Published online Feb 7, 2018. doi: 10.3748/wjg.v24.i5.573
Peer-review started: November 6, 2017
First decision: November 22, 2017
Revised: December 3, 2017
Accepted: December 12, 2017
Article in press: December 12, 2017
Published online: February 7, 2018
Processing time: 86 Days and 10.4 Hours
To detect abnormal microRNA (miRNA) expression in type 1 gastric neuroendocrine neoplasms (g-NENs) and find potential target genes.
Tumour tissues from patients with type 1 g-NENs were used as experimental samples, and gastric mucosal tissues from the same patients obtained during gastroscopy review after several months were used as control samples. miRNA expression was examined with Agilent human miRNA chips and validated via RT-PCR. Three types of target gene prediction software (TargetScan, PITA, and microRNAorg) were used to predict potential target genes of the differentially expressed miRNAs, and a dual-luciferase reporter assay system was used for verification.
Six miRNAs were significantly upregulated or downregulated in the tumours compared to the control samples. Among them, miR-202-3p was extraordinarily upregulated. RT-PCR of seven sample sets confirmed that miR-202-3p was upregulated in tumour tissues. In total, 215 target genes were predicted to be associated with miR-202-3p. Among them, dual-specificity phosphatase 1 (DUSP1) was reported to be closely related to tumour occurrence and development. The dual-luciferase reporter assay showed that miR-202-3p directly regulated DUSP1 in 293T cells.
miR-202-3p is upregulated in type 1 g-NEN lesions and might play important roles in the pathogenesis of type 1 g-NENs by targeting DUSP1.
Core tip: In this study, we have innovatively used chip technology to study microRNAs in type 1 gastric neuroendocrine neoplasms. We found miR-202-3p was extraordinarily upregulated in the tumours compared to the control samples. Interestingly, although miR-202 belongs to let-7, a famous cancer-suppressing family, some studies have reported its oncogenic potential in some tumours. Then, we found that DUSP1 could be a target gene of miR-202-3p and was closely related to tumour occurrence and development. Finally, we successfully showed that the miR-202-3p directly regulated DUSP1 in tool cells.
- Citation: Dou D, Shi YF, Liu Q, Luo J, Liu JX, Liu M, Liu YY, Li YL, Qiu XD, Tan HY. Hsa-miR-202-3p, up-regulated in type 1 gastric neuroendocrine neoplasms, may target DUSP1. World J Gastroenterol 2018; 24(5): 573-582
- URL: https://www.wjgnet.com/1007-9327/full/v24/i5/573.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i5.573
Gastric neuroendocrine neoplasms (g-NENs) are a rare malignancy mainly derived from enterochromaffin-like (ECL) cells and occasionally derived from other cells that secrete somatostatin, auxin, or serotonin[1]. Based on the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) cancer registry in the United States, the annual age-adjusted incidence of neuroendocrine tumours (NETs) was 1.09 per 100000 persons in 1973 and increased to 6.98 per 100000 persons by 2012[2]. The proportion of g-NENs among all NENs was 6%[3], 23%[4], 5%[5], and 7.4%[6] in the United States, Austria, Canada, and Taiwan, respectively.
Patients with g-NENs can be subdivided into four types according to their specific tumour aetiology, pathogenesis, and pathology. The clinicopathological features, treatment, and prognosis of different types are completely different[7,8]. The type 1 g-NEN is a well differentiated g-NEN related to chronic atrophic gastritis, with patients exhibiting hypergastrinaemia and achlorhydria. It is the most common type of g-NEN and accounts for approximately 70%-80% of g-NENs[9]. Metastatic type 1 g-NENs are extremely rare[10]; however, the median relapse-free survival is only 8 mo after endoscopic submucosal dissection (ESD) or endoscopic mucosal resection (EMR)[11]. These characteristics make the type 1 g-NEN a stubborn disease. Nevertheless, the molecular mechanism of type 1 g-NEN recurrence is not well understood, and the treatment targets are limited.
MicroRNAs (miRNAs) are small endogenous non-coding RNAs that can regulate target genes through translational repression or mRNA transcript destabilization at the post-transcriptional level. It is estimated that approximately 300 miRNAs (1%-4% of the expressed human genes) exist in the human genome, and a single miRNA can regulate as many as 200 mRNAs[12]. miRNA regulation is linked to various tumours and plays different roles in different diseases, including both oncogenic and tumour suppressor roles[13,14]. miRNAs have the potential to act as biomarkers for patient stratification into different prognostic groups and may also be able to guide treatment decisions. Furthermore, the molecular mechanisms of tumour recurrence and metastasis can often be explained by miRNA activity[7,15,16].
At present, because of the low incidence of NENs, miRNA research related to this disease is not as common as that for other tumour types. However, several studies have shown a variety of results with regard to pancreatic and small intestinal NENs and small cell lung cancer. Roldo et al[17] reported differences in miRNA expression between pancreatic NENs and pancreatic acinar cell tumours. Li et al[18] reported that the progression of small intestinal NENs is related to a significant increase in miR-96/182/183/196/200. Miller et al[19] reported that miR-1 and miR-143-3p gene targets were upregulated in an existing small bowel NET dataset, which could contribute to disease progression, and showed that these miRNAs directly regulate FOSB and NUAK2 oncogenes.
There are a few previous reports regarding type 1 g-NEN-associated miRNAs, including a study from Professor Pritchard et al, who reported that miR-222 expression was increased in the serum and gastric corpus mucosa of hypergastrinaemic INS-GAS mice and hypergastrinaemic patients with autoimmune atrophic gastritis and type 1 gastric NETs. miR-222 expression decreased in these patients following treatment with the CCK2R antagonist netazepide (YF476)[20].
Although there have been a few previous miRNA studies about (or including) type 1 g-NENs, there are still many other miRNAs and target genes waiting to be discovered and studied. The molecular mechanism of this disease remains largely unknown. This study assessed differences in miRNA expression between type 1 g-NEN tissues and non-tumour tissues and identified relevant target genes, with an aim to discover the possible molecular mechanism of type 1 g-NEN recurrence.
This study was approved by the Ethics Committee of China-Japan Friendship Hospital. All patient procedures were performed after obtaining written informed consent.
Tissue samples were collected from patients with type 1 g-NENs at our hospital, and patients were included in the study based on the following criteria: (1) a clear pathological diagnosis of g-NENs; and (2) compliance with all of the following clinical characteristics of type 1 g-NENs: hypergastrinaemia, achlorhydria, and chronic atrophic gastritis[9].
To observe differences in miRNA expression between tumour and non-tumour tissues, tumour tissue obtained from the first endoscopic biopsy (before ESD or EMR) was selected for use as experimental samples and gastric mucosal tissue obtained from gastroscopy review (an average of 9 mo after ESD or EMR) was used as control samples. To ensure that the gastric mucosal tissue was tumour-free, the gastroscopy review had to show no recurrence based on both endoscopic and pathologic assessments. Each pair of experimental and control samples was taken from the same patient, reducing the possible impact of individual differences.
Sections were prepared from each paraffin-embedded specimen. The specimens were prepared from tumour tissues or non-tumour tissues removed from the patients described above via endoscopic biopsy. Total RNA was extracted from the specimens using a RecoverAll Total Nucleic Acid Isolation Kit for FFPE (OE Biotech, Shanghai, China) according to the manufacturer’s specifications. The RNA yield was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, United States) and an Agilent Bioanalyzer 2100 (Agilent Technologies Inc., Santa Clara, United States), and the integrity was evaluated using agarose gel electrophoresis with ethidium bromide staining.
The miRNAs in the total RNA were labelled using a miRNA complete Labeling and Hyb Kit (Agilent Technologies Inc., Santa Clara, United States). Then, the miRNAs were hybridized at 55 °C for 20 h with an Agilent human miRNA microarray (8*60 K, Design ID: 070156), which included all mature human miRNAs available in the latest version of the miRBase database (Release 20). After the microarray was washed, the fluorescence intensity of the samples was scanned using a microarray scanner (Agilent p/n G4900DA), and the results were transformed into quantitative data. The normalized value was calculated as the ratio of the expression of each target miRNA to that of the reference 5S in the same sample. Fold changes in miRNA expression were obtained by comparing the ratio of miRNA expression in the tumour samples to that in the control samples. Three biological replicates were examined.
The expression of miRNAs chosen for further study was validated via qRT-PCR analysis. Total RNA from the samples was purified in the manner described above, and the quality of total RNA was also monitored.
Quantification was performed via a two-step reaction process: reverse transcription (RT) and PCR. Each RT reaction consisted of 1 μg of RNA, 4 μL of miScriptHiSpec Buffer, 2 μL of Nucleotide Mix, and 2 μL of miScript Reverse Transcriptase Mix (Qiagen, GER) in a total volume of 20 μL. Reactions were performed on a GeneAmp PCR System 9700 (Applied Biosystems, United States) for 60 min at 37 °C, followed by heat inactivation of RT for 5 min at 95 °C. The 20 μL RT reaction mix was then diluted 5-fold in nuclease-free water and stored at -20 °C.
Real-time PCR was performed using a LightCycler 480 II Real-time PCR Instrument (Roche, SWI) with 10 μL of PCR reaction mixture that included 1 μL of cDNA, 5 μL of 2 × LightCycler 480 SYBR Green I Master (Roche, SWI), 0.2 μL, of universal primer (Qiagen, GER), 0.2 μL of miRNA-specific primer, and 3.6 μL of nuclease-free water. Reactions were incubated in a 384-well optical plate (Roche, SWI) at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Each sample was run in triplicate for analysis. At the end of the PCR cycles, a melting curve analysis was performed to validate the specific generation of the expected PCR product. The miRNA-specific primer sequences were designed and synthesized by Generay Biotech (Shanghai, China) based on the miRNA sequences obtained from the miRBase database (Release 20.0) and are shown in Table 1. Other primer sequences used (not shown in Table 1) are the universal primers from the kit (Qiagen, GER). We used 5S rRNA as an endogenous reference gene. The miRNA expression levels were normalized to that of 5S rRNA and calculated using the 2-ΔΔCt method.
| Primer sequence | |
| Hsa-miR-202-3p | 5’-AGAGGTATAGGGCATGGGAA-3’ |
| 5S rRNA | 5’-GGAGACCGCCTGGGAATA-3’ |
MiRNA target prediction can be performed through computational algorithms due to the base-pairing rules between miRNA and mRNA target sites, location of binding sequences within the target 3’-untranslated region (UTR), and conservation of target binding sequences within related genomes. We searched potential targets of certain miRNAs in TargetScan (http://www.targetscan.org/vert_71/), PITA (http://www.pita.ps/), and microRNAorg (http://www.microrna.org/microrna/home.do). Overlapping target genes predicted by the three different databases were further studied. By reviewing the published literature to understand the function of all the overlapping predicted target genes, we selected the target genes that may be associated with type 1 g-NENs for validation.
We then used DAVID Bioinformatics Resources (http://david.abcc.ncifcrf.gov/home.jsp) to perform GO analysis, which is used to predict the potential functions of the gene products, including molecular function, biological process, and cellular component categories. Pathway analysis of the target genes was performed using the KEGG database, and a statistical test was used to calculate the significance of target gene enrichment in each pathway and obtain the FDR_bhvalue (P-value corrected by Benjamini-Hochberg method). This value can be used to provide some hints for selecting the target genes if P < 0.05 in any term.
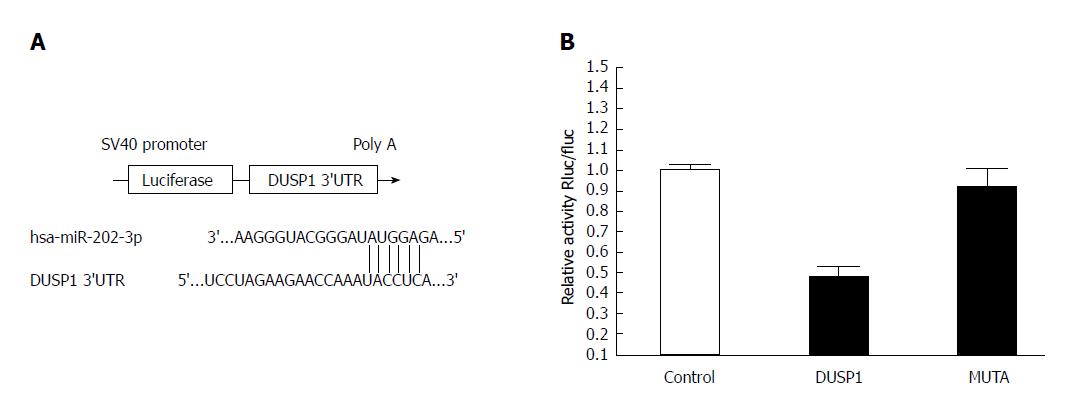
The putative interaction site between hsa-miR-202-3p and the dual-specificity phosphatase 1 (DUSP1) 3′-UTR was searched using TargetScan and microRNA.org. The 388 bp 3′UTR region of the DUSP1 gene, including the binding site for miR-202, was amplified from 293T cells. The amplified fragment was cloned into a psiCHECK2 luciferase reporter vector (Promega, USA) at the Xba I site. A deletion in the miR-202-3p binding site of the DUSP1 gene 3′-UTR was introduced using a Quik-Change Site-Directed Mutagenesis Kit (Stratagene, United States) following the manufacturer’s instructions. The group design is shown in Table 2. The luciferase activity was corrected for transfection efficiency using a Renilla luciferase vector (Promega, United States).
| Control | DUSP1 3’-UTR | Non-targeting control |
| WT | DUSP1 3’-UTR | miR-202-3p |
| MUTA | DUSP1 3’-UTR-muta | miR-202-3p |
In accordance with the manufacturer’s instructions, Lipofectamine 2000 was used to cotransfect 293T cells with the miR-202-3p/non-targeting control mimic (purchased from Hanbio, Shanghai) using a psiCHECK2 vector containing either the wild-type or mutant-type 3′-UTR of DUSP1. After incubation in 5% CO2 at 37 °C for 48 h, a luciferase reporter assay was performed using the Promega dual-luciferase reporter assay system according to the manufacturer’s instructions. Each assay was performed in triplicate.
Statistical analyses of the qRT-PCR results were performed using SPSS software (Version 20.0; SPSS Inc., Chicago, IL, United States). All the data were from at least two independent experiments, with triplicate samples tested in each experiment, and the results are expressed as the means ± SD. The differences between groups were analysed by single-factor analysis of variance (ANOVA). Differences were considered statistically significant when P < 0.05.
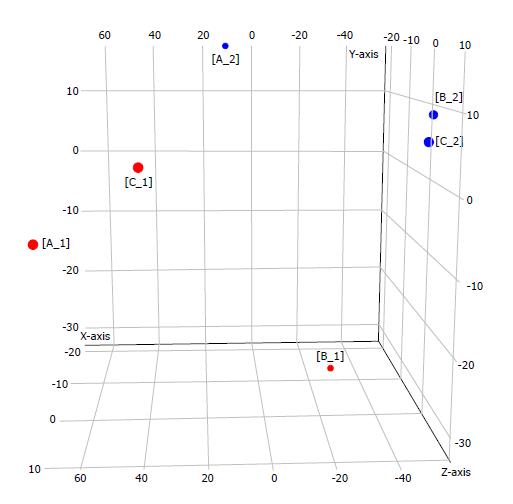
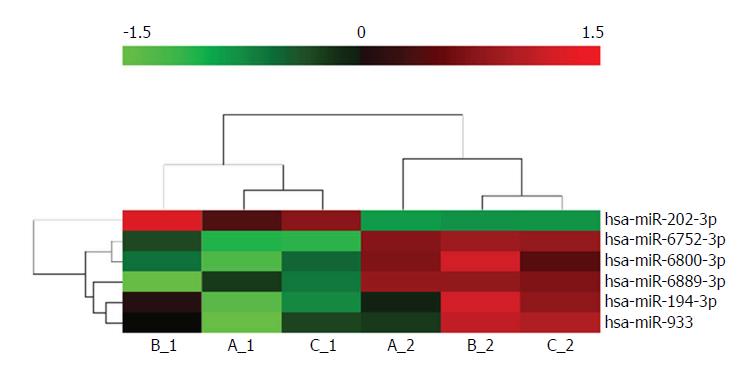
Samples from three patients (marked as A, B, and C) with type 1 g-NENs were collected following the procedures described in the ‘Materials and Methods’ section. Thus, we obtained three pairs of samples: three tumour samples (marked as A1, B1, and C1) and the corresponding three tumour-free samples (marked as A2, B2, and C2). An Agilent human miRNA microarray was used to evaluate the miRNA expression profiles in type 1 g-NEN and NGM tissues. We found one upregulated miRNA and five downregulated miRNAs (log2-fold change (FC) > 1.5; adjusted P < 0.05) in type 1 g-NEN vs NGM tissue (Table 3, Figures 1 and 2).
| Systematic_Name | P value | FC (abs) | Regulation |
| Hsa-miR-194-3p | 0.022978 | 4.278965 | Down |
| Hsa-miR-202-3p | 0.014934 | 12.12983 | Up |
| Hsa-miR-6752-3p | 0.012949 | 6.846719 | Down |
| Hsa-miR-6800-3p | 0.026902 | 5.196331 | Down |
| Hsa-miR-6889-3p | 0.039335 | 15.72488 | Down |
| Hsa-miR-933 | 0.008580 | 4.18559 | Down |
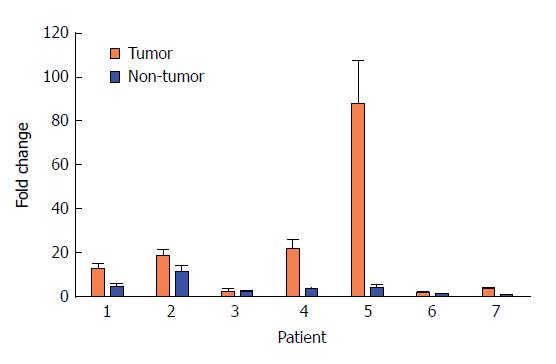
Because we were mainly looking for upregulated miRNAs in type 1 g-NENs, we chose miRNA-202 for the follow-up study. Seven pairs of samples obtained from different patients were used for the qRT-PCR analysis of miRNA-202-3p. The results validated our miRNA expression profile microarray results (Figure 3). Each pair of samples showed the same trend as the results mentioned above: miRNA-202-3p is upregulated in type 1 g-NENs.
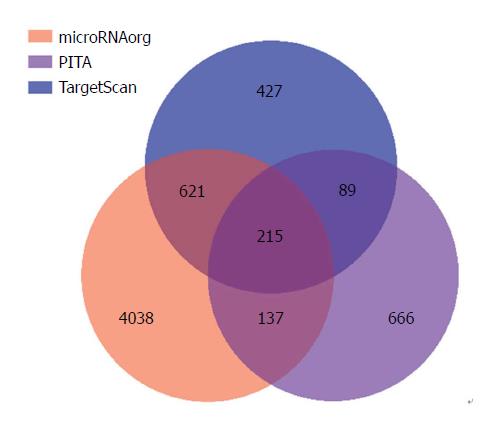
Next, miR-202-3p target genes were predicted using three online available databases, and 427, 4038, and 666 potential target genes were predicted with TargetScan, microRNA.org, and PITA, respectively. There were 215 genes at the intersection of the three predicted target gene sets (Figure 4). We considered potential target genes to exhibit an expression trend opposite to that of the miRNA, in accordance with the antiregulation paradigm (i.e., upregulated miRNA and downregulated mRNA)[21]. As miR-202-3p is overexpressed in type 1 g-NENs, we speculated that it may play a role in promoting cancer by downregulating specific tumour suppressor genes. Enrichment analyses of GO terms and KEGG pathways were also performed. Unfortunately, the bioinformatic analysis did not show any terms with an FDR_bh less than 0.05, and thus it failed to provide hints for selecting target genes.
We searched a large number of studies on the 215 potential target genes mentioned above and found that DUSP1 was reported to be a tumour suppressor gene in a variety of diseases and was associated with gastric tumours[22-26]. Therefore, we chose this gene for further validation experiments.
To verify whether miR-202-3p directly targets DUSP1, luciferase reporter assays were conducted. The results of a luciferase reporter assay are shown in Figure 5 and indicate that 293T cells cotransfected with the miR-202-3p mimic and wild-type 3′-UTR of DUSP1 showed a notable decrease in luciferase activity compared with the control group (P < 0.05). However, 293T cells cotransfected with the miR-202-3p mimic and mutant-type 3′-UTR of DUSP1 had the same luciferase activity as the control group. These results indicate that DUSP1 is a target gene of miR-202-3p, and its expression can be negatively regulated by miR-202-3p.
miR-202 is located at 10q26 (position 135061015 -135061124 on chromosome 10), and its mature single-stranded miRNA sequence is 5’-UUCCUAUGCAUAUAC UUCUUUG-3’[27]. The mature sequence is highly conserved in vertebrates, such as humans, rats, and mice. The two arms of a pre-miRNA are named -3p and -5p, and their properties and functions are basically the same when they are processed to produce miRNAs.
miR-202 belongs to the let-7 family, which was discovered by Reinhart et al in Caenorhabditis elegans. It is developed from a precursor molecule of approximately 70 nucleotides with a 21 nt-long stem-loop structure. According to current studies, let-7 is highly conserved, and its expression is often controlled in a temporal and tissue specific manner. There are currently 13 types of miRNAs in the let-7 family, including let-7a-1, let-7a-2, let-7a-3, let-7b, let-7c, let-7d, let-7e, let-7f -1, let-7f-2, let-7g, let-7i, miR-98, and miR-202. The main physiological functions of let-7 include regulating the growth and development of cells and organs, regulating cell proliferation and apoptosis, and participating in metabolic and stress responses[28]. Above all, let-7 is a widely accepted tumour suppressor miRNA. The expression of let-7 family members is downregulated in many carcinoma types[29].
Interestingly, miRNAs may play a diametrically opposed role in different tumours[30]. Although miR-202 belongs to this famous cancer-suppressing family and has been reported as an anti-oncomir in various tumours, such as lung and liver cancer, some studies have reported its oncogenic potential in other tumours in recent years. Researchers from Henan University reported that miR-202 played a promotive role in endometrial cell proliferation[31]. Yu et al reported that miR-202 was highly expressed in peripheral blood monocytes in patients with multiple myeloma[32]. Researchers in Washington state found that in eight cases of prostate cancer specimens, miR-202 was more highly expressed than in benign prostatic hyperplasia tissues[33]. British researchers observed 102 cases of breast cancer patients, benign breast disease patients, and healthy people and reported that the expression of miR-202 in tumour tissue was significantly higher and was associated with a worse prognosis[34]. The Lancet Cancer Journal reported upregulation of miR-202 in gastric cancer. The researchers compared 160 pairs of gastric cancer tissues and matched paracancerous tissues. Finally, four miRNAs were found to be dramatically upregulated in intestinal-type gastric carcinoma, including miR-202[35].
Our results have shown that DUSP1 is a target gene of miR-202-3p, and its expression can be negatively regulated by miR-202-3p. DUSP1, also known as mitogen-activated protein kinase phosphatase-1 (MKP-1), is an archetypal member of the dual-specificity phosphatases, which play an important role in inactivating different isoforms of mitogen-activated protein kinases (MAPKs)[36]. In recent years, DUSP1 has been studied in many fields. The functions of DUSP1 focus on cell proliferation, differentiation and transformation, stress responses, inflammation, cycle arrest, and apoptosis mainly by regulating MAPK signalling[37]. An increasing number of studies have discovered that its effects in tumours may be varied and complex. The role of DUSP1 can be oncogenic in some tumours, while it can be anti-oncogenic in other tumours[38].
According to the literature, DUSP1, which is considered an oncogene, is upregulated in lung cancer[39], cholangiocarcinoma[40], and colorectal cancer[41], while in many other tumour types, such as endometrial cancer[24], liver cancer[22], and head and neck squamous cell carcinoma[25], it acts as an anti-oncogene. Researchers have found that total DUSP1 protein levels were decreased in 63.7% of breast cancer tissues compared with matched noncancerous breast tissues. Decreased DUSP1 protein levels were correlated with increased tumour stage, positive recurrence, and poor survival, even when using a multivariate Cox regression model[23]. An experiment showed that most of the apparently normal glands, benign prostatic hyperplasia, and low-grade prostatic intraepithelial neoplasia samples showed high DUSP1 expression. By contrast, DUSP1 expression levels were lower or even absent in high-grade prostatic intraepithelial neoplasia and prostatic adenocarcinoma samples. The researchers also found an inverse correlation between DUSP1 expression and activation of both p65/NF-κa and p38 MAPK, and DUSP1 promoted apoptosis through a p38 MAPK-dependent mechanism[26].
Because our experiments demonstrated that DUSP1 and miR-202-3p are inversely regulated and miR-202-3p is highly expressed in g-NENs, we can speculate that DUSP1 expression is also low in type 1 g-NENs, and it is likely a tumour suppressor gene in these tumours.
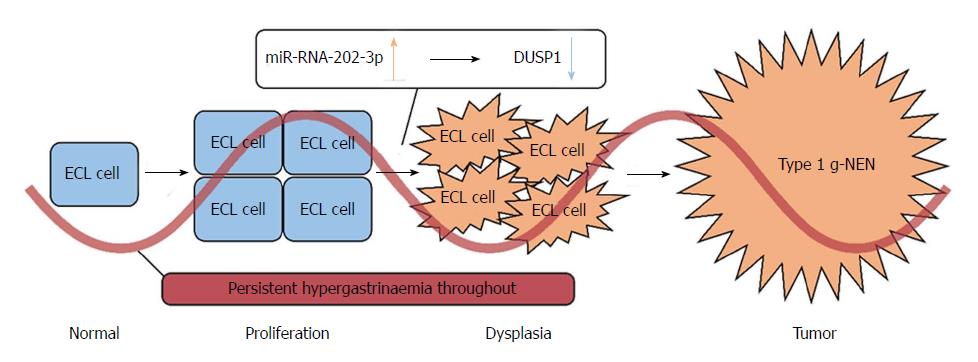
Type 1 g-NETs arise in patients who have autoimmune atrophic gastritis. This kind of gastritis causes atrophy and a reduced number of acid secreting gastric parietal cells. This leads to achlorhydria and corresponding symptoms such as bloating, indigestion, and constipation. The high intra-gastric pH level triggers negative feedback that stimulates G cells to over-secrete gastrin and leads to hypergastrinaemia. Since gastrin has a nutritional effect on ECL cell growth, hypergastrinaemia can lead to ECL cell proliferation. In some patients, ECL cell proliferation will further develop into dysplasia and ultimately the formation of a type 1 g-NET[42-44].
Nevertheless, only a few autoimmune atrophic gastritis patients eventually develop type 1 g-NETs[45]. This indicates that there are still other factors in addition to hypergastrinaemia that promote progression from proliferation and dysplasia to tumour formation. Thus, many factors that may contribute to tumour formation have been proposed, such as mutations in BCL-2[46], Reg[47], Mcl-2[48], and MEN-1[49] genes, growth factor regulation, and bacterial infection. These factors may affect apoptosis, autophagy, proliferation, and differentiation, thereby promoting tumour formation[50].
In our study, all the control group samples were tumour-free gastric mucosa, but they all exhibited ECL cell proliferation. Therefore, we speculate that miR-202-3p/DUSP1 plays a role in the process of ECL cell dysplasia and tumour formation (Figure 6). Because high miR-202-3p expression has been found in type 1 g-NETs, we can safely conclude that miR-202-3p acts as a tumour-promoting miRNA in the development of these tumours. The dual-luciferase reporter assay showed the relationship between miR-202-3p and DUSP1, confirming the negative regulation between them.
Moreover, the studies mentioned above have shown that DUSP1 has a significant antitumour effect in a variety of tumours. Therefore, we speculate that DUSP1 is a tumour suppressor gene in type 1 g-NENs. In normal situations, although hypergastrinaemia leads to ECL cell proliferation, the cells will be prevented from dysplasia if normal DUSP1 expression is maintained. However, when DUSP1 is downregulated by high miR-202 expression, ECL cells will have more opportunities to develop type 1 g-NETs.
MiRNA-mRNA interactions occur in a context-dependent, cell-type-specific manner[51,52]. Since g-NEN is a rare disease, no ready-made cell lines can be found, and thus, we had to validate the interaction between miRNA-202-3p and DUSP1 in 293T cells (a well-established tool cell for dual-luciferase experiments). Our next step is to culture primary ECL cells to further validate our findings.
As type 1 g-NENs are tumours with a high recurrence rate and a short recurrence time, what concerns researchers the most is how to prevent this disease from recurrence. Because the recurrence mechanism is similar to the pathogenesis of type 1 g-NETs, we believe that treatment targeting miR-202-3p/DUSP1 may help reduce the recurrence rate. In our hospital, we often use Chinese herbal medicine for patients with type 1 g-NETs after ESD/EMR to prevent recurrence. Our previous clinical observation has found that Chinese herbal medicine can extend the median disease-free survival to 15 mo (Tan HY, unpublished data). However, the mechanism of this phenomenon is unclear. We also plan to explore whether Chinese herbal medicine can regulate the expression of miRNA-202-3p/DUSP1 using cell models.
Type 1g-NEN is a kind of rare malignant tumor. Because its recurrence rate is relatively high, the molecular mechanism of this disease urgently needs to be explored. MiRNAs play important roles in the occurrence and development of tumors. At present, studies on the role of miRNAs in type 1 g-NEN are quite few. This study may provide some potential therapeutic targets for the prevention of type 1 g-NEN recurrence.
The main topic of this study is the molecular mechanism of type 1 g-NENs. The key issue to be solved is which miRNAs and their target genes could affect the process of tumor recurrence. In future research, our study may help to explain the mechanism of some existing treatment and provide new therapeutic targets for type 1 g-NEN therapy.
The main objective of this study was to discover some type 1 g-NEN associated miRNAs and find their target genes. In this study, the differential miRNA expression between tumor lesions and tumor-free gastric mucosa was described, and the target gene of miRNA-202-3p was found. These may provide a basis for further revealing the molecular mechanism of type 1 g-NEN recurrence in the future.
Four main technologies were used in this study. First, we used Agilent human miRNA chips to find the differential miRNA expression between tumor lesions and tumor-free gastric mucosa. This kind of chip is expensive, but covers all known human miRNAs. Second, the results of chips were validated via RT-PCR, which is a proven and reliable experimental method to obtain a more accurate conclusion. Third, we used bioinformatics to look for target genes on web (TargetScan, PITA, and microRNAorg). This technique can quickly help us narrow down the scope of target genes. Last, a dual-luciferase reporter assay system was used for verification of the target gene. This system can show clearly the influence of miRNAs on its target gene. All the above research methods have been rarely applied to g-NENs before.
The high expression of miR-202-3p in type 1 g-NENs was found, which indicates that miR-202-3p acts as a tumor-promoting miRNA. The dual-luciferase reporter assay system showed the relationship between miR-202-3p and DUSP1, confirming that the high expression of miR-202-3p leads to the downregulation of DUSP1. Therefore, we speculate that DUSP1 is a tumor suppressor gene in type 1 g-NENs. Since g-NEN is a rare disease, there are no ready-made cell lines and we had to conduct the dual-luciferase experiment in 293T cells (a well-established tool cell for dual-luciferase experiment). Our next step is to carry out the primary culture of ECL cells to further validate our findings.
In this study, we summarized the existing mechanisms of type 1 g-NENs and confirmed the difference of miRNA expression between tumor and non-tumor gastric mucosa. Our study found that miRNA-202-3p was overexpressed in type 1 g-NENs and DUSP1 was its target gene and put forward an assumption: In normal situation, although hypergastrinaemia leads to ECL cell proliferation, the cells could be prevented from dysplasia if DUSP1 is expressed normally. However, when the DUSP1 is down-regulated by the highly expressed miR-202, ECL cells will have more opportunities to develop to type 1 g-NENs. This will help clinicians to further understand the molecular mechanism of the disease.
Our next step is to carry out the primary culture of ECL cells to further validate our findings. Our previous clinical study has found that Chinese herbal medicine can extend the median disease-free survival (DFS). So, using cell models to explore whether Chinese herbal medicine can regulate the expression of miRNA-202-3p/DUSP1 is the best method for the future research.
The authors thank Hanbio and Oebiotech for their excellent technical assistance and the patients for providing the tissues.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Aoyagi K, Link A, Yoshiyama H S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Modlin IM, Shapiro MD, Kidd M. Siegfried Oberndorfer: origins and perspectives of carcinoid tumors. Hum Pathol. 2004;35:1440-1451. [PubMed] |
| 2. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2489] [Article Influence: 311.1] [Reference Citation Analysis (4)] |
| 3. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3246] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 4. | Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 5. | Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (1)] |
| 6. | Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in Taiwan: a nation-wide cancer registry-based study. PLoS One. 2013;8:e62487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Zhang P, Zhang Y, Zhang C, Shi Y, Liu J, Liu Q, Yu L, Wang M, Zou G, Lou J. [Subtype classification and clinicopathological characteristics of gastric neuroendocrine neoplasms: an analysis of 241 cases]. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19:1241-1246. [PubMed] |
| 8. | Park SC, Chun HJ. Clinical aspects of gastric and duodenal neuroendocrine neoplasms. J Gastroenterol Hepatol Res. 2012;8:139-146. |
| 9. | Delle Fave G, O’Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 10. | Grozinsky-Glasberg S, Thomas D, Strosberg JR, Pape UF, Felder S, Tsolakis AV, Alexandraki KI, Fraenkel M, Saiegh L, Reissman P. Metastatic type 1 gastric carcinoid: a real threat or just a myth? World J Gastroenterol. 2013;19:8687-8695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 11. | Merola E, Sbrozzi-Vanni A, Panzuto F, D’Ambra G, Di Giulio E, Pilozzi E, Capurso G, Lahner E, Bordi C, Annibale B. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology. 2012;95:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5384] [Cited by in RCA: 5607] [Article Influence: 295.1] [Reference Citation Analysis (0)] |
| 13. | Rushworth SA. Targeting the oncogenic role of miRNA in human cancer using naturally occurring compounds. Br J Pharmacol. 2011;162:346-348. [PubMed] |
| 14. | Endo Y, Toyama T, Takahashi S, Yoshimoto N, Iwasa M, Asano T, Fujii Y, Yamashita H. miR-1290 and its potential targets are associated with characteristics of estrogen receptor α-positive breast cancer. Endocr Relat Cancer. 2013;20:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Wang F, Zhou J, Zhang Y, Wang Y, Cheng L, Bai Y, Ma H. The Value of MicroRNA-155 as a Prognostic Factor for Survival in Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS One. 2015;10:e0136889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Calatayud D, Dehlendorff C, Boisen MK, Hasselby JP, Schultz NA, Werner J, Immervoll H, Molven A, Hansen CP, Johansen JS. Tissue MicroRNA profiles as diagnostic and prognostic biomarkers in patients with resectable pancreatic ductal adenocarcinoma and periampullary cancers. Biomark Res. 2017;5:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677-4684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 592] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 18. | Li SC, Essaghir A, Martijn C, Lloyd RV, Demoulin JB, Oberg K, Giandomenico V. Global microRNA profiling of well-differentiated small intestinal neuroendocrine tumors. Mod Pathol. 2013;26:685-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Miller HC, Frampton AE, Malczewska A, Ottaviani S, Stronach EA, Flora R, Kaemmerer D, Schwach G, Pfragner R, Faiz O. MicroRNAs associated with small bowel neuroendocrine tumours and their metastases. Endocr Relat Cancer. 2016;23:711-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Lloyd KA, Moore AR, Parsons BN, O’Hara A, Boyce M, Dockray GJ, Varro A, Pritchard DM. Gastrin-induced miR-222 promotes gastric tumor development by suppressing p27kip1. Oncotarget. 2016;7:45462-45478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Frampton AE, Castellano L, Colombo T, Giovannetti E, Krell J, Jacob J, Pellegrino L, Roca-Alonso L, Funel N, Gall TM. MicroRNAs cooperatively inhibit a network of tumor suppressor genes to promote pancreatic tumor growth and progression. Gastroenterology. 2014;146:268-277.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 22. | Calvisi DF, Pinna F, Meloni F, Ladu S, Pellegrino R, Sini M, Daino L, Simile MM, De Miglio MR, Virdis P. Dual-specificity phosphatase 1 ubiquitination in extracellular signal-regulated kinase-mediated control of growth in human hepatocellular carcinoma. Cancer Res. 2008;68:4192-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Hou MF, Chang CW, Chen FM, Wang SN, Yang SF, Chen PH, Su JH, Yeh YT. Decreased total MKP-1 protein levels predict poor prognosis in breast cancer. World J Surg. 2012;36:1922-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Gao BR, Yao YY, Chen YH, Li XP, Wang JL, Wei LH. [Relationship between the expression of dual specificity phosphatase-1 and the prognosis of endometrioid adenocarcinoma]. Zhonghua Yi Xue Za Zhi. 2013;93:2493-2495. [PubMed] |
| 25. | Zhang X, Hyer JM, Yu H, D’Silva NJ, Kirkwood KL. DUSP1 phosphatase regulates the proinflammatory milieu in head and neck squamous cell carcinoma. Cancer Res. 2014;74:7191-7197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Gil-Araujo B, Toledo Lobo MV, Gutiérrez-Salmerón M, Gutiérrez-Pitalúa J, Ropero S, Angulo JC, Chiloeches A, Lasa M. Dual specificity phosphatase 1 expression inversely correlates with NF-κB activity and expression in prostate cancer and promotes apoptosis through a p38 MAPK dependent mechanism. Mol Oncol. 2014;8:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | He J, Li Y, Huang G, Cui M, Zhu J, Gu Y. Analysis on biological information of has-miR-202. Beijing Biomed Eng. 2013;32:78-82. |
| 28. | Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 1100] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 29. | Boyerinas B, Park SM, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer. 2010;17:F19-F36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 519] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 30. | Manikandan J, Aarthi JJ, Kumar SD, Pushparaj PN. Oncomirs: the potential role of non-coding microRNAs in understanding cancer. Bioinformation. 2008;2:330-334. [PubMed] |
| 31. | Zhang D, Li Y, Tian J, Zhang H, Wang S. MiR-202 promotes endometriosis by regulating SOX6 expression. Int J Clin Exp Med. 2015;8:17757-17764. [PubMed] |
| 32. | Yu J, Qiu X, Shen X, Shi W, Wu X, Gu G, Zhu B, Ju S. miR-202 expression concentration and its clinical significance in the serum of multiple myeloma patients. Ann Clin Biochem. 2014;51:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130-6135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 702] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 34. | Joosse SA, Müller V, Steinbach B, Pantel K, Schwarzenbach H. Circulating cell-free cancer-testis MAGE-A RNA, BORIS RNA, let-7b and miR-202 in the blood of patients with breast cancer and benign breast diseases. Br J Cancer. 2014;111:909-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 676] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 36. | Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol. 2002;3:REVIEWS3009. [PubMed] |
| 37. | Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203-3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 640] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 38. | Shen J, Zhang Y, Yu H, Shen B, Liang Y, Jin R, Liu X, Shi L, Cai X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016;5:2061-2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 39. | Moncho-Amor V, Ibañez de Cáceres I, Bandres E, Martínez-Poveda B, Orgaz JL, Sánchez-Pérez I, Zazo S, Rovira A, Albanell J, Jiménez B. DUSP1/MKP1 promotes angiogenesis, invasion and metastasis in non-small-cell lung cancer. Oncogene. 2011;30:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Leelawat K, Udomchaiprasertkul W, Narong S, Leelawat S. Induction of MKP-1 prevents the cytotoxic effects of PI3K inhibition in hilar cholangiocarcinoma cells. J Cancer Res Clin Oncol. 2010;136:1537-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Montagut C, Iglesias M, Arumi M, Bellosillo B, Gallen M, Martinez-Fernandez A, Martinez-Aviles L, Cañadas I, Dalmases A, Moragon E. Mitogen-activated protein kinase phosphatase-1 (MKP-1) impairs the response to anti-epidermal growth factor receptor (EGFR) antibody cetuximab in metastatic colorectal cancer patients. Br J Cancer. 2010;102:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Robinson M. Review article: current perspectives on hypergastrinaemia and enterochromaffin-like-cell hyperplasia. Aliment Pharmacol Ther. 1999;13 Suppl 5:5-10. [PubMed] |
| 43. | D'Elios MM, Bergman MP, Azzurri A, Amedei A, Benagiano M, De Pont JJ, Cianchi F, Vandenbroucke-Grauls CM, Romagnani S, Appelmelk BJ. H(+),K(+)-atpase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001;120:377-386. [PubMed] |
| 44. | Macukanović-Golubović L, Katić V, Rancić G, Milenović M, Marjanović G, Golubović Z. [Study on histogenesis of enterochromaffin-like carcinoid in autoimmune atrophic gastritis associated with pernicious anemia]. Vojnosanit Pregl. 2007;64:543-548. [PubMed] |
| 45. | Annibale B, Azzoni C, Corleto VD, di Giulio E, Caruana P, D’Ambra G, Bordi C, Delle Fave G. Atrophic body gastritis patients with enterochromaffin-like cell dysplasia are at increased risk for the development of type I gastric carcinoid. Eur J Gastroenterol Hepatol. 2001;13:1449-1456. [PubMed] |
| 46. | Bordi C, D’Adda T, Azzoni C, Ferraro G. Pathogenesis of ECL cell tumors in humans. Yale J Biol Med. 1998;71:273-284. [PubMed] |
| 47. | Higham AD, Bishop LA, Dimaline R, Blackmore CG, Dobbins AC, Varro A, Thompson DG, Dockray GJ. Mutations of RegIalpha are associated with enterochromaffin-like cell tumor development in patients with hypergastrinemia. Gastroenterology. 1999;116:1310-1318. [PubMed] |
| 48. | Pritchard DM, Berry D, Przemeck SM, Campbell F, Edwards SW, Varro A. Gastrin increases mcl-1 expression in type I gastric carcinoid tumors and a gastric epithelial cell line that expresses the CCK-2 receptor. Am J Physiol Gastrointest Liver Physiol. 2008;295:G798-G805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | D'Adda T, Keller G, Bordi C, Höfler H. Loss of heterozygosity in 11q13-14 regions in gastric neuroendocrine tumors not associated with multiple endocrine neoplasia type 1 syndrome. Lab Invest. 1999;79:671-677. [PubMed] |
| 50. | Burkitt MD, Pritchard DM. Review article: Pathogenesis and management of gastric carcinoid tumours. Aliment Pharmacol Ther. 2006;24:1305-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Erhard F, Haas J, Lieber D, Malterer G, Jaskiewicz L, Zavolan M, Dölken L, Zimmer R. Widespread context dependency of microRNA-mediated regulation. Genome Res. 2014;24:906-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 52. | Kedde M, Strasser MJ, Boldajipour B, Oude Vrielink JA, Slanchev K, le Sage C, Nagel R, Voorhoeve PM, van Duijse J, Ørom UA. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell. 2007;131:1273-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 558] [Article Influence: 32.8] [Reference Citation Analysis (0)] |