouch mucosa was stained with hematoxylin and eosin and occludin protein levels were measured by immunohistochemistry.
- Citation: Xu YY, He AQ, Liu G, Li KY, Liu J, Liu T. Enteral nutrition combined with glutamine promotes recovery after ileal pouch-anal anastomosis in rats. World J Gastroenterol 2018; 24(5): 583-592
- URL: https://www.wjgnet.com/1007-9327/full/v24/i5/583.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i5.583
Proctocolectomy with ileal pouch-anal anastomosis (IPAA) has become the gold-standard surgical treatment for ulcerative colitis (UC)[1]. However, studies have shown that some patients who have undergone IPAA still have postoperative problems, such as malnutrition, frequent defecation, and severe pouchitis[2], which can cause surgical failure if the pouch needs to be discarded. Therefore, ways of providing nutrition and energy, promoting early recovery of patients, and maintaining the integrity of the pouch mucosa barrier and function have thus become key considerations for surgical treatment of UC.
In recent years, the IPAA rat model established by Chen et al[3] has shown many characteristics similar to human IPAA, and has become an effective and important in vivo model for the study of recovery and defense as well as immune mechanisms after IPAA.
Nutritional support includes parenteral nutrition (PN) and enteral nutrition (EN). At present, numerous studies have reported that giving EN to patients at an early stage following surgery or severe trauma can rapidly restore the integrity of the digestive tract. This prevents intestinal mucosal atrophy, enhances intestinal barrier function, promotes rehabilitation, and reduces complications and mortality[4,5]. In addition, enteric bacteria and endotoxins are prone to translocation if patients fast too long postoperatively[6]. Early EN is therefore recommended in patients with IPAA. However, general EN may lack certain specific nutrients and its ability to regulate immune function is thus limited, and the supplementation of nutritional therapy with specific nutrients with certain pharmacological effects may improve the patient’s nutritional status, while protecting the integrity of mucosal barrier function.
Glutamine is a non-essential amino acid that can act as an energy source for the proliferation of intestinal lymphocytes, mucosal cells, and fibroblasts[7]. It also has many important physiological properties, including restoring intestinal permeability, preventing intestinal mucosal atrophy, protecting the barrier function of the intestinal mucosa, and improving nitrogen balance. Glutamine can also stimulate immune cells in a specific way, enhance immune function, and maintain a moderate immune response, which is therefore referred to as ‘immune nutrition’. Li et al[8] reported that glutamine may be an effective intestinal mucosal protective agent when supplemented into EN. Rogero et al[9] also suggested that glutamine plays an important role in maintaining the integrity of the intestinal epithelial structure. Fujita et al[10] found that glutamine-supplemented EN reduced the translocation of bacteria in the intestinal contents and enhanced the barrier function of the intestinal mucosa in a pig model.
Clinical research into nutritional support for UC patients after IPAA is currently lacking; however, it is important to explore appropriate postoperative nutritional support to address the issue of postoperative malnutrition. The purpose of this study was to investigate the effects of EN supplemented with different nutrients on recovery, nutritional status, and mucosal barrier function of the ileal pouch in IPAA rats. These results will provide an experimental basis for nutritional treatment of UC patients after IPAA.
Specific pathogen-free (SPF) male Sprague-Dawley (SD) rats aged 10-12 wk and weighing 320-350 g were purchased from the Laboratory Animal Center of the Military Medical Science Academy of the Chinese People’s Liberation Army. They were housed in controlled environmental conditions of ventilation (wind speed, 0.1-0.2 m/s), room temperature (20-25 °C), and humidity (40%-70%) for 1 wk. Rats had access to natural light and were provided with standard rat chow and running water ad libitum prior to surgery. The animal use protocol was reviewed and approved by the Animal Ethical and Welfare Committee.
All rats underwent IPAA using a microsurgical technique. They were then divided into three groups (n = 8 each): a control group fed standard rat chow and tap water (The detailed compositions of standard rat chow were rice, bran, corn, soybean cake, vitamins, minerals, salt, etc), an EN group fed short peptide EN (Milupa GmbH, Germany), and an IN group fed short peptide EN combined with 0.4 g/(kg/d) glutamine (YaoYou Pharmaceutical Co., Ltd, ChongQing, China), ad libitum. All groups were given from the third postoperative day.
The rats’ general status was observed and recorded daily from the first to the 30th postoperative day. Observations included mental state (burnout, laziness, and/or irritability) and fur condition (glossiness and messiness). Body weight was measured at 10 am every day to produce a weight-change curve. Stool characteristics were evaluated and feces were scored using the Bristol Stool Form Scale[11]: type 1, separate hard lumps, like nuts; type 2, sausage-shaped but lumpy; type 3, like a sausage or snake but with cracks on its surface; type 4, like a sausage or snake, smooth and soft; type 5, soft blobs with clear-cut edges; type 6, fluffy pieces with ragged edges, a mushy stool; and type 7, watery, no solid pieces. The fecal score was summarized every 5 d.
Ileal pouch tissue was harvested from rats under anesthesia on the 30th postoperative day, with specimens taken from the same location in the pouches in all rats. Specimens were rinsed with ice-cold saline and then fixed in 4% neutral formalin solution (Jiayu Chemical Co. Ltd, Jinan, China). Blood samples (3 mL) were taken from the abdominal aorta using a 5 mL syringe, placed in an anticoagulant biochemical tube, reversed, and mixed. After centrifugation, the supernatant was collected, packed separately, and stored at -20 °C in a refrigerator for cryopreservation.
Serum levels of total protein (TP), albumin (ALB), prealbumin (PA), and transferrin (TF) were detected in defrosted blood samples using an automatic biochemical analyzer (Johnson and Johnson, United States).
Histological scoring was performed by hematoxylin and eosin (HE) staining. Tissues previously fixed in formaldehyde solution were cut into 0.5 cm pieces, dehydrated in graded ethanol solutions, embedded in paraffin, and stained with HE. The ileal pouch tissue was then evaluated according to the histopathological scoring criteria described by Shebani et al[12] (Table 1).
| Score | Erosion | Ulceration | Villous atrophy | Edema in the lamina propria |
| 0 | Negative | None | None | None |
| 1 | Focal erosion | Focal ulceration of the mucosa at ½ superficial regions | Mild | Positive |
| 2 | Erosion is observed In many regions | Total mucosal ulceration at multiple foci | Moderate | |
| 3 | Extensive erosion | Extensive mucosal ulceration extending to muscularis mucosa or beyond | Severe, villous flattening |
Occludin protein in the ileal pouch mucosa was detected by immunohistochemical staining. Paraffin sections (approximately 5 μm thick) were dewaxed, hydrated, and immersed in boiling citrate buffer (Scientan, Beijing, China; 0.01 mol/L, pH 6.0) for thermal antigen repair. They were then cooled and washed twice with phosphate buffered solution (PBS; Scientan, Beijing, China; 0.01 mol/L, pH 7.4). Sections were immersed in 5% bovine serum albumin and then incubated with rabbit anti-occludin antibody (bs-10011R; Bioss, Beijing, China), followed by incubation with biotinylated goat anti-mouse IgG. A DAB kit (AR1022; Boster, Wuhan, China) was used for color rendering, and sections were lightly stained with hematoxylin (Hua Liang, Fushan, China) and finally observed under a microscope.
The optical density of the immunohistochemical images was measured using Image-Pro Plus 6.0 software, according to the Chinese reference guidelines. The ‘irregular’ tool was used to delineate the measuring area, and the results were then calculated and analyzed.
All statistical analyses were performed using SPSS 19.0. Measurement data are presented as mean ± SD. Data analysis was carried out using one-way ANOVA, and comparisons between two among the three groups were made using the Student-Newman-Keuls method. A P-value < 0.05 was considered statistically significant.
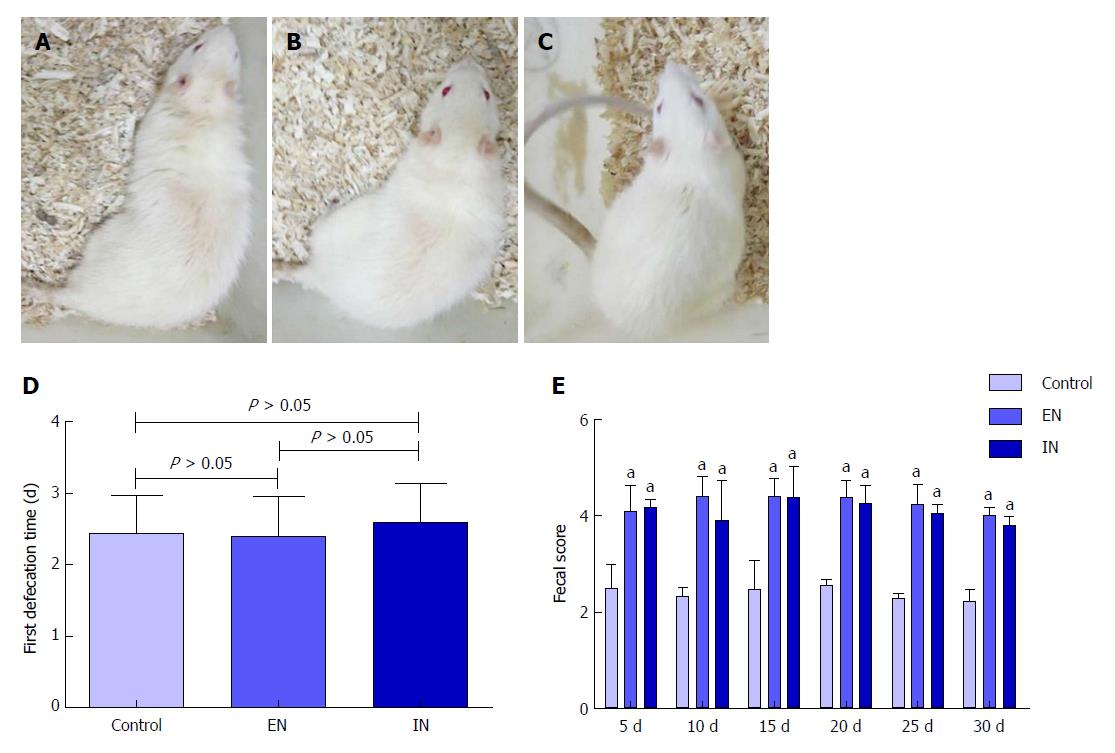
Rats in all groups responded well to surgery, with no signs of burnout, laziness, or irritability (Figure 1A-C). The time to first defecation in the control, EN, and IN groups was 2.43 ± 0.53, 2.40 ± 0.55, and 2.60 ± 0.54 d after IPAA, respectively. There was no significant difference in the first defecation time among the three groups (F = 0.32, P = 0.73) (Figure 2D). The fecal scores in the EN and IN groups were significantly higher than those in the control group at 5, 10, 15, 20, 25, and 30 d postoperatively (P < 0.05 for all), but there were no differences between the EN and IN groups (P > 0.05 for all).
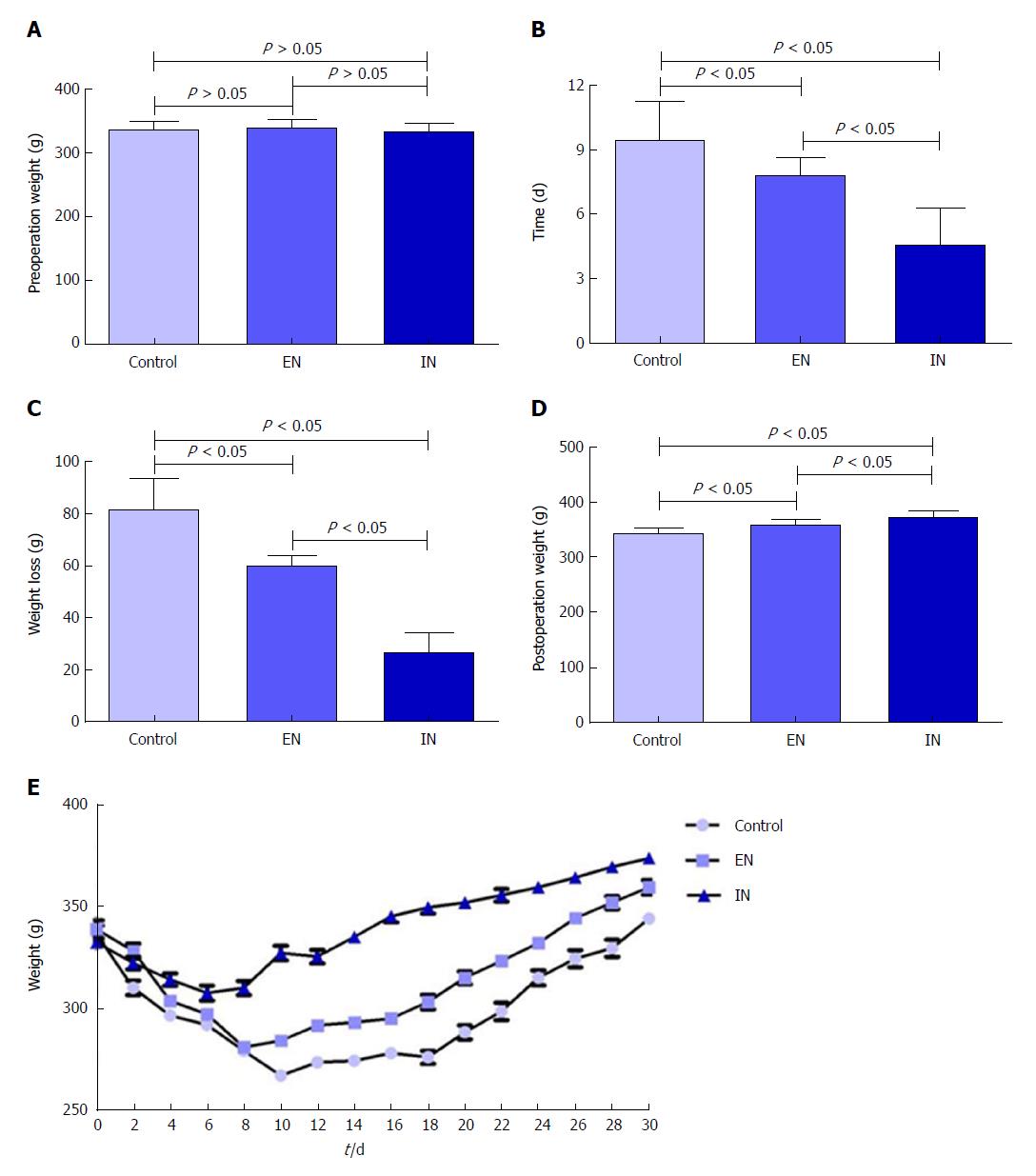
There were no significant differences in body weight among the three groups pre-operatively (F = 0.57, P = 0.57) (Figure 2A), but body weight showed a short-term decrease postoperatively. The lowest body weight was reached at 9.43 ± 1.81, 7.80 ± 0.84, and 4.60 ± 1.71 d postoperatively in the control, EN, and IN groups, respectively (F = 20.98, P < 0.01), and the weight decreases were 81.29 ± 12.30, 59.81 ± 4.15, and 26.26 ± 8.04 g, respectively (F = 79.18, P < 0.01). The time to the lowest body weight and body weight decrease were both greater in the control compared with the EN (P < 0.05) and IN (P < 0.05) groups, and were both greater in the EN compared with the IN group (P < 0.05) (Figure 2B and C). Body weights of rats in the control, EN, and IN groups recovered to 344.00 ± 9.66, 359.20 ± 10.06, and 373.60 ± 9.86 g, respectively, on the 30th day postoperatively (F = 18.02, P < 0.01). Body weight was significantly lower in the control group than in the EN an IN groups (P < 0.05), and in the EN group than in the IN group (P < 0.05) (Figure 2D).
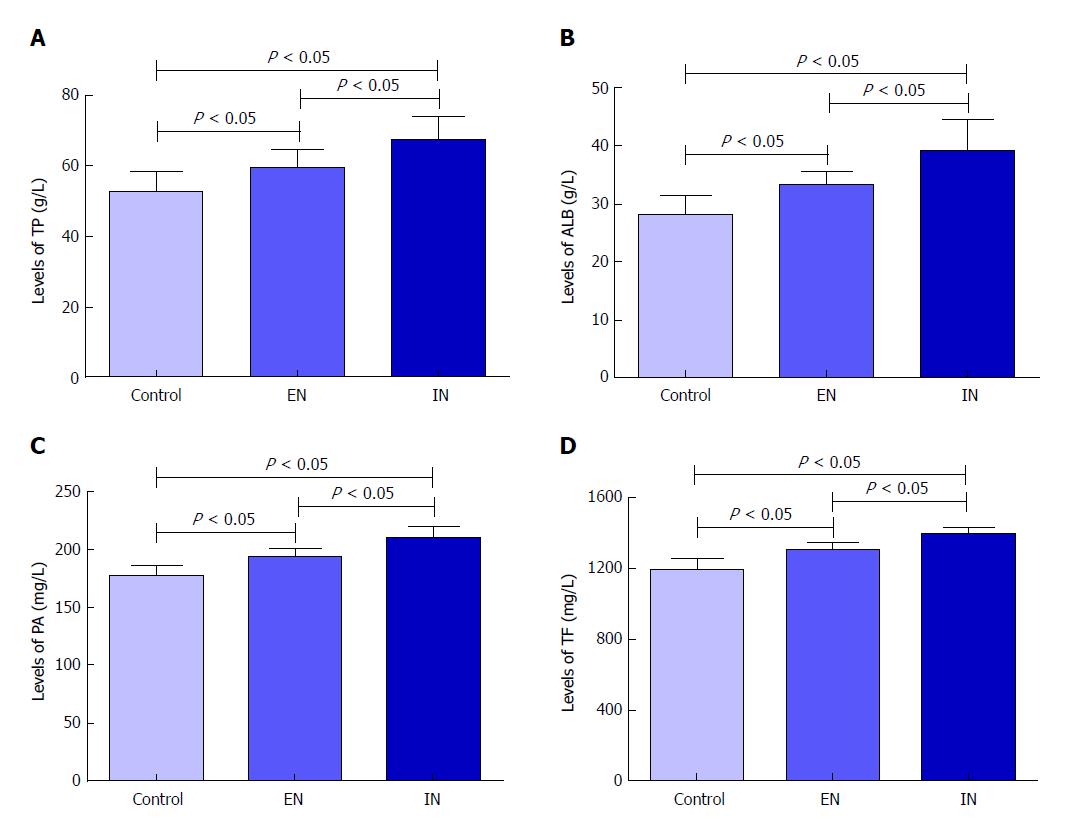
Serum levels of TP, ALB, PA, and TF were significantly different among the three groups (F = 13.51, P < 0.01; F = 17.25, P < 0.01; F = 37.98, P < 0.01; F = 36.41, P < 0.01, respectively). Levels in the control group were significantly higher than those in the EN (P < 0.05 for all) and IN (P < 0.05 for all) groups, while levels in the EN group were higher than those in the IN group (P < 0.05 for all) (Figure 3).
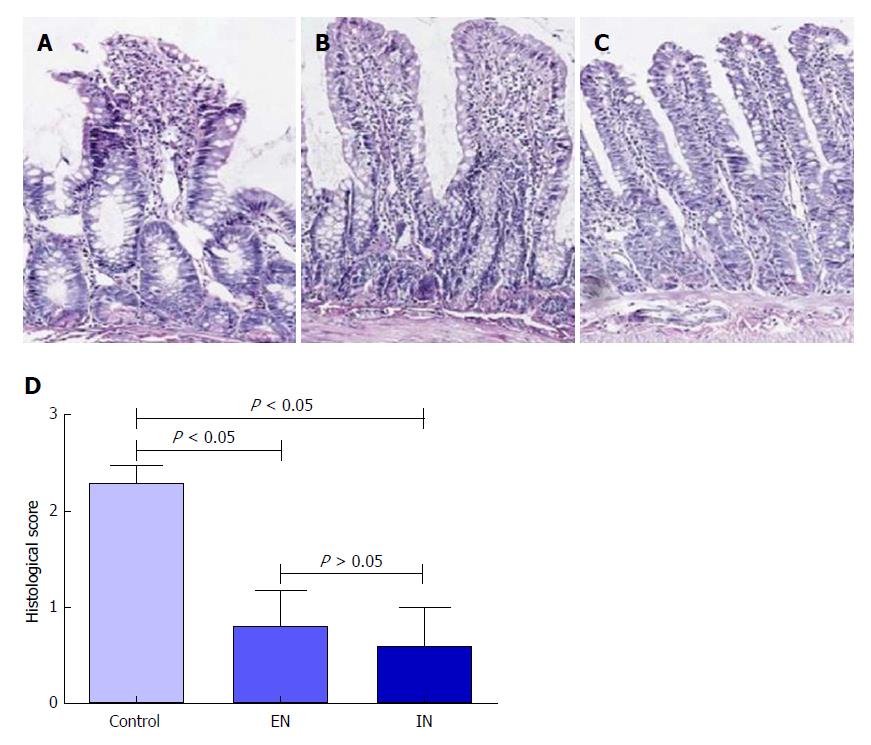
HE staining showed that the integrity of the mucosal villi in the ileal pouch was disrupted in the control group. The villus stroma was loose and irregular, and the villus epithelium showed necrosis, shedding, and atrophy, as well as edema in the lamina propria (Figure 4A). The morphology of the pouch mucosa in the EN (Figure 4B) and IN (Figure 4C) groups was largely normal: the villus structure was intact and its arrangement was neat, the epithelial cells were arranged regularly, and there was occasional interstitial edema. The pathological scores in the EN (0.80 ± 0.37) and IN (0.60 ± 0.40) groups were significantly higher than that in the control group (2.29 ± 0.18, F = 62.15, P < 0.01) (P < 0.05 for both). However, there was no significant difference between the EN and IN groups (P > 0.05) (Figure 4D).
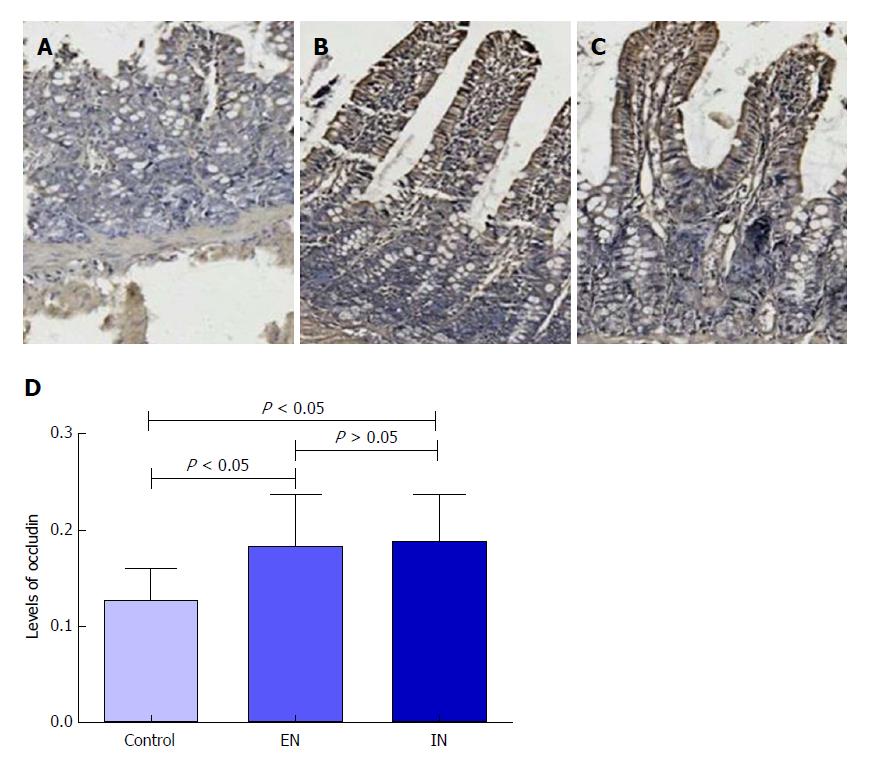
Immunohistochemical staining revealed that expression levels of occludin protein in the ileal pouch mucosa in the EN (0.182 ± 0.054) and IN (0.188 ± 0.048) groups were significantly higher than that in the control group (0.127 ± 0.032, F = 4.34, P = 0.02) (P < 0.05 for both), but there was no significant difference between the EN and IN groups (P > 0.05) (Figure 5).
UC patients lose nutrition because of the nature of the disease and its related clinical manifestations. In addition, restrictions on the types of food that can be eaten safely can result in food intolerances. As a result, about 23.4% of UC patients are malnourished[13]. During IPAA, the colon and rectum are removed and the ileal pouch is connected to the anus[3]. However, although the digestive tract is reconstructed, its digestive and absorptive functions are impaired, while the patient’s nutritional status is further worsened by stress caused by the surgery and anesthesia[14]. Effective means of providing nutritional support for UC patients and thus accelerating their recovery after IPAA have thus become an important question. The current study, based on a stable model of IPAA in rats fed different EN diets, showed that short peptide EN and glutamine could effectively improve nutritional status, protect the mucosal barrier of the ileal pouch, and accelerate the recovery of rats after IPAA operation.
The results of this study showed that the time to first defecation and mental state of rats fed short peptide EN with different nutrients after IPAA were similar to those of control rats, but the fecal scores were higher. This effect may be associated with the increased absorption efficiency of short peptide nutrients in the small intestine[15]. Glutamine can serve as an energy source for intestinal epithelial cells and provide nutrients[7], while short peptide nutrition has the characteristics of low residue, defecation balance, and a low need for digestive juices[16].
The levels of plasma proteins play an important role in the assessment of nutrition[17]. The main indices include TP, ALB, and protein A/G; however, progress in the development of detection technologies means that other visceral proteins, such as PA and TF, have also emerged as candidates for assessing nutritional status[18]. TP can reflect malnutrition caused by chronic disease, while ALB is an important indicator of nutritional status that can effectively reflect disease severity and the relationship between protein consumption and intake. PA is a relatively sensitive indicator of acute changes in nutritional status in the short term, and is the gold standard for monitoring and evaluating the nutritional status of patients[19,20]. TF often falls along with decreases in PA and ALB in the acute reaction phase, and can be used as an indicator of nutritional status[21,22].
The results of this study showed that serum levels of TP, PA, ALB, and TF were significantly improved in the IN group postoperatively, compared with the control and EN groups. Changes in body weight, which is another indicator of nutritional status, are consistent with serum protein levels. These findings demonstrated that glutamine could effectively improve nutritional status after IPAA in rats. Glutamine is a non-essential amino acid in the body in the stress state; it can prevent the excessive decomposition of muscle, promote protein synthesis, and protect the intestinal mucosa. After IPAA, the rats were stressed and the demand for nutrients was thus greatly increased, but the synthesis of nutrients could not meet their needs. EN and postoperative diets generally have a lack of glutamine, and supplementing the diet with glutamine after IPAA could increase the synthesis of tissue proteins, meet the nutritional needs, and improve nutritional status. Liu et al[23] found that glutamine supplementation improved the nutritional status of patients with acute pancreatitis, while Wischmeyer et al[24] also reported that glutamine had a positive effect on recovery after IPAA. Our results are consistent with these previous reports.
In addition to the functions of digestion, absorption, and peristalsis, the intestinal tract is also involved in immune regulation[25,26], hormone secretion[27], and mucosal barrier function. The intestinal barrier plays an important role in the maintenance of intestinal function[28]. Occludin is an intercellular tight junction protein, and numerous studies have shown that occludin plays an important role in the intestinal mucosal barrier[29,30] and can be used as an indicator of mucosal barrier function in the ileal pouch[31]. In our study, occludin levels were higher in the EN and IN groups compared with the control group, suggesting that short peptide EN and glutamine could enhance the mucosal barrier function of the ileal pouch by increasing the expression of occludin.
In addition, the histological score and occludin level in the IN group were higher than those in the EN group, indicating that glutamine could increase the expression level of occludin. Numerous animal experiments have demonstrated that glutamine supplementation can prevent intestinal mucosal atrophy, as well as restore intestinal villus height and crypt depth. Wang et al[7] reported that glutamine could regulate the expression of tight junction proteins. A possible mechanism for this is as follows: (1) glutamine can supply energy for the proliferation and differentiation of intestinal epithelial cells[32]; (2) stimulate heat shock proteins and thus promote cell growth[33]; and (3) promote cell proliferation by regulating the ERK1 and the JNK signaling pathways[34].
In conclusion, feeding short peptide EN supplemented with glutamine can accelerate postoperative recovery after IPAA in rats. It can also improve nutritional status, which has important implications for the nutritional support of patients with UC after IPAA. In terms of nutritional support, EN can be used in patients with UC at the early postoperative stage, and glutamine may be added as appropriate to improve the nutritional status of patients and speed up their recovery.
Glutamine is a nutrient active in the immune system, and may influence recovery after surgery. However, clinical research into nutritional support for ulcerative colitis (UC) patients after ileal pouch-anal anastomosis (IPAA) is currently lacking. Therefore, it is important to explore appropriate postoperative nutritional support to address the issue of postoperative malnutrition.
The purpose of this study was to investigate the effects of enteral nutrition (EN) supplemented with different nutrients on recovery, nutritional status, and mucosal barrier function of the ileal pouch in IPAA rats. These results will provide an experimental basis for nutritional treatment of ulcerative colitis (UC) patients after IPAA.
To assess the effect of EN supplemented with glutamine on recovery after IPAA in rats, to provide an experimental basis for nutritional support in patients with UC after IPAA.
Male Sprague-Dawley (SD) rats were randomly divided into three groups (n = 8) after IPAA operation using a microsurgical technique. From the third day postoperatively, rats in the control group, EN group, and immune nutrition (IN) group were fed standard rat chow, short peptide EN, and short peptide EN combined with glutamine ad libitum, respectively. The rats’ general condition was observed throughout the study. Serum levels of total protein, albumin, prealbumin, and transferrin were detected on the 30th day postoperatively, using an automatic biochemical analyzer. The ileal pouch mucosa was stained with hematoxylin and eosin, and occludin protein levels were detected by immunohistochemistry.
The body weight of rats in the EN group was significantly higher than that in the control group (P < 0.05) and lower than that in the IN group (P < 0.05) on the 30th day postoperatively. The levels of serum TP, ALB, PA, and TF in the EN group were significantly higher than those in the control group (P < 0.01 for all) and lower than those in the IN group (P < 0.05 for all). Histopathological scores and expression levels of occludin protein were significantly lower in the control group compared with the EN and IN groups (P < 0.05 for all), but there were no significant differences between the latter two groups (P > 0.05 for all).
Feeding short peptide EN supplemented with glutamine can accelerate postoperative recovery after IPAA in rats. It can also improve nutritional status, which has important implications for the nutritional support of patients with UC after IPAA. In terms of nutritional support, EN can be used in patients with UC at the early postoperative stage, and glutamine may be added as appropriate to improve the nutritional status of patients and speed up their recovery.
In the future, we will further study on the nutritional support following IPAA procedure, such as enteral nutrition supplemented with probiotics, to improve the postoperative life quality.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Limketkai B, Madnani MA S- Editor: Cui LJ L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Mark-Christensen A, Erichsen R, Brandsborg S, Pachler FR, Nørager CB, Johansen N, Pachler JH, Thorlacius-Ussing O, Kjaer MD, Qvist N. Pouch failures following ileal pouch-anal anastomosis for ulcerative colitis. Colorectal Dis. 2018;20:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Kühn F, Klar E. Surgical Principles in the Treatment of Ulcerative Colitis. Viszeralmedizin. 2015;31:246-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Chen CN, McVay LD, Batlivala ZS, Hendren SK, Swain GP, Salzman N, Williams NN, Rombeau JL. Anatomic and functional characteristics of the rat ileal pouch. Am J Surg. 2002;183:464-470. [PubMed] |
| 4. | Qiang H, Hang L, Shui SY. The curative effect of early use of enteral immunonutrition in postoperative gastric cancer: a meta-analysis. Minerva Gastroenterol Dietol. 2017;63:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Luo Z, Wang J, Zhang Z, Li H, Huang L, Qiao Y, Wang D, Huang J, Guo L, Liu J. Efficacy of Early Enteral Immunonutrition on Immune Function and Clinical Outcome for Postoperative Patients With Gastrointestinal Cancer. JPEN J Parenter Enteral Nutr. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Sugita T, Watarida S, Katsuyama K, Nakajima Y, Yamamoto R, Matsuno S, Tabata R, Mori A. Endotoxemia after elective surgery for abdominal aortic aneurysm and the effect of early oral feeding. J Cardiovasc Surg (Torino). 1998;39:547-549. [PubMed] |
| 7. | Wang B, Wu G, Zhou Z, Dai Z, Sun Y, Ji Y, Li W, Wang W, Liu C, Han F. Glutamine and intestinal barrier function. Amino Acids. 2015;47:2143-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 8. | Li Y, Xu B, Liu F, Tan L, Li J. The effect of glutamine-supplemented total parenteral nutrition on nutrition and intestinal absorptive function in a rat model. Pediatr Surg Int. 2006;22:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Rogero MM, Borelli P, Vinolo MA, Fock RA, de Oliveira Pires IS, Tirapegui J. Dietary glutamine supplementation affects macrophage function, hematopoiesis and nutritional status in early weaned mice. Clin Nutr. 2008;27:386-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Fujita T, Sakurai K. Efficacy of glutamine-enriched enteral nutrition in an experimental model of mucosal ulcerative colitis. Br J Surg. 1995;82:749-751. [PubMed] |
| 11. | Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 2105] [Article Influence: 75.2] [Reference Citation Analysis (1)] |
| 12. | Shebani KO, Stucchi AF, Fruin B, McClung JP, Gee D, Beer ER, LaMorte WW, Becker JM. Pouchitis in a rat model of ileal J pouch-anal anastomosis. Inflamm Bowel Dis. 2002;8:23-34. [PubMed] |
| 13. | Csontos ÁA, Molnár A, Piri Z, Pálfi E, Miheller P. Malnutrition risk questionnaire combined with body composition measurement in malnutrition screening in inflammatory bowel disease. Rev Esp Enferm Dig. 2017;109:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale R. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr. 2017;36:623-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 1062] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 15. | Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806-815. [PubMed] |
| 16. | Nguyen DL. Guidance for supplemental enteral nutrition across patient populations. Am J Manag Care. 2017;23:S210-S219. [PubMed] |
| 17. | Wunderlich SM. Using plasma proteins for nutrition assessment. J Am Diet Assoc. 1989;89:1236. [PubMed] |
| 18. | Pencharz PB, Azcue M. Use of bioelectrical impedance analysis measurements in the clinical management of malnutrition. Am J Clin Nutr. 1996;64:485S-488S. [PubMed] |
| 19. | Wang F, Hou MX, Wu XL, Bao LD, Dong PD. Impact of enteral nutrition on postoperative immune function and nutritional status. Genet Mol Res. 2015;14:6065-6072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Devoto G, Gallo F, Marchello C, Racchi O, Garbarini R, Bonassi S, Albalustri G, Haupt E. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem. 2006;52:2281-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Brugler L, Stankovic A, Bernstein L, Scott F, O’Sullivan-Maillet J. The role of visceral protein markers in protein calorie malnutrition. Clin Chem Lab Med. 2002;40:1360-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | López-Hellin J, Baena-Fustegueras JA, Schwartz-Riera S, García-Arumí E. Usefulness of short-lived proteins as nutritional indicators surgical patients. Clin Nutr. 2002;21:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Liu X, Sun XF, Ge QX. The role of glutamine supplemented total parenteral nutrition (TPN) in severe acute pancreatitis. Eur Rev Med Pharmacol Sci. 2016;20:4176-4180. [PubMed] |
| 24. | Wischmeyer PE, Dhaliwal R, McCall M, Ziegler TR, Heyland DK. Parenteral glutamine supplementation in critical illness: a systematic review. Crit Care. 2014;18:R76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1024] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 26. | Dahan S, Roth-Walter F, Arnaboldi P, Agarwal S, Mayer L. Epithelia: lymphocyte interactions in the gut. Immunol Rev. 2007;215:243-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Ahlman H, Nilsson . The gut as the largest endocrine organ in the body. Ann Oncol. 2001;12 Suppl 2:S63-S68. [PubMed] |
| 28. | Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 810] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 29. | Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 659] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 30. | Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1210] [Cited by in RCA: 1114] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 31. | Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C, Mrsny RJ. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J Biol Chem. 2000;275:29816-29822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Zou XP, Chen M, Wei W, Cao J, Chen L, Tian M. Effects of enteral immunonutrition on the maintenance of gut barrier function and immune function in pigs with severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2010;34:554-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Marc Rhoads J, Wu G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids. 2009;37:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 235] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Rhoads JM, Argenzio RA, Chen W, Rippe RA, Westwick JK, Cox AD, Berschneider HM, Brenner DA. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997;272:G943-G953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |