Published online Dec 28, 2018. doi: 10.3748/wjg.v24.i48.5415
Peer-review started: September 28, 2018
First decision: October 23, 2018
Revised: October 27, 2018
Accepted: November 8, 2018
Article in press: November 8, 2018
Published online: December 28, 2018
Processing time: 90 Days and 14 Hours
Nonalcoholic fatty liver disease (NAFLD) is the commonest chronic liver disease in high-income countries and is associated with increased morbidity and mortality. Macrophages appear to play an important role in the development and progression of hepatic fibrosis in patients with NAFLD. Accordingly, modulation of macrophage trafficking might represent an attractive therapeutic strategy in this population. Cenicriviroc is an oral inhibitor of the chemokine ligand 2/C-C chemokine receptor 2 pathway, which plays an important role in the hepatic recruitment of the macrophages. Preclinical studies and a phase 2b study in humans suggest that this agent might hold promise in the management of NAFLD.
Core tip: Macrophages appear to play an important role in the development and progression of hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). Accordingly, modulation of macrophage trafficking might represent an attractive therapeutic strategy in this population. Cenicriviroc is an oral inhibitor of the chemokine ligand 2/C-C chemokine receptor 2 pathway, which plays an important role in the hepatic recruitment of the macrophages. Preclinical studies and a phase 2b study in humans suggest that this agent might hold promise in the management of NAFLD.
- Citation: Neokosmidis G, Tziomalos K. Role of cenicriviroc in the management of nonalcoholic fatty liver disease. World J Gastroenterol 2018; 24(48): 5415-5417
- URL: https://www.wjgnet.com/1007-9327/full/v24/i48/5415.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i48.5415
The prevalence of nonalcoholic fatty liver disease (NAFLD) is increasing worldwide and NAFLD has become the most predominant liver disease in Western countries due to the pandemic of obesity, metabolic syndrome and type 2 diabetes[1]. The histopathological spectrum of NAFLD ranges from isolated steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis, and NAFLD is associated with increased risk for hepatocellular carcinoma (HCC)[2].
The mainstay of treatment of NAFLD is weight loss through diet and exercise[2]. However, many patients cannot achieve or sustain weight loss[2]. Accordingly, pharmacotherapy is required in a considerable proportion of patients with NAFLD, particularly those with advanced fibrosis, who are at higher risk for liver-related complications[2,3].
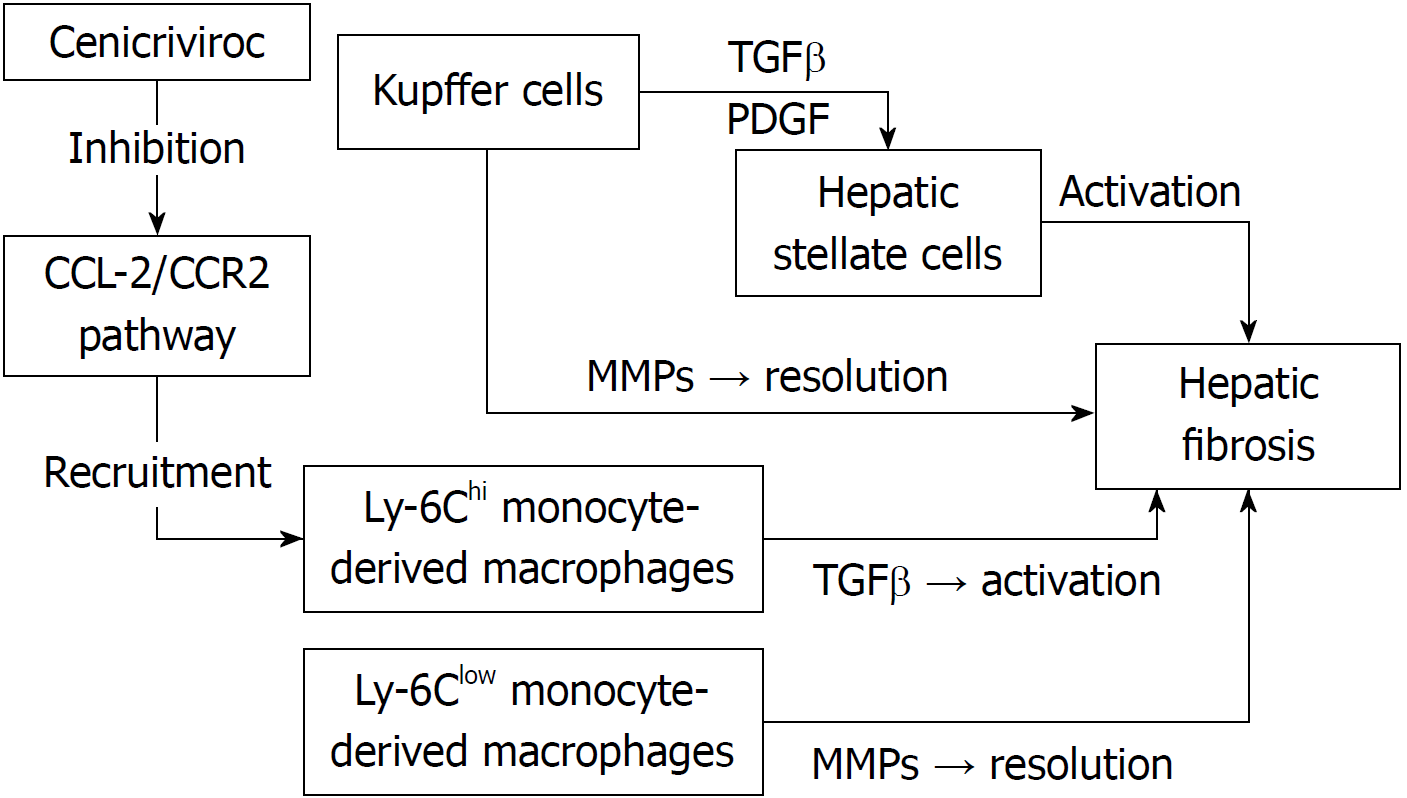
Hepatic macrophages play an important role in the pathogenesis of acute and chronic liver injury[4,5]. Hepatic macrophages are a heterogenous population, consisting of Kupffer cells (KCs) and monocyte-derived macrophages (MoMF), which are recruited from the circulation to the liver[4,5]. KCs activate hepatic stellate cells (HSCs) through the production of pro-fibrotic cytokines transforming growth factor β (TGFβ) and platelet-derived growth factor (PDGF)[4,5]. On the other hand, KCs also secrete matrix metalloproteinases (MMPs), resulting in resolution of fibrosis[4,5]. Therefore, hepatic macrophages appear to exert both pro- and anti-fibrotic functions[4,5]. In cases of hepatic injury, Ly-6Chi monocytes differentiate into proinflammatory macrophages, which interact with HSCs to promote fibrosis through the production of TGFβ[4,5]. The chemokine (C-C motif) ligand (CCL) 2/C-C chemokine receptor 2 (CCR2) pathway plays an important role in the hepatic recruitment of the Ly-6Chi monocytes[4,5]. On the other hand, a recent study showed that phagocytosis of cellular debris facilitates a phenotypic switch from Ly-6Chi macrophages to Ly-6Clow macrophages[4,5]. The Ly-6Clow monocyte is the main source of MMPs and promotes fibrosis resolution[4,5] (Figure 1).
Given the important role of hepatic macrophages in the pathogenesis of liver fibrosis, targeting these cells might represent a promising approach in the management of NAFLD. Krenkel et al[6] recently reported an increased number of CCR2+ macrophages in patients with NASH and either advanced fibrosis (stage 3) or cirrhosis (stage 4). It was also shown that cenicriviroc (CVC), an oral CCR2/CCR5 antagonist, prevents macrophage trafficking and hepatic infiltration by CCR2+ MoMFs and might therefore be useful in the prevention and/or resolution of hepatic fibrosis in patients with NAFLD[6].
Several animal studies evaluated the antifibrotic potential of CVC. Kruger et al[7] administered CVC 10 mg/(kg•d) or 30 mg/(kg•d) for 4 wk and 20 mg/(kg•d) or 30 mg/(kg•d) for 14 wk in mice fed with a choline-deficient, L-amino acid- defined, high-fat diet (CDAHFD). A second group of mice was fed with standard chow and did not receive CVC. At 4 and 14 wk, livers were harvested for histology and flow cytometric analyses of intrahepatic cells. Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels were normal in the standard chow group but were elevated in CDAHFD-fed, untreated control mice. In contrast, the CDAHFD-fed mice that were treated with CVBC 10 mg/(kg•d) and 30 mg/(kg•d) for 4 wk experienced a reduction in ALT levels. Moreover, after 4 wk of high-dose CVC treatment, a significant decrease in the number of intrahepatic Ly6-Chigh macrophages was observed relative to the vehicle control. The intrahepatic CCR2+ macrophages also decreased with 4 wk of CVC treatment whereas CCR5+ macrophages were not affected. Therefore, CVC appears to have greater affinity for CCR2 than for CCR5 in mice. Kruger et al[7] also noticed a significant decrease in hepatic fibrosis in mice that received high-dose CVC treatment for 14 wk compared with vehicle controls. In another study, CVC reduced monocyte/macrophage recruitment at doses > 20 mg/(kg•d) in a mouse model of thiaglycalate-induced peritonitis[8]. At these doses, CVC also prevented hepatic fibrosis in a rat model of thioacetamide-induced liver fibrosis and in a mouse model of diet-induced NASH. A reduction in NAFLD activity score (NAS) and in plasma ALT levels was also observed with CVC treatment[8]. In another animal model of carbon tetrachloride-induced acute liver injury, mice received CVC or a vehicle control solution orally before the administration of carbon tetrachloride and after 12 h and 24 h[9]. CVC treatment reduced the number of F4/80 positive macrophages in the liver, particularly in the periportal and necrotic areas. Reductions in ALT levels and in the necrotic area at 36 h were also observed[9].
Recently, the results of the CENTAUR study were published[10]. This was a phase 2b, double-blind, placebo-controlled, multinational study that randomized 289 patients with NASH, NAS ≥ 4 with ≥ 1 in each component and stage 1-3 liver fibrosis to receive CVC 150 mg per os once daily or placebo. After 1 year of treatment, the primary endpoint (≥ 2-point improvement in NAS with ≥ 1-point reduction in either lobular inflammation or hepatocellular ballooning and no worsening of fibrosis) in the intent-to-treat population was achieved in a similar proportion of subjects on CVC and placebo (16% vs 19%, respectively; P = 0.52). Resolution of steatohepatitis and no worsening of fibrosis, a key secondary outcome, was also observed in similar rates in the 2 groups (8% vs 6%, respectively; P = 0.49). However, improvement in fibrosis by ≥ 1 stage and no worsening of steatohepatitis, another key secondary outcome, was observed in more patients on CVC than placebo (20% vs 10%, respectively; P = 0.02). Patients with higher NAS, prominent hepatocellular ballooning, higher fibrosis stage, mild or no portal inflammation and with higher body mass index showed greater improvements with CVC treatment. The levels of biomarkers of systemic inflammation (high-sensitivity C-reactive protein, interleukin-6 and 1β, and fibrinogen) and of monocyte activation (sCD14) decreased in patients treated with CVC. On the other hand, CCL-2 and -4 increased in CVC-treated patients, confirming CCR2 and CCR5 blockade by CVC. Importantly, safety and tolerability of CVC were comparable to placebo[10]. AURORA, a phase III study, will evaluate the effects of CVC on hepatic fibrosis in 2000 patients with NASH and is expected to be completed in 2019[11].
Cenicriviroc appears to represent a promising tool in the management of patients with NAFLD. However, larger studies are needed to clearly define the safety and efficacy of this novel agent in this highly prevalent disease.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kim DJ, Sherif Z, Zhu HF S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7536] [Article Influence: 837.3] [Reference Citation Analysis (0)] |
| 2. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2613] [Article Influence: 201.0] [Reference Citation Analysis (1)] |
| 3. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1703] [Article Influence: 170.3] [Reference Citation Analysis (1)] |
| 4. | Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 410] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 5. | Raeman R, Anania FA. Therapy for steatohepatitis: Do macrophages hold the clue? Hepatology. 2018;67:1204-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, Liepelt A, Lefebvre E, Luedde T, Hellerbrand C. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 404] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 7. | Kruger AJ, Fuchs BC, Masia R, Holmes JA, Salloum S, Sojoodi M, Ferreira DS, Rutledge SM, Caravan P, Alatrakchi N. Prolonged cenicriviroc therapy reduces hepatic fibrosis despite steatohepatitis in a diet-induced mouse model of nonalcoholic steatohepatitis. Hepatol Commun. 2018;2:529-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PLoS One. 2016;11:e0158156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 9. | Puengel T, Krenkel O, Kohlhepp M, Lefebvre E, Luedde T, Trautwein C, Tacke F. Differential impact of the dual CCR2/CCR5 inhibitor cenicriviroc on migration of monocyte and lymphocyte subsets in acute liver injury. PLoS One. 2017;12:e0184694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 533] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 11. | Phase 3 Study for the Efficacy and Safety of CVC for the Treatment of Liver Fibrosis in Adults With NASH. In: Clinical Trials.gov [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT03028740?term=cenicrivirocrank=10 Clinical Trials.gov Identifier: NCT03028740. |