Published online Dec 14, 2018. doi: 10.3748/wjg.v24.i46.5215
Peer-review started: August 29, 2018
First decision: October 14, 2018
Revised: October 24, 2018
Accepted: November 2, 2018
Article in press: November 2, 2018
Published online: December 14, 2018
Processing time: 106 Days and 22.3 Hours
Hepatocellular carcinomas (HCCs) frequently recur despite initial successful surgical resection or local ablation therapy. Diagnostic methods for small HCCs have improved with the introduction of gadoxetic acid-enhanced liver magnetic resonance imaging and diffusion-weighted imaging (DWI). Currently, sub-centimeter recurrent nodules showing typical hallmark imaging findings of HCC are frequently detected in patients with a treatment history for HCC. With five typical magnetic resonance findings, including arterial enhancement, washout on portal or transitional phase, high signal intensity on both T2-weighted image and DWI, and low signal intensity on hepatobiliary phase, sub-centimeter recurrent HCC can be diagnosed with high accuracy. Although more information is needed to determine the treatment of choice, local ablation therapy under fusion imaging and/or contrast-enhanced ultrasound guidance or cone-beam computed tomography-guided chemoembolization seem to be promising as they are effective and safe for the management of sub-centimeter recurrent HCCs.
Core tip: Sub-centimeter recurrent nodules can be diagnosed as hepatocellular carcinomas (HCC) in patients with a history of HCC using five typical magnetic resonance findings, including arterial enhancement, washout on portal or transitional phase, high signal intensity on both T2-weighted image and diffusion-weighted imaging, and low signal intensity on hepatobiliary phase. Local ablation therapy under fusion imaging and/or contrast-enhanced ultrasound guidance or cone-beam computed tomography-guided chemoembolization seems to be promising as they are effective and safe. Further comparative studies are warranted to determine the best treatment options.
- Citation: Lee MW, Lim HK. Management of sub-centimeter recurrent hepatocellular carcinoma after curative treatment: Current status and future. World J Gastroenterol 2018; 24(46): 5215-5222
- URL: https://www.wjgnet.com/1007-9327/full/v24/i46/5215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i46.5215
Hepatocellular carcinoma (HCC) is characterized by frequent recurrence despite the initial success of curative treatments, such as surgical resection and local ablation therapy, with recurrence rates 5 years after treatment ranging from 73% to 100%[1-4]. For patients with a treatment history of HCC, instead of ultrasound (US), computed tomography (CT) or magnetic resonance imaging (MRI) is typically used for follow-up[5]. Recent advances in CT and MRI technology have facilitated earlier detection of recurrent HCCs, and currently, small hepatic lesions, even sub-centimeter nodules, are more frequently detected than before by using gadoxetic acid-enhanced MRI and diffusion-weighted imaging (DWI)[6,7]. As tumor size is one of the important predictive factors affecting treatment outcomes of recurrent HCC[8], early diagnosis is crucial for favorable prognosis in patients with recurrent HCC.
Noninvasive diagnostic criteria for sub-centimeter HCC have yet to be established. According to the guidelines of the American Association for the Study of Liver Disease (AASLD) and the European Association for the Study of the Liver-European Organization for Research and Treatment of Cancer (EASL-EORTC), imaging diagnosis for HCC is applicable only for nodules larger than 1 cm in patients with risk factors for HCC[9-11]. In contrast, guidelines from other associations allow noninvasive diagnosis of sub-centimeter HCC based on typical imaging hallmarks in patients with chronic liver disease or cirrhosis[11-16], even allowing the treatment of such sub-centimeter nodules in patients with a history of HCC[17,18].
Percutaneous radiofrequency (RF) ablation has been widely accepted as a valuable first-line treatment option for HCCs 3 cm or smaller[9,19]. The outcome of RF ablation for sub-centimeter HCCs is expected to be excellent, as tumor size is a major determinant of local tumor control with RF ablation[19]. Meanwhile, if RF ablation for sub-centimeter HCCs is to be performed successfully, tumors need to be identifiable by using guiding modalities, such as CT or US. However, small HCCs are sometimes challenging to detect using unenhanced CT or conventional B-mode US[20,21]. In terms of US guidance, fusion imaging of conventional B-mode US with pre-acquired CT/MR images and/or contrast-enhanced US (CEUS) improves tumor visibility and technical feasibility of RF ablation for HCC[22-27].
Unlike other malignant tumors, HCC can be diagnosed by non-invasive imaging criteria of intense contrast uptake during the arterial phase followed by contrast washout during the venous phases in contrast-enhanced CT or MRI[10,28,29]. In terms of tumor size, however, Western guidelines, such as the AASLD and EASL guidelines, recommend US follow up at 3-4 mo intervals for sub-centimeter nodules instead of instantaneous diagnosis of HCC. This policy is based on the beliefs that confident diagnosis of sub-centimeter HCC is almost impossible with current imaging techniques. Even if a definitive diagnosis of HCC for such small nodules is possible, over diagnosis may cause more harm than good[28]. Meanwhile, according to the Liver Imaging Reporting and Data System (LI-RADS) classification, which endorses a conservative approach, even though a definitive diagnosis of HCC cannot be established, “probable” HCC can be given for sub-centimeter nodules with typical imaging hallmarks of HCC. Interestingly, the LI-RADS system also integrates imaging features not related to tumor enhancement patterns, such as presence of a tumor capsule or threshold tumor growth over time[30]. Furthermore, Eastern guidelines, such as the Asian-Pacific Association for the Study of the Liver, the Korean Liver Cancer Study Group-National Cancer Center, and the Japan Society of Hepatology, allow imaging diagnosis of sub-centimeter HCC[13,14,31].
The diagnostic algorithm in most imaging-based guidelines for HCC in treatment-naïve patients begins with nodule detection performed by US during HCC surveillance[10,29,31]. On the other hand, diagnosis of recurrent HCC in patients with a treatment history of HCC has yet to be clearly established. Given that recurrent HCCs are common due to either metastasis from original tumor or de novo carcinogenesis, early diagnosis of recurrent HCC and application of appropriate rescue therapy are crucial for improved survival outcomes. For this reason, CT or MRI is frequently used for follow up after treatment. However, according to a recent meta-analysis[32], diagnosis of sub-centimeter HCC using either CT or MRI may be challenging, and per-lesion sensitivity was significantly lower for sub-centimeter HCCs than that for HCCs 1 cm or larger. Currently, gadoxetic acid is commonly used as a contrast agent for liver MRI and has shown significantly higher per-lesion sensitivity than MRI performed using other contrast agents[32]. This improved diagnostic performance is primarily attributed to hepatobiliary phase images in which most HCCs are seen as nodules with hypo-intensity[33].
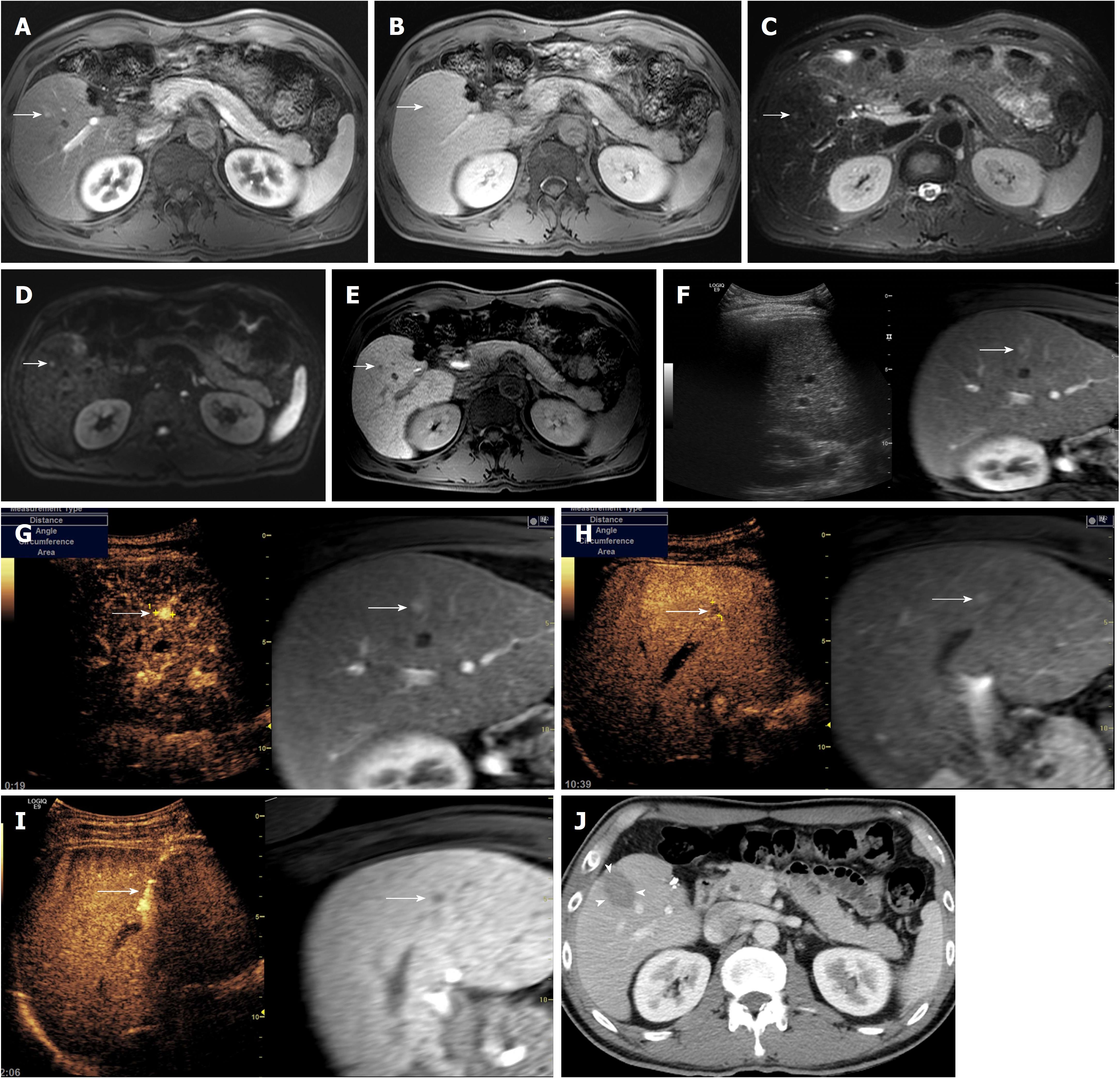
Recent studies using gadoxetic acid-enhanced MRI suggest that sub-centimeter recurrent HCC can be diagnosed based on five typical MR findings, including arterial enhancement, washout on portal or transitional phase, high signal intensity on both T2-weighted image and DWI, and low signal intensity on hepatobiliary phase (Figure 1)[17,18]. In a previous study, the progression rate of sub-centimeter nodules fulfilling the aforementioned five MR findings for overt HCC larger than 1 cm within 12 mo was as high as 89.9% (33/36) in patients with a treatment history of HCC[17]. Interestingly, the initial size of the sub-centimeter nodules was an important predictor for progression to overt HCC, with an optimal cut-off value of 5.5 mm. Similar results were found in a recent study in which 95.8% (23/24) of sub-centimeter nodules with the aforementioned five MR findings increased in size larger than 1 cm at follow up CT or MRI, eventually fulfilling the Western diagnostic criteria for HCC. In addition, all three sub-centimeter nodules that were managed with surgical resection or liver transplantation were pathologically confirmed to be HCCs. These findings suggest that there is very little false positivity for HCC when sub-centimeter nodules show the typical five MR findings of HCC in patients with a treatment history for HCC. In other words, sub-centimeter recurrent nodules in the liver after treatment for HCC can be accurately diagnosed as recurrent tumors when the lesions exhibit typical hallmark imaging features of HCC on gadoxetic acid-enhanced liver MRI. This implies that sub-centimeter recurrent HCCs can be treated as soon as they are detected[34].
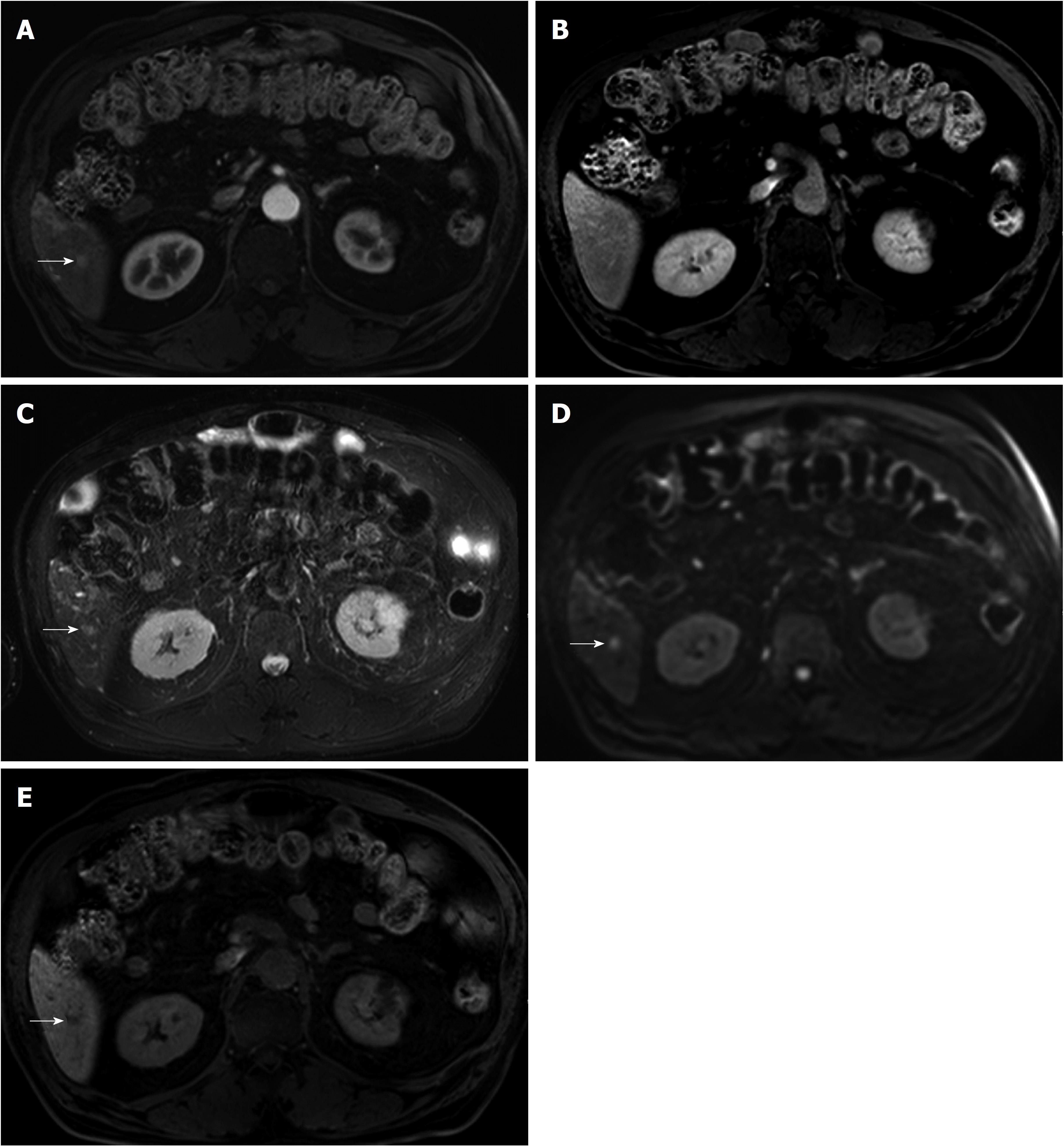
In a recent study[35], 81.3% (13/16) of sub-centimeter nodules meeting the typical five MR findings in patients without a history of HCC were eventually confirmed as HCC. This result suggests that sub-centimeter nodules exhibiting all five diagnostic hallmarks of HCCs on MRI are highly likely to progress to HCC in high-risk patients without a treatment history for HCC. However, great caution is required when applying MRI-based diagnosis of sub-centimeter HCCs to retain high specificity and to avoid overtreatment (Figure 2). Since there are currently no universally accepted imaging criteria for the diagnosis of sub-centimeter HCC, the diagnostic criteria using gadoxetic acid-enhanced MRI may warrant further validation, especially in patients without a treatment history of HCC. Nevertheless, it is our understanding that sub-centimeter recurrent HCCs can be accurately classified as HCCs based on five typical MR findings in patients with a treatment history of HCC[17,18].
There is controversy regarding the optimal management of sub-centimeter nodules showing typical imaging patterns consistent with HCC[29]. According to updated EASL practice guidelines[29], the panel recommends local multidisciplinary board discussion for the management of patients with sub-centimeter lesions showing typical imaging features of HCCs. Given that the incidence of microscopic vascular invasion or satellite nodules would be rare for sub-centimeter HCC, a wait-and-see policy may be chosen until the tumor grows larger than 1 cm if the patient has no treatment history of HCC. However, it is noteworthy that the cost of intense imaging follows up and patient anxiety cannot be ignored when the wait-and-see strategy is adopted.
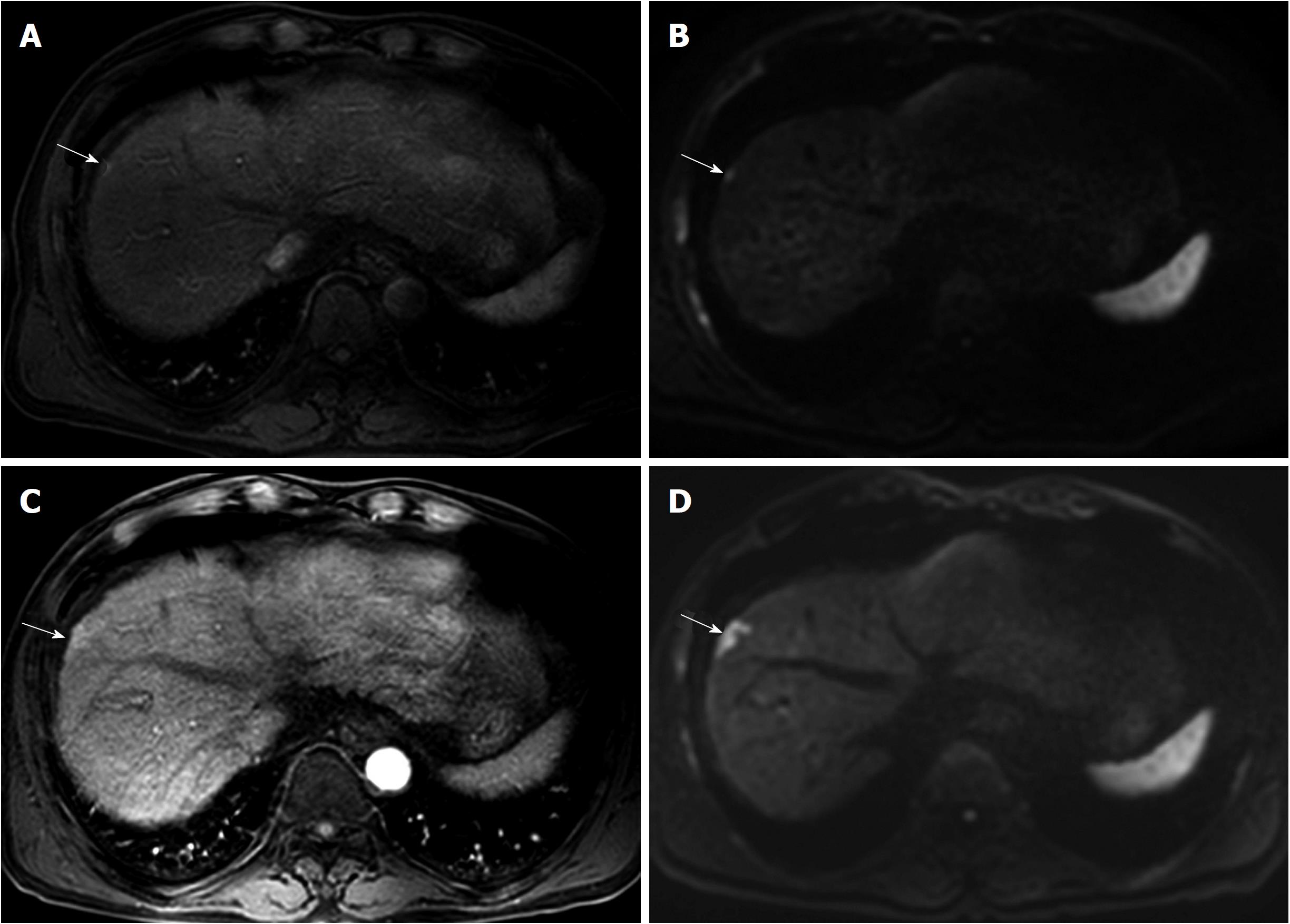
In contrast to treating naïve patients, a wait-and-see policy may not always lead to favorable clinical outcomes in patients with a treatment history of HCC if the recurrent tumor exhibits aggressive tumor biology (Figure 3). Rapid tumor progression with portal vein invasion can occur in patients with sub-centimeter recurrent HCC[17]. As with every tumor, it is preferable to treat recurrent HCC as soon as possible if early treatment can offer better therapeutic outcomes.
Until now, there have been no solid data assessing whether immediate treatment or a wait-and-see strategy should be followed to achieve better outcomes in patients with sub-centimeter recurrent HCC. According to a recent study[36], recurrence-free survival in patients with sub-centimeter HCC did not vary between immediate treatment and wait-and-see groups. However, the previous study is limited by its retrospective study design and small sample size. Of note, treatment outcomes of the immediate treatment group may be affected by lead time bias, meaning that if survival is counted from the date of diagnosis, moving the date of HCC diagnosis earlier artificially inflates patient survival, despite the treatment being ineffective. Therefore, optimal treatment time of sub-centimeter recurrent HCCs warrants prospective evaluation in a well-designed study to determine whether immediate treatment produces better therapeutic outcomes compared to a wait-and-see approach.
If sub-centimeter recurrent HCCs are going to be managed immediately after detection, treatment should be both effective and safe. Until now, treatment algorithms for recurrent sub-centimeter HCC have not been documented in most guidelines on HCC management, as studies dealing with therapeutic efficacy and safety are rare. Applying surgical resection to such small recurrent tumors may be too invasive, especially in patients with a previous history of surgical resection, portal hypertension or poor performance status[37]. In addition, patients with multifocal HCCs may not benefit from surgical resection due to the risk of liver failure.
In contrast to surgical resection, percutaneous RF ablation may play an important role in the management of sub-centimeter recurrent HCCs, as it is effective and safe for the management of patients with small HCCs. However, it is challenging to localize sub-centimeter HCCs under B-mode US guidance, one of the most widely used guiding modalities[38,39]. Currently, more advanced US technologies, such as fusion imaging and CEUS, which are useful for guiding local ablation therapy of small HCCs with poor B-mode US conspicuity, are available in our clinical practice[24,25,27,40,41]. A recent article addressed the technical feasibility and therapeutic outcomes of percutaneous fusion imaging-guided RF ablation for sub-centimeter recurrent HCC[18]. The feasibility of percutaneous RF ablation for sub-centimeter HCCs was 65.7% (138/210). Among 125 patients who were treated with RF ablation, both technical success and technique efficacy rates were as high as 98.4% (123/125). The rates of major complication and local tumor progression were 2.5% (3/119) and 7.3% (9/123), respectively. These results indicate that percutaneous fusion imaging-guided RF ablation is safe and effective for sub-centimeter recurrent HCCs.
However, fusion imaging with or without CEUS is not always satisfactory to localize sub-centimeter HCCs. According to a previous study[18], percutaneous RF ablation was not feasible in 34.3% (72/210) of sub-centimeter recurrent HCCs primarily due to poor lesion conspicuity. Although RF electrodes may be positioned based on anatomic landmarks, such as hepatic vascular structures, technical feasibility of fusion imaging-guided RF ablation is a predictive factor for local tumor progression[24]. Along with US, CT is also widely used for guidance of RF ablation. However, unenhanced CT guidance is of little value in accurately localizing sub-centimeter HCCs[20]. MRI may be used as an alternative guiding modality for sub-centimeter HCCs as it has several advantages, such as near real-time guiding capability using fluoroscopic imaging, higher sensitivity in depicting small hepatic lesions, free selection of imaging planes, monitoring tissue temperature, and absence of ionizing radiation hazard[42,43]. Moreover, according to a recent study[44], planning, applicator placement and therapy monitoring are feasible even without contrast enhancement. For these reasons, MR guidance seems to be promising for the treatment of sub-centimeter HCCs. Feasibility and therapeutic outcomes of MR-guided local ablation therapy of sub-centimeter recurrent HCCs warrant further investigation.
Recently, Hyun et al[45] reported that combined transarterial chemoembolization (TACE) and RF ablation is an effective treatment for HCCs that are not suitable for US-guided RF ablation. Theoretically, intratumoral accumulation of radio-opaque iodized oil used in TACE can make the target tumor visible by either fluoroscopy or CT images. TACE also increases the echogenicity and conspicuity of small HCC on US, as microbubbles generated during emulsion preparation might act as strong sonic reflectors, such as sonographic contrast agents[46]. Therefore, placement of RF electrodes into the tumor would be possible under US, CT, or fluoroscopy guidance. In a previous study[45], as expected, the technical success rate of combined treatment was as high as 99% (93/94) in HCCs 3 cm or smaller. However, more data are needed to assess whether combined treatment is also effective for sub-centimeter HCCs.
Cone-beam CT-guided TACE can also be used as an alternative locoregional therapy for sub-centimeter recurrent HCCs since it provides a high degree of technical feasibility and is safe[37]. High technical feasibility of cone-beam CT-guided TACE may be attributed to its high spatial resolution and capability to select intra-arterial bolus injection of contrast media during CT angiography. Surprisingly, Choi et al[37] reported that initial follow up CT images after cone-beam CT-guided TACE showed complete response in all 57 patients with probable sub-centimeter HCCs. In addition, the 1-, 2-, and 3-year local tumor progression rates were 10.4%, 21.7%, and 35.7%, respectively. Furthermore, cone-beam CT hepatic angiography provides higher detectability of small hypervascular HCCs than does multi-detector CT, and additional small hypervascular HCCs missed on CT are frequently detected with cone-beam CT hepatic angiography[37,47]. The high technical feasibility and detectability of obscured lesions using cone-beam CT are major advantages over percutaneous US-guided RF ablation, in which technical feasibility is affected by tumor location and lesion conspicuity on US. Further studies are warranted to evaluate the therapeutic efficacy of cone-beam CT-guided TACE compared with other treatment options, such as RF ablation.
Stereotactic body radiation therapy (SBRT) may also be used as an alternative locoregional therapy for patients with small HCCs. Lewandowski et al[48] recently reported that this method provides response rates, tumor control, and survival outcomes comparable to curative-intent treatments for selected patients with early-stage HCC who have preserved liver function. Until now, however, there have been insufficient clinical data regarding treatment outcomes after SBRT for sub-centimeter HCCs in the literature.
Sub-centimeter recurrent tumors that develop after successful curative treatment of HCC can now be accurately diagnosed based on typical hallmark findings using gadoxetic acid-enhanced liver MRI. Local ablation therapy using fusion imaging and/or CEUS guidance is effective and safe for the management of sub-centimeter recurrent HCC. However, local ablation therapy is not always feasible due to tumor location and lesion conspicuity on US. Cone-beam CT-guided TACE may also play an important role for the treatment of sub-centimeter recurrent HCC due to its high technical feasibility and safety profile. Although there are insufficient data, other treatment modalities, such as SBRT, may be applicable for the management of patients with sub-centimeter recurrent HCCs with curative intent. Further comparative studies are warranted to determine the best treatment of choice for recurrent sub-centimeter HCCs and to establish treatment algorithms for recurrent sub-centimeter HCC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kim JH, Pan GD, Sartori S S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Guglielmi A, Ruzzenente A, Valdegamberi A, Pachera S, Campagnaro T, D’Onofrio M, Martone E, Nicoli P, Iacono C. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2008;12:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi K, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology. 2008;55:2171-2174. [PubMed] |
| 3. | Lupo L, Panzera P, Giannelli G, Memeo M, Gentile A, Memeo V. Single hepatocellular carcinoma ranging from 3 to 5 cm: radiofrequency ablation or resection? HPB (Oxford). 2007;9:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, Yang J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RKG, Ehman EC, Fowler KJ, Hussain HK, Jha RC. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology. 2018;286:29-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 227] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 6. | Kim JE, Kim SH, Lee SJ, Rhim H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:W758-W765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, Choi D, Rhim H. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Yagi R, Midorikawa Y, Moriguchi M, Nakayama H, Aramaki O, Yamazaki S, Higaki T, Takayama T. Liver resection for recurrent hepatocellular carcinoma to improve survivability: a proposal of indication criteria. Surgery. 2018;163:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016;22:7-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 10. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 11. | European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4519] [Article Influence: 347.6] [Reference Citation Analysis (2)] |
| 12. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3596] [Article Influence: 276.6] [Reference Citation Analysis (4)] |
| 13. | Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015;9:267-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 15. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 841] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 16. | Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 363] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 17. | Song KD, Kim SH, Lim HK, Jung SH, Sohn I, Kim HS. Subcentimeter hypervascular nodule with typical imaging findings of hepatocellular carcinoma in patients with history of hepatocellular carcinoma: natural course on serial gadoxetic acid-enhanced MRI and diffusion-weighted imaging. Eur Radiol. 2015;25:2789-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Song KD, Lee MW, Rhim H, Kang TW, Cha DI, Sinn DH, Lim HK. Percutaneous US/MRI Fusion-guided Radiofrequency Ablation for Recurrent Subcentimeter Hepatocellular Carcinoma: Technical Feasibility and Therapeutic Outcomes. Radiology. 2018;288:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Kim YS, Lim HK, Rhim H, Lee MW. Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Park BJ, Byun JH, Jin YH, Won HJ, Shin YM, Kim KW, Park SJ, Kim PN. CT-guided radiofrequency ablation for hepatocellular carcinomas that were undetectable at US: therapeutic effectiveness and safety. J Vasc Interv Radiol. 2009;20:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Lee MW, Rhim H, Cha DI, Kim YJ, Lim HK. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1-3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol. 2013;24:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Song KD, Lee MW, Rhim H, Cha DI, Chong Y, Lim HK. Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol. 2013;201:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Toshikuni N, Takuma Y, Tomokuni J, Yamamoto H. Planning Sonography Using Real-time Virtual Sonography and Contrast-enhanced Sonography for Radiofrequency Ablation of Inconspicuous Hepatocellular Carcinoma Nodules. Hepatogastroenterology. 2015;62:661-666. [PubMed] |
| 24. | Ahn SJ, Lee JM, Lee DH, Lee SM, Yoon JH, Kim YJ, Lee JH, Yu SJ, Han JK. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017;66:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Mauri G, Cova L, De Beni S, Ierace T, Tondolo T, Cerri A, Goldberg SN, Solbiati L. Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol. 2015;38:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 26. | Masuzaki R, Shiina S, Tateishi R, Yoshida H, Goto E, Sugioka Y, Kondo Y, Goto T, Ikeda H, Omata M. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Lee MW, Lim HK, Rhim H, Cha DI, Kang TW, Song KD, Min JH, Gwak GY, Kim S, Lu DSK. Percutaneous Radiofrequency Ablation of Small (1-2 cm) Hepatocellular Carcinomas Inconspicuous on B-Mode Ultrasonographic Imaging: Usefulness of Combined Fusion Imaging with MRI and Contrast-Enhanced Ultrasonography. Can J Gastroenterol Hepatol. 2018;2018:7926923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4102] [Article Influence: 586.0] [Reference Citation Analysis (6)] |
| 29. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6056] [Article Influence: 865.1] [Reference Citation Analysis (3)] |
| 30. | Elsayes KM, Hooker JC, Agrons MM, Kielar AZ, Tang A, Fowler KJ, Chernyak V, Bashir MR, Kono Y, Do RK. 2017 Version of LI-RADS for CT and MR Imaging: An Update. Radiographics. 2017;37:1994-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 31. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1643] [Article Influence: 205.4] [Reference Citation Analysis (0)] |
| 32. | Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, Han JK, Choi BI. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 393] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 33. | Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW, Han JK, Choi BI. Small (≤ 1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014;271:748-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Heverhagen JT, Kudrnová S. Subcentimeter Hepatocellular Carcinoma: A New Paradigm for Treatment with Percutaneous Radiofrequency Ablation? Radiology. 2018;288:887-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Park CJ, An C, Park S, Choi JY, Kim MJ. Management of subcentimetre arterially enhancing and hepatobiliary hypointense lesions on gadoxetic acid-enhanced MRI in patients at risk for HCC. Eur Radiol. 2018;28:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Woo JH, Song KD, Kim SH. Subcentimeter hypervascular nodules with typical imaging findings of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: Outcomes of early treatment and watchful waiting. Eur Radiol. 2017;27:4406-4414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Choi JW, Kim HC, Lee JH, Yu SJ, Cho EJ, Kim MU, Hur S, Lee M, Jae HJ, Chung JW. Cone Beam CT-Guided Chemoembolization of Probable Hepatocellular Carcinomas Smaller than 1 cm in Patients at High Risk of Hepatocellular Carcinoma. J Vasc Interv Radiol. 2017;28:795-803.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Kim PN, Choi D, Rhim H, Rha SE, Hong HP, Lee J, Choi JI, Kim JW, Seo JW, Lee EJ. Planning ultrasound for percutaneous radiofrequency ablation to treat small (≤ 3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: a multicenter prospective study to assess factors affecting ultrasound visibility. J Vasc Interv Radiol. 2012;23:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Lee MW, Kim YJ, Park HS, Yu NC, Jung SI, Ko SY, Jeon HJ. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010;194:W396-W400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Lee MW, Rhim H, Cha DI, Kim YJ, Choi D, Kim YS, Lim HK. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol. 2012;198:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014;33:227-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Rempp H, Loh H, Hoffmann R, Rothgang E, Pan L, Claussen CD, Clasen S. Liver lesion conspicuity during real-time MR-guided radiofrequency applicator placement using spoiled gradient echo and balanced steady-state free precession imaging. J Magn Reson Imaging. 2014;40:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Anzidei M, Napoli A, Sandolo F, Marincola BC, Di Martino M, Berloco P, Bosco S, Bezzi M, Catalano C. Magnetic resonance-guided focused ultrasound ablation in abdominal moving organs: a feasibility study in selected cases of pancreatic and liver cancer. Cardiovasc Intervent Radiol. 2014;37:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Hoffmann R, Rempp H, Keßler DE, Weiß J, Pereira PL, Nikolaou K, Clasen S. MR-guided microwave ablation in hepatic tumours: initial results in clinical routine. Eur Radiol. 2017;27:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Hyun D, Cho SK, Shin SW, Park KB, Lee SY, Park HS, Do YS. Combined transarterial chemoembolization and radiofrequency ablation for small treatment-naïve hepatocellular carcinoma infeasible for ultrasound-guided radiofrequency ablation: long-term outcomes. Acta Radiol. 2018;59:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Lee MW, Kim YJ, Park SW, Yu NC, Park HS, Jung SI, Jeon HJ. Sequential changes in echogenicity and conspicuity of small hepatocellular carcinoma on gray scale sonography after transcatheter arterial chemoembolization. J Ultrasound Med. 2010;29:1305-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Choi YR, Chung JW, Yu MH, Lee M, Kim JH. Diagnostic accuracy of contrast-enhanced dynamic CT for small hypervascular hepatocellular carcinoma and assessment of dynamic enhancement patterns: Results of two-year follow-up using cone-beam CT hepatic arteriography. PLoS One. 2018;13:e0203940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, Kulik L, Ganger D, Desai K, Thornburg B. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology. 2018;287:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (0)] |