Published online Nov 28, 2018. doi: 10.3748/wjg.v24.i44.5046
Peer-review started: August 17, 2018
First decision: October 14, 2018
Revised: October 27, 2018
Accepted: November 9, 2018
Article in press: November 9, 2018
Published online: November 28, 2018
Processing time: 102 Days and 18.9 Hours
To investigate the prognostic value of preoperative fibrinogen concentration (FIB) and D-dimer-fibrinogen ratio (DFR) in gastrointestinal stromal tumors (GISTs).
The purpose of this study was to retrospectively analyze 170 patients with GISTs who were admitted to our hospital from January 2010 to December 2015. The optimal cutoff values of related parameters were estimated by receiver operating characteristic (ROC) curve analysis. The recurrence free survival (RFS) rate was evaluated using Kaplan-Meier curves. Univariate analysis and multivariate Cox regression models were used to analyze the prognostic factors of GISTs. The relationship between the FIB, D-dimer, DFR, platelet count (PLT), and the clinicopathological features of GISTs was described by the chi-square test or nonparametric rank sum test (Mann-Whitney test).
In ROC analysis, the optimal cutoff values of FIB, D-dimer, DFR, and PLT were 3.24 g/L, 1.24 mg/L, 0.354, and 197.5 (× 109/L), respectively. Univariate analysis and the Kaplan-Meier survival curve showed that FIB, D-dimer, DFR, PLT, National Institutes of Health (NIH) risk category, tumor size, tumor location, and mitotic index were significantly relevant to the 3-year and 5-year survival rate of patients (P < 0.05). Cox multivariate regression analysis illustrated that FIB (RR: 0.108, 95%CI: 0.031-0.373), DFR (RR: 0.319, 95%CI: 0.131-0.777), and NIH risk category (RR: 0.166, 95%CI: 0.047-0.589) were independent prognostic factors of the RFS rate (P < 0. 05). Moreover, FIB, D-dimer, DFR, and PLT were correlated with the clinical features of GISTs.
FIB, D-dimer, DFR, and PLT are all related to the prognosis of GISTs. Moreover, FIB and DFR may be independent risk factors for predicting the prognosis of resectable GISTs.
Core tip: For patients with gastrointestinal stromal tumors (GISTs), postoperative recurrence and metastasis are the main factors affecting survival. Moreover, recurrence and metastasis mainly occur in moderate and high-risk patients. Therefore, it is necessary to screen these patients for adjuvant treatment at an early stage. Fibrinogen (FIB) and D-dimer were reported to be associated with the prognosis of many tumors. The purpose of this study was to investigate the value of preoperative FIB, D-dimer, the D-dimer-fibrinogen ratio (DFR), and platelets in the prognosis of GISTs. The results showed that FIB and DFR were independent risk factors for predicting the prognosis of primary resectable GIST.
- Citation: Cai HX, Li XQ, Wang SF. Prognostic value of fibrinogen and D-dimer-fibrinogen ratio in resectable gastrointestinal stromal tumors. World J Gastroenterol 2018; 24(44): 5046-5056
- URL: https://www.wjgnet.com/1007-9327/full/v24/i44/5046.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i44.5046
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms originating from the gastrointestinal tract[1,2]. GISTs may occur anywhere in the digestive tract, including outside the gastrointestinal tract[1]. The incidence of GISTs is 60%-70% in the stomach, and 30% in the small intestine[3]. However, GISTs occurring in areas such as the colon, rectum, and esophagus are rare. The main treatment for primary localized GISTs is margin negative complete resection[1]. However, the recurrence or metastasis of the original disease after the operation are an obstacle to prolonging the survival period. It was reported that approximately 50% of the patients who undergo surgery alone will have tumor recurrence, and the median survival after recurrence is less than 2 years[4]. Unfortunately, if GISTs recur or are metastatic, the value of the operation is low. In addition, the expert consensus on GISTs in China indicates that patients in the intermediate category or high category who meet the 2008 revision risk classification standard should carry out corresponding auxiliary treatment[3]. Therefore, early screening of middle and high category patients with adjuvant therapy can significantly improve the prognosis of patients. However, the current accepted risk classification criteria for predicting the prognosis of GIST patients require pathological results to be obtained. In this case, it is important to develop some simple, noninvasive methods to accurately screen high-risk populations of GISTs and provide early adjuvant therapy to improve their prognosis.
Thrombocytopenia and coagulation abnormalities are very common in cancer patients. Research shows that cancer is a prothrombotic state, and much evidence points to a role for the fibrinogen-platelet axis in tumor biology[4]. Fibrinogen (FIB) is a glycoprotein produced mainly by liver cells, which is an important coagulation factor and contributes to the regulation of blood coagulation pathways[5]. Moreover, FIB can promote cell adhesion and inflammation in the process of coagulation. Additionally, recent evidence suggests that tumors with elevated FIB levels are more likely to develop invasion and metastasis[6,7], including esophageal, gastric, and colorectal carcinomas[8]. In the coagulation system, FIB can be transformed into fibrin by thrombin, and the end product D-dimer of fibrin is increased in cases such as colorectal cancer, lymph node metastasis, and vascular invasion[9]. In addition, it was reported that the combination of FIB and the neutrophil-lymphocyte ratio could predict tumor progression and prognosis in gastric cancer patients[8]. At the same time, D-dimer can also help predict the prognosis of metastatic gastric cancer after chemotherapy[10].
Based on the above results, we hypothesized that plasma FIB, D-dimer, and platelet count (PLT) may be associated with clinical outcomes in patients with cancer. However, as far as we know, studies assessing the prognostic value of plasma FIB, D-dimer, and PLT in patients with GISTs are rarely reported.
The aim of this study was to investigate the relationship between preoperative FIB, D-dimer, the D-dimer-FIB ratio (DFR), PLT values, and clinicopathologic characteristics and to evaluate the prognostic value of these markers in GISTs.
The research institution of this study was the First Affiliated Hospital of Xi’an Jiaotong University. One hundred and seventy patients with GISTs were treated at our department from January 1, of 2010 to December 31, 2015. Clinicopathological parameters and follow-up data were assessed for all the GIST patients (91 men and 79 women) who received initial curative surgical resection. All the patients were pathologically confirmed with GISTs. The demographic data and clinicopathologic features of each patient were collected. The average age of the patients was 61 years.
All enrolled patients must meet the following criteria: (1) the first diagnosis was primary resectable GIST; (2) complete blood test results can be obtained before treatment; (3) surgical treatment was performed, and imatinib was not administered preoperatively; and (4) there were complete postoperative follow-up data.
Patients with hematological diseases, other tumor types, the use of coagulation and anticoagulation drugs for 8 weeks, incomplete blood test results, or with myocardial infarction, cerebral infarction, and other diseases were excluded.
The preoperative assessment of GISTs was performed by abdominal or pelvic CT, magnetic resonance imaging (MRI), gastrointestinal endoscopy, or endoscopic ultrasound. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University, and all the patients signed an informed consent form before the operation.
We collected the data of all patients in the study, including patient demographics (age and gender), clinical and pathological features, comorbidities, FIB, D-dimer, PLT, operative factors (type of surgery and extent of lymph node dissection), and tumor characteristics (location, size, lymph node metastasis, mitotic number, distant metastasis, and risk category).
Data collected on preoperative plasma FIB, D-dimer, PLT, and other laboratory indexes are the closest to the results of a test of the time of surgery. It was most important that all the laboratory data were obtained from each patient before breakfast. Venous blood with no evidence of infection was collected. The DFR was calculated as D-dimer (mg/L) divided by fibrinogen concentration (g/L).
Patients were followed once every 3 mo for 2 years, every 6 mo between 2 and 5 years, and then every year thereafter. Follow-ups were either by outpatient or inpatient review, or by contacting patients or their relatives by telephone. For the follow-up of GIST patients after surgery, chest and abdominal CT scan, abdominal (liver and adrenal) ultrasound scan, bone marrow scan and endoscopic biopsy, and positron emission computerized tomography (PET) to exclude recurrence and metastasis were utilized. According to the follow-up program, all patients were followed until the deadline of December 1, 2016 or the death of the patient. Metastatic recurrence revealed by imaging and death were considered as the end point events.
The frequency and percentage are used to represent the patient’s baseline characteristics. The optimal values of FIB, D-dimer, DFR, and PLT were determined using receiver operating characteristic (ROC) curves[1]. The patients were divided into high and normal groups by the optimal values. The area under the curve was determined by ROC curve analysis, and the 95% confidence interval (CI) was determined. The correlation of tests, including binary classification variables, was performed using the Chi-square test or nonparametric rank sum test (Mann-Whitney test). Recurrence free survival (RFS) was defined as the time from the surgery to clinical or imaging evidence of recurrence for the first time. The Kaplan-Meier method was used to estimate the survival curve of RFS, and the log-rank test was used to evaluate the difference between groups. Univariate analysis was used to analyze the risk factors influencing the RFS of GISTs. A multivariate Cox proportional hazards regression model was used to identify the independent risk factors affecting RFS, with the risk ratio (RR) and the corresponding 95%CI calculated. The meaningful indicators found in univariate analysis were further evaluated by a multivariate Cox proportional hazards regression model (Forward stepwise method - conditional likelihood ratio). The Cox regression equation was as follows: h (t, x) = h0 (t) exp (β1x1 + β2x2 + … + βpxp), where x is covariate with time, h (t, x) is a risk function that is the individual with a covariate x function of risk on the t moment, h0 (t) is the baseline hazard function, and βI ( I = 1, 2 ,…, p) is population regression. The prognostic index (PI) model was as follows: PI = β1x1 + β2x2 + … + βpxp.
All data were statistically analyzed using SPSS software (version 18.0; SPSS Inc., Chicago, IL, United States). All tests were two-sided, and P-values below 5% were considered statistically significant.
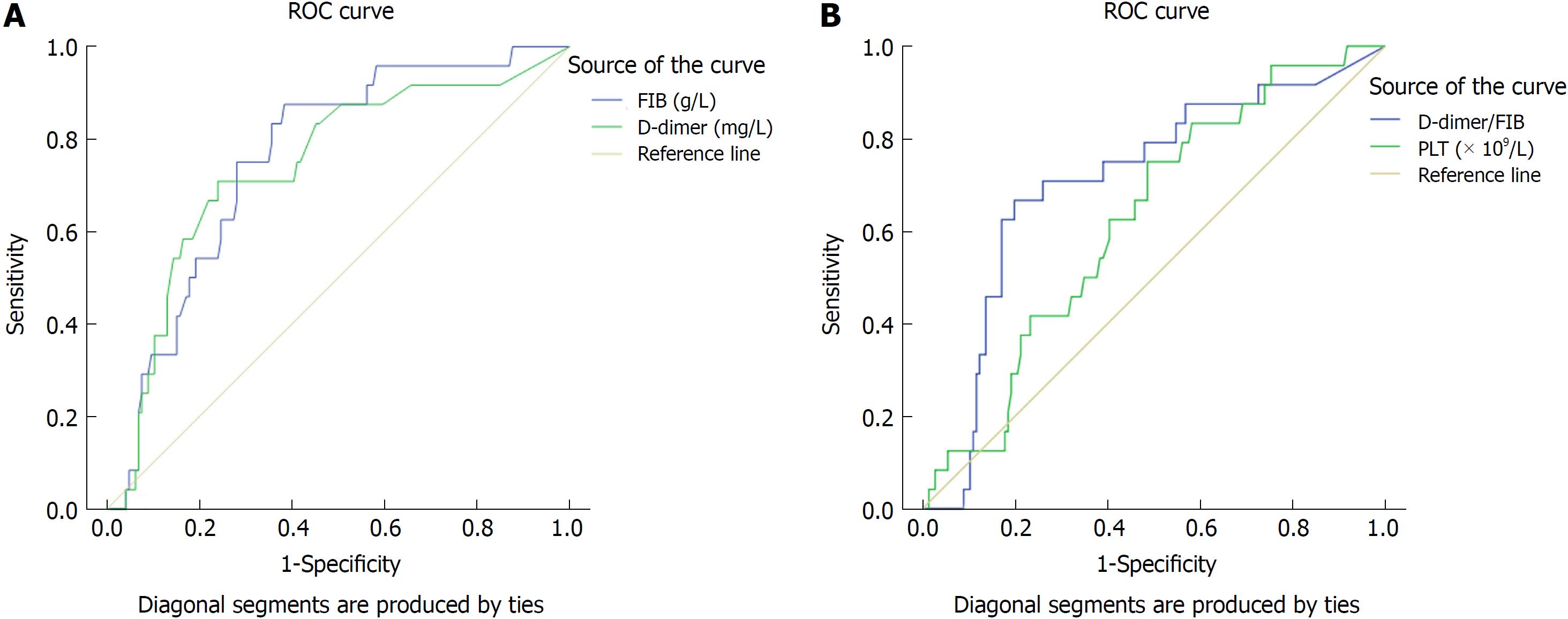
The optimal cutoff values of FIB, D-dimer, DFR, and PLT were determined using ROC curves. The areas under the ROC curves for FIB, D-dimer, DFR and PLT were 0.758 (95%CI: 0.666-0. 850; P < 0.01), 0.739 (95%CI: 0.629-0. 850; P < 0.01), 0.709 (95%CI: 0.596-0. 822; P = 0.001), and 0.625 (95%CI: 0.517-0. 733; P = 0.050), respectively (Figure 1). For all GIST patients, FIB = 3.24 g/L, D-dimer = 1.24 mg/L, DFR = 0.354, and PLT = 197.5 (× 109/L) had the highest sensitivity (87.5%, 70.8%, 66.7%, and 75%) and specificity (61.6%, 76%, 80.1%, and 51.4%), respectively (Table 1).
| Variable | Area | SE | P value | 95%CI |
| FIB (g/L) | 0.758 | 0.047 | < 0.01 | 0.666-0.850 |
| D-dimer (mg/L) | 0.739 | 0.056 | < 0.01 | 0.629-0.850 |
| D-dimer/FIB | 0.709 | 0.058 | 0.001 | 0.596-0.822 |
| PLT (109/L) | 0.625 | 0.055 | 0.05 | 0.517-0.733 |
All 170 patients in this study were confirmed with GISTs by pathology. The age of the study population ranged from 19 to 80 years, with a median age of 61 years. In all patients, there were 91 male cases, accounting for 53.5%; the male to female ratio was 1.15:1. The most common site of GISTs was the stomach (122 cases), accounting for 71.76%, followed by the small intestine (20%; 34/170), colon and rectum (5.88%; 10/170), pelvic cavity (1.76%; 3/170), and esophagus (0.59%; 1/170). The median diameter of tumors in this study was 5 cm, with a minimum of 0.5 cm and a maximum of 29 cm. According to the revised NIH (National Institutes of Health) risk category of 2008[10], of the total 170 patients, 18 were at very low risk (10.59%), 65 at low risk (38.24%), 37 at intermediate risk (21.76%), and 50 at high risk (29.41%). The mitotic count was more than 5/50 high power fields (HPFs) in 125 (73.53%) patients. There were 48 (55.17%, 48/87) patients receiving adjuvant imatinib following surgery in the intermediate and high risk categories. During follow-up, 24 patients showed recurrence or metastasis, 15 patients suffered from GIST related deaths, and 1 patient died in a car accident. The 3- and 5-year RFS rates in the 170 patients with GISTs were 85% and 75%, respectively.
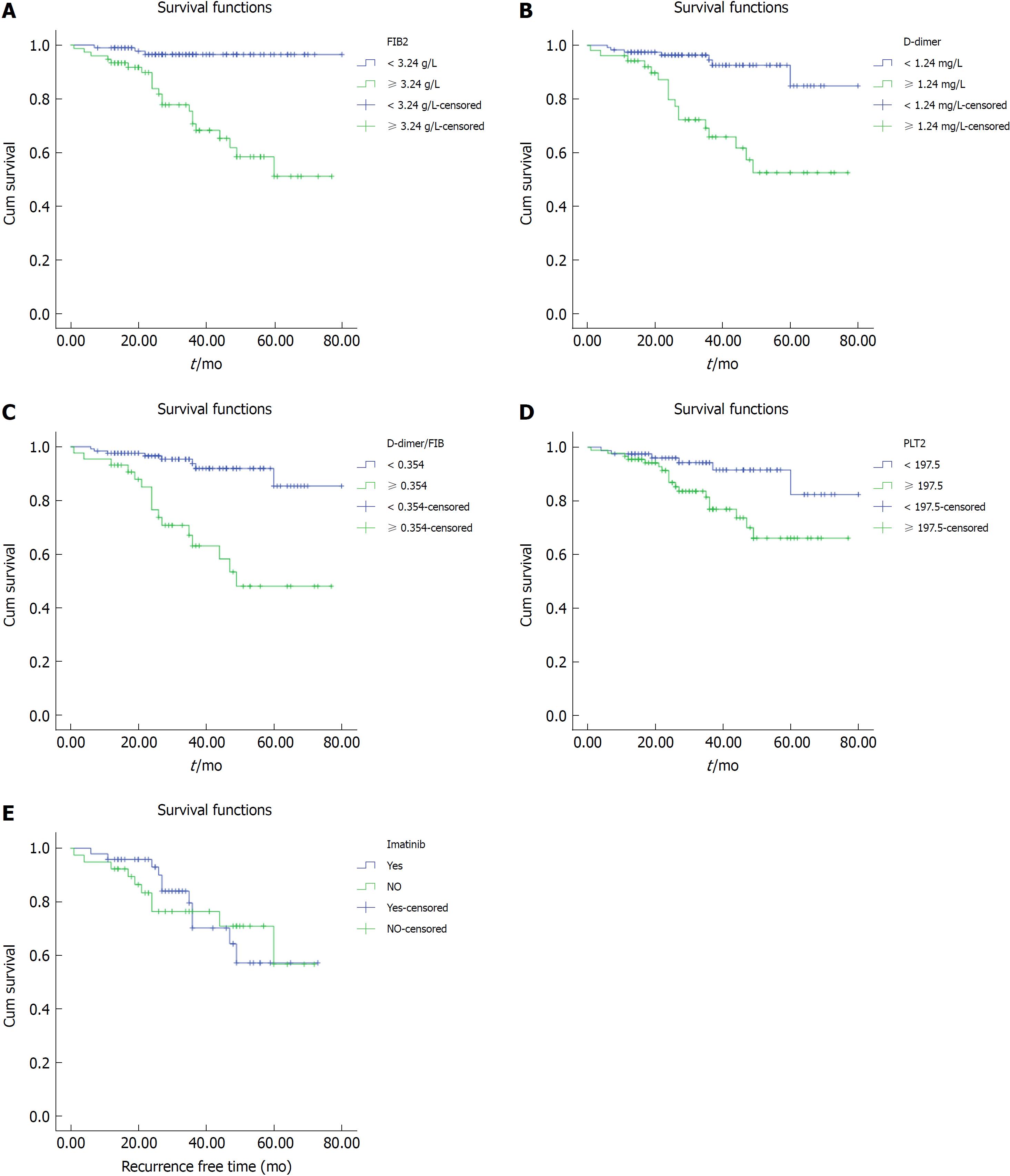
Of the 170 patients in this study, the median RFS survival time was 32 mo (1 mo to 80 mo) postoperatively. Univariate analyses of demographic and clinicopathologic factors were performed to assess the prognostic factors associated with survival. Univariate analysis showed that gender (male and female) and age (< 61 years vs ≥ 61 years) were not relevant to the prognosis of patients with GISTs (P > 0.05). However, other demographic and clinical pathology factors were risk factors that affected the prognosis of the patients. These risk factors included FIB (≥ 3.24 g/L vs < 3.24 g/L, P < 0.01), D-dimer (≥ 1.24 mg/L vs < 1.24 mg/L, P < 0.01), DFR (< 0.354 vs ≥ 0.354, P < 0.01), PLT (≥ 197. 5 × 109/L vs < 197.5 × 109/L, P = 0.014), mitotic index (≤ 5/50 HPFs vs > 5/50 HPFs, P < 0.01), tumor size (≤ 5 cm vs > 5 cm, P < 0.01), tumor location (gastric vs extragastric, P = 0.001), and NIH risk category (very low and low vs intermediate and high, P < 0.01). The results of these univariate analyses are shown in Table 2.
| Characteristic | Number | 3-yr RFS (%) | 5-yr RFS (%) | 95%CI | P value |
| Gender | |||||
| Male | 91 | 80 | 75 | 61.170-72.86 | 0.498 |
| Female | 79 | 90 | 80 | 56.828-65.996 | |
| Age (yr) | |||||
| < 61 | 78 | 85 | 81 | 58.018-66.650 | 0.406 |
| ≥ 61 | 92 | 83 | 78 | 61.508-73.445 | |
| NIH risk category | |||||
| Very low, low | 83 | 97 | 95 | 73.805-80.329 | < 0.01 |
| Intermediate, high | 87 | 80 | 65 | 50.488-62.245 | |
| Tumor size (cm) | |||||
| ≤ 5 | 97 | 95 | 95 | 75.130-80.262 | < 0.01 |
| > 5 | 73 | 63 | 55 | 49.388-63.256 | |
| Tumor location | |||||
| Gastric | 122 | 92 | 85 | 67.968-76.637 | 0.001 |
| Extragastric | 48 | 76 | 55 | 42.703-56.229 | |
| FIB (g/L) | |||||
| < 3.24 | 95 | 95 | 95 | 75.248-80.253 | < 0.01 |
| ≥ 3.24 | 75 | 65 | 50 | 48.878-62.958 | |
| D-dimer (mg/L) | |||||
| < 1.24 | 118 | 95 | 88 | 70.09-88.603 | < 0.01 |
| ≥ 1.24 | 52 | 65 | 55 | 46.163-62.647 | |
| DFR | |||||
| < 0.354 | 126 | 95 | 85 | 70.237-78.220 | < 0.01 |
| ≥ 0.354 | 44 | 70 | 50 | 43.262-61.237 | |
| PLT (× 109/L) | |||||
| < 197.5 | 81 | 92 | 85 | 68.060-78.540 | 0.014 |
| ≥ 197.5 | 89 | 76 | 68 | 55.307-67.619 | |
| Mitotic index | |||||
| ≤ 5/50 HPFs | 125 | 95 | 90 | 70.431-77.808 | < 0.01 |
| > 5/50 HPFs | 45 | 65 | 35 | 39.663-55.513 | |
| Adjuvant imatinib1 | |||||
| Yes | 48 | 70 | 55 | 48.468-64.287 | 0.940 |
| No | 39 | 75 | 55 | 41.127-64.055 |
We divided the patients into a normal group and a high group based on serum FIB level, D-dimer, DFR, and PLT, respectively, and investigated the relationship between the above parameters and the prognosis of GIST patients. We found that patients with high preoperative FIB, D-dimer, DFR, or PLT had a shorter RFS than normal controls (Figure 2A-D). In the first 3 years, the survival time of patients who received the adjuvant imatinib was significantly longer than that of the untreated in the intermediate and high risk category group (Figure 2E).
In addition, extragastric tumors, tumors larger than 5 cm, intermediate-high NIH risk category, and mitotic image count above 5/50 HPFs were associated with a poorer prognosis (log-rank, P < 0.01) (Table 2).
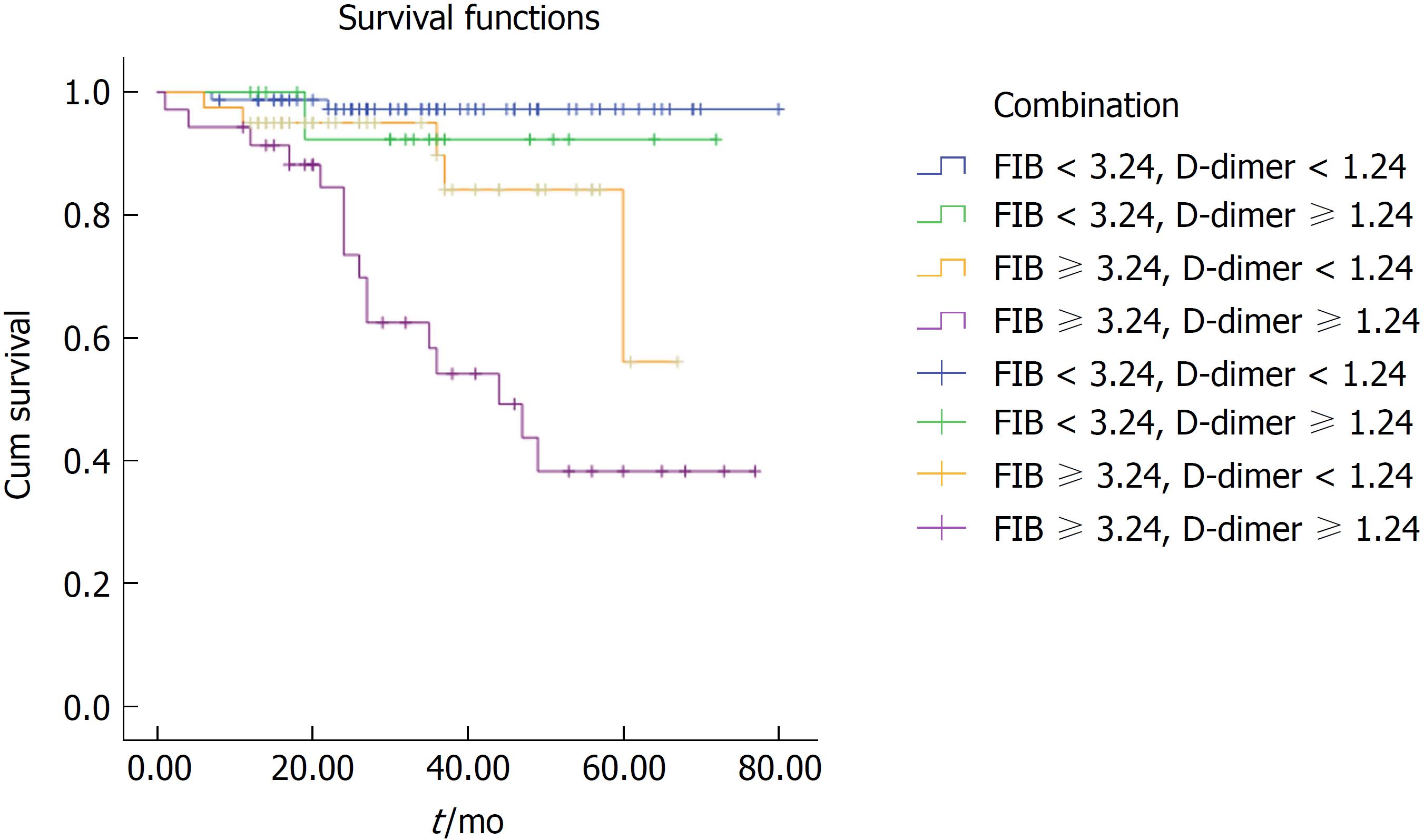
This study also evaluated the prognostic value of FIB concentration combined with D-dimer in patients with GISTs, as well as its clinical applicability and clinical value. We divided GIST patients into four groups based on the following criteria: low FIB and low D-dimer; low FIB and high D-dimer; high FIB and low D-dimer; and high FIB and high D-dimer. The prognosis of the high FIB and high D-dimer group was significantly worse than that of the low FIB and/or low D-dimer group (38% vs 95% vs 92% vs 82%, P < 0. 01, Figure 3).
To determine the independent risk factors for GIST patients, we used the Cox proportional hazard model to assess the outcome.
Multivariate analysis was used to further analyze the risk factors affecting the prognosis of GISTs in univariate analysis. Factors in the multivariate analysis included FIB levels, D-dimer levels, DFR, PLT, tumor size, tumor location, and NIH risk category. The results showed that FIB (RR: 0.131, 95%CI: 0.039-0.443, P = 0.001), DFR (RR: 0.334, 95%CI: 0.139-0.802, P = 0.014), and NIH risk category (RR: 0.206, 95%CI: 0.059-0.711, P = 0.012) were independent risk factors for the prognosis of GISTs (Table 3).
| B | SE | Wald | P value | RR (95%CI) | |
| FIB | -2.035 | 0.622 | 10.692 | 0.001 | 0.131 (0.039-0.443) |
| Risk | -1.582 | 0.633 | 6.249 | 0.012 | 0.206 (0.059-0.711) |
| DFR | -1.097 | 0.447 | 6.022 | 0.014 | 0.334 (0.139-0.802) |
The Cox regression formula for the present study was h (t, x ) = h0 (t) exp (2.035 FIB + 1. 097 DFR + 1.582 NIH risk category). The PI of the present study was PI = 2.035 FIB + 1.097 DFR + 1.582 NIH risk category.
To exclude confounding the analyses by the treatment of GIST patients with the tyrosine kinas inhibitor imatinib, we recalculated the RFS of GISTs by a hierarchy of whether patients received adjuvant imatinib or not after surgery. This multivariate analysis indicated that FIB, DFR, and NIH risk category (RR: 0.108, 95%CI: 0.031-0.373, P < 0. 01; RR: 0.319, 95%CI: 0.131-0.777, P = 0.012; RR: 0.166, 95%CI: 0.047-0.589, P = 0.005; respectively) were still independent risk factors associated with GIST prognosis, as shown in Table 4. The Cox proportional regression model was h (t, x) = h0 (t) exp (2.223 FIB + 1.141 DFR + 1.795 NIH risk category). The PI of the present study was PI = 2.223 FIB + 1.141 DFR + 1.795 NIH risk category.
| B | SE | Wald | P value | RR (95%CI) | |
| FIB | -2.223 | 0.632 | 12.385 | 0.000 | 0. 108 (0.031-0.373) |
| Risk | -1.795 | 0.645 | 7.736 | 0.005 | 0.166 (0.047-0.589) |
| DFR | -1.141 | 0.454 | 6.325 | 0.012 | 0.319 (0.131-0.777) |
The clinicopathologic features of high and low FIB, D-dimer, DFR and PLT GIST patients were analyzed and are summarized in Tables 5-8, respectively. The results showed that age, sex, tumor location, tumor size, NIH risk category, and mitotic index were correlated with the above indexes (P < 0.05). This finding indicated that the correlation between the above parameters and prognosis may be attributed to their correlation with tumor size, mitotic index, and NIH risk category.
| Characteristic | Low FIB (< 3.24 g/L) | High FIB (≥ 3.24 g/L) | P value |
| Age (yr) | < 0.01 | ||
| < 61 | 78 | 0 | |
| ≥ 61 | 17 | 75 | |
| Gender | < 0.01 | ||
| Male | 91 | 0 | |
| Female | 4 | 75 | |
| Location | < 0.01 | ||
| Gastric | 95 | 27 | |
| Nongastric | 0 | 48 | |
| Tumor size (cm) | < 0.01 | ||
| ≤ 5 | 95 | 2 | |
| > 5 | 0 | 73 | |
| NIH risk category | < 0.01 | ||
| Very low | 18 | 0 | |
| Low | 65 | 0 | |
| Intermediate | 12 | 25 | |
| High | 0 | 50 | |
| Mitotic index | < 0.01 | ||
| ≤ 5/50 HPFs | 95 | 30 | |
| > 5/50 HPFs | 0 | 45 |
| Characteristic | Low D-dimer (< 1.24 mg/L) | High D-dimer (≥ 1.24 mg/L) | P value |
| Age (yr) | < 0.01 | ||
| < 61 | 78 | 0 | |
| ≥ 61 | 40 | 52 | |
| Gender | < 0.01 | ||
| Male | 91 | 0 | |
| Female | 27 | 52 | |
| Location | < 0.01 | ||
| Gastric | 118 | 4 | |
| Nongastric | 0 | 48 | |
| Tumor size (cm) | < 0.01 | ||
| ≤ 5 | 97 | 0 | |
| > 5 | 21 | 52 | |
| NIH risk category | < 0.01 | ||
| Very low | 18 | 0 | |
| Low | 65 | 0 | |
| Intermediate | 35 | 2 | |
| High | 0 | 50 | |
| Mitotic index | < 0.01 | ||
| ≤ 5/50 HPFs | 118 | 7 | |
| > 5/50 HPFs | 0 | 45 |
| Characteristic | Low DFR (< 0.354) | High DFR (≥ 0.354) | P value |
| Age (yr) | < 0.01 | ||
| < 61 | 78 | 0 | |
| ≥ 61 | 48 | 44 | |
| Gender | < 0.01 | ||
| Male | 91 | 0 | |
| Female | 35 | 44 | |
| Location | < 0.01 | ||
| Gastric | 122 | 0 | |
| Nongastric | 4 | 44 | |
| Tumor size (cm) | < 0.01 | ||
| ≤ 5 | 97 | 0 | |
| > 5 | 29 | 44 | |
| NIH risk category | < 0.01 | ||
| Very low | 18 | 0 | |
| Low | 65 | 0 | |
| Intermediate | 37 | 0 | |
| High | 6 | 44 | |
| Mitotic index | < 0.01 | ||
| ≤ 5/50 HPFs | 125 | 0 | |
| > 5/50 HPFs | 1 | 44 |
| Characteristic | Low PLT (< 197.5 × 109/L) | High PLT (≥ 197.5 × 109/L) | P value |
| Age (yr) | < 0.01 | ||
| < 61 | 78 | 0 | |
| ≥ 61 | 3 | 89 | |
| Gender | < 0.01 | ||
| Male | 81 | 10 | |
| Female | 0 | 79 | |
| Location | < 0.01 | ||
| Gastric | 81 | 41 | |
| Nongastric | 0 | 48 | |
| Tumor size (cm) | < 0.01 | ||
| ≤ 5 | 81 | 16 | |
| > 5 | 0 | 3 | |
| NIH risk category | < 0.01 | ||
| Very low | 18 | 0 | |
| Low | 63 | 2 | |
| Intermediate | 0 | 37 | |
| High | 0 | 50 | |
| Mitotic index | < 0.01 | ||
| ≤ 5/50 HPFs | 81 | 44 | |
| > 5/50 HPFs | 0 | 45 |
It was reported that FIB could strongly predict the prognosis of various malignant tumors, such as lung, stomach, and pancreatic cancer[11]. D-dimer was related to the stage of the tumor in patients with prostate, lung, cervix, ovary, breast, or colorectal cancer[12-14]. PLT was associated with the prognosis of epithelial ovarian carcinoma and pancreatic cancer[4,15]. However, the data for FIB, D-dimer, and PLT predicting the prognosis of primary resectable GISTs are still very limited. We found only one study that examined the role of D-dimer in primary GISTs[16]. That study found that a baseline D-dimer level greater than 1000 ng/mL was inversely associated with total GIST survival rate and progression-free survival rate. Recently, another study found that high levels of FIB were associated with decreased overall survival (OS) and RFS in patients with GISTs[17]. However, as far as we know, there is little research on the value of these markers in the prognosis of patients with GISTs.
In the present study, we explored the clinical association of FIB, D-dimer, DFR, and PLT with pathological features and the prognosis of GISTs. Our purpose was to determine whether the above parameters could be associated with the prognosis of GISTs. We found that preoperative plasma FIB, D-dimer, PLT, NIH risk category, tumor location, tumor size, and mitotic index were associated with RFS in GIST patients who underwent surgical excision. To eliminate the interference of various factors on the predicted results of FIB and D-dimer, we calculated the DFR and found that DFR was also a prognostic indicator of GISTs. In addition, we combined FIB and D-dimer and divided the patients into four groups to show that the prognosis of the high D-dimer and high FIB group was significantly poorer than that of others. By inputting the statistically significant indexes that were found in the univariate analysis results into the Cox proportional hazards models, multiple factor regression analysis indicated that elevated FIB, DFR, and high NIH risk category were independent risk factors for poor prognosis of GISTs. There was also a correlation between the preoperative FIB, D-dimer, DFR, PLT and the clinicopathologic features of GISTs. If we can predict the prognosis of GISTs by using hematology markers such as FIB, D-dimer, DFR, and PLT, especially FIB and DFR, the analysis will be more convenient. We can easily monitor and predict the recurrence and metastasis of patients.
It is well known that the failure of many cancer treatments is closely related to tumor metastasis. A positive correlation between coagulation and tumor metastasis was observed many years ago[18]. Coagulation system abnormalities are usually associated with tumor progression, and the coagulation cascade is often amplified in cancer patients. Approximately 50% of local tumor patients and most metastatic tumor patients have several coagulation factor abnormalities[9,19].
FIB is a plasma protein that plays an important role in the process of coagulation. Some studies have shown that FIB is positively related to cancer progression[20]. The elevated FIB is associated with distant tumor metastasis, suggesting that FIB plays an important role in the adhesion of tumor and vascular metastasis of the target organ. In addition, FIB plays an important role in angiogenesis and tumor cell growth, which may be associated with the promotion of growth factor fusion and cell adhesion, proliferation, and migration. Moreover, platelet fibrin malignancies play an important role in the early development of tumor cells, avoiding the host’s autoimmune surveillance by providing protection[20]. Other research evidence indicates that the FIB fragment promotes tumor progression and metastasis by inhibiting tumor angiogenesis and binding and downregulating the expression of vascular endothelial cells[20]. However, the exact mechanism of the role of FIB in the progression of tumor remains unclear and requires further study.
D-dimer is not only a product of fibrin degradation that is found in blood after the coagulation cascade but also a specific marker for fibrinolysis. Similarly, D-dimer is an important marker of coagulation abnormalities. Studies have indicated that coagulation disorders exist in 90% of cancer patients, such as shortened prothrombin and partial thromboplastin time, and increased factors II, V, VIII, IX, XI, XII, FIB, and fibrin degradation products[11,13]. There are other studies determining whether the level of D-dimer significantly increased in patients with disseminated intravascular coagulation (DIC), deep vein thrombosis, pulmonary embolism, myocardial infarction, cerebral infarction, and other thromboembolic events[18].
In addition, we speculate that another cause of the increase in FIB and D-dimer may be related to the liver because the blood clotting related indicators are produced by the liver. Furthermore, it is not ruled out that the prognostic effect of FIB and DFR on GISTs may be due to the correlation between these indexes and the clinicopathologic features of GISTs.
The relationship between PLT and tumors can be understood by the fact that PLT plays a very important role in tumor vascular growth through various platelet-derived vascular growth factors, and platelet-derived transforming growth factor β (TGF-β) can coactivate TGF-β/Smad and NF-κB pathways in cancer cells, thus promoting tumor metastasis[21].
At present, the prognostic factors of GISTs, such as mitotic count, tumor location, size, rupture, and metastasis[2,22], all depend on postoperative pathological results. Biomarkers to screen for the recurrence or progression of GISTs are still limited, but many studies support the notion that the prognosis of tumors can be judged using tumor biomarkers[13]. For example, marker CA-153 is associated with the recurrence and metastasis of breast cancer, CEA is used to monitor the treatment of colorectal cancer and gastric cancer, and CA-199 is associated with ovarian cancer.
Moreover, in most hospitals, the tests for plasma FIB, D-dimer, and PLT have been included in conventional preoperative coagulation and routine blood tests, and the values are easy to obtain. Based on this, it is easy for clinicians to use FIB, D-dimer, and PLT as prognostic markers in GIST patients. Therefore, before an operation, we could roughly predict the prognosis of GISTs by hematological indicators, to give early intervention therapy, and thus obtain a better prognosis. Especially for those patients who cannot obtain pathological results, it is more significant to use these hematological indicators to predict the prognosis of GISTs. Furthermore, combining these hematological parameters with the clinical features of GISTs can significantly improve the prognostic evaluation of GISTs.
This study has some limitations. First, it is a single center, retrospective, nonrandomized, controlled study. More prospective research is needed to verify the predictive value of these indicators. Second, its small sample size may cause some bias; therefore, a larger sample size study is required to further validate our results. Third, this study also included a number of deleted cases that did not survive for 5 years after surgery, which may lead to bias in the survival analysis. Fourth, some of the NIH risk categories of high risk GIST patients in this study failed to receive adjuvant treatment or to complete adjuvant treatment as a result of their high drug costs or adverse drug reactions. Furthermore, we only analyzed the predictive value of these indicators in primary resectable and preoperative GISTs without adjuvant medication, and the predictive value of these markers in patients receiving adjuvant imatinib and nonoperative GISTs needs further study.
However, considering the low cost and ease of operation of hematological testing, plasma FIB, D-dimer, DFR, and PLT should be considered as prognostic indicators for GIST patients, especially in developing countries. It is known that clinicians are better at screening patients requiring adjuvant therapy and formulating targeted general treatment and monitoring programs. Nevertheless, this finding also requires a large, prospective study to further confirm.
In light of these results, it is concluded that preoperative plasma FIB, D-dimer, DFR, and PLT can be used as effective hematological biomarkers for monitoring the prognosis of GIST patients that do not require special measuring devices. It was also indicated that clinicians should obtain FIB, D-dimer, DFR, and PLT values as part of routine care. In particular, the value of FIB and DFR in predicting the prognosis of GISTs should be emphasized. These values should be added to the currently accepted preoperative risk categories, such as size, primary tumor site, and genetic mutation.
It is well known that moderate and high-risk gastrointestinal stromal tumor (GIST) patients have a high recurrence rate and need adjuvant targeted therapy to improve prognosis. Therefore, early screening of this protion of patients to give adjuvant treatment is particularly important. At present, the prediction of GISTs is obtained by postoperative pathology, and there is no effective index to predict the prognosis of GISTs before operation. The purpose of this retrospective study was to investigate the role of fibrinogen (FIB), D-dimer-fibrinogen ratio (DFR) in the prognosis of GISTs before operation.
The increase of blood coagulation indexes such as fibrinogen content and D-dimer before operation can predict the adverse prognosis of many kinds of cancer, but there is little discussion about the relationship between these indexes and GISTs.
The retrospective study analyzed the role of FIB, D-dimer, DFR, and platelet count (PLT) in the prognosis of GISTs before operation.
This study included 170 patients with GISTs who met the criteria. The data of all the patients were collected before and after operation to make statistical analysis. All data were analyzed using the SPSS 18.0 statistical software, with a P-value below 0.05 considered statistically significant.
In the present study, univariate analysis showed that FIB, D-dimer, DFR, and PLT were correlated with the 3- and 5-year survival rates of GIST patients. In addition, there was a correlation between the clinical features and FIB, D-dimer, DFR, and PLT in GISTs. Moreover, multivariate analysis showed that FIB and DFR had an independent effect on the prognosis of GIST patients.
This retrospective study showed that FIB, D-dimer, DFR, and PLT were the prognostic factors of GISTs, but there was an independent correlation between FIB and DFR and GIST prognosis. These factors may help screen out high-risk patients early and to administer adjuvant intervention as soon as possible.
For patients with GISTs, the prognosis can be preliminarily estimated according to hematological indexes such as FIB and DFR before the operation, and adjuvant therapy can be given early to improve the prognosis of patients. Of course, the results of this study need to be further verified by a large sample size prospective study.
ACKNOWLEDGMENTS
Thank you to all those who contributed to the design and modification of this article. The authors are responsible for obtaining written permission to use any copyrighted text and/or illustrations.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Grau JM, Lee SW, Negreanu L S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Yin SY
| 1. | Goh BK, Chok AY, Allen JC Jr, Quek R, Teo MC, Chow PK, Chung AY, Ong HS, Wong WK. Blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independent prognostic factors for surgically resected gastrointestinal stromal tumors. Surgery. 2016;159:1146-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Joensuu H, Eriksson M, Hall KS, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer. 2014;120:2325-2333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, He Y, Liang X, Liu X, Zhou Y. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. 2017;29:281-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 4. | Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Lee SE, Lee JH, Ryu KW, Nam BH, Cho SJ, Lee JY, Kim CG, Choi IJ, Kook MC, Park SR. Preoperative plasma fibrinogen level is a useful predictor of adjacent organ involvement in patients with advanced gastric cancer. J Gastric Cancer. 2012;12:81-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Jiang Z, Zhang J, Li Z, Liu Y, Wang D, Han G. A meta-analysis of prognostic value of KIT mutation status in gastrointestinal stromal tumors. Onco Targets Ther. 2016;9:3387-3398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Zhu JF, Cai L, Zhang XW, Wen YS, Su XD, Rong TH, Zhang LJ. High plasma fibrinogen concentration and platelet count unfavorably impact survival in non-small cell lung cancer patients with brain metastases. Chin J Cancer. 2014;33:96-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Arigami T, Uenosono Y, Matsushita D, Yanagita S, Uchikado Y, Kita Y, Mori S, Kijima Y, Okumura H, Maemura K. Combined fibrinogen concentration and neutrophil-lymphocyte ratio as a prognostic marker of gastric cancer. Oncol Lett. 2016;11:1537-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Kilic L, Yildiz I, Sen FK, Erdem MG, Serilmez M, Keskin S, Ciftci R, Karabulut S, Ordu C, Duranyildiz D. D-dimer and international normalized ratio (INR) are correlated with tumor markers and disease stage in colorectal cancer patients. Cancer Biomark. 2015;15:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Go SI, Lee MJ, Lee WS, Choi HJ, Lee US, Kim RB, Kang MH, Kim HG, Lee GW, Kang JH. D-Dimer Can Serve as a Prognostic and Predictive Biomarker for Metastatic Gastric Cancer Treated by Chemotherapy. Medicine (Baltimore). 2015;94:e951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Yu W, Wang Y, Shen B. An elevated preoperative plasma fibrinogen level is associated with poor overall survival in Chinese gastric cancer patients. Cancer Epidemiol. 2016;42:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 865] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 13. | Tekeşin K, Bayrak S, Esatoğlu V, Özdemir E, Özel L, Melih Kara V. D-Dimer and Carcinoembryonic Antigen Levels: Useful Indicators for Predicting the Tumor Stage and Postoperative Survival. Gastroenterol Res Pract. 2016;2016:4295029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Liu L, Zhang X, Yan B, Gu Q, Zhang X, Jiao J, Sun D, Wang N, Yue X. Elevated plasma D-dimer levels correlate with long term survival of gastric cancer patients. PLoS One. 2014;9:e90547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Wang H, Gao J, Bai M, Liu R, Li H, Deng T, Zhou L, Han R, Ge S, Huang D. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets. 2014;25:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Afshar M, Hamilton P, Seligmann J, Lord S, Baxter P, Marples M, Stark D, Hall PS. Can D-Dimer Measurement Reduce the Frequency of Radiological Assessment in Patients Receiving Palliative Imatinib for Gastrointestinal Stromal Tumor (GIST)? Cancer Invest. 2015;33:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Lu J, Chen S, Li X, Qiu G, He S, Wang H, Zhou L, Jing Y, Che X, Fan L. Gastrointestinal stromal tumors: Fibrinogen levels are associated with prognosis of patients as blood-based biomarker. Medicine (Baltimore). 2018;97:e0568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Diao D, Wang Z, Cheng Y, Zhang H, Guo Q, Song Y, Zhu K, Li K, Liu D, Dang C. D-dimer: not just an indicator of venous thrombosis but a predictor of asymptomatic hematogenous metastasis in gastric cancer patients. PLoS One. 2014;9:e101125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Zhang W, Dang S, Hong T, Tang J, Fan J, Bu D, Sun Y, Wang Z, Wisniewski T. A humanized single-chain antibody against beta 3 integrin inhibits pulmonary metastasis by preferentially fragmenting activated platelets in the tumor microenvironment. Blood. 2012;120:2889-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Lee JH, Hyun JH, Kim DY, Yoo BC, Park JW, Kim SY, Chang HJ, Kim BC, Kim TH, Oh JH. The role of fibrinogen as a predictor in preoperative chemoradiation for rectal cancer. Ann Surg Oncol. 2015;22:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1402] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 22. | Li J, Zhang H, Chen Z, Su K. Clinico-pathological characteristics and prognostic factors of gastrointestinal stromal tumors among a Chinese population. Int J Clin Exp Pathol. 2015;8:15969-15976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |