Published online Nov 28, 2018. doi: 10.3748/wjg.v24.i44.4989
Peer-review started: August 24, 2018
First decision: October 5, 2018
Revised: November 7, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: November 28, 2018
Processing time: 95 Days and 17.4 Hours
To investigate the distribution and function of interstitial cells of Cajal (ICCs) and platelet-derived growth factor receptor-α positive (PDGFRα+) cells in the proximal and distal colon.
The comparison of colonic transit in the proximal and distal ends was performed by colonic migrating motor complexes (CMMCs). The tension of the colonic smooth muscle was examined by smooth muscle spontaneous contractile experiments with both ends of the smooth muscle strip tied with a silk thread. Intracellular recordings were used to assess electrical field stimulation (EFS)-induced inhibitory junction potentials (IJP) on the colonic smooth muscle. Western blot analysis was used to examine the expression levels of ICCs and PDGFRα in the colonic smooth muscle.
Treatment with NG-nitro-L-arginine methyl ester hydrochloride (L-NAME) significantly increased the CMMC frequency and spontaneous contractions, especially in the proximal colon, while treatment with MRS2500 increased only distal CMMC activity and smooth muscle contractions. Both CMMCs and spontaneous contractions were markedly inhibited by NPPB, especially in the proximal colon. Accordingly, CyPPA sharply inhibited the distal contraction of both CMMCs and spontaneous contractions. Additionally, the amplitude of stimulation-induced nitric oxide (NO)/ICC-dependent slow IJPs (sIJPs) by intracellular recordings from the smooth muscles in the proximal colon was larger than that in the distal colon, while the amplitude of electric field stimulation-induced purinergic/PDGFRα-dependent fast IJPs (fIJPs) in the distal colon was larger than that in the proximal colon. Consistently, protein expression levels of c-Kit and anoctamin-1 (ANO1) in the proximal colon were much higher, while protein expression levels of PDGFRα and small conductance calcium-activated potassium channel 3 (SK3) in the distal colon were much higher.
The ICCs are mainly distributed in the proximal colon and there are more PDGFRα+ cells are in the distal colon, which generates a pressure gradient between the two ends of the colon to propel the feces to the anus.
Core tip: The different distributions of interstitial cells of Cajal (ICCs) and platelet-derived growth factor receptor-α positive (PDGFRα+) cells in the different parts of the colon result in a pressure gradient in the colon that propels the feces to the anus. Nitric oxide (NO) is mainly involved in the regulation of contraction through ICCs, and purine mainly participates in relaxation through PDGFRα+ cells. The regulation of the inhibitory transmitter NO and purine through the two kinds of interstitial cells (ICCs and PDGFRα+ cells) is very important for rhythmic colonic migrating motor complexes, which are the main driving force underlying colon transit.
- Citation: Lu C, Huang X, Lu HL, Liu SH, Zang JY, Li YJ, Chen J, Xu WX. Different distributions of interstitial cells of Cajal and platelet-derived growth factor receptor-α positive cells in colonic smooth muscle cell/interstitial cell of Cajal/platelet-derived growth factor receptor-α positive cell syncytium in mice. World J Gastroenterol 2018; 24(44): 4989-5004
- URL: https://www.wjgnet.com/1007-9327/full/v24/i44/4989.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i44.4989
In the gastrointestinal (GI) tract, smooth muscle cell/interstitial cell of Cajal/platelet-derived growth factor receptor-α positive cell (SIP) syncytium consists of interstitial cells of Cajal (ICCs), platelet-derived growth factor receptor-α positive cells (PDGFRα+ cells), and smooth muscle cells (SMCs). ICCs and PDGFRα+ cells are involved in the smooth muscle contraction. Remodeling or damaging of these cells can result in a variety of motor disorders[1]. Cells in the SIP syncytium express many kinds of receptors and ion channels, and conductance changes in any type of cell can induce the excitability or relaxation of the smooth muscle. Although there have been several studies of ICCs and PDGFRα+ cells in the GI tract regarding their location, morphology, function and more, most studies have focused on the small intestine and stomach, with few concerning the colon[2,3]. Therefore, in the present study, we explored the distributions of the two kinds of interstitial cells along with their ion channels and transmission mechanisms for inducing contraction and diastolic reactions at both ends of the colon.
ICCs were first suggested to be GI pacemaker cells in the late 1970s based on electrophysiological and ultrastructural observations[4]. Anoctamin-1 (ANO1), a very important functional protein in ICCs, is a calcium-activated chloride channel that produces pacemaker currents[5]. Another interstitial cell type, PDGFRα+ cells, referred to as “fibroblast-like” cells, express specific small conductance calcium-activated potassium channel 3 (SK3)[6]. When the SK3 channel is activated, large amounts of K+ flow out, causing hyperpolarization and subsequent relaxation of PDGFRα+ cells and downstream SMCs[7]. Consequently, the motility of smooth muscles depends on the balance between excitatory regulation from ICCs and inhibitory regulation from PDGFRα+ cells.
Colonic motility requires the coordination of the enteric nervous system (ENS) and SIP syncytium. The ENS, consisting of excitatory motor neurons and inhibitory motor neurons (IMNs), including nitric oxide synthase (NOS) and purine neurons, is involved in the regulation of colonic movement[8-10]. Mañé et al[11] have reported that nitric oxide (NO) is responsible for continuous smooth muscle relaxation, while purine neurons are responsible for transient relaxation, which may be dominant in colonic propulsion. In addition, colonic migrating motor complexes (CMMCs) known as the main form of the colonic transmission, have been demonstrated that their generation require two conditions: the activation of cholinergic motor neurons and the release of inhibitory neurotransmitters (mainly NO and purines), both of which can act on the SIP syncytium to regulate colonic transmission[12].
It has been reported that electrical field stimulation (EFS) can induce changes in the membrane potential mediated by inhibitory neurotransmitters called the inhibitory junction potential (IJP), and subsequent relaxation reaction[13,14]. IJPs are composed of two components: a fast-transient hyperpolarization (fIJP) and a subsequent slow and sustained hyperpolarization (sIJP)[6,12]. The release of NO is closely related to ICCs, which can influence the generation of spontaneous contractions and the initiation of sIJPs through ICCs[15-17]. On the other hand, fIJPs have been shown to be mediated by purines specifically through the P2Y1 receptor on PDGFRα+ cells[7,14].
Based on these previous reports, we sought to characterize the mechanism of the ENS and SIP in the regulation of colon motility, especially distributions of ICCs and PDGFRα+ cells in the proximal and distal colon according to the response of CMMC, smooth muscle contraction, and intracellular recoding for the blockers of neurotransmitters.
All animals were obtained from the Experimental Animal Center of Shanghai Jiao Tong University School of Medicine. This research rigorously complied with the rules of the Guide for the Care and Use of Laboratory Animals of the Science and Technology Commission of China (STCC Publication No. 2, revised 1988). The protocol was approved by the Committee on the Ethics of Animal Experiments of Shanghai Jiao Tong University School of Medicine (Permit Number: Hu 686-2009). All operations were performed under anesthesia using isopentane, and every manipulation of the experimental animals was performed while simultaneously attempting to maximally relieve any suffering.
Adult male ICR mice were obtained from the Experimental Animal Center of Shanghai Jiao Tong University School of Medicine. The mice, aged 35 d and weighing approximately 30 g, were housed at 22 °C under a 12 h light/dark cycle with free access to water and food.
Mice were sacrificed under general anesthesia induced by inhalant isoflurane overdose followed by cervical dislocation. Then, the abdomen was opened along the ventral midline, and the colon was exposed, removed quickly, and placed into Krebs solution continuously bubbling with a carbonated mixture (5% CO2 and 95% O2). The Krebs solution contained the following components (all concentrations in mmol/L): NaCl, 121.9; NaHCO3, 15.5; KCl, 5.9; MgSO4, 1.2; KH2PO4, 1.2; glucose, 11.5; and CaCl2, 2.4. The entire colon was fixed in a Sylgard base dish with impalpable steel pins. The mesentery was carefully removed along the boundary line of the enterocoel under a dissecting microscope. All fecal pellets in the colon were artificially expelled with a 1-mL injector; this procedure was repeated to expel every pellet. This procedure must be performed with care to minimize intestinal damage. The empty colon was gently washed with 5 mL of warm Krebs solution, and a glass capillary tube was inserted through the lumen and linked to an artificial fecal pellet. The capillary was attached to the floor of the silica gel plate using U-shaped pins at the oral and anal end. A rectangular organ filled with 20 mL of warm Krebs solution (36.5 ± 0.5 °C) was also constantly inundated with carbon-oxygen gas. Then, the colon specimen was gently perfused with warm Krebs solution and left to stabilize for 30-40 min to secure the recovery of the colonic contraction activity. A silk thread (USP 5/0) was attached to both ends of the colon. The mechanical activity of the CMMCs was recorded using an isometric force transducer (RM6240C, Chengdu Instrument Factory, China) linked to an amplifier device. A tension of 0.1 g was applied to the empty colon, which was equilibrated for at least 40 min before the addition of the experimental drugs.
The entire colon full of fecal pellets was isolated as described above. The colon was cut along the mesentery, which is on the colonic circular axis; the pellets were flushed out with Krebs solution; the colon was pinned to a Sylgard dish with the mucosa facing upwards; and the mucosa and submucosa were removed carefully under a dissecting microscope. Smooth muscle strips (approximately 2 mm × 8 mm) were obtained by cutting along the circular axis from the fresh smooth muscle tissue. A silk thread (USP 5/0) was attached to both ends of the muscle strips and attached along the circular axis into 10 mL organ baths containing warm (37 °C) Krebs solution filled with 95% O2 and 5% CO2. The recording device was the same as that for the CMMCs above. A tension of 0.3 g was applied to the muscle strip, and it was equilibrated for at least 40 min before the recovery of its contraction activity.
Protein samples were extracted from the colonic smooth muscle tissues and lysed in radioimmunoprecipitation assay (RIPA) buffer (1:10; P0013, Beyotime Chemical Co., Jiangsu, China) and PMSF (1:100) solution. The suspended material was centrifuged at 12000 rpm for 15 min at 4 °C, mixed with 4 × loading buffer, and then boiled for 5 min in a 100 °C water bath. The protein concentration of the supernatant was calculated using the bicinchoninic acid (BCA) protein assay method (P0010, Beyotime Chemical Co., Jiangsu, China). Protein (30 μg/lane) was subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred from the polyacrylamide gel to polyvinylidene difluoride (PVDF) membranes using an E-Blotter unit (Bio-Rad) for 120 min. Then, the PVDF membranes were blocked in 5% nonfat milk for 2 h and incubated with rabbit c-Kit and PDGFR-α monoclonal antibodies (1:1000; #3074, #3174, Cell Signaling Technology, United States) and with mouse anti-tubulin antibody (1:1000; AT819, Beyotime Chemical Co., Jiangsu, China) overnight at 4 °C. The blots were then washed five times (5 min per wash) with Tris-HCl-buffered saline including 0.1% Tween-20 (TBST) and then incubated with secondary antibodies, including either anti-rabbit IgG HRP-linked antibody (1:1000; 7074; Cell Signaling Technology) or anti-mouse IgG HRP-linked antibody (1:1000; 7076; Cell Signaling Technology) for 2 h at room temperature. Protein signal detection was performed using an enhanced chemiluminescence agent (ECL reagents). The signals of the blots were analyzed using Quantity One software.
Based on the operation above, smooth muscle tissue (approximately 20 mm × 8 mm) was isolated from the empty colon, fixed facing forward and up onto the base of a Sylgard-covered chamber, and constantly filled with warm (37 °C) Krebs solution, 95% O2, and 5% CO2. The tissue was equilibrated for approximately 2 h before the recording started. The muscle tissue was maintained at 37 ± 0.5 °C through continuous perfusion with warm Krebs solution. Experimental procedures were carried out in the presence of nifedipine (1 μmol/L) to minimize the muscle contraction and maintain the cellular implements. Circular muscle cells were inserted using glass microelectrodes (80-100 MΩ) filled with KCl. Membrane potential was recorded with a Duo 773 (WPI Inc., Sarasota, FL, United States). EFS was made under a consistent voltage, pulse width, and duration (50 V, 0.5 ms, and 5 s, respectively) by two parallel platinum electrodes using a square-wave stimulator (YC-2 stimulator; Chengdu, China). The sIJPs and fIJPs of the smooth muscle were recorded in the presence and absence of various drugs, such as receptor antagonists or agonists in Krebs solution.
Tetrodotoxin (TTX) was obtained from Absin Biochemical Company. Atropine was purchased from Sigma-Aldrich. Apamin, NG-Nitro-L-arginine methyl ester hydrochloride (L-NAME), NPPB, MRS2500, and CyPPA were obtained from Tocris Bioscience (Ellisville, MO, United States).
The data are described as the mean ± SE. The analysis of data differences between groups was performed using one-way analysis of variance (ANOVA), followed by the Bonferroni’s post-hoc test or using the Student’s unpaired t-test when needed. P-values less than 0.05 were considered to represent significant differences between groups, and n-values correspond to the number of animals that were used in the indicated experiments.
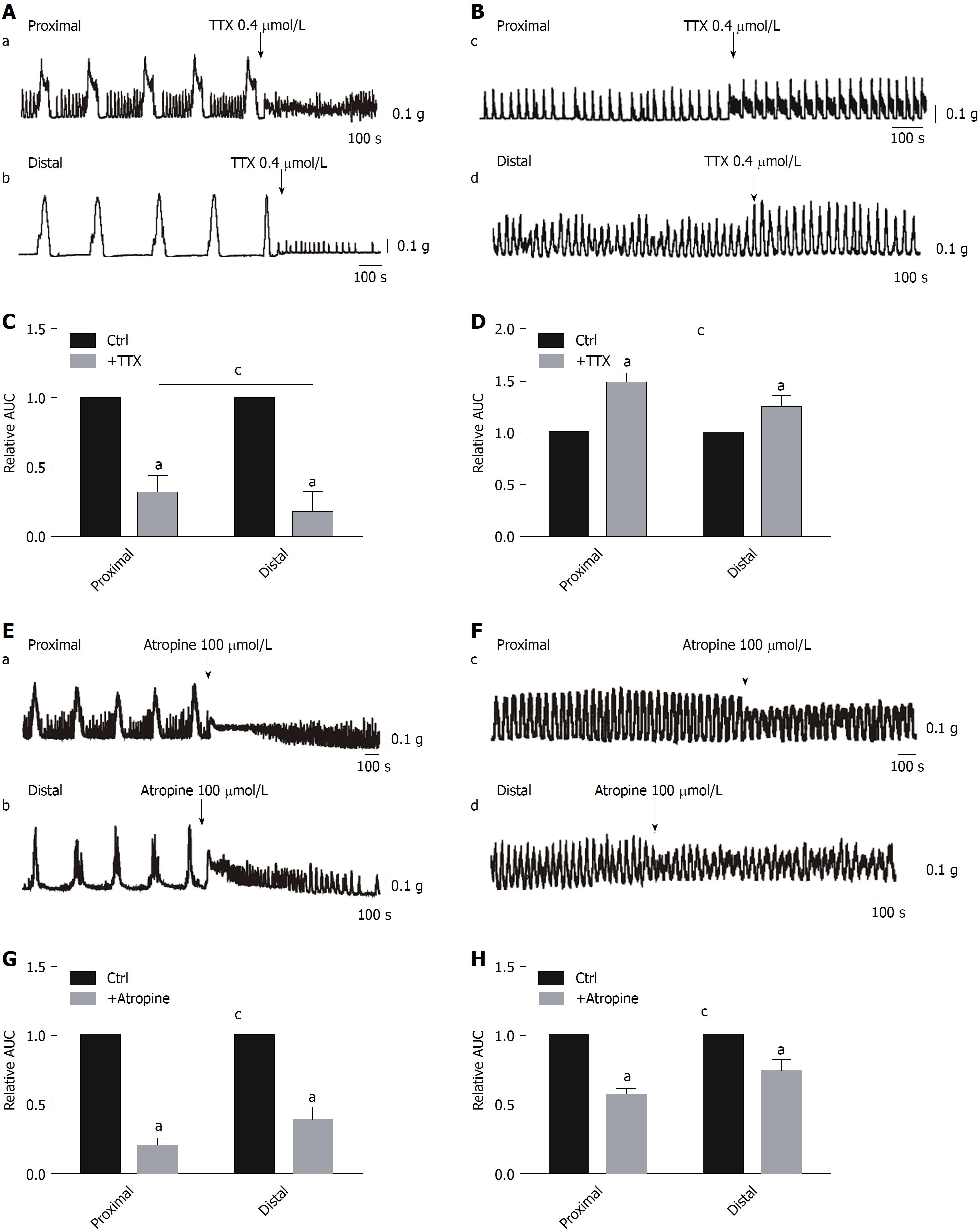
Effects of TTX and atropine on CMMCs and spontaneous contractions: To demonstrate the role of the ENS in colonic transit, we used a Na+ channel antagonist, TTX (0.4 μmol/L), to block neurons. TTX abolished the large amplitude contractions of CMMCs, and only the fast oscillating contractions remained. The contractions were decreased from 100% in controls to 30% ± 2.6% in the proximal colon and 21% ± 4.7% in the distal part (aP < 0.05; n = 6, meaning the number of animals used and the same below; Figure 1A and C). Interestingly, the basal tone and frequencies of the burst-like contractions in both ends of the colon were markedly increased by TTX, indicating that the generation of CMMCs in the colon was significantly mediated by the ENS, while the increased basal tone and frequencies of the remaining contractions in the presence of TTX are likely generated directly by ICCs because the dominant effects of the ENS on ICCs were limited by TTX. In addition, the effects of TTX were significantly different between the proximal and distal colon. To further confirm the effects of TTX on the colonic contractions, we observed that TTX significantly increased the rate of spontaneous contractions from 100% in controls to 149% ± 4.2% in the proximal colon and 124% ± 5.4% in the distal part (aP < 0.05; n = 7; Figure 1B and D). Moreover, there were significant differences in proximal and distal colon with TTX administration, respectively (cP < 0.05; Figure 1A-D).
To explain the differences of TTX above, we further compared the roles of the excitatory neurons and inhibitory neurons in regulating colonic motility. First, atropine was employed to block acetylcholine (Ach), released by cholinergic neurons. We found that, similar to the effects of TTX, atropine (100 μmol/L) abolished the large amplitude contractions of CMMCs, and only the burst-like contractions (e.g., ICC pacemaking activity) remained in both parts of the colon. Overall, CMMCs were significantly suppressed, from 100% in controls to 21% ± 1.9% in the proximal colon and 39% ± 3.7% in the distal part (aP < 0.05; n = 5; Figure 1E and G). Similarly, spontaneous contractions were also partially decreased by atropine treatment, from 100% in controls to 57% ± 1.8% in the proximal colon and 74% ± 3.9% in the distal part (aP < 0.05; n = 7; Figure 1F and H). Interestingly, significant differences remained between the proximal and distal ends treated with atropine in the CMMC and muscle strip experiments (cP < 0.05; Figure 1E-H). These results suggest that Ach may play a regulatory role for ICCs.
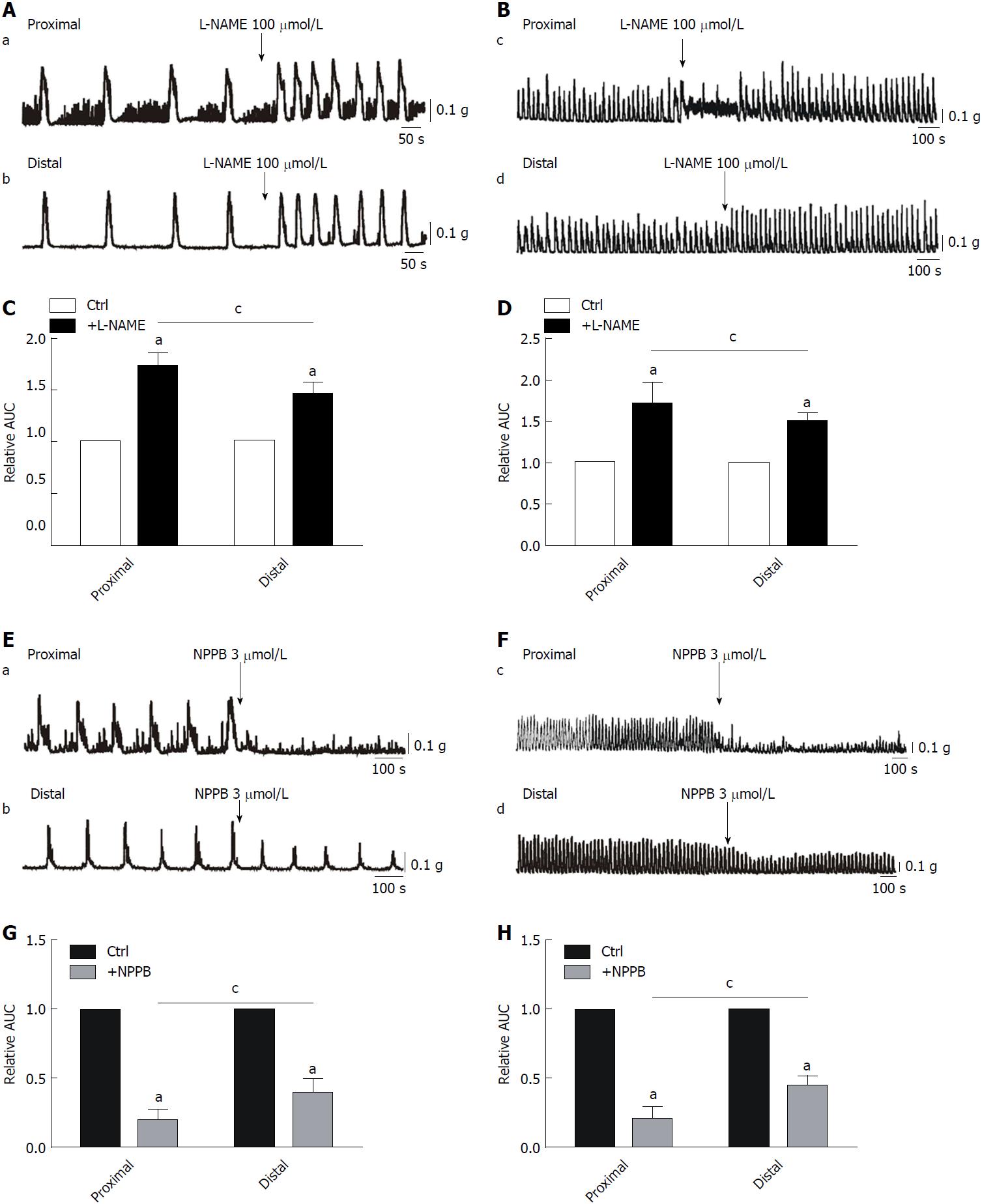
Effects of L-NAME and NPPB on CMMCs and spontaneous contractions: To characterize the effects of NO on colonic motility, L-NAME (100 μmol/L), a nonspecific inhibitor of NOS, was employed in the present study. We found that CMMCs, especially their frequency, were significantly enhanced by L-NAME, from 100% in controls to 172% ± 4.6% in the proximal colon and 145% ± 4.4% in the distal part (aP < 0.05; n = 7; Figure 2A and C). Similarly, spontaneous contractions became larger and faster after administration with L-NAME, from 100% in controls to 170% ± 11.5% in the proximal colon and 150% ± 4.0% in the distal part (aP < 0.05; n = 7; Figure 2E-H). Importantly, we also found that the effects of L-NAME on both CMMCs and spontaneous contractions were more significant in the proximal colon than in the distal part, respectively (cP < 0.05; Figure 2E-H). Based on these results, we confirmed that NO contributes substantially to the regulation of CMMC frequency and suggest that there may be more ICCs in the proximal colon than in the distal part.
To further confirm that ICCs have distinct distributions between different parts of the colon, NPPB, a blocker of ANO1 channels, was applied. NPPB treatment (3 μmol/L) significantly inhibited CMMCs, from 100% in controls to 22% ± 2.7% in the proximal colon and 46 ± 4.3% in the distal part (aP < 0.05; n = 5; Figure 2E and G). The effects of NPPB were much more significant in the proximal part than in the distal part (cP < 0.05; n = 5; Figure 2E and G). Furthermore, spontaneous contractions were also decreased by NPPB treatment, from 100% in controls to 19% ± 3.4% in the proximal colon and 48% ± 3.7% in the distal part (aP < 0.05; n = 5; Figure 2F and H). However, the inhibitory effect of NPPB was much more significant in the proximal part than in the distal part of the colon (cP < 0.05; n = 5; Figure 2F and H).
Regulatory effects of purine for SK3 channels in PDGFRα+ cells on CMMCs and spontaneous contractions
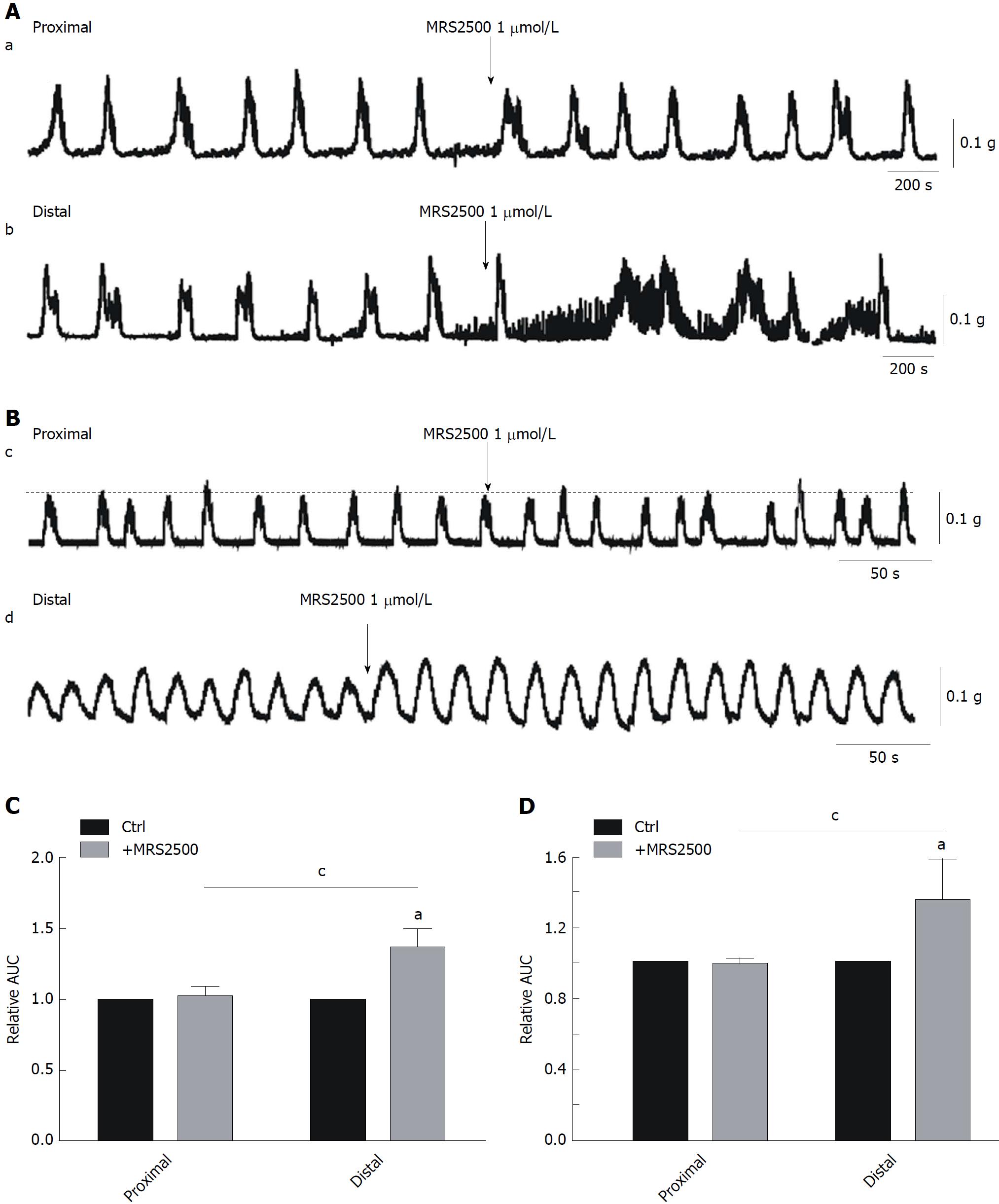
Effects of MRS-2500 treatment on CMMCs and spontaneous contractions: In subsequent experiments, to further investigate the role of PDGFRα+ cells in the regulation of colonic motility. First, MRS2500 was used (1 μmol/L; an antagonist of P2Y1) on CMMCs and spontaneous contractions. We found that in the proximal colon, MRS2500 had no significant effect on either CMMCs or spontaneous contractions (Figure 3A and B). However, in the distal colon, both CMMCs and spontaneous contractions were increased by MRS2500, reaching 143% ± 2.4% for CMMCs and 130% ± 4.2% for spontaneous contractions (aP < 0.05; n = 8; Figure 3A-D). MRS2500 had a markedly stronger effect in the distal colon compared to the proximal part (cP < 0.05; n = 8; Figure 3A-D), which indicates that there is an obvious difference between the two ends. Similarly, this effect may result from different distributions of PDGFRα+ cells in these colonic regions.
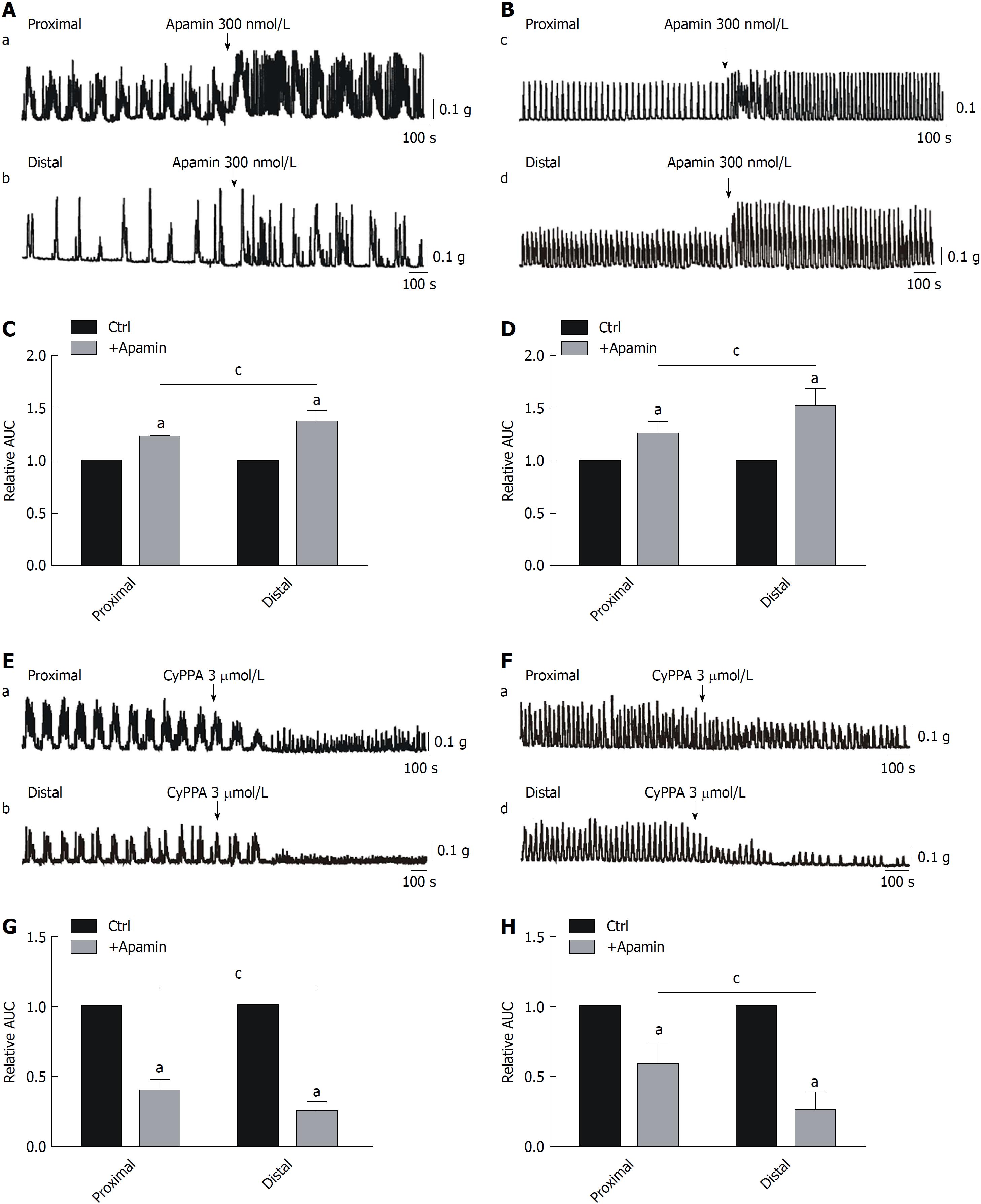
Effects of apamin and CyPPA on CMMCs and spontaneous contractions: To test the above hypothesis, we determined the effects of apamin (300 nmol/L; an SK3 channel antagonist) on colonic motility. We can observe that apamin significantly enhanced CMMCs, from 100% in controls to 126% ± 0.4% in the proximal colon and 148% ± 2.1% in the distal part (aP < 0.05; n = 7; Figure 4A and C). Similar results were obtained for spontaneous contractions, which were increased from 100% in controls to 127% ± 1.6% in the proximal colon and 159% ± 3.4% in the distal part (aP < 0.05; n = 7; Figure 4B and D). Similar to MRS2500 treatment, the effects of apamin in the distal colon were stronger than those in the proximal part (cP < 0.05; n = 7; Figure 4A-D).
Subsequently, CyPPA (3 μmol/L), an SK3 channel agonist, was also used in the present study. We found that the effects of CyPPA were completely opposite of those observed with apamin treatment. CyPPA markedly inhibited CMMCs, from 100% in controls to 39% ± 1.7% in the proximal colon and 25% ± 2.2% in the distal part (aP < 0.05; n = 7; Figure 4E and G). Clearly, the effects of CyPPA were much more significant in the distal part than in the proximal part of the colon (cP < 0.05; n = 7; Figure 4E and G). Moreover, spontaneous contractions were also inhibited by CyPPA, from 100% in controls to 54% ± 4.1% in the proximal colon and 19% ± 3.6% in the distal part (aP < 0.05; n = 7; Figure 4F and H). Therefore, CyPPA significantly inhibited both CMMCs and spontaneous contractions in both ends. Moreover, the effects of this drug in the distal colon were markedly stronger than those in the proximal part (cP < 0.05; n = 8; Figure 4E-H).
Effects of ICC/ANO1 and PDGFRα+/SK3 on colonic membrane potentials of smooth muscle tissue
Effects of ANO1 and SK3 antagonists on resting membrane potentials of colonic smooth muscle tissues: It is well-established that smooth muscle contraction and relaxation result from membrane depolarization and hyperpolarization[18]. To further evaluate the distributions of the two interstitial cell type, we first detected the average resting membrane potential (RMP) of colonic circular muscle cells in the proximal colon, which was 42.2 ± 1.92 mV, less than that in the distal part, which was 53.2 ± 2.86 mV (aP < 0.05; n = 12; Figure 5A). Furthermore, NPPB induced membrane hyperpolarization in both parts of the colon, showing a greater extent in the proximal colon than in the distal part (9.17 ± 1.19 mV in the proximal colon and 4.67 ± 0.56 mV in the distal colon, aP < 0.05; n = 6, Figure 5B and D). Nevertheless, apamin induced membrane depolarization, which was more marked in the distal colon than in the proximal part (7.10 ± 1.36 mV in the proximal colon and 11.07 ± 1.01 mV in the distal colon, aP < 0.05; n = 5; Figure 5C and E).
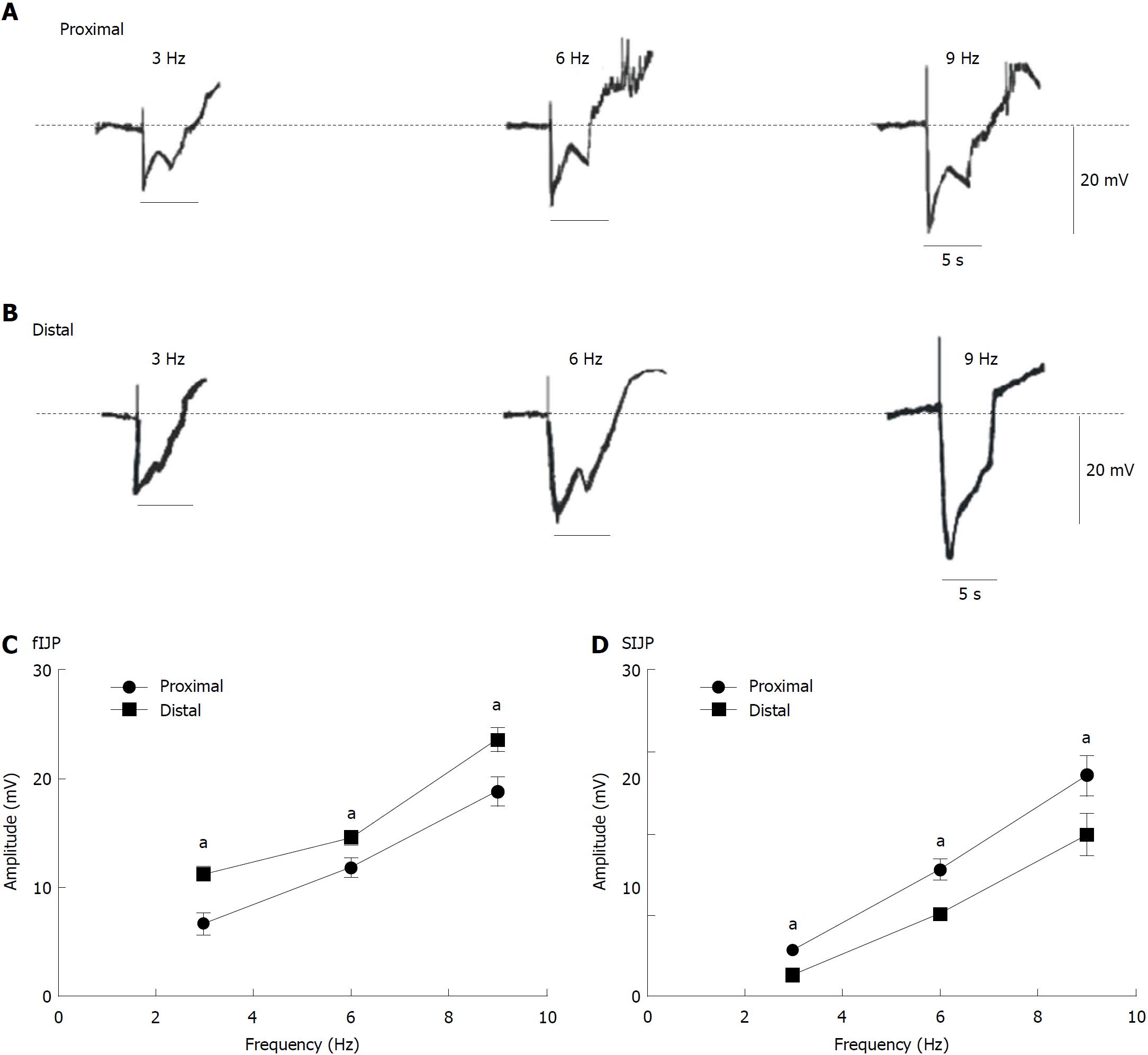
Postjunctional potentials evoked by EFS in colonic smooth muscle tissues: Consequently, we used EFS to evaluate the response of membrane potentials to determine whether there were differences between the proximal and distal colon. We observed that EFS (50 V; 3, 6, and 9 Hz; 5 s) induced IJPs including fIJPs and sIJPs in both ends of the colon. The average amplitude of the fIJPs was larger in the distal colon than in the proximal part (6.70 ± 0.39, 11.8 ± 0.33, and 18.86 ± 0.53 mV in the proximal colon, n = 8; 11.24 ± 0.35, 14.58 ± 0. 34, and 23.60 ± 0.45 mV in the distal colon, n = 8; aP < 0.05, Figure 6A-C), while the amplitude of sIJPs was more obvious in the proximal colon than in the distal parts (2.94 ± 0.17, 7.8 ± 0.25, and 3.60 ± 0.51 mV in the proximal colon, n = 8; 1.34 ± 0. 24, 5.14 ± 0.15, and 9.98 ± 0.56 mV in the distal part, n = 8; aP < 0.05, Figure 6A, B, and D). These results further confirm that there is indeed a difference in the distribution of ICC and PDGFRα+ cells in the proximal and distal colon.
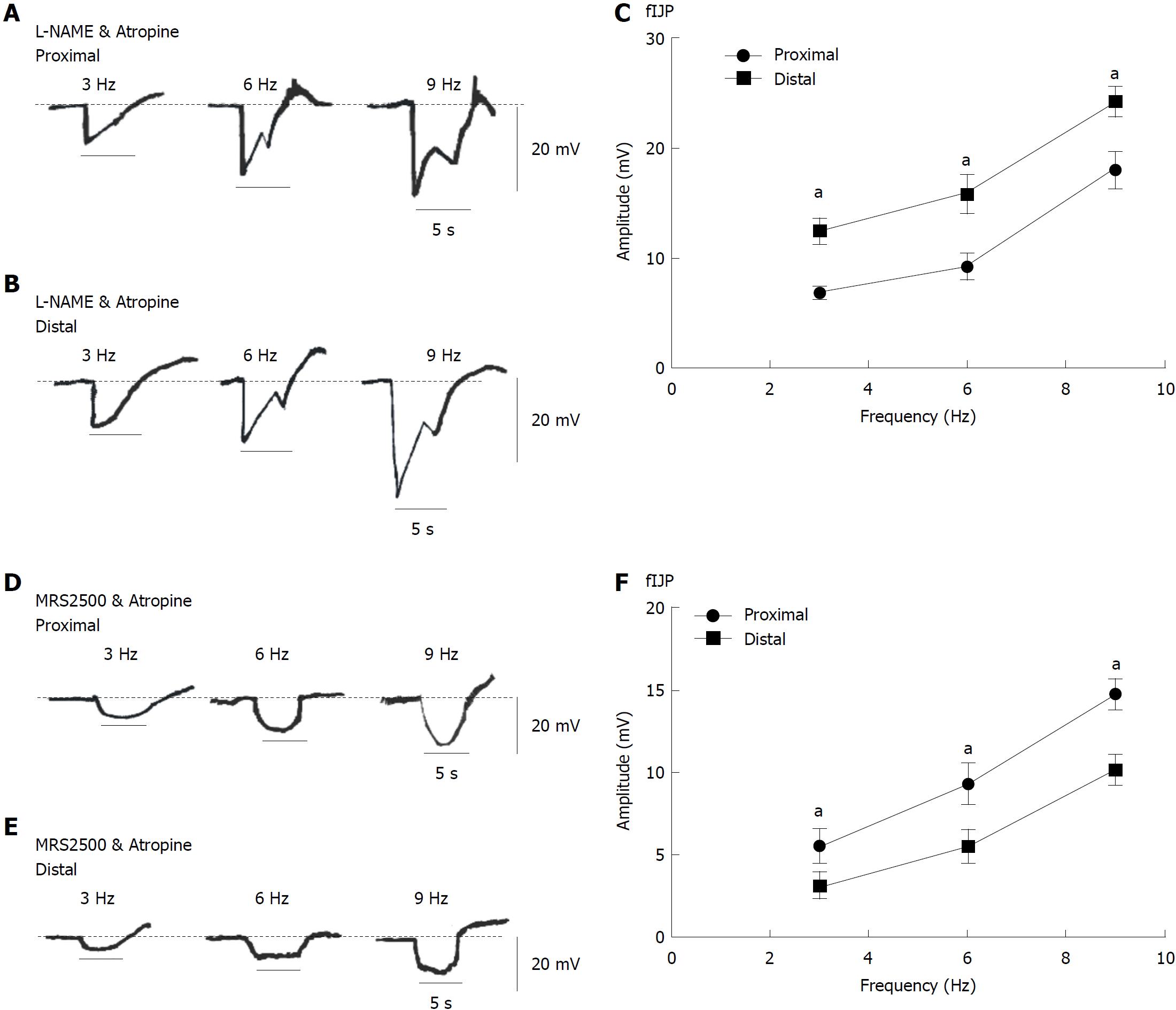
Changes in purine-dependent fIJPs and NO-dependent sIJPs in colonic smooth muscle tissues: For the purpose of illustrating the distribution of PDGFRα+ cells at both ends of the colon, L-NAME (100 μmol/L) and atropine (1 μmol/L) were used to block NO and Ach to solely observe the EFS response to purine. We found that the amplitude of the sIJPs significantly attenuated and almost all that remains were the fIJPs. The amplitudes of fIJPs in the distal colonic muscles remained stronger than those in the proximal colon (i.e., 6.78 ± 0. 27, 9.20 ± 0. 49, and 18.00 ± 0.71 mV; aP < 0.05; n = 7; Figure 7A and C). In the distal colon, the mean amplitudes were separately 12.44 ± 0. 47, 15.80 ± 0.73, and 24.22 ± 0.54 mV (aP < 0.05; n =8; Figure 7B and C). These results indicate that the distal colon has more PDGFRα+ cells and stronger fIJPs primarily elicited by purine and induces relaxation.
To explore the distribution of ICCs at both ends of the colon, MRS2500 (1 μmol/L) and atropine (1 μmol/L) were used to block purines and Ach to solely observe the EFS response to NO. The addition of MRS2500 and atropine almost fully eliminated the fIJPs, leaving only the sIJPs. The amplitudes of sIJPs (with EFS 3, 6, and 9 Hz) in the proximal colon appeared much larger than those in the distal colon (i.e., 3.12 ± 0.33, 5.54 ± 0.39, and 10.18 ± 0.40 mV, Figure 7E and F); in the proximal colon, the mean amplitudes of sIJPs were 5.54 ± 0. 43, 9.32 ± 0.53, and 14.8 ± 0.37 mV, respectively (aP < 0.05; n = 7; Figure 7D and F). These results confirm that the proximal colon has a greater distribution of ICCs and stronger sIJPs elicited by NO, inducing stimulation by ICCs.
Expression levels of c-Kit and PDGFRα in colonic smooth muscle tissues
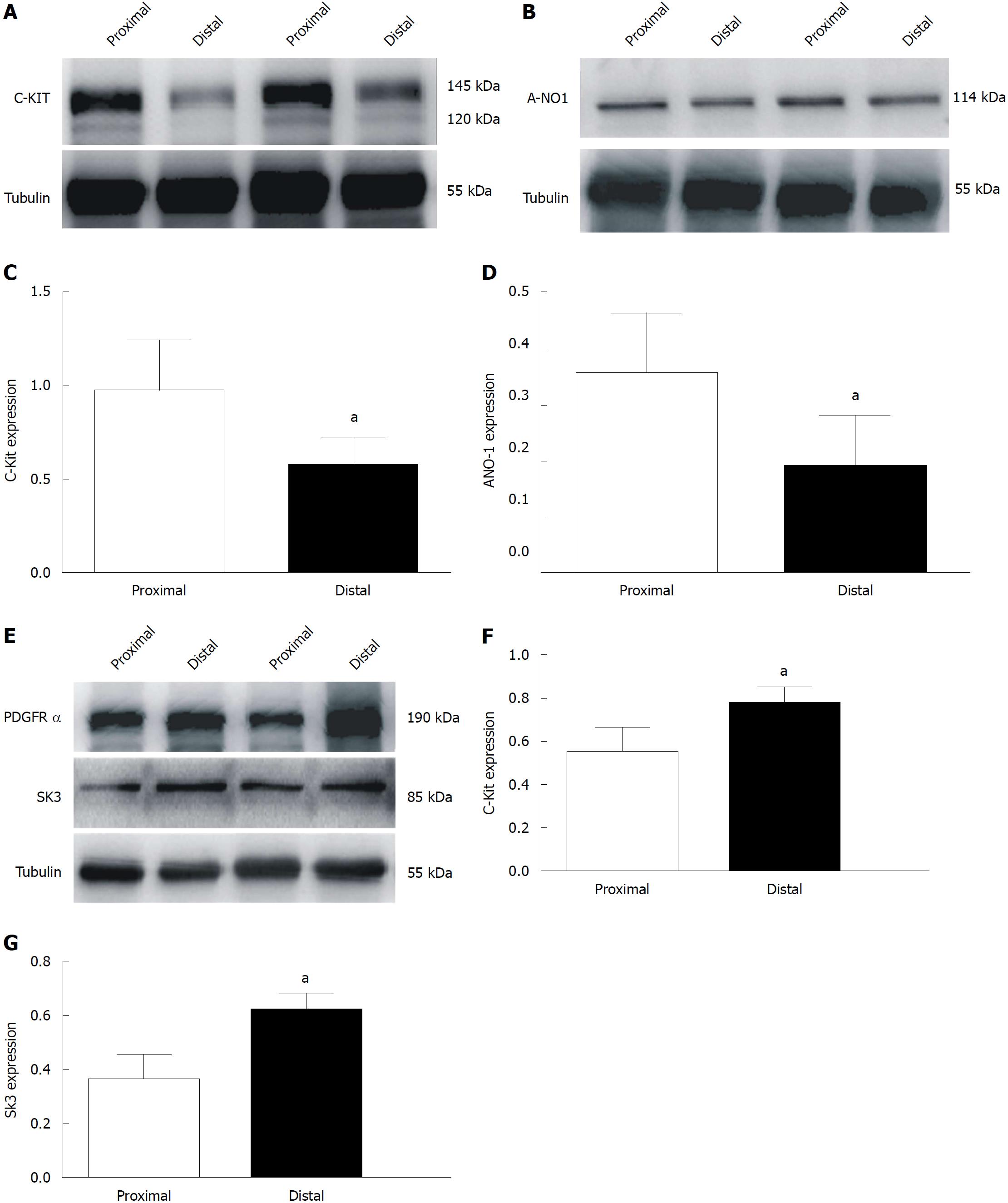
Finally, we examined the density of ICC, ANO1, PDGFRα, and SK3 in the smooth muscle tissues of the colon. We found that the expression levels of c-Kit and ANO1 were much higher in the proximal colon (97.7% ± 10.9% and 35.7% ± 4.3%, respectively), while those in the distal end were lower (58.2% ± 6.5% and 19% ± 3.6%, respectively [aP < 0.05; n = 8; Figure 8A and C (c-Kit) and Figure 8B and D (ANO1)]. Additionally, the expression of the PDGFRα protein in the colonic muscle layer was significantly increased, from 54.8% ± 5.0% to 77.6% ± 3.2% in the proximal and distal ends, respectively; similarly, the expression of SK3 in the colonic muscle layer ranged from 36.2% ± 4.1% to 61.4% ± 3.1%, respectively, in the proximal and distal ends [aP < 0.05; n = 8; Figure 8E and F (PDGFRα) and Figure 8E and G (SK3)]. These protein expression data directly show that the proximal colon has more ICCs and that the distal colon has more PDGFRα+ cells.
This study focused on the distribution and function of ICCs and PDGFRα+ cells at both ends of the colon and the role of NO and purines on colonic dynamic transmission. This finding may have clinical implications in studying and treating colonic dysmotility of inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). It has previously been established that the large intestine has many different motor patterns, including segmental activity, antiperistaltic and peristaltic waves, and tonic inhibition[19,20]. Regardless of transmission form, it is necessary to produce pressure gradient to ensure that feces are propelled from the proximal to distal colon. Therefore, we divided the colon into two segments to separately study its transmitting mechanism. Based on previous reports, the speed of fecal pellet propulsion along the murine colon is the same as the conduction velocity of the CMMC contraction, indicating that CMMCs drive the pellets forward[21].
In our study, we found that the large amplitude contraction of CMMCs was almost completely inhibited when the ENS or cholinergic neurons were blocked; instead, irregular contractions with much higher frequencies were recorded. Notably, in the CMMC experiments (treatment with TTX), the distal colon had more obvious drug effect, while in smooth muscle contraction experiment, the drug effect of proximal colon was more prominent in the presence of TTX or atropine (Figure 1). These results suggested that even though CMMC was almost abolished by TTX, the spontaneous contractions of the smooth muscle were significantly increased by TTX treatment. Therefore, we speculate that in the ENS, inhibitory neurons play a dominant role in spontaneous contractions of the colon. Moreover, the small irregular contraction waves in atropine may result from the pacemaker activity of ICCs and CMMCs are initiated by excitatory cholinergic neurons through ICCs. In the past several years, pacemaker function of ICCs and the ENS network have generally been considered as separate systems in colonic transit. However, new findings have reported that the pacemaker network in the colon consists of both ICC-MY (interstitial cells of Cajal of myenteric plexus) and ICC-IM (intramuscular interstitial cells of Cajal) and that this network is activated via cholinergic KV7.5 channel inhibition in ICC-IM[22]. Even though colonic motor transit has been confirmed by the cooperation of ICC networks with the ENS[23], information regarding the transit mechanism by which ICCs sequentially function remain unclear. Therefore, we primarily focused on the regulation of murine colonic contractile activity by nitrergic and purinergic neurons.
Based on the finding of nitrergic signaling through ICCs to regulate colonic spontaneous contraction and CMMCs (described above), we used L-NAME and NPPB to block NO and ANO1 in ICCs, respectively, to observe whether there are functional differences between the two ends of the colon (Figure 2). Our results showed that the AUC (equaling the frequencies and amplitudes) of CMMCs and spontaneous contractions in the smooth muscle were significantly increased in the presence of L-NAME or NPPB, indicating that NO is involved in regulating the frequency of CMMC through ICCs to maintain normal colonic transit. Moreover, the proximal colon has larger amplitude and higher frequencies in the presence of these two drugs, indicating that there are more ICCs in this part of the colon.
In addition to NO, purine is also considered to be involved in inhibitory regulation of smooth muscle contractions through another kind of interstitial cells-PDGFRα+ cells[24]. Burnstock et al[7] first proposed that adenosine triphosphate (ATP) or purine was a main inhibitory neurotransmitter in the GI tract in 1970. Additionally, it may promote the release of glutamate (an inhibitory neurotransmitter) by binding to presynaptic metabotropic P2Y receptors[25]. Recent studies have proven that purinergic receptors (P2Y1) and SK3 channels are expressed in PDGFRα+ cells responsible for nerve-mediated relaxation in the GI tract[24]. Compared to other classical neurotransmitters, the role of purinergic signaling in colonic transmission is not well understood or appreciated. Therefore, our results (Figures 3 and 4) proved that the amplitudes and frequencies of CMMCs and spontaneous contractions in the distal colon were markedly changed when P2Y1 receptors and SK3 channels were blocked by MRS2500 and apamin, respectively, or SK3 was activated by CyPPA, illustrating that purines help to maintain regular colonic transmission by inhibiting contractions. Moreover, the distal colon showing stronger contractile activity indicates that there may be more PDGFRα+ cells, resulting from the inhibitory role of purine on PDGFRα+ cells being eliminated by MRS2500 treatment.
Based on CMMC and smooth muscle contraction experiments, we speculated that ICCs and PDGFRα+ cells may have different distributions at different ends of the colon. To confirm this hypothesis, we used intracellular recordings to detect whether there exists a difference in the electrophysiological properties between the two ends of the colon. Previous studies have shown that GI motility is regulated by excitatory and IMNs, among which IMNs evoke IJPs, causing muscle relaxation. In the colon, neurotransmitters released by the ENS could activate the conductance of interstitial cells (i.e., ANO1 in ICCs or SK3 channels in PDGFRα+ cells) to regulate the excitability of SMCs through gap junctions to meditate colonic motility[23,26]. ANO1 regulates sensory signal transduction and smooth muscle contractile activity to generate rhythmic contraction, which is supported by the absence of slow waves in ANO1 knockout mice[27-29]. Additionally, SK3 channels in PDGFRα+ cells are activated by purines and produce apamin-sensitive outward currents[30]. These SK3 channels are believed to be involved in inducing membrane hyperpolarization and IJPs. In GI smooth muscle tissues, IJPs have bipolar phases and consist of two main components: fIJP, induced by the release of purines in PDGFRα+ cells, and sIJP arising from NO through ICCs[31,32], which was also proved in this study (Figures 5-7). More importantly, we also found that the amplitudes of fIJPs induced by purinergic neurons through PDGFRα+ cells were much higher in the distal colon than in the proximal segment. Furthermore, the amplitude of sIJPs induced mainly by NO through ICCs was much higher in the proximal colon than in the distal part. These results provide concrete functional evidence that ICCs and PDGFRα+ cells have a different distribution at both ends of the colon.
Finally, we confirmed that more ICCs were distributed in the proximal colon, whereas more PDGFRα+ cells were distributed in the distal colon using Western blot analysis (Figure 8). We speculate that this distribution difference is responsible for the stronger contractions in the proximal colon and the relatively weaker contractions in the distal part, forming a pressure gradient from the proximal colon to the distal part to propel feces smoothly forward to the anus.
In summary, our findings in the present study provide clear evidence that the ENS regulates colonic motility through cholinergic and nitrergic neurons regulating the spontaneous rhythmic pacemaker activity of ICCs and through purinergic neurons mediating inhibitory effects on smooth muscle contractions by acting on P2Y1 receptors in PDGFRα+ cells to suppress colonic transit. We further found that inhibitory neuromodulation has a leading role in colonic transmission, which may be associated with the formation of fecal pellets in the colon and full absorption of water and nutrients. Furthermore, the distribution characteristics of more ICCs in the proximal and more PDGFRα+ cells in the distal colon contribute to the formation of a pressure gradient from the oral to anal ends of the colon, which is associated with the roles of ENS-NO/ICCs and purine/PDGFRα+ cells in the regulation of colonic motility. Consequently, an in-depth understanding of the mechanism of colon motility may provide a new direction and target for the study and treatment of diarrhea or constipation in colon motility disorder, such as IBD and IBS.
Interstitial cells of Cajal (ICCs) and PDGFRα+ cells are mainly located in the smooth muscle layer of the colon, which is widely believed to play a critical role in the generation of colonic transit motility. Large numbers of studies have shown that ICCs are mainly responsible for the contraction response of the smooth muscle, while PDGFRα+ cells mainly regulate the relaxation of the smooth muscle. Inhibitory neuronal transmitters [nitric oxide (NO) and purine] can respectively act on ICCs and PDGFRα+ cells to mediate colonic transit, thus propelling feces into the rectum and the anus. However, the distributions of ICC and PDGFRα+ in the colon remain unclear. Therefore, it is an important scientific problem to study the transit mechanism of the proximal and distal colon.
In recent years, the incidence of colonic dysmotility diseases has been increasing year by year, such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and complications of some diseases (e.g., diabetes-induced slow transit constipation), whose main clinical symptoms are diarrhea or constipation. The smooth muscle layer, as the main source of colon power, consists of ICCs, PDGFRα+ cells, and smooth muscle cells (SMCs) contacted by the gap junction. Therefore, it is very significant to study the differential distributions of ICCs and PDGFRα+ cells in the two ends of the colon.
In this study, we for the first time researched the distributions and functions of ICCs and PDGFRα+ cells in the proximal and distal colon. Then, we studied the roles of inhibitory neurotransmitters NO for ICCs and purine for P2Y1 receptor on PDGFRα+ cells in colonic motility transit. This study may represent a future strategy for therapeutic intervention in disorders of colonic motility, such as IBD and IBS, by understanding the distributions and function of NO-ICCs and purine-PDGFRα+ cells at both ends of the colon.
First, we compared the isolated colonic transit differences between the proximal and distal colon using colonic migrating motor complexes (CMMCs). Then, we used the smooth muscle contraction experiment, which is the major source of colon motility, to compare the drug differences between the proximal and distal colon by adding the blockers and agonists of anoctamin-1 (ANO1) channels on ICCs and small conductance calcium-activated potassium channel 3 (SK3) channels on PDGFRα+ cells. Subsequently, we compared the membrane potentials of the proximal and distal colon by intracellular recordings. Later, Western blot analysis was used to detect the protein expression of c-Kit, ANO1, PDGFRα, and SK3 in the colon. Finally, we added immunofluorescence methods to visually describe the distributions of ICC and PDGFRα+ cells in the proximal and distal colon.
Treatment with tetrodotoxin (TTX) to block the enteric nervous system (ENS) in the CMMC experiment almost completely blocked colonic transit. However, in the smooth muscle contraction experiment, when the ENS was blocked, the contraction of the colon was enhanced, suggesting that inhibitory nerve regulation plays critical roles in the transmission of the colon. In addition, when the ANO1 channel on ICCs was blocked by NPPB, the proximal colon showed a more obvious inhibitory role. While the SK3 channels on PDGFRα+ cells were blocked by apamin, there was a more obvious drug effect in the distal colon, indicating that the proximal colon might distribute more ICCs, and the distal colon has more PDGFRα+ cells. Intracellular electrical recording experiments indicated that slow inhibitory junction potentials (sIJP) mediated by the NO-ICC-ANO1 signal pathway was more obvious in the proximal colon, while fast inhibitory junction potentials (fIJP) mediated by purine-PDGFRα+-SK3 was more prominent in the distal colon, indicating that there are more ICCs in the proximal colon and more PDGFRα+ cells in the distal colon from the membrane potential level.
In this study, we demonstrated that NO has a more obvious effect on the ICCs in the proximal colon, while purine has a more prominent effect on the distal PDGFRα+ cells, indicating that there are more ICC cells in the proximal colon and more PDGFRα+ cells in the distal colon. In addition, NO and purine acting on the SMC/ICC/PDGFRα+ cell (SIP) syncytium (consisting of SMCs, ICCs, and PDGFRα+ cells) are both inhibitory neurotransmitters, suggesting that colonic transit is mainly dominated by inhibitory neuromodulation. Although the stomach and small intestine are dominated by excitatory neuromodulation, this feature of the colon may contribute to the adequate absorption of nutrients and the formation of feces.
This is the first study to report that the differential distributions of ICCs and PDGFRα+ cells at the two ends of the colon. Our findings have highlighted the effects of inhibitory neuromodulation NO-ICC-ANO1 and purine-PDGFRα+ cells-SK3 on colonic transit. In order to maintain regular colonic transit, the perfect cooperation of NO-ICC and purine-PDGFRα+ cells is required. Therefore, in the future study of colon dynamic disorder such as IBD or IBS, we can start from whether the distribution and function of ICC and PDGFRα+ cells have changed, and then extend to the effect of the ENS, especially inhibitory neuromodulation, on colonic transmission disorder to find the pathogenesis of colonic transit dysfunction. These findings in the present study may provide new insights and strategies for the diagnosis and treatment of gastrointestinal motility disorders.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu S, Rolle U S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Yin SY
| 1. | Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 2. | Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 447] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 3. | Huizinga JD, Chen JH. Interstitial cells of Cajal: update on basic and clinical science. Curr Gastroenterol Rep. 2014;16:363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Faussone Pellegrini MS, Cortesini C, Romagnoli P. [Ultrastructure of the tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called Cajal’s interstitial cells]. Arch Ital Anat Embriol. 1977;82:157-177. [PubMed] |
| 5. | Bernstein K, Vink JY, Fu XW, Wakita H, Danielsson J, Wapner R, Gallos G. Calcium-activated chloride channels anoctamin 1 and 2 promote murine uterine smooth muscle contractility. Am J Obstet Gynecol. 2014;211:688.e1-688.10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Song NN, Lu HL, Lu C, Tong L, Huang SQ, Huang X, Chen J, Kim YC, Xu WX. Diabetes-induced colonic slow transit mediated by the up-regulation of PDGFRα+ cells/SK3 in streptozotocin-induced diabetic mice. Neurogastroenterol Motil. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Jiménez M. Platelet-derived growth factor receptor-α-positive cells: new players in nerve-mediated purinergic responses in the colon. J Physiol. 2015;593:1765-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Qu ZD, Thacker M, Castelucci P, Bagyánszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Foong JP, Hirst CS, Hao MM, McKeown SJ, Boesmans W, Young HM, Bornstein JC, Vanden Berghe P. Changes in Nicotinic Neurotransmission during Enteric Nervous System Development. J Neurosci. 2015;35:7106-7115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Lu HL, Huang X, Wu YS, Zhang CM, Meng XM, Liu DH, Kim YC, Xu WX. Gastric nNOS reduction accompanied by natriuretic peptides signaling pathway upregulation in diabetic mice. World J Gastroenterol. 2014;20:4626-4635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Mañé N, Gil V, Martínez-Cutillas M, Clavé P, Gallego D, Jiménez M. Differential functional role of purinergic and nitrergic inhibitory cotransmitters in human colonic relaxation. Acta Physiol (Oxf). 2014;212:293-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Durnin L, Lees A, Manzoor S, Sasse KC, Sanders KM, Mutafova-Yambolieva VN. Loss of nitric oxide-mediated inhibition of purine neurotransmitter release in the colon in the absence of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2017;313:G419-G433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Hibberd TJ, Costa M, Travis L, Brookes SJH, Wattchow DA, Feng J, Hu H, Spencer NJ. Neurogenic and myogenic patterns of electrical activity in isolated intact mouse colon. Neurogastroenterol Motil. 2017;29:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Lomax AE, Paterson WG. P2Y1 receptors mediate apamin-sensitive and -insensitive inhibitory junction potentials in murine colonic circular smooth muscle. J Pharmacol Exp Ther. 2010;333:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Lies B, Beck K, Keppler J, Saur D, Groneberg D, Friebe A. Nitrergic signalling via interstitial cells of Cajal regulates motor activity in murine colon. J Physiol. 2015;593:4589-4601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Kaji N, Horiguchi K, Iino S, Nakayama S, Ohwada T, Otani Y, Firman , Murata T, Sanders KM, Ozaki H. Nitric oxide-induced oxidative stress impairs pacemaker function of murine interstitial cells of Cajal during inflammation. Pharmacol Res. 2016;111:838-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Smith TK, Koh SD. A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: the role of serotonin. Am J Physiol Gastrointest Liver Physiol. 2017;312:G1-G14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil. 2014;20:294-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Costa M, Dodds KN, Wiklendt L, Spencer NJ, Brookes SJ, Dinning PG. Neurogenic and myogenic motor activity in the colon of the guinea pig, mouse, rabbit, and rat. Am J Physiol Gastrointest Liver Physiol. 2013;305:G749-G759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Costa M, Wiklendt L, Simpson P, Spencer NJ, Brookes SJ, Dinning PG. Neuromechanical factors involved in the formation and propulsion of fecal pellets in the guinea-pig colon. Neurogastroenterol Motil. 2015;27:1466-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology. 2009;136:1328-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Wright GW, Parsons SP, Loera-Valencia R, Wang XY, Barajas-López C, Huizinga JD. Cholinergic signalling-regulated KV7.5 currents are expressed in colonic ICC-IM but not ICC-MP. Pflugers Arch. 2014;466:1805-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Huizinga JD, Martz S, Gil V, Wang XY, Jimenez M, Parsons S. Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front Neurosci. 2011;5:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Lee H, Koh BH, Peri LE, Sanders KM, Koh SD. Purinergic inhibitory regulation of murine detrusor muscles mediated by PDGFRα+ interstitial cells. J Physiol. 2014;592:1283-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Patti L, Raiteri L, Grilli M, Parodi M, Raiteri M, Marchi M. P2X(7) receptors exert a permissive role on the activation of release-enhancing presynaptic alpha7 nicotinic receptors co-existing on rat neocortex glutamatergic terminals. Neuropharmacology. 2006;50:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1068] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 27. | Hwang SJ, Blair PJ, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887-4904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 344] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 28. | Cipriani G, Serboiu CS, Gherghiceanu M, Faussone-Pellegrini MS, Vannucchi MG. NK receptors, Substance P, Ano1 expression and ultrastructural features of the muscle coat in Cav-1(-/-) mouse ileum. J Cell Mol Med. 2011;15:2411-2420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci USA. 2009;106:21413-21418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 30. | Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch Histol Cytol. 2009;72:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Lang RJ. Do ‘fibroblast-like cells’ intercede during enteric inhibitory motor neurotransmission in gastrointestinal smooth muscles? J Physiol. 2011;589:453-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Yeoh JW, Corrias A, Buist ML. A mechanistic model of a PDGFRα(+) cell. J Theor Biol. 2016;408:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |