Published online Dec 28, 2018. doi: 10.3748/wjg.v24.i48.5462
Peer-review started: August 20, 2018
First decision: October 8, 2018
Revised: December 5, 2018
Accepted: December 19, 2018
Article in press: December 19, 2018
Published online: December 28, 2018
Processing time: 129 Days and 17.7 Hours
To study the counteraction of perforated cecum lesion using BPC 157 and nitric oxide (NO) system agents.
Alongside with the agents’ application (after 1 min, medication (/kg, 10 mL/2 min bath/rat) includes: BPC 157 (10 μg), L-NAME (5 mg), L-arginine (100mg) alone or combined, and saline baths (controls)) on the rat perforate cecum injury, we continuously assessed the gross reappearance of the vessels (USB microcamera) quickly propagating toward the defect at the cecum surface, defect contraction, bleeding attenuation, MDA- and NO-levels in cecum tissue at 15 min, and severity of cecum lesions and adhesions at 1 and 7 d.
Post-injury, during/after a saline bath, the number of vessels was significantly reduced, the defect was slightly narrowed, bleeding was significant and MDA-levels increased and NO-levels decreased. BPC 157 bath: the vessel presentation was markedly increased, the defect was noticeably narrowed, the bleeding time was shortened and MDA- and NO-levels remained normal. L-NAME: reduced vessel presentation but not more than the control, did not change defect and shortened bleeding. L-arginine: exhibited less vessel reduction, did not change the defect and prolonged bleeding. In combination, mutual counteraction occurred (L-NAME + L-arginine) or the presentation was similar to that of BPC 157 rats (BPC 157 + L-NAME; BPC 157 + L-arginine; BPC 157 + L-NAME + L-arginine), except the defect did not change. Thereby at day 1 and 7, saline, L-NAME, L-arginine and L-NAME + L-arginine failed (defect was still open and large adhesions present).
The therapeutic effect was achieved with BPC 157 alone or in combination with L-NAME and L-arginine as it was able to consolidate the stimulating and inhibiting effects of the NO-system towards more effective healing recruiting vessels.
Core tip: In rats, the cecum was exposed, and a perforation (5-mm diameter) was made at the ventral face of the basal region of the cecum close to the largest curvature. After 1 min, a bath (1 mL/rat) containing BPC 157, L-NAME, L-arginine, saline (controls) was directly applied to the perforated cecum, alone or in combination. Previously, BPC 157 rapidly activated the collateral circulation from existing vessels in ischemic colitis or inferior caval vein occlusion (by passing through the arcade vessels or the left ovarian vein and other veins) to reestablish blood flow and exert its free radical scavenger effect in both ischemia and reperfusion.
- Citation: Drmic D, Samara M, Vidovic T, Malekinusic D, Antunovic M, Vrdoljak B, Ruzman J, Milkovic Perisa M, Horvat Pavlov K, Jeyakumar J, Seiwerth S, Sikiric P. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol 2018; 24(48): 5462-5476
- URL: https://www.wjgnet.com/1007-9327/full/v24/i48/5462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i48.5462
In cecum perforation studies[1-3] the immediate post-perforation threat is rarely studied, particularly regarding: the rapid disappearance of blood vessels in the cecum serosa that are instantly emptied and thereby “disappear”, perforation defect enlargement, bleeding and fluid leakage, increased oxidative stress and disturbed nitric oxide (NO) levels in cecum tissue. Likewise, the possible effect of cytoprotective agents, known to act as a class on endothelium maintenance in the gastrointestinal tract (and, thereby, mucosal maintenance)[4-9], especially the stable gastric pentadecapeptide BPC 157 as a cytoprotective agent rapidly acting on endothelium integrity maintenance[10-19], has been not investigated.
We focused on a perforated cecum, the stable gastric pentadecapeptide BPC 157, the NOS-blocker L-NAME, and the NOS-substrate L-arginine, as well as on the initial post-perforation period, rapid disappearance of blood vessels at the cecum serosa (emptied/disappeared), a large immediate defect, bleeding, leakage of fluid, increased oxidative stress and disturbed NO levels in cecum tissue. The rationale was the beneficial effects in rats with ischemic colitis and inferior caval vein occlusion syndrome[20,21] and the particular effect of BPC 157 on blood vessels[20,21]. Namely, we recently demonstrated that BPC 157 rapidly activated the collateral circulation from existing vessels in rats with ischemic colitis or inferior caval vein occlusion[20,21]. In ischemic colitis (i.e., segment of the left colic artery and vein excluded by two ligations) we proved effectiveness by passing through arcade vessels[20]. Antagonizing the syndrome of inferior caval vein infrarenal occlusion, we evidenced by passing through the left ovarian vein and other veins[21]. These accentuated the specific action of BPC 157 to reestablish blood flow, and exert its free radical scavenger effect in both ischemia and reperfusion[20,21].
In addition, BPC 157, as a novel mediator of Robert’s cytoprotection[4,10-19], also affects several other molecular pathways[21-27]. It is native and stable in human gastric juice and maintains gastrointestinal mucosal integrity[10-19], and it represents a prototype of a more effective class of cytoprotective agents with both prophylactic and therapeutic abilities[5,17] (unlike standard cytoprotective agents that exhibit only prophylactic effectiveness (shared limitation of activity))[4-9]. BPC 157 (used in ulcerative colitis and now in multiple sclerosis trials) also counteracts colitis (in various models of colitis)[20,28-33] and its complications, such as fistulas[34-38], failed healing of anastomoses[28,29,39,40] and other gastrointestinal lesions[10-19], given parenterally or orally. Furthermore, BPC 157 is known to counteract thrombosis, not only after abdominal aorta anastomosis[41] or inferior caval vein ligation[21], but also after bleeding prolongation upon amputation and the administration of anticoagulants and aspirin[42,43] or inferior caval vein ligation[21]. This effect is related to its endothelial maintenance[21,41-43] and its interaction with the NO system, which provides the counteraction of both the opposite effects of L-NAME (prothrombotic) and L-arginine (antithrombotic) application[43]. Otherwise, bleeding from the perforated lesion and perforated lesion outcome might be complicated with these innate L-NAME and NOS substrate L-arginine effects[43].
In practice, as described previously[20,21], our approach to cure a perforated cecum begins with the original understanding of stomach cytoprotection (a very rapid protection of the stomach endothelium and epithelium against diverse direct injuries; endothelium maintenance → epithelium maintenance), regularly shown with cytoprotective agents[4-9]. This was further perceived as an extended cytoprotection background[20,21] (endothelium maintenance → epithelium maintenance = blood vessel recruitment and activation towards the site of injury), also described as “bypassing” occlusion via alternative ways, as demonstrated with BPC 157[20,21], which can likely cure rats with a perforated cecum and thereafter. Furthermore, antagonization of two distinct detrimental obstructive events (i.e., left colic artery and vein ligation-colitis and inferior caval vein-infrarenal ligation-full syndrome)[20,21] appears. Likewise, positive outcome matching also appears, providing a special effect: blood vessel recruitment to organize bypassing of vessel occlusions and reestablishment of blood flow by BPC 157 therapy[20,21]. Thus, we can make the argument that, under analogous or more severe conditions (i.e., perforated cecum), a similar positive outcome will appear. If this occurs, we can attenuate cecum perforation syndrome; in BPC 157-treated rats, therapy may lead to attenuation and reversal of the consequent tissue damage. Specifically, a reversal of vessel disappearance by vessels “running” toward the defect (vessels filled/reappeared), transition of defect enlargement to defect narrowing (note, fistula defects healing)[34-38], change from prolonged bleeding to bleeding attenuation[21,42,43], induced reduction of increased MDA values and normalization of NO values in cecum tissue[20,21]. In subsequent days and weeks, this may lead to a closed cecum defect and attenuation of adhesion formation.
For further clarification and to demonstrate a direct beneficial effect, medication (BPC 157, L-NAME, and L-arginine, alone and/or together) was applied directly to the injury[20,21] once during surgery, as a bath to the perforated cecum, the solution then spread throughout the abdominal cavity. Consequently, the beneficial effects are directly related to the reversal of perforated injury outcome consequences, in both the early and later periods. These effects may be triggered shortly after injury initiation, or reverse an already advanced injury course.
Male Albino Wistar rats, 200 g b.w., were randomly assigned (7 rats per group) and used for the experiments, which were approved by the local ethics committee. The perforation procedure was performed in rats that had food and water ad libitum before the procedure and until the end of the experiment.
Pentadecapeptide Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val, M.W. 1419, named BPC 157, a part of the sequence of human gastric juice protein encoded by BPC, freely soluble in water at pH 7.0 and in saline, was prepared (Diagen, Ljubljana, Slovenia) as described previously[10-21]. The peptide with 99% high-pressure liquid chromatography (HPLC) purity, 1-des-Gly peptide as a biologically inactive impurity, was used[10-21]. L-NAME and L-arginine were commercially purchased (Sigma, United States).
In deeply anaesthetized rats (thiopental (Rotexmedica, Germany) 40 mg/kg ip, apaurin (Krka, Slovenia) 10 mg/kg ip), the cecum was exposed, and a perforation (5-mm diameter) was made at the ventral aspect of the basal region of the cecum close to the largest curvature; the rats were monitored for the next 15 min.
After 1 min, a bath (1 mL/rat) containing BPC 157 (0.01 mg/kg), NOS blocker L-NAME (5 mg/kg), or NOS substrate L-arginine (100 mg/kg) was directly applied to the perforated cecum, alone or combined, and spread through the abdominal cavity, whereas controls accordingly received a saline bath of equal volume. Using a USB microscope camera (Veho discovery VMS-004D-400x USB microscope; Veho®, United Kingdom), from the point immediately before therapy, we recorded and assessed the blood vessels (emptied/disappeared; refilled/reappeared) (total % of cecum vessel augmentation/reduction from proximal to distal end = [number of blood vessels (10 vessels assessed) /100] × %] in terms of augmentation/reduction of each vasa recta (0 as the point immediately before therapy) and defect closing or widening (as % of presentation immediately before therapy) and at particular time points: A-after perforation (1 min), B-during application (2 min), C-period after application (2 min), D-next 5 min period and E-period until the end of observation (15 min). The bleeding time (s) throughout that period was also assessed; at 15 min, oxidative stress was measured by quantifying thiobarbituric acid (TBA) reactivity as malondialdehyde (MDA) equivalents, and nitric oxide determination in cecum tissue was also carried out.
At day 1 and day 7, rats were relaparotimiezed, and we assessed the serosal/mucosal defect (longest lesion diameter, mm) and adhesion severity scoring (adhesions of the abdominal wall-1 point; adhesions of the intestine-1 point; adhesions of the cecal area: ventral side-1 point/dorsal side-1 point; adhesions involving both the intestine and colon-3 points) (resulting in a total maximum score of 7). Representative tissue sections were processed for further histological analysis as described previously[10-21].
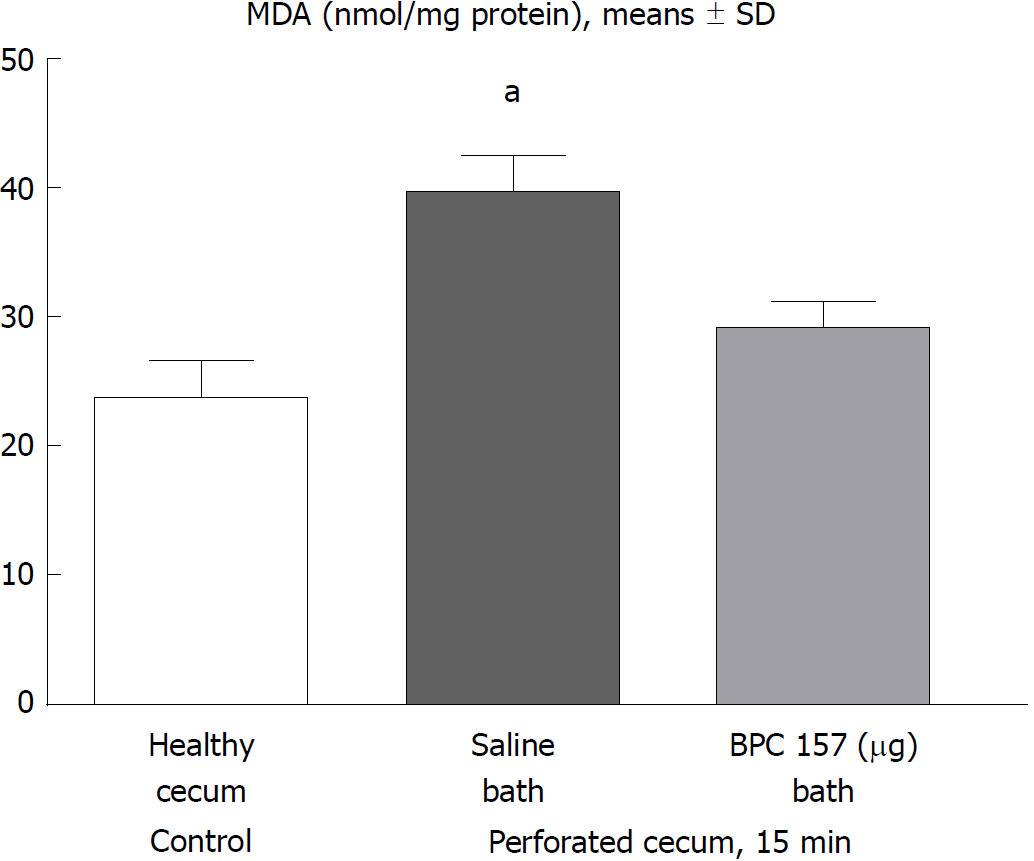
At 15 min post-injury, oxidative stress in the tissue samples (1 cm2 around the defect) was assessed by quantifying thiobarbituric acid (TBA) reactivity as malondialdehyde (MDA) equivalents. Trichloroacetic acid (TCA) was added to homogenize the tissue samples, which were then centrifuged (3000 rpm, 5 min), and the supernatant was collected. Thereafter, 1% TBA was added, and the samples were boiled (95 °C, 60 min). The tubes were kept on ice for 10 min, and the absorbance was determined at the wavelengths of 532 and 570 nm. The concentration of MDA was read from a standard calibration curve plotted using 1,1,3,3’-tetra-ethoxy propane (TEP). The extent of lipid peroxidation was expressed as MDA using a molar extinction coefficient for MDA of 1.56 × 105 mol/(L•cm). The results are expressed in nmol/mg of protein[20,21].
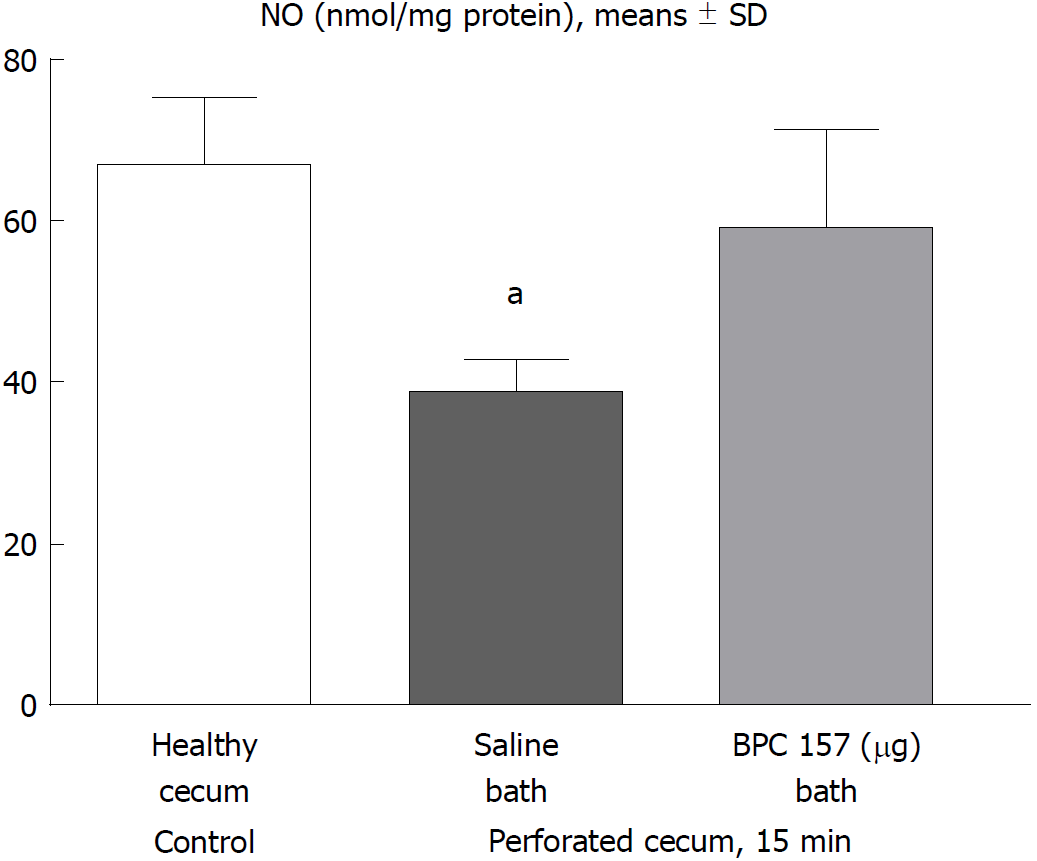
At 15 min post-injury, we determined the nitric oxide (NO) levels in cecum tissue samples using the Griess reaction (Griess Reagent System, Promega, United States). Sulfanilamide was then incubated with the homogenized tissue, and then, N-1-naphthylethylenediamine dihydrochloride was added. The Griess reaction is based on a diazotization reaction in which acidified nitrite reacts with diazonium ions and, in a further step, are coupled to N-1-naphthylethylenediamine dihydrochloride, forming a chromophoric azo derivate. The absorbance was measured at 540 nm using sodium nitrite solution as the standard. NO levels are reported in μmol/mg protein. The protein concentrations were determined using a commercial kit (BioRad Protein DR Assay Reagent Kit, United States)[20,21].
Statistical analysis was performed by parametric one-way ANOVA with the post-hoc Newman-Keuls test and non-parametric Kruskal-Wallis and subsequent Mann-Whitney U-tests to compare the groups. The values are represented as means ± SD and minimum/median/maximum. The results with P < 0.05 were considered significant.
After direct administration of the agents to the site of injury one minute after cecum perforation, the results obtained can clearly describe the ongoing events. Of note, after the administration of the drug directly to the lesion, the solution spread through the abdominal cavity; however, its effect can still be directly correlated with the injury course (aggravated, regular, reversed), particularly in the earliest period, and then, the final outcome is seen at one week after surgery and medication application.
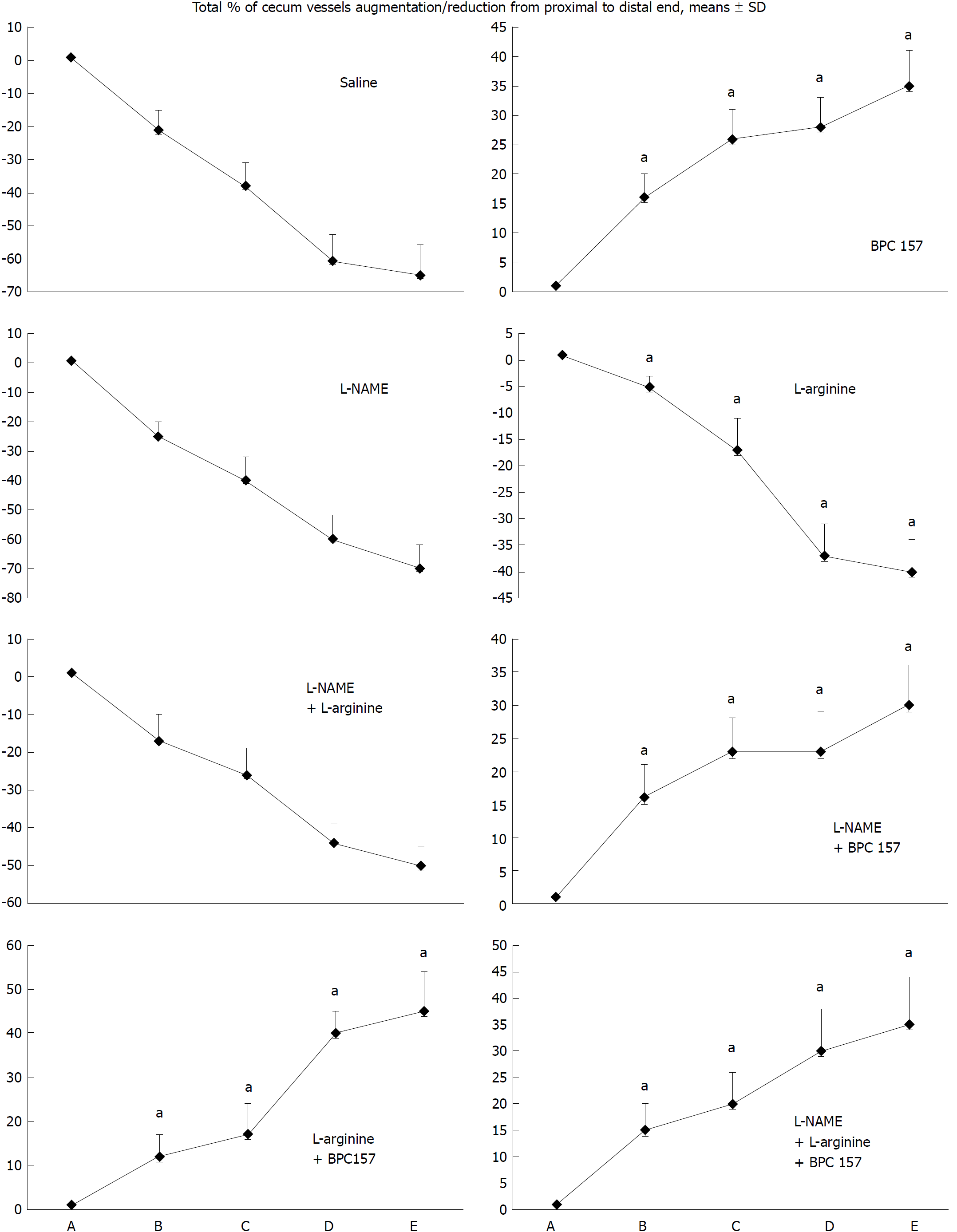
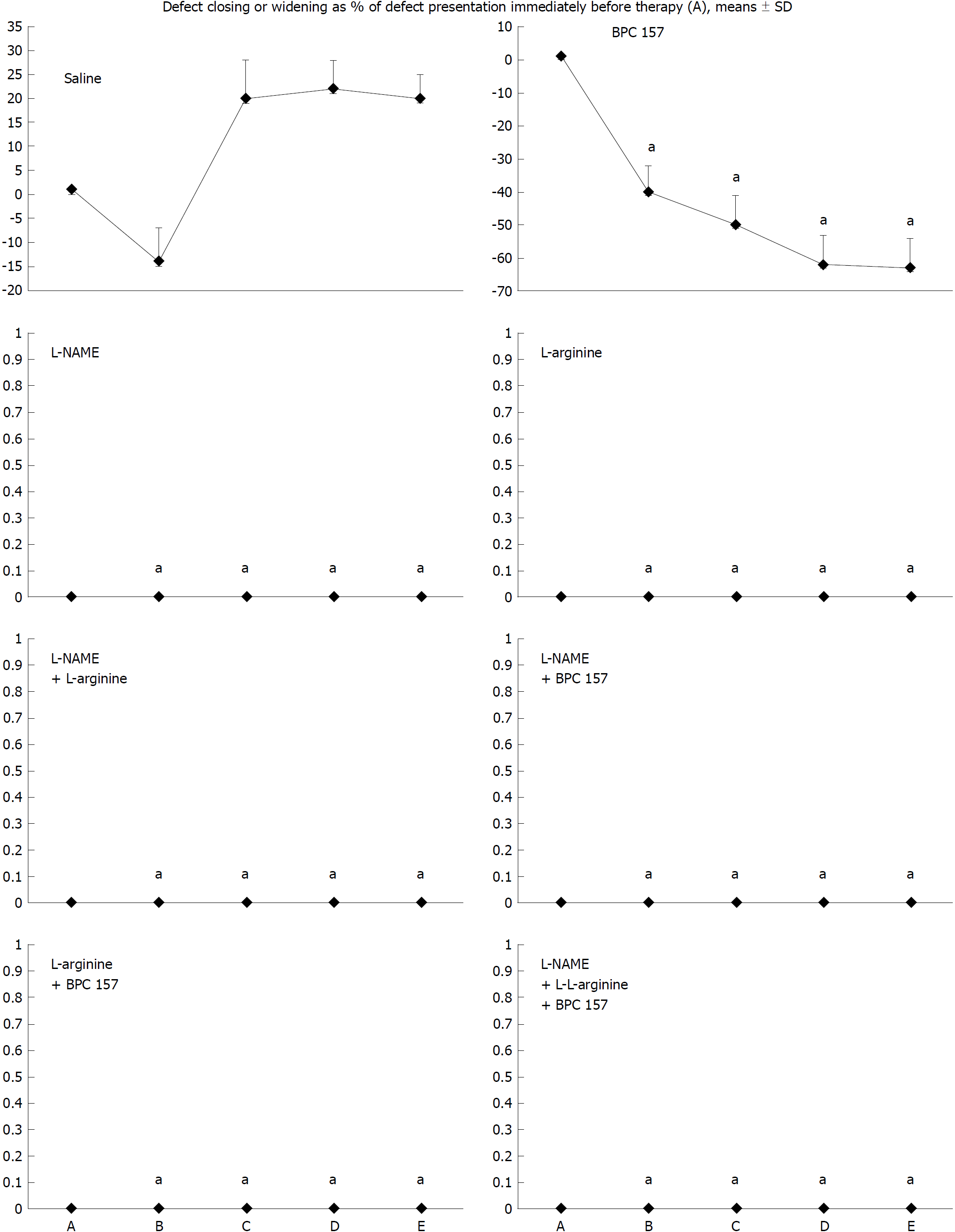
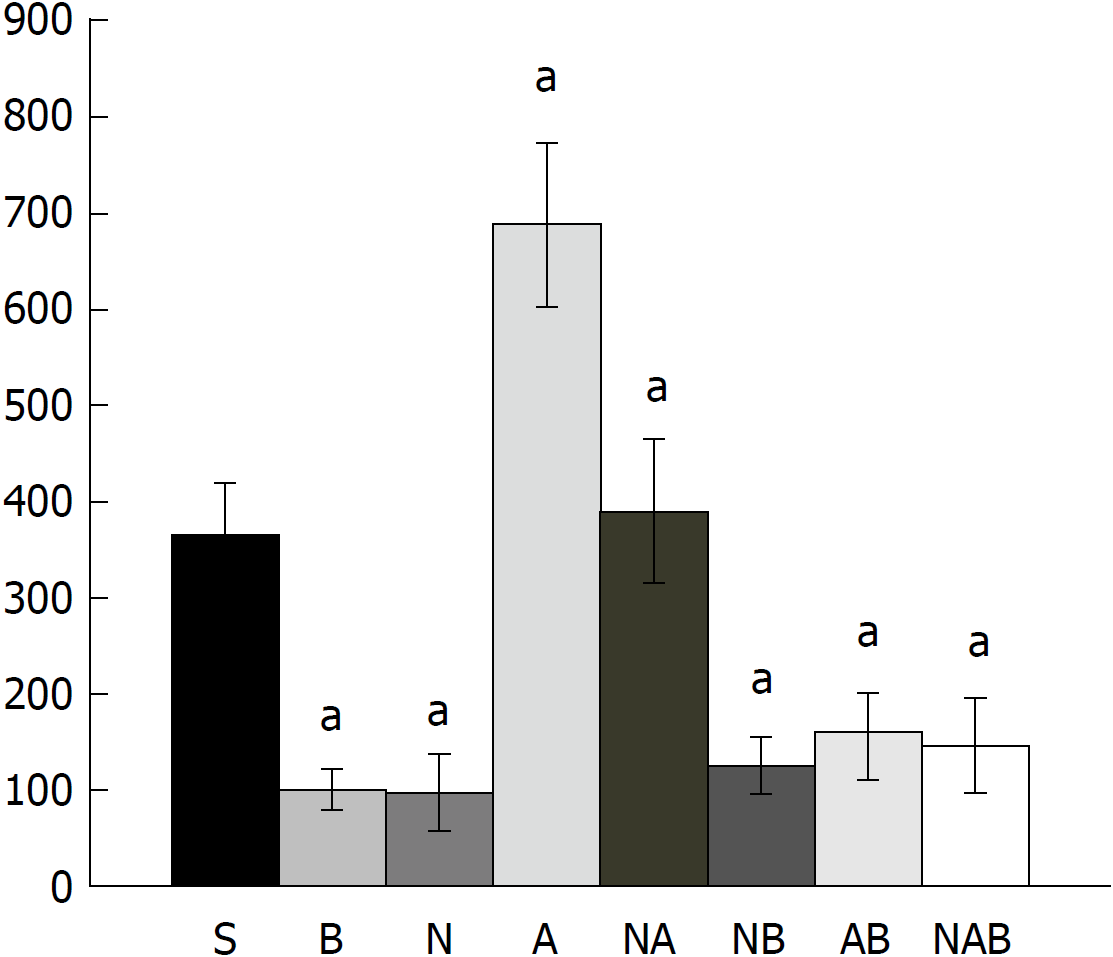
The numerous negative changes [i.e., initial course: vessel presentation (Figure 1), defect closing or widening (Figure 2), bleeding time (Figure 3), NO-cecum level (Figure 4), MDA-cecum level (Figure 5); and final course of the injury: cecum defect and adhesions at 1 or 7 days (Table 1)] are significant in several respects.
| Medication (/kg, 10 ml/2 min bath/rat) | Assessed cecum lesion (sum of longest diameters, mm, means ± SD) and adhesion severity (score 0-9, Min/Med/Max) | |||
| Day 1 | Day 7 | |||
| Perforated cecum lesion | Adhesion severity | Perforated cecum lesion | Adhesion severity | |
| 0.9%NaCl (control) | 5.0 ± 0.5 (M) | 3/3/3 | 5.0 ± 0.5 (M) | 3/4/4 |
| 5.0 ± 0.5 (S) | 6.0 ± 0.5 (S) | |||
| BPC 157 (10 µg) | 3.0 ± 0.0 (M)a | 1/1/1a | 0.0 ± 0.0 (M)a | 1/1/2a |
| 3.0 ± 0.0 (S)a | 0.0 ± 0.0 (S)a | |||
| L-NAME 5 mg | 7.5 ± 0.4 (M)a | 5/6/6a | 8.0 ± 0.4 (M)a | 6/6/6a |
| 7.5 ± 0.5 (S)a | 8.8 ± 0.5 (S)a | |||
| L-arginine 100 mg | 7.0 ± 0.0 (M)a | 4/5/5a | 7.5 ± 0.3 (M)a | 5/6/6a |
| 7.0 ± 0.0 (S) | 8.0 ± 0.4 (S)a | |||
| L-NAME 5 mg + | 7.3 ± 0.5 (M) | 5/6/6a | 8.0 ± 0.3 (M) | 5/6/6a |
| L-arginine 100 mg | 7.3 ± 0.5 (S) | 8.2 ± 0.5 (S) | ||
| L-NAME 5 mg + BPC 157 10 μg | 3.0 ± 0.0 (M)a | 1/1/1a | 0.0 ± 0.0 (M)a | 1/1/2a |
| 3.0 ± 0.0 (S)a | 0.0 ± 0.0 (S)a | |||
| L-arginine 100 mg + BPC 157 10 μg | 3.0 ± 0.0 (M)a | 1/1/1a | 0.0 ± 0.0 (M)a | 1/1/2a |
| 3.0 ± 0.0 (S)a | 0.0 ± 0.0 (S)a | |||
| L-NAME 5 mg + L-arginine 100 mg + BPC 157 10 μg | 3.0 ± 0.0 (M)a | 0/1/1a | 0.0 ± 0.0 (M)a | 1/1/2a |
| 3.0 ± 0.0 (S)a | 0.0 ± 0.0 (S)a | |||
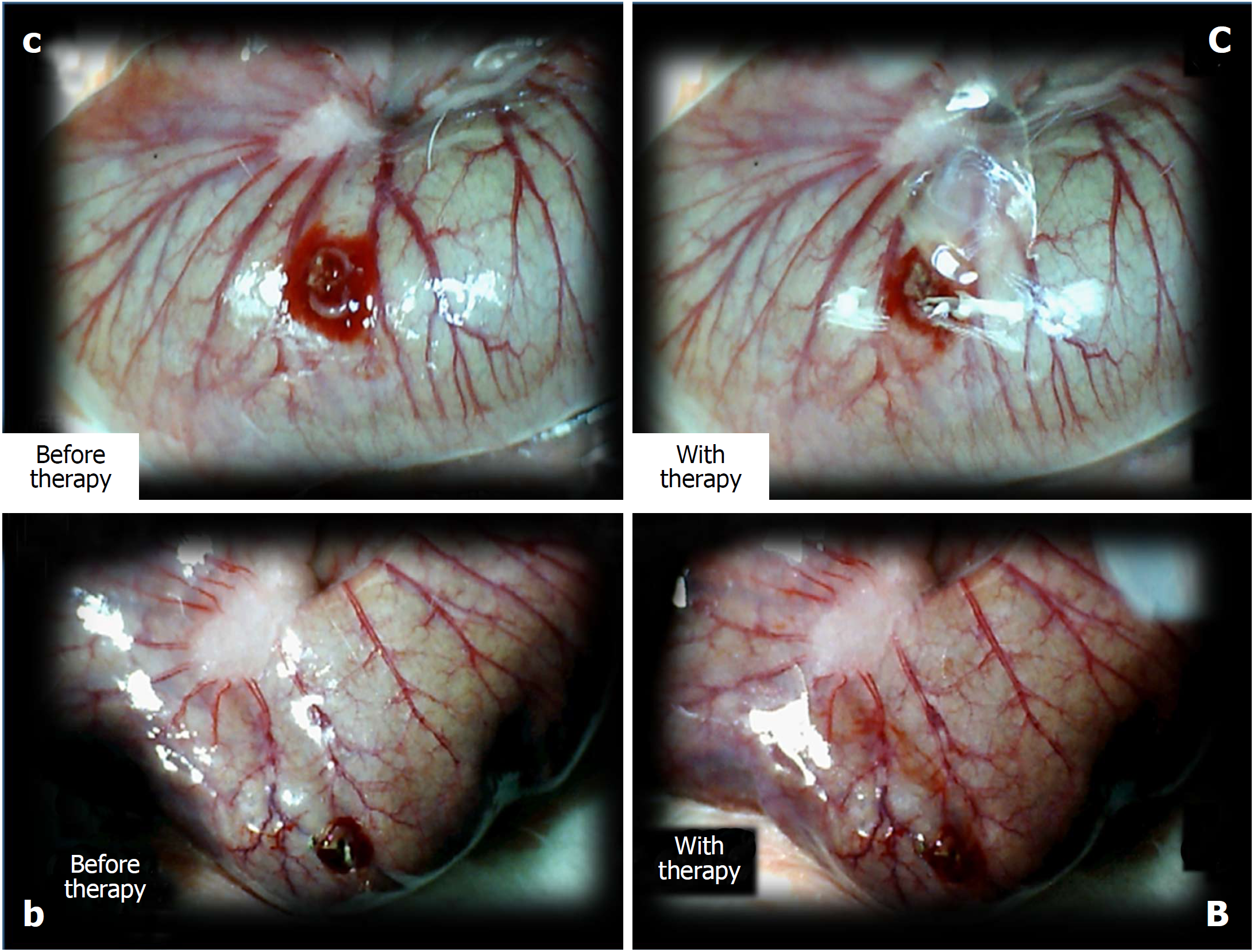
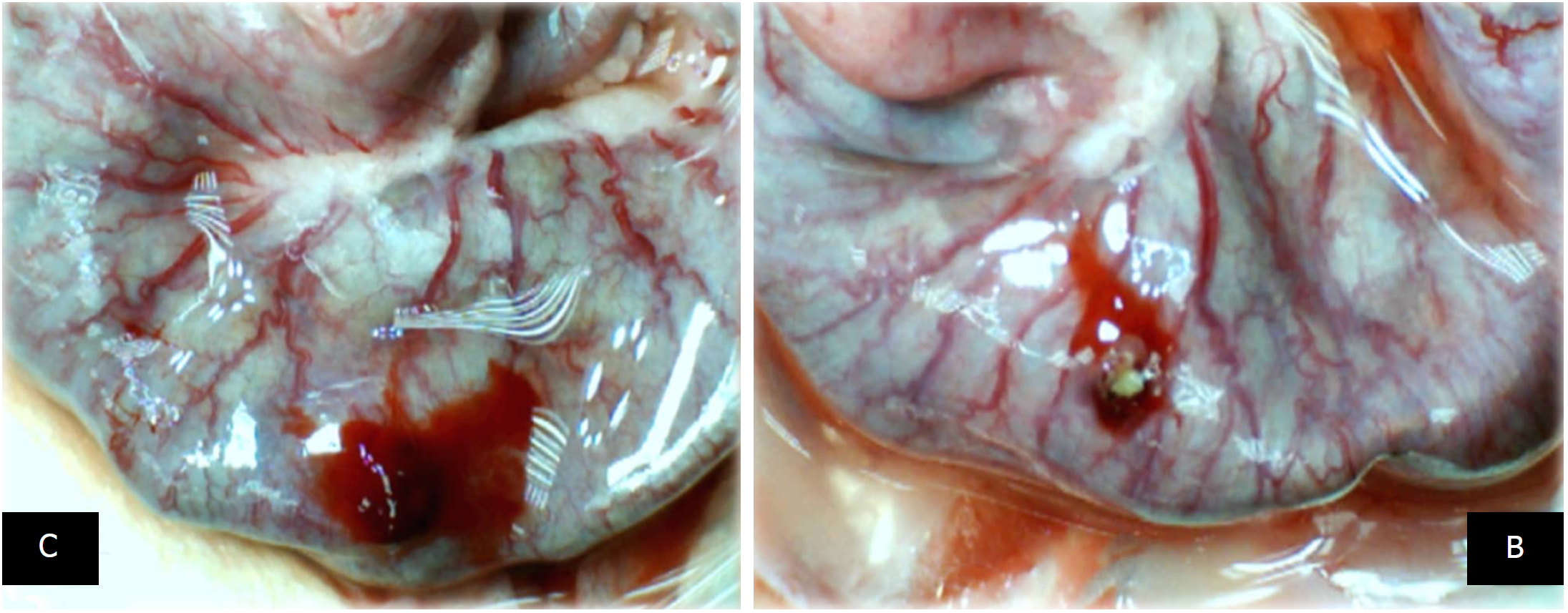
Depending on the agent applied, the initial defensive response confers blood vessel presentation (emptied/disappeared; refilled/reappeared) and augmentation/reduction (Figures 1, 6 and 7), defect closing or widening (Figure 2) and bleeding prolongation or shortening (Figures 3 and 7). MDA oxidative stress (Figure 5) and NO levels (Figure 4) were determined in the cecum tissue as well. They permit combining of initial response to the final response (cecum defect and adhesions at 1 or 7 days (Table 1)].
Controls: In rats that received a saline bath, the initial course was progressive vessel disappearance and very poor vessel branching, which occurred during application (Figures 1 and 6). Thereafter, the defect only had a small initial contraction (Figure 2) and then continuous widening. Additionally, there was a significant bleeding period (Figures 3 and 7).
BPC 157 therapy: Rats with a perforated cecum that underwent a BPC 157 bath exhibited progressive vessel presentation toward the injury (i.e., a network of small vessels branching around the injury) (Figures 1 and 6). The defect contracted during application (Figure 2) and the bleeding period was significantly shortened thereafter (Figure 3).
L-arginine therapy: After application of L-arginine, rats with a perforated cecum exhibited less vessel disappearance (Figure 1). We noted counteracted defect widening (Figure 2), and the bleeding period was significantly prolonged (Figure 3).
L-NAME therapy: After application of L-NAME, rats with a perforated cecum exhibited vessel disappearance similar to controls (Figure 1). There was counteraction of defect widening (Figure 2), and the bleeding period was shortened (Figure 3).
Combination therapy of L-arginine and L-NAME: After application of L-arginine and L-NAME, rats with a perforated cecum exhibited a combined effect, with less vessel disappearance (Figure 1), counteracted defect widening (Figure 2) and a bleeding period in the range of that for the control animals (Figure 3).
Combined therapy of L-arginine and/or L-NAME with BPC 157: After application of BPC 157 with L-arginine and/or L-NAME, rats with a perforated cecum that underwent combined BPC 157 therapy (L-NAME + BPC 157; L-arginine + BPC 157; L-NAME + L-arginine + BPC 157) regularly exhibited progressive vessel presentation (Figure 1) and a shortened bleeding time (Figure 3), similar to the rats that received BPC 157 alone. All rats exhibited counteraction of defect widening (Figure 2).
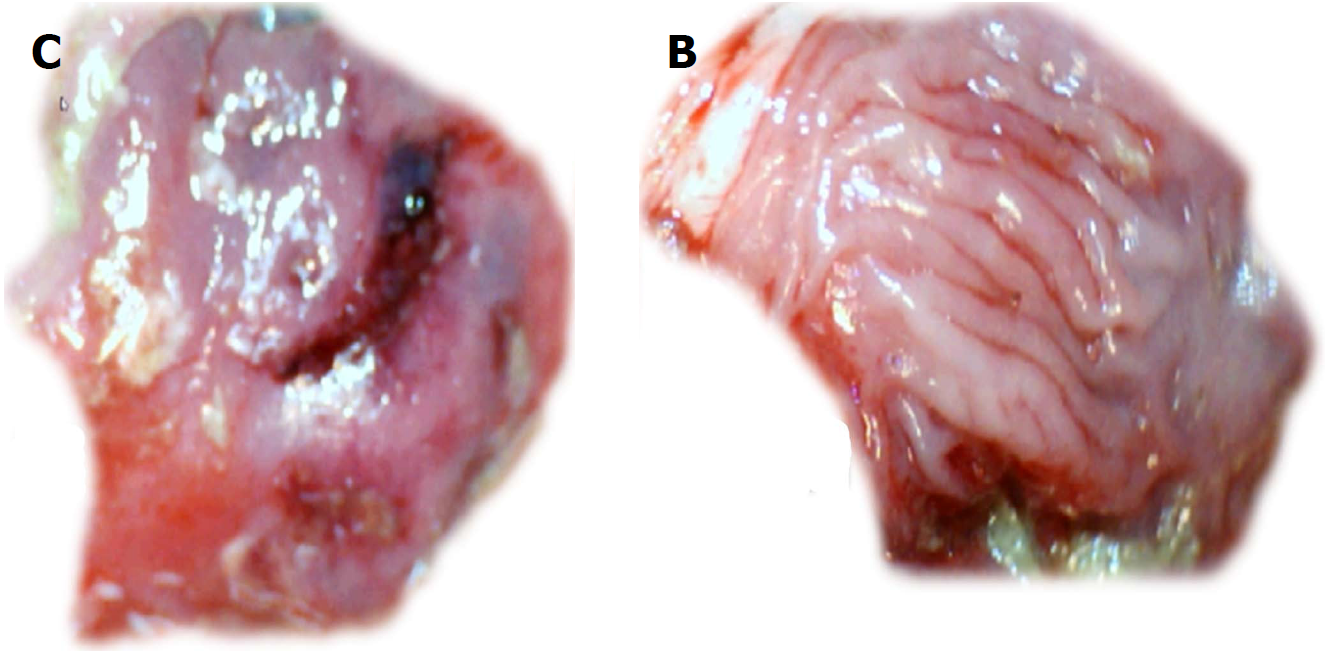
Controls: Regularly, after one day or one week, rats that underwent cecum perforation presented with a significant defect (Table 1, Figure 8) that remained open and showed significant adhesions (Table 1).
BPC 157 therapy: Rats with a perforated cecum that underwent a BPC 157 bath exhibited an attenuated defect and finally, no gross defect (Table 1, Figure 8), and markedly fewer adhesions were present.
L-arginine therapy: Rats with a perforated cecum that underwent an L-arginine bath initially exhibited an open defect larger than in the controls, and markedly more adhesions were present (Table 1).
L-NAME therapy: Rats with a perforated cecum that underwent an L-NAME bath exhibited an open defect larger than that in the controls, and markedly more adhesions were present (Table 1).
Combined therapy of L-arginine and L-NAME: Rats with a perforated cecum that underwent this combined therapy exhibited an open defect that was found to be larger than that in the controls, and markedly more adhesions were present (Table 1).
Combined therapy of L-arginine and/or L-NAME with BPC 157: BPC 157 with L-arginine and/or L-NAME (L-NAME+BPC 157; L-arginine+BPC 157; L-NAME+L-arginine+BPC 157) led to defect attenuation and eventually no defect grossly present with fewer adhesions, similar to the rats that received BPC 157 alone (Table 1).
NO level
In the early term, NO level assessment demonstrated a reduction in the levels in rats that underwent cecum perforation, but the NO values in rats with a perforated cecum that underwent BPC 157 medication were similar to those in healthy rats (Figure 4).
In the early stages, lipid peroxidation assessment demonstrated a huge increase in rats that underwent cecum perforation but no MDA oxidative stress in rats with a perforate cecum that underwent BPC 157 medication (Figure 5).
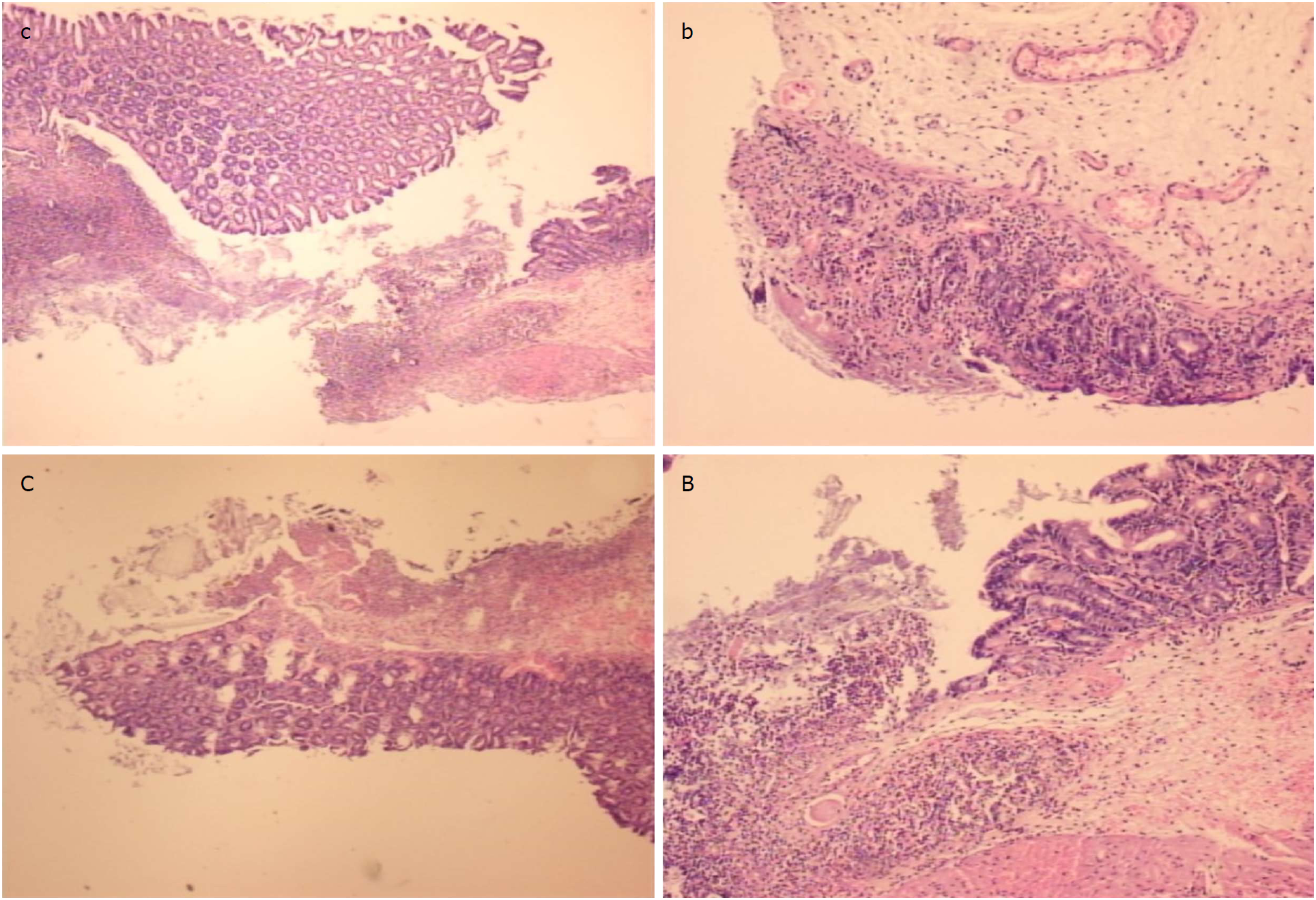
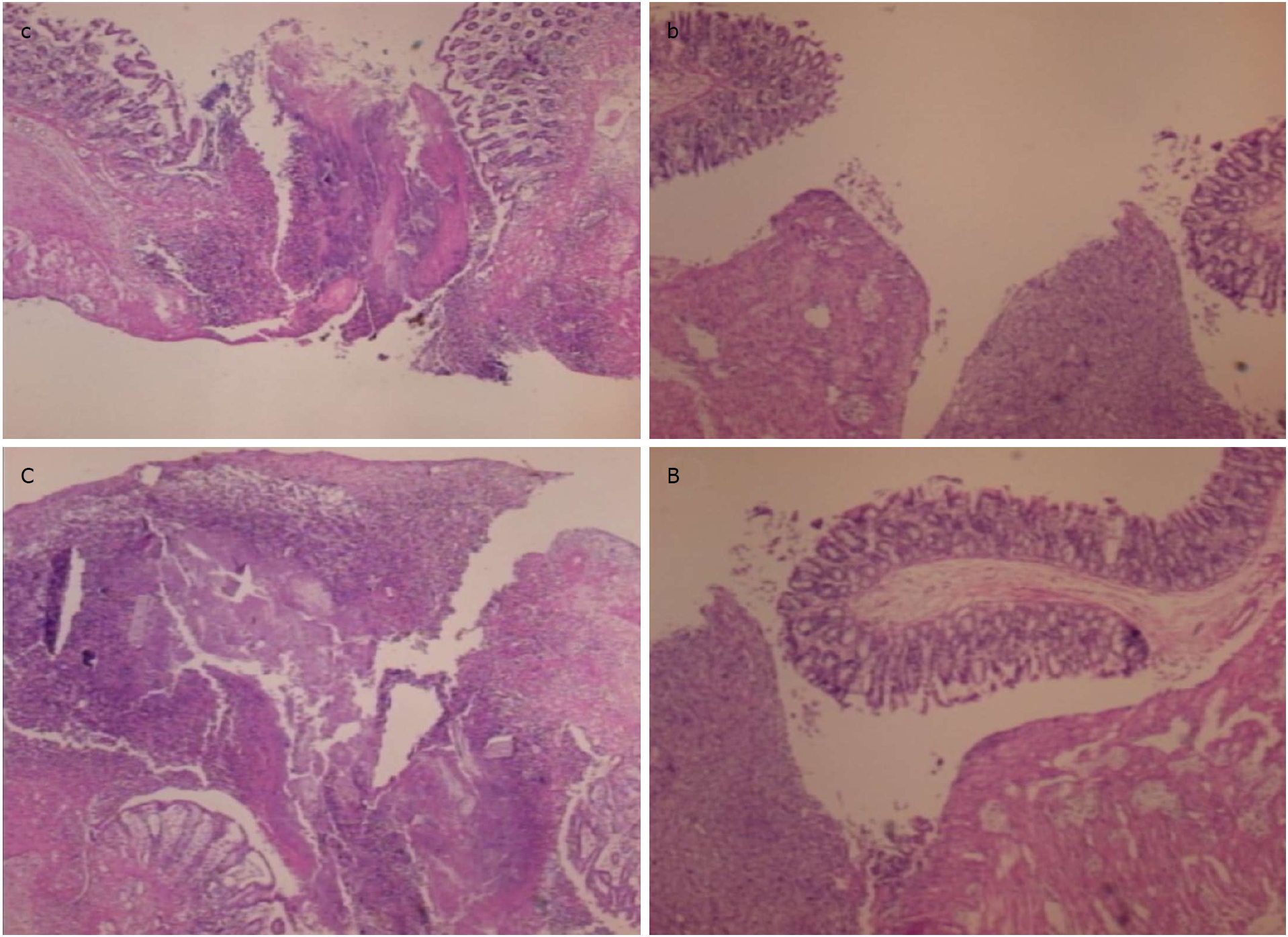
At the first day post-injury, controls exhibited intensive edema, loss of glands in the vicinity of the perforation, scarce inflammation, and neutrophils. By contrast, BPC 157-treated animals had much less edema, intensive inflammation (mixed neutro and mono) and a fibrin cloth attached to the defect (‘holding’ the edges) (Figure 9).
In control animals, after 7 d, the perforation showed communication between the lumen and peritoneal cavity that was partly closed by a loose clot with much debris and many inflammatory cells (Figure 10). In treated animals, the defect was sealed with well-formed granulation tissue. The surrounding mucosa in controls was edematous, and the epithelium showed practically no regenerative activity. However, in treated animals, the edema was much less pronounced, and the surface epithelium began to migrate over the defect.
However, no lethal outcome was noted during the experiment.
Major disturbances in an immediate post-injury period also entails the possible injury reversal in studies of ischemic colitis and inferior caval vein occlusion[20,21]. Thus, to reverse the immediate post-perforation threat in the cecum (and, therefore, the complete downhill course) we focused on the immediate post-perforation period. We also addressed the novel aspect of vascular rescue through the application of cytoprotective agents, known, as a class, to function in endothelium maintenance in the gastrointestinal tract (and, thus, mucosal maintenance)[4-9]. Instant application of the stable gastric pentadecapeptide BPC 157 was used as a prototype agent rapidly acting on endothelium integrity maintenance[10-19], which is known to be of therapeutic value in rats with ischemic colitis and inferior caval vein ligation[20,21].
Of note, the whole initial syndrome (in particular, instantly emptied blood vessels at the cecum serosa and thus the rapid “disappearance” of existing vessels and failure of vessel function upon injury) and, consequently, perforation defect enlargement and fluid leakage, profuse bleeding, increased oxidative stress and disturbed NO levels in cecum tissue were accordingly affected.
We suggest that this effect should be seen, based on evidence of BPC 157 rapidly activating the collateral circulation from existing vessels in rats with ischemic colitis[20] or inferior caval vein occlusion[21]. Thus, the essential findings (i.e., cecum perforation syndrome occurring in BPC 157-treated rats shows attenuation and reversal of the consequent tissue damages) indicate the rapidly activated bypassing loops (i.e., through arcade vessels or the left ovarian vein and other veins)[20,21], corresponding to the prompt reperfusion seen after perforation. Reperfusion may be observed by vessels “running” toward the defect (vessels filled/reappeared), and a small-vessel network appearing around the perforated defect with BPC 157 bath administration. Each considered a break of blood flow, this suggests that the therapy resolving the defect (i.e., cecum defect enlargement reversed to defect contraction in BPC 157-treated rats) may be a result of the reestablishment of blood flow. The bleeding time from the perforated cecum is thereby shortened, as demonstrated in rats with amputation, anticoagulant application, or vein obstruction[21,42,43]. Furthermore, defect closing corresponds to the closing of various fistula defects, which were surgically made by particular defect creations in corresponding organs[34-38].
In addition, the free radical scavenger effect and normalization of NO tissue values noted in rats with ischemic colitis or inferior vein obstruction in colon and venous tissues in both ischemia and reperfusion[20,21] correspond to the reduction in increased MDA values and normalization of NO values in cecum tissue in perforation studies. Heavy loss of endothelium cells from the vascular wall and, therefore, less eNOS NO production ability[44] would explain increased MDA values and decreased NO levels, which were regularly found in the cecum. Additionally, various free radical-induced lesions in other organs were counteracted by BPC 157 administration[20,21,45-48].
The importance of these beneficial effects was verified by the final complete closing of the defect, both grossly and microscopically, with the surface epithelium beginning to migrate over the defect and a lower adhesion severity score. It may also be that BPC 157 exhibits an additional beneficial effect because it counteracts peritonitis and adhesion formation along with beneficial effects in diverse intestinal lesion models[34-36,39,40].
Thus, it may be generally observed, that BPC 157 promptly facilitates and extends the cytoprotection background (endothelium maintenance → epithelium maintenance = blood vessel recruitment and activation toward injury, providing also described “bypassing” of occlusion through alternative ways)[20,21]; thus, all chains of events were accordingly and promptly involved to rapidly initiate and achieve a full healing effect.
This specific level of healing (initial and final) can be not obtained from the effect of NOS-blockade (L-NAME) or NOS-substrate (L-arginine), two pharmacologically distinct mechanisms with opposite effects on the same signaling NO-pathway[13]. Initially, L-arginine reduced vessel disappearance and counteracted defect widening, and prolonged the bleeding period. With L-NAME, vessel disappearance was similar to that in the controls; however, there was counteraction of defect widening, and the bleeding period was shortened. These would both be inadequate, though NO-specific, responses [given together, L-NAME+L-arginine regularly reversed each other’s initial responses, being not different from the controls, with a bleeding period similar to the controls (L-NAME+L-arginine)]. Namely, they both eventually aggravated perforated lesions in the cecum, a non-NO-specific effect (given together, they did not reverse each other’s final responses: L-NAME+L-arginine rats still had an open defect larger than that in the controls with adhesions of higher score). By contrast, as a proof of concept regarding the beneficial effects of BPC 157, rats in experiments with perforated cecum and colitis syndromes[20] showed the counteraction of the worsening of the course of injury after L-NAME and L-arginine application as individual agents (L-NAME rats; L-arginine rats → L-NAME+BPC 157 rats, L-arginine+BPC 157 rats ≈ BPC 157 rats) . When given together, the counteraction of the remaining extensive pathology was observed regardless of whether L-NAME and L-arginine reversed each other’s worsening responses (L-NAME + L-arginine rats → L-NAME + L-arginine + BPC 157 rats ≈ BPC 157 rats)[20].
In conclusion, the remaining extensive pathology in the L-NAME+L-arginine animals (both in early and final terms) means that other systems (i.e., the BPC 157 system) may function along with the NO system (previously supposed to be immobilized by the mutual actions of combined L-NAME and L-arginine)[13]. In the case of the effectiveness of BPC 157, the normalized MDA values and NO levels (always with progressive vessel presentation, a shortened bleeding time, closure of the defect, surface epithelium migration over the defect and fewer adhesions) indicated effectiveness over the background of the NO-system, whether immobilized (L-NAME + L-arginine), overstimulated (L-arginine) or blocked (L-NAME)[13]. Thus, BPC 157 could consolidate the NO system’s stimulating and inhibiting effects toward more effective healing (i.e., revascularization (vessel “running” toward the defect and small vessel network around the perforated defect) appearing with BPC 157 bath administration)[13]. Furthermore, this additional rapid cytoprotective vascular recovery and presentation leads to a consequent strong angiogenic effect in subsequent days[12,18,23-27,49,50], more profound than the angiogenesis of standard anti-ulcer agents[50] because of its interaction with several molecular pathways[21-27]. Illustratively, in other studies, BPC 157 induced an acceleration of blood flow recovery and vessel number within days in rats with hind limb ischemia[25].
Additionally, in practice, this means distinct endpoints should be overwhelmed to achieve the presentation seen in rats that underwent BPC 157 application. Finally, in addition to counteracting the parallel (worsening) effect of L-NAME/L-arginine on ischemic colitis and cecum perforation syndromes[21], BPC 157 counteracts the common parallel L-NAME/L-arginine activity points in other assays (i.e., magnesium intoxication and miosis/mydriasis)[51,52]. Notably, BPC 157 instantly prevents and reverses L-NAME-induced hypertension and L-NAME-induced thrombocytopenia as well as L-arginine-induced hypotension and L-arginine-induced prolonged bleeding and thrombocytopenia[13].
Therefore, BPC 157, with certain vascular effects[20,21] and NO-effect[13] can be used in perforated cecum and bleeding therapy.
To illustrate the background, present status, and significance of the study we should emphasize that in cecum perforation studies the immediate post-perforation threat is rarely studied. On the other hand, we recently claimed that treatment with the prototype cytoprotective agent, stable gastric pentadecapeptide BPC 157, induces bypassing of occlusions in rats that underwent vessel occlusions through the rapid presentation of collaterals, and exert its free radical scavenger effect in both ischemia and reperfusion. As a consequence, in this study, we focused on the resolving of the cecum perforation lesion, particularly the rapid disappearance of blood vessels in the cecum serosa that are instantly emptied and thereby “disappear”, perforation defect enlargement, bleeding and fluid leakage, increased oxidative stress and disturbed NO levels in cecum tissue.
The main topics, the key problems to be solved, and the significance of solving these problems for further research are related to reverse the immediate post-perforation threat in the cecum (and, therefore, the complete downhill course). We focused on perforated cecum, the stable gastric pentadecapeptide BPC 157, the NOS-blocker L-NAME, and the NOS-substrate L-arginine, as well as on the initial post-perforation period, rapid disappearance of blood vessels at the cecum serosa (emptied/disappeared), a large immediate defect, bleeding, leakage of fluid, increased oxidative stress and disturbed NO levels in cecum tissue. The rationale was that BPC 157 rapidly activated the collateral circulation from the existing vessels in rats with ischemic colitis or inferior caval vein occlusion. This was further perceived as an extended cytoprotection background (endothelium maintenance → epithelium maintenance = blood vessel recruitment and activation toward injury), also described as “bypassing” occlusion via alternative ways, as demonstrated with BPC 157, which, in principle, can likely cure rats with perforated cecum and thereafter.
The main objectives, the objectives that were realized, and the significance of these objectives for future research could be summarized as follows. In practice, as described previously, our approach to cure perforated cecum begins with the original understanding of stomach cytoprotection (a very rapid protection of the stomach endothelium and epithelium against diverse direct injuries; endothelium maintenance → epithelium maintenance), as “bypassing” occlusion via alternative ways, as demonstrated with BPC 157. Furthermore, we used these findings as the general argument that under analogous or even worse conditions (i.e., perforated cecum) a similar positive outcome can appear. Consequently, BPC 157 can attenuate cecum perforation syndrome; in BPC 157-treated rats, therapy may lead to attenuation and reversal of the consequent tissue damage. Especially, reversal of vessel disappearance to vessel “running” toward the defect (vessel filled/reappeared), reversal of defect enlargement to defect narrowing, reversal of prolonged bleeding to bleeding attenuation can induce reduction in the increased MDA values and normalization of NO values in cecum tissue. In subsequent days and weeks, this may lead to a closed cecum defect and attenuation of adhesion formation.
As an advantageous principle, methodology includes direct monitoring of the events occurring in rats during perforation of the cecum and immediately thereafter with the application of the stable gastric pentadecapeptide BPC 157, as a cytoprotective agent, and NO-agents, L-NAME and L-arginine. In deeply anaesthetized rats the cecum was exposed, and a perforation (5-mm diameter) was made at the ventral face of the basal region of the cecum close to the largest curvature; the rats were monitored for the next 15 min. The agents’ application (after 1 minute, medication (/kg, 10 mL/2min bath/rat) includes: BPC 157 (10 μg), L-NAME (5 mg), L-arginine (100 mg) alone or combined, and saline baths (controls)) on the rat perforate caecum injury. Alongside with the agents’ application, we continuously assessed the gross reappearance of the vessels (USB microcamera) quickly propagating toward the defect at the caecum surface, defect contraction, bleeding attenuation, MDA- and NO-levels in colon tissue at 15 min, and, in repalaratomized rats severity of colon lesions and adhesions at 1 and 7 days.
The study of the counteraction of perforated cecum lesions in rats and the effects of pentadecapeptide BPC 157, L-NAME and L-arginine merits several emphasizes to summarize the research findings, their contribution to the research in the field, and the problems that remain to be solved. First, as an issue so far not fully addressed, to reverse the immediate post-perforation threat in the cecum (and, therefore, the complete downhill course) we focused on this immediate post-perforation period. We also addressed the novel aspect of vascular rescue through the application of cytoprotective agents, known, as a class, to function in endothelium maintenance in the gastrointestinal tract (and, thus, mucosal maintenance). Of note, with applied BPC 157 therapy, the whole initial syndrome (in particular, instantly emptied blood vessels at the cecum serosa (thus rapid “disappearance” of the existing vessels and vessel function failure upon injury)) and, consequently, perforation defect enlargement and fluid leakage, profuse bleeding, increased oxidative stress and disturbed NO levels in cecum tissue were accordingly affected. This specific level of healing (initial and final) can be not obtained from the effect of NOS-blockade (L-NAME) or NOS-substrate (L-arginine).
As the new findings, we suggest that this recovering of the perforated cecum should be an activity much like an activity seen when BPC 157 rapidly activated the collateral circulation from existing vessels in rats with ischemic colitis or inferior caval vein occlusion. Thus, the essential findings (i.e., cecum perforation syndrome occurs in BPC 157-treated rats with attenuation and reversal of the consequent tissue damages) indicate the rapidly activated bypassing loops (i.e., through arcade vessels or the left ovarian vein and other veins, corresponding to the prompt particular vascular rescue seen after perforation). Vascular rescue may be vessels “running” toward the defect (vessel filled/reappeared), and a small-vessel network appears around the perforated defect with BPC 157 bath administration. Each considered a break of blood flow, suggests that the therapy resolving the defect (i.e., cecum defect enlargement reversed to defect contraction in BPC 157-treated rats) may be reestablishing blood flow. That explanation represents the newest theoretical extension of cytoprotection theory background (endothelium maintenance → epithelium maintenance = blood vessel recruitment and activation toward injury, providing also described “bypassing” of occlusion through alternative ways). Since all chains of events could be accordingly and promptly involved to rapidly initiate and achieve a full healing effect. The bleeding time from the perforated cecum is thereby shortened, as previously demonstrated in rats with amputation, anticoagulant application, or vein obstruction. Furthermore, defect closing corresponds to the previously demonstrated closing of various fistula defects, which were surgically made by particular defect creations in corresponding organs. The implications of this study for clinical practice in the future can be that BPC 157, with certain vascular effects and NO-effect, can be used in the perforated cecum and bleeding therapy.
The main experiences and lessons that can be learnt from this study may be the evidence that BPC 157 promptly facilitates and extends the cytoprotection background (endothelium maintenance → epithelium maintenance = blood vessel recruitment and activation toward injury, providing also described “bypassing” of occlusion through alternative ways). Thus, the evidence was obtained that all chains of events were accordingly and promptly involved to rapidly initiate and achieve a full healing effect, and with the respect to the corresponding positive effects in two other studies (ischemic/reperfusion colitis; inferior caval vein occlusion), the beneficial effect in rats with perforated cecum may be generally observed. Thereby, the future research should be related to the similar and/or even worse condition, to verify full significance of the presented beneficial findings seen in rats after cecum perforation and BPC 157 therapy application.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Николаевич YN, Ricci G S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
| 1. | Aslan MK, Boybeyi O, Soyer T, Senyücel MF, Ayva S, Kısa U, Cesur O, Cakmak M. Evaluation of omental inflammatory response with P-/E-selectin levels and histopathologic findings in experimental model. J Pediatr Surg. 2012;47:2050-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Liu J, Shi B, Shi K, Ma G, Zhang H, Lou X, Liu H, Wan S, Liang D. Ghrelin upregulates PepT1 activity in the small intestine epithelium of rats with sepsis. Biomed Pharmacother. 2017;86:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Santiago MB, Vieira AA, Giusti-Paiva A. Impaired chemoreflex sensitivity during septic shock induced by cecal ligation and perforation. Can J Physiol Pharmacol. 2013;91:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761-767. [PubMed] |
| 5. | Sikiric P, Seiwerth S, Grabarevic Z, Petek M, Rucman R, Turkovic B, Rotkvic I, Jagic V, Duvnjak M, Mise S. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides. Life Sci. 1994;54:PL63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Szabo S. Gastric Cytoprotection [Internet]. 1. st. Boston, MA: Springer US; 1989; Available from: http://link.springer.com/10.1007/978-1-4684-5697-4. |
| 7. | Szabo S, Trier JS, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88:228-236. [PubMed] |
| 8. | Szabo S, Trier J. Pathogenesis of acute gastric mucosal injury: Sulfhydrils as a protector, adrenal cortex as a modulator, and vascular endothelium as a target. Allen A, Flemstrom G, Garner A, Silen W, Turnberg L. Mechanism of mucosal protection in the upper gastrointestinal tract. New York: Raven; 1984; 387-393. |
| 9. | Trier JS, Szabo S, Allan CH. Ethanol-induced damage to mucosal capillaries of rat stomach. Ultrastructural features and effects of prostaglandin F2 beta and cysteamine. Gastroenterology. 1987;92:13-22. [PubMed] |
| 10. | Sikiric P, Seiwerth S, Rucman R, Drmic D, Stupnisek M, Kokot A, Sever M, Zoricic I, Zoricic Z, Batelja L. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr Pharm Des. 2017;23:4012-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Sikiric P, Seiwerth S, Rucman R, Kolenc D, Vuletic LB, Drmic D, Grgic T, Strbe S, Zukanovic G, Crvenkovic D. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr Neuropharmacol. 2016;14:857-865. [PubMed] |
| 12. | Seiwerth S, Brcic L, Vuletic LB, Kolenc D, Aralica G, Misic M, Zenko A, Drmic D, Rucman R, Sikiric P. BPC 157 and blood vessels. Curr Pharm Des. 2014;20:1121-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20:1126-1135. [PubMed] |
| 14. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19:76-83. [PubMed] |
| 15. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Focus on ulcerative colitis: Stable gastric pentadecapeptide BPC 157. Curr Med Chem. 2012;19:126-132. [PubMed] |
| 16. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17:1612-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Sikiric P, Seiwerth S, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des. 2010;16:1224-1234. [PubMed] |
| 18. | Sikiric P, Seiwerth S, Brcic L, Blagaic AB, Zoricic I, Sever M, Klicek R, Radic B, Keller N, Sipos K. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response. Inflammopharmacology. 2006;14:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Sikirić P, Petek M, Rucman R, Seiwerth S, Grabarević Z, Rotkvić I, Turković B, Jagić V, Mildner B, Duvnjak M. A new gastric juice peptide, BPC. An overview of the stomach-stress-organoprotection hypothesis and beneficial effects of BPC. J Physiol Paris. 1993;87:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Duzel A, Vlainic J, Antunovic M, Malekinusic D, Vrdoljak B, Samara M, Gojkovic S, Krezic I, Vidovic T, Bilic Z. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J Gastroenterol. 2017;23:8465-8488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Vukojević J, Siroglavić M, Kašnik K, Kralj T, Stanćić D, Kokot A, Kolarić D, Drmić D, Sever AZ, Barišić I. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul Pharmacol. 2018;106:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Cesarec V, Becejac T, Misic M, Djakovic Z, Olujic D, Drmic D, Brcic L, Rokotov DS, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol. 2013;701:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol (1985). 2011;110:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Chang CH, Tsai WC, Hsu YH, Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19:19066-19077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Hsieh MJ, Liu HT, Wang CN, Huang HY, Lin Y, Ko YS, Wang JS, Chang VH, Pang JS. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl). 2017;95:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Huang T, Zhang K, Sun L, Xue X, Zhang C, Shu Z, Mu N, Gu J, Zhang W, Wang Y. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther. 2015;9:2485-2499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Tkalcević VI, Cuzić S, Brajsa K, Mildner B, Bokulić A, Situm K, Perović D, Glojnarić I, Parnham MJ. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol. 2007;570:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Klicek R, Kolenc D, Suran J, Drmic D, Brcic L, Aralica G, Sever M, Holjevac J, Radic B, Turudic T. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol. 2013;64:597-612. [PubMed] |
| 29. | Lojo N, Rasic Z, Zenko Sever A, Kolenc D, Vukusic D, Drmic D, Zoricic I, Sever M, Seiwerth S, Sikiric P. Effects of diclofenac, L-NAME, L-arginine, and pentadecapeptide BPC 157 on gastrointestinal, liver, and brain lesions, failed anastomosis, and intestinal adaptation deterioration in 24 hour-short-bowel rats. PLoS One. 2016;11:e0162590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Sandor Z, Vincze A, Jadus M, Dohoczky C, Erceg D, Brajsa K, Kolega M, Szabo S. The protective effect of newly isolated peptide PL-10 in the iodoacetamide colitis model in rats. Gastroenterology. 1997;112:A400. |
| 31. | Sikiric P, Seiwerth S, Grabarevic Z, Balen I, Aralica G, Gjurasin M, Komericki L, Perovic D, Ziger T, Anic T. Cysteamine-colon and cysteamine-duodenum lesions in rats. Attenuation by gastric pentadecapeptide BPC 157, cimetidine, ranitidine, atropine, omeprazole, sulphasalazine and methylprednisolone. J Physiol Paris. 2001;95:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Sikiric P, Seiwerth S, Aralica G, Perovic D, Staresinic M, Anic T, Gjurasin M, Prkacin I, Separovic J, Stancic-Rokotov D. Therapy effect of antiulcer agents on new chronic cysteamine colon lesion in rat. J Physiol Paris. 2001;95:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Veljaca M, Lesch C, Sanchez B, Low J, Guglietta A. Protection of BPC-15 on TNBS-induced colitis in rats: possible mechanisms of action. Gastroenterology. 1995;108:936. |
| 34. | Baric M, Sever AZ, Vuletic LB, Rasic Z, Sever M, Drmic D, Pavelic-Turudic T, Sucic M, Vrcic H, Seiwerth S. Stable gastric pentadecapeptide BPC 157 heals rectovaginal fistula in rats. Life Sci. 2016;148:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Grgic T, Grgic D, Drmic D, Sever AZ, Petrovic I, Sucic M, Kokot A, Klicek R, Sever M, Seiwerth S. Stable gastric pentadecapeptide BPC 157 heals rat colovesical fistula. Eur J Pharmacol. 2016;780:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Klicek R, Sever M, Radic B, Drmic D, Kocman I, Zoricic I, Vuksic T, Ivica M, Barisic I, Ilic S. Pentadecapeptide BPC 157, in clinical trials as a therapy for inflammatory bowel disease (PL14736), is effective in the healing of colocutaneous fistulas in rats: role of the nitric oxide-system. J Pharmacol Sci. 2008;108:7-17. [PubMed] |
| 37. | Skorjanec S, Kokot A, Drmic D, Radic B, Sever M, Klicek R, Kolenc D, Zenko A, Lovric Bencic M, Belosic Halle Z. Duodenocutaneous fistula in rats as a model for “wound healing-therapy” in ulcer healing: the effect of pentadecapeptide BPC 157, L-nitro-arginine methyl ester and L-arginine. J Physiol Pharmacol. 2015;66:581-590. [PubMed] |
| 38. | Skorjanec S, Dolovski Z, Kocman I, Brcic L, Blagaic Boban A, Batelja L, Coric M, Sever M, Klicek R, Berkopic L. Therapy for unhealed gastrocutaneous fistulas in rats as a model for analogous healing of persistent skin wounds and persistent gastric ulcers: stable gastric pentadecapeptide BPC 157, atropine, ranitidine, and omeprazole. Dig Dis Sci. 2009;54:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Sever M, Klicek R, Radic B, Brcic L, Zoricic I, Drmic D, Ivica M, Barisic I, Ilic S, Berkopic L. Gastric pentadecapeptide BPC 157 and short bowel syndrome in rats. Dig Dis Sci. 2009;54:2070-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Vuksic T, Zoricic I, Brcic L, Sever M, Klicek R, Radic B, Cesarec V, Berkopic L, Keller N, Blagaic AB. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL14736, Pliva, Croatia) heals ileoileal anastomosis in the rat. Surg Today. 2007;37:768-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Hrelec M, Klicek R, Brcic L, Brcic I, Cvjetko I, Seiwerth S, Sikiric P. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J Physiol Pharmacol. 2009;60 Suppl 7:161-165. [PubMed] |
| 42. | Stupnisek M, Franjic S, Drmic D, Hrelec M, Kolenc D, Radic B, Bojic D, Vcev A, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb Res. 2012;129:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Stupnisek M, Kokot A, Drmic D, Hrelec Patrlj M, Zenko Sever A, Kolenc D, Radic B, Suran J, Bojic D, Vcev A. Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin, L-NAME and L-arginine. PLoS One. 2015;10:e0123454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Berra-Romani R, Avelino-Cruz JE, Raqeeb A, Della Corte A, Cinelli M, Montagnani S, Guerra G, Moccia F, Tanzi F. Ca2+-dependent nitric oxide release in the injured endothelium of excised rat aorta: a promising mechanism applying in vascular prosthetic devices in aging patients. BMC Surg. 2013;13 Suppl 2:S40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Belosic Halle Z, Vlainic J, Drmic D, Strinic D, Luetic K, Sucic M, Medvidovic-Grubisic M, Pavelic Turudic T, Petrovic I, Seiwerth S. Class side effects: decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Ilic S, Drmic D, Zarkovic K, Kolenc D, Coric M, Brcic L, Klicek R, Radic B, Sever M, Djuzel V. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J Physiol Pharmacol. 2010;61:241-250. [PubMed] |
| 47. | Luetic K, Sucic M, Vlainic J, Halle ZB, Strinic D, Vidovic T, Luetic F, Marusic M, Gulic S, Pavelic TT. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology. 2017;25:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Sikiric P, Seiwerth S, Grabarevic Z, Rucman R, Petek M, Rotkvic I, Turkovic B, Jagic V, Mildner B, Duvnjak M. Hepatoprotective effect of BPC 157, a 15-amino acid peptide, on liver lesions induced by either restraint stress or bile duct and hepatic artery ligation or CCl4 administration. A comparative study with dopamine agonists and somatostatin. Life Sci. 1993;53:PL291-PL296. [PubMed] |
| 49. | Brcic L, Brcic I, Staresinic M, Novinscak T, Sikiric P, Seiwerth S. Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. J Physiol Pharmacol. 2009;60 Suppl 7:191-196. [PubMed] |
| 50. | Sikiric P, Separovic J, Anic T, Buljat G, Mikus D, Seiwerth S, Grabarevic Z, Stancic-Rokotov D, Pigac B, Hanzevacki M. The effect of pentadecapeptide BPC 157, H2-blockers, omeprazole and sucralfate on new vessels and new granulation tissue formation. J Physiol Paris. 1999;93:479-485. [PubMed] |
| 51. | Medvidovic-Grubisic M, Stambolija V, Kolenc D, Katancic J, Murselovic T, Plestina-Borjan I, Strbe S, Drmic D, Barisic I, Sindic A. Hypermagnesemia disturbances in rats, NO-related: pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology. 2017;25:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 52. | Kokot A, Zlatar M, Stupnisek M, Drmic D, Radic R, Vcev A, Seiwerth S, Sikiric P. NO system dependence of atropine-induced mydriasis and L-NAME- and L-arginine-induced miosis: Reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur J Pharmacol. 2016;771:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |