Published online Nov 14, 2018. doi: 10.3748/wjg.v24.i42.4809
Peer-review started: July 4, 2018
First decision: August 25, 2018
Revised: October 19, 2018
Accepted: October 26, 2018
Article in press: October 26, 2018
Published online: November 14, 2018
Processing time: 133 Days and 1.7 Hours
To determine the usefulness of assigning narrow-band imaging (NBI) scores for predicting tumor grade and invasion depth in colorectal tumors.
A total of 161 colorectal lesions were analyzed from 138 patients who underwent endoscopic or surgical resection after conventional colonoscopy and magnifying endoscopy with NBI. The relationships between the surface and vascular patterns of the lesions, as visualized with NBI, and the tumor grade and depth of submucosa (SM) invasion were determined histopathologically. Scores were assigned to distinct features of the surface microstructures of tubular and papillary-type lesions. Using a multivariate analysis, a model was developed for predicting the tumor grade and depth of invasion based on NBI-finding scores.
NBI findings that correlated with a high tumor grade were associated with the “regular/irregular” (P < 0.0001) surface patterns and the “avascular area” pattern (P = 0.0600). The vascular patterns of “disrupted vessels” (P = 0.0714) and “thick vessels” (P = 0.0133) but none of the surface patterns were associated with a depth of invasion of ≥ 1000 μm. In our model, a total NBI-finding score ≥ 1 was indicative of a high tumor grade (sensitivity: 0.97; specificity: 0.24), and a total NBI-finding score ≥ 9 (sensitivity: 0.56; specificity: 1.0) was predictive of a SM invasion depth ≥ 1000 μm. Scores less than these cutoff values signified adenomas and a SM invasion depth < 1000 μm, respectively. Associations were also noted between selected NBI findings and tumor tissue architecture and histopathology.
Our multivariate statistical model for predicting tumor grades and invasion depths from NBI-finding scores may help standardize the diagnosis of colorectal lesions and inform therapeutic strategies.
Core tip: While magnifying endoscopy with narrow-band imaging (NBI) has been integrated into diagnostic histopathology, universal standardized criteria for differentiating non-neoplastic lesions, benign adenomas, and malignant neoplasms using NBI are urgently needed. We propose a multivariate statistical model for predicting the tumor grade and invasion depth from NBI finding scores. A total NBI-finding score ≥ 1 is indicative of a high tumor grade (sensitivity: 0.97; specificity: 0.24), while a score ≥ 9 (sensitivity: 0.56; specificity: 1.0) is predictive of a submucosa invasion depth ≥ 1000 μm. Our model may help to standardize the diagnosis of colorectal lesions and inform therapeutic strategies.
- Citation: Maeyama Y, Mitsuyama K, Noda T, Nagata S, Nagata T, Yoshioka S, Yoshida H, Mukasa M, Sumie H, Kawano H, Akiba J, Araki Y, Kakuma T, Tsuruta O, Torimura T. Prediction of colorectal tumor grade and invasion depth through narrow-band imaging scoring. World J Gastroenterol 2018; 24(42): 4809-4820
- URL: https://www.wjgnet.com/1007-9327/full/v24/i42/4809.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i42.4809
Colorectal cancer is one of the leading causes of cancer-related morbidity and mortality worldwide. Early diagnosis and patient stratification can greatly improve disease outcomes[1,2]. Colonoscopy remains a mainstay in the screening and diagnosis of colorectal cancer and plays an important role in determining the course of treatment[3]. The majority of colorectal cancers are known to evolve from preexisting adenomas according to the adenoma-carcinoma sequence[4]. Therefore, when performing a colonoscopy to diagnose colorectal lesions, it is essential to determine whether a given lesion is a non-neoplastic lesion, benign adenoma, or malignant neoplasm[5]. It is also important to evaluate the depth of tumor invasion into the submucosa (SM), as this factor has been shown to predict the risk of local recurrence and nodal metastasis[6,7]. Thus, colorectal cancers limited to the mucosa are unlikely to metastasize, whereas those invading the SM are more likely to metastasize to the lymph nodes and adjacent organs. Additionally, cancers with an SM invasion depth of 1000 μm rarely metastasize and are amenable to endoscopic resection, whereas those with an SM invasion depth ≥ 1000 μm are more likely to metastasize and require surgery[8-11].
Recent advances in endoscopic techniques, including the development of chromoendoscopy and magnifying endoscopy with narrow-band imaging (NBI), have attempted to overcome the limitations of conventional colonoscopy and thus yield improvements in the detection, diagnosis, and resection of colorectal lesions. Kudo et al[12,13] employed chromoendoscopy to visualize pit patterns on the surfaces of colorectal mucosal lesions and established a classification of this feature as a reliable diagnostic criterion with which to distinguish between non-neoplastic lesions, benign adenomas, and malignant neoplasms and assign tumor grades[12-14]. Tobaru et al[15] and Kanao et al[16] used magnifying chromoendoscopy to classify lesions with a V1 pit pattern into distinct subtypes with respect to the depth of tumor invasion into the SM layer, thus consolidating the value of classifying pit patterns as a surrogate measure of tumor invasiveness.
The application of an NBI filter set (415 ± 30 nm) during endoscopic imaging was shown to markedly enhance the contrast of vascular patterns in the superficial layer of tissues, compared with images obtained under broadband illumination[17]. Since this seminal observation, magnifying endoscopy with NBI has been widely integrated into diagnostic histopathology. NBI has several advantages over chromoendoscopy. For example, it allows the acquisition of endoscopic images with a uniform mucosal pattern across images without dye-spraying. Furthermore, it enables enhanced visualization of vascular features on the mucosal surfaces of lesions that would be difficult to observe by chromoendoscopy, thus augmenting the diagnostic accuracy[18-21]. To date, many reports have highlighted the clinical usefulness, improved diagnostic accuracy, and increased sensitivity of NBI magnification for observing the surface microstructure (i.e., surface pattern) together with the surface microvessels (i.e., vascular pattern) of colorectal lesions, thus enabling the diagnoses of histologic grade and invasion depth[22-25]. Indeed, NBI magnification was shown to be effective for determining the surface and vascular patterns of small and diminutive colonic polyps (< 10 mm), thus underscoring the potential of this technique for the accurate diagnosis of the invasion depths of early lesions[20,26]. This is particularly important, given that early detection is key to successful treatment.
Many of the above reports merely described and categorized various endoscopic observations. Several experts in the field have therefore highlighted the need to universally standardize NBI observation criteria and implement a simple system with which to classify NBI findings for the diagnosis of colorectal lesions[27-29]. However, the multitude of emergent diagnostic classification systems renders it difficult for physicians with insufficient experience to use such reports to inform precise endoscopic diagnosis in a clinical setting and may lead to subjective variability and a lack of standardization in histopathology reporting.
The present study was undertaken to develop a statistical model for predicting the tumor grade and invasion depth using magnifying endoscopy-NBI in patients harboring suspicious colorectal lesions. We devised a novel scoring system to evaluate the surface and vascular patterns in NBI images and assessed the sensitivity and specificity of our model for distinguishing malignant from non-malignant colorectal lesions. Furthermore, we investigated for the first time the association of the NBI findings of a range of colorectal tumors with tumor tissue architecture and histopathology.
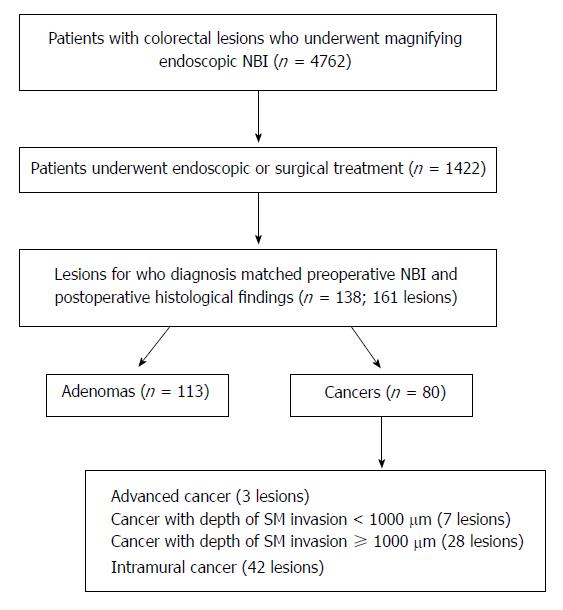
Between February 2007 and March 2013, 4762 patients diagnosed with colorectal lesions underwent magnifying endoscopy-NBI at Kurume University Hospital (Japan). Of these, 1422 patients were endoscopically or surgically treated. We retrospectively analyzed the postoperative histopathological data and included 161 lesions from 138 patients for whom the preoperative magnifying endoscopy-NBI findings corresponded with the postoperative histopathological findings. Of the 138 patients, 113 underwent endoscopic lesion resection and the remaining 25 underwent surgical resection (Figure 1). The patient population comprised 85 male and 53 female patients with a mean [± standard deviation (SD)] age of 65.7 ± 10.1 years. The study protocol was approved by the Ethics Committee of Kurume University Hospital, and an informed consent waiver was granted for the retrospective analysis of endoscopic findings. All samples were anonymized and de-identified prior to analysis to preserve patient confidentiality. All patients had provided informed written consent prior to treatment.
For bowel preparation prior to the colonoscopy, patients were instructed to ingest 2 L of a polyethylene glycol-electrolyte solution on the morning of the procedure. Unless contraindicated, scopolamine butylbromide (10 mg) was administered via intravenous injection prior to the procedure to minimize bowel spasms during the colonoscopy. Both models of high-resolution video endoscopes (CF-FH260AZI and CF-H260AZI; Olympus Medical Systems Corporation, Tokyo, Japan) used in this study exhibit the capacity to magnify images 75 times. The acquired endoscopic images were evaluated retrospectively and independently by two experienced physicians (Maeyama Y and Tsuruta O) who specialize in endoscopy.
Based on the NBI findings, colorectal lesions were broadly divided into tubular or papillary types according to their histopathologic appearance and proliferation pattern. The surface and vascular patterns of the lesions were subsequently analyzed as detailed below.
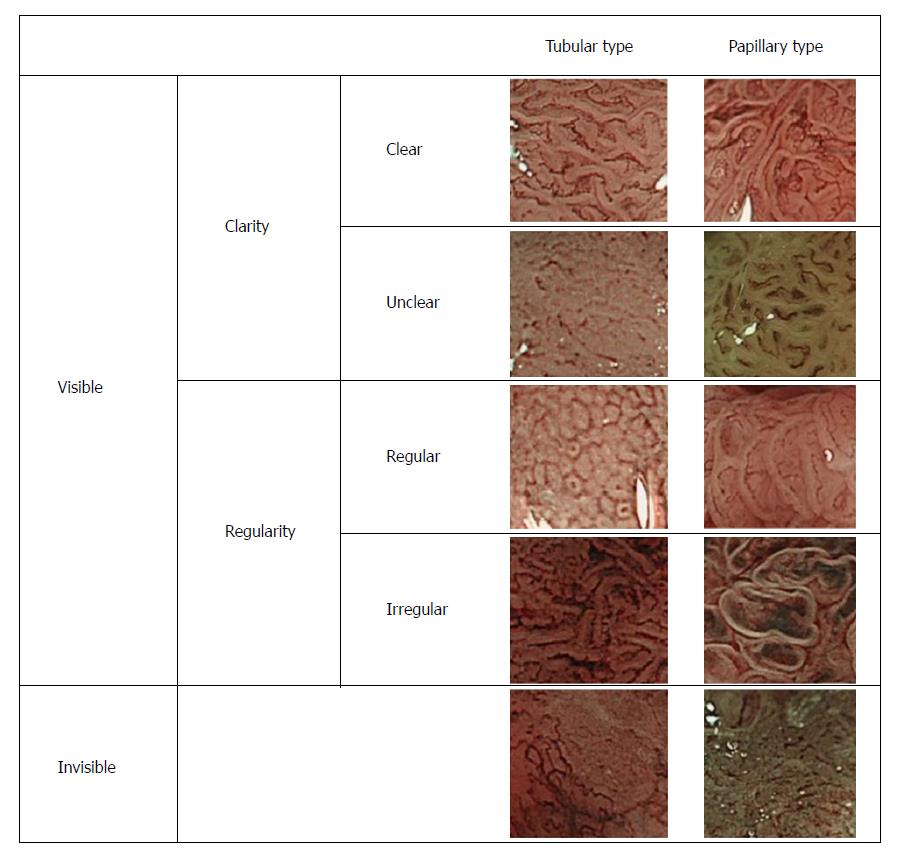
The surface microstructures of the colorectal lesions were classified as “visible” and “invisible” according to the degree of elevation or flatness of the pit structures on the lesion surface, as well as the relative ease of discerning surface pit patterns. The “visible” types were further subdivided into “clear” and “unclear” subtypes according to clarity, and into “regular” and “irregular” types according to the regularity of glandular structures on the lesion surface. Lesions with a surface microstructure that could be visualized clearly were designated as “clear-type” lesions, whereas those with a visible but poorly-defined surface microstructure were designated as “unclear-type” lesions. Furthermore, lesions in which the surface microstructure exhibited a regular size and arrangement were defined as the “regular” type, whereas those in which the surface microstructure exhibited an irregular size and arrangement were defined as the “irregular” type. Representative images of the different types of lesions, classified according to their surface pattern as described above, are shown in Figure 2.
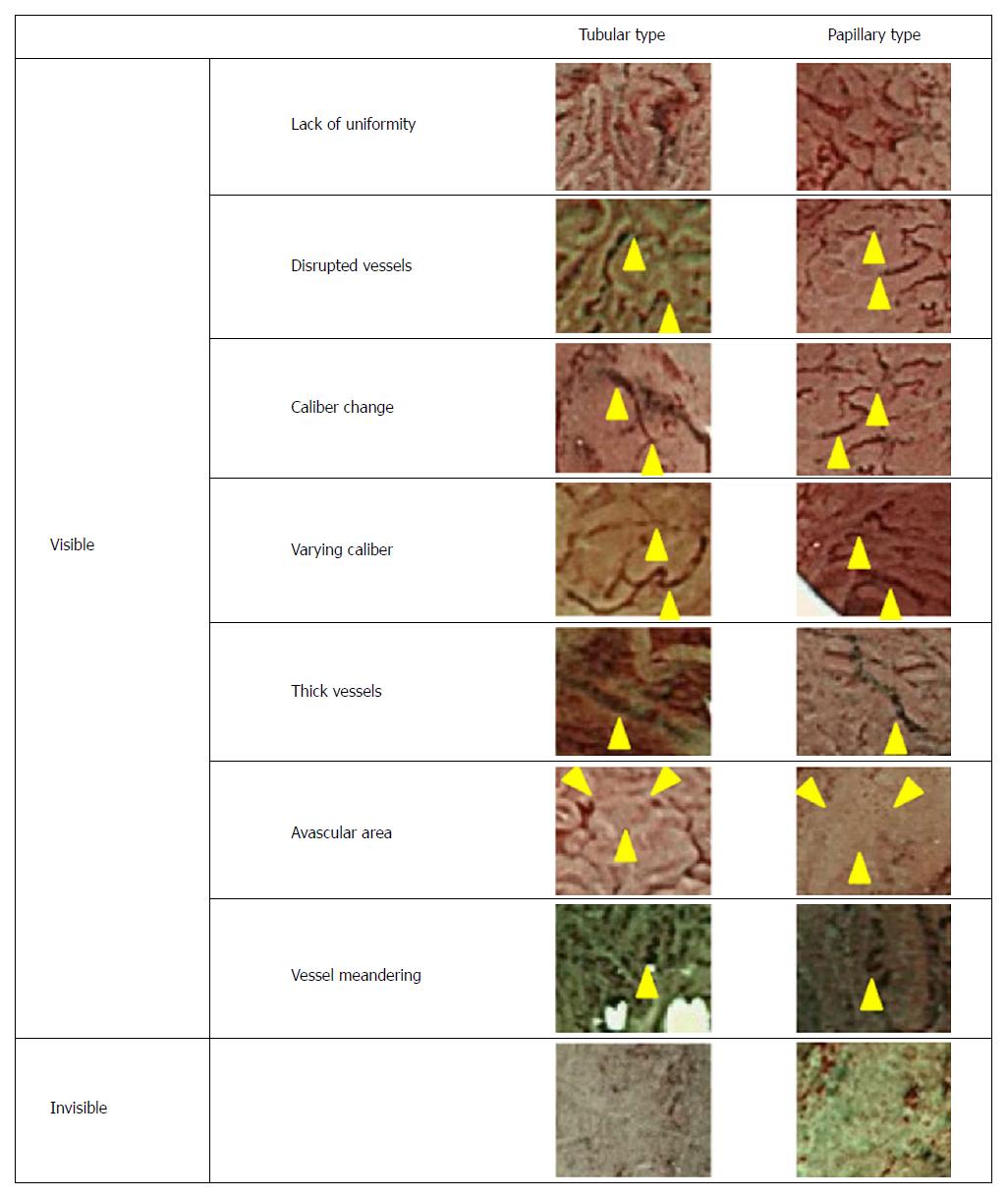
The vessel microstructures on the surfaces of colorectal lesions were classified as “visible” and “invisible,” and the “visible” type was further subdivided into “disturbed arrangement,” “disrupted vessels,” “varying caliber,” “size irregularity,” “thick vessels,” “avascular area”, and “vessel meandering” (Figure 3). “Disturbed arrangement” was defined as a disturbance in the form, size, and arrangement of the microvessels. “Disrupted vessels” was defined as evident disruption of the microvessels, “varying caliber” was defined as variance in the caliber of the same vessel, “size irregularity” was defined as variance in caliber among different vessels, “thick vessels” was defined as markedly green-colored vessels, “avascular area” was defined as an area with no visible vessels, and “vessel meandering” was defined as evident meandering of the blood vessels, whereby tortuous vessels exhibited a twisting and winding path.
Endoscopically or surgically resected specimens were immediately fixed in 40 g/L formaldehyde and subsequently stained with hematoxylin and eosin. All histopathological specimens were evaluated by an experienced gastrointestinal pathologist who was blinded to the endoscopic diagnosis.
Evaluation of the depth of invasion of tumors: The vertical depth of invasion of each lesion into the SM was measured as described by Kitajima et al[9]. The lesions were divided into two groups: lesions with a depth of invasion < 1000 μm (SM cancer with < 1000 μm invasion) and those with a depth of invasion ≥ 1000 μm (SM cancer with ≥ 1000 μm invasion).
Evaluation of the histological architecture of the tumor: Based on a report by Tobaru et al[15], the tumor tissue architecture was evaluated with respect to the degeneration/prolapse of the epithelial lining, variation in vessel caliber and/or disturbed arrangement of the glandular ducts, histological differentiation grade of the tumor, degree of superficial desmoplastic reaction, degree of loss of the muscularis mucosae (residual muscularis), and presence/absence of granuloma formation.
The associations of the surface and vascular patterns of colorectal lesions, as revealed by NBI, with the tumor grade and depth of invasion were determined through a multivariate analysis based on a logistic regression model. This analysis was followed by a stepwise selection of variables related to the NBI-findings and the determination of scores based on the estimated statistical values.
A multivariate analysis (logistic regression) included selected variables from among the explained variables (from endoscopic findings) that were found to correlate with the objective variables (pathological diagnosis, invasion depth) during the univariate analysis. The adjusted value (z score or t score) was obtained by dividing the partial regression coefficient by the standard error of the mean to yield the maximum possible difference, after which the sums of the extracted endoscopic scores were considered. Receiver operating characteristic (ROC) curve modeling was used to determine approximate cut-off values. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, United States) and R version 3.1.2[30]. The statistical analyses in this study were performed and reviewed by Yuko Araki and Tatsuyuki Kakuma from the Biostatistics Center of the Kurume University School of Medicine (Kurume, Japan).
The 161 specimens excised from 138 patients in whom diagnosed colorectal lesions correlated directly with pre-operative NBI findings included 81 adenomas and 80 cancers (intramural cancer, 42 lesions; cancer with a depth of SM invasion < 1000 μm, 7 lesions; cancer with a depth of SM invasion ≥ 1000 μm, 28 lesions; advanced cancer infiltrated beyond the muscular layer, 3 lesions; Figure 1).
First, a multivariate analysis was conducted to determine the association of NBI findings with the tumor grade and depth of invasion into the SM (Table 1). NBI findings that correlated with a diagnosis of a high tumor grade were associated with an “irregular” surface pattern, irrespective of whether the lesion was tubular (P < 0.0001) or papillary (P < 0.0001), as well as an “avascular area” vascular pattern (P = 0.0600). Regarding predictions of the depth of invasion, the vascular patterns of “disrupted vessels” (P = 0.0714) and “thick vessels” (P = 0.0133) were associated with a depth of invasion of ≥ 1000 μm, whereas no surface patterns were associated with this parameter.
| Dependent variable | Independent variable1 | Univariate analysis | Multivariate analysis | |||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |||
| Tumor grade | Probability of cancer | Irregular (tubular type) | 0.034 [0.013, 0.088] | < 0.0001 | 46.81 [14.55, 184.48] | < 0.0001 |
| AUC = 0.89 | Irregular (papillary type) | 0.034 [0.013, 0.088] | < 0.0001 | 47.21 [7.51, 512.05] | < 0.0001 | |
| Avascular area | 0.048 [0.006, 0.376] | < 0.0001 | 16.3 [1.69, 396.35] | 0.06 | ||
| Invasion depth | Probability of SM deep invasive cancer2 | Disrupted vessels | 0.169 [0.058, 0.488] | 0.0004 | 3.63 [0.98, 17.69] | 0.0714 |
| AUC = 0.70 | Thick vessels | 0.509 [0.127, 2.037] | 0.3325 | 22.86 [2.55, 537.18] | 0.0133 | |
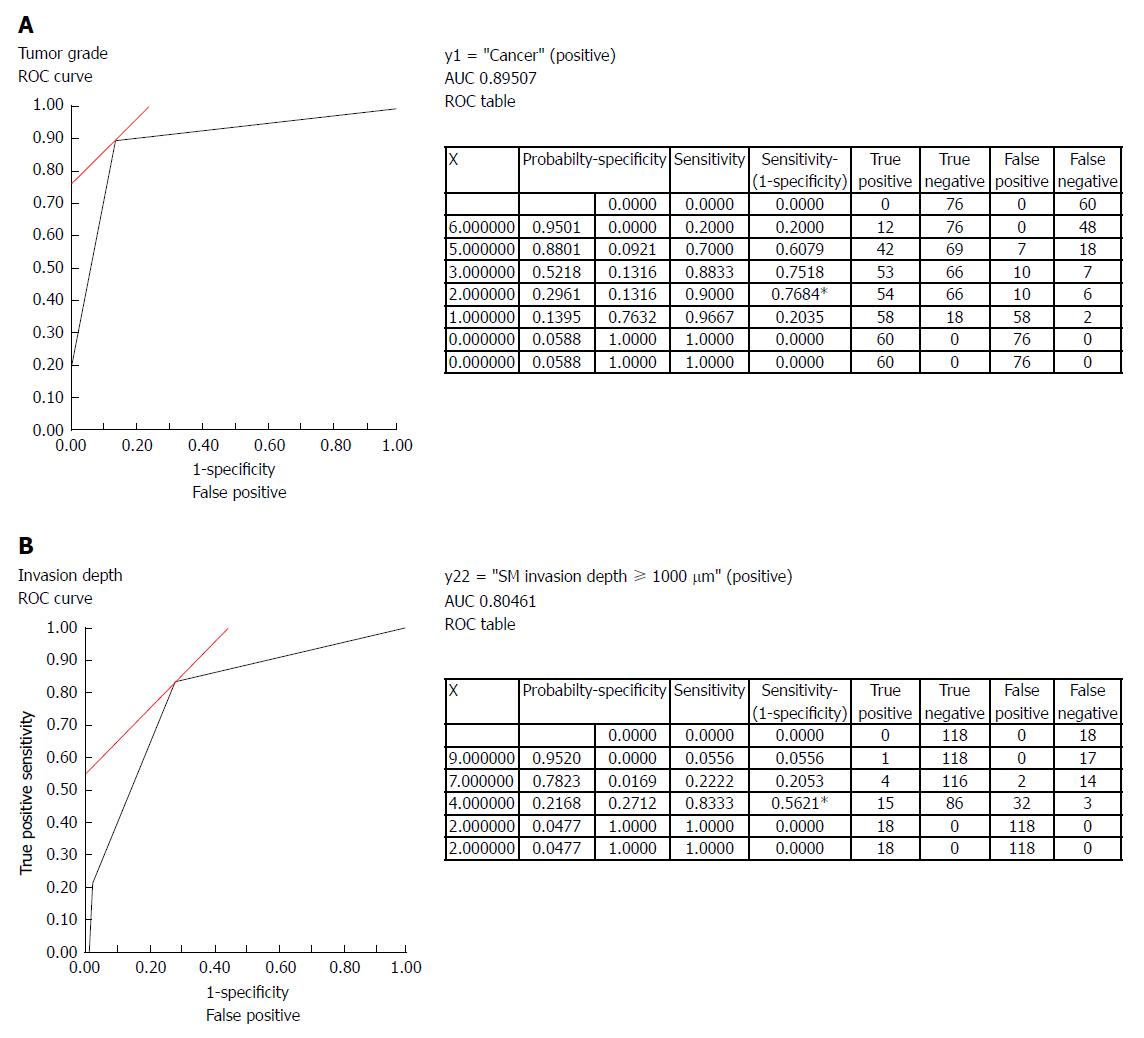
The scores for each of the above-mentioned NBI findings were assigned based on the estimated statistical values and were followed by the development of a model for independently predicting the tumor grade and depth of invasion (Table 2). In this model, a total tumor grade score of ≥ 1 had a sensitivity of 0.97 and specificity of 0.24 for a diagnosis of cancer (Figure 4A). Similarly, a total depth of tumor invasion score of ≥ 9 had a sensitivity of 0.56 and specificity of 1.0 for tumors with an SM invasion depth ≥ 1000 μm (Figure 4B). NBI findings with scores less than these cut-off values were considered adenomas and to have a SM invasion depth < 1000 μm, respectively.
| Variable | Value3 | Score4 | |
| Tumor grade1 | Surface pattern = regular/irregular | Irregular (tubular type) | 5 |
| Regular (tubular type) | 1 | ||
| Irregular (papillary type) | 3 | ||
| Regular (papillary type) | 0 | ||
| Vascular pattern = avascular area | Present | 1 | |
| Absent | 0 | ||
| Invasion depth2 | Vascular pattern = disrupted vessels | Present | 3 |
| Absent | 1 | ||
| Vascular pattern = thick vessels | Present | 6 | |
| Absent | 1 |
Table 3 shows the relationship of the NBI findings used to predict the tumor grade and invasion depth with the tumor tissue architecture. Of the surface pattern categories, the “regular/irregular” pattern was associated with degeneration/prolapse of the lining epithelium, regularity of glandular duct arrangement, and degree of loss of the muscularis mucosae in both the tubular and papillary types (P < 0.05) and with the presence/absence of granuloma formation only in papillary-type lesions.
| Variable | Classification1 | Tissue architecture | ||||||
| Lining epithelium degeneration/ prolapse | Glandular duct arrangement | Superficial differentiation | Superficial desmoplastic reaction | Muscularis mucosae | Granuloma formation | |||
| Surface pattern | Visible/Invisible | Tubular | a | a | a | a | a | a |
| Papillary | b | c | c | a | a | a | ||
| Clarity | Tubular | a | a | c | c | a | a | |
| Papillary | a | a | c | n.a. | a | a | ||
| Regular/Irregular | Tubular | a | a | b | c | a | b | |
| Papillary | a | a | c | n.a. | a | a | ||
| Vascular pattern | Disturbed arrangement | Tubular | a | a | a | a | a | a |
| Papillary | a | a | b | c | a | a | ||
| Disrupted vessels | Tubular | a | a | c | a | a | a | |
| Papillary | a | a | c | a | a | a | ||
| Varying caliber | Tubular | a | a | c | a | a | b | |
| Papillary | c | c | c | a | b | b | ||
| Size irregularity | Tubular | a | a | a | a | a | a | |
| Papillary | a | a | b | c | a | a | ||
| Thick vessels | Tubular | a | a | b | a | a | c | |
| Papillary | a | c | c | c | c | b | ||
| Avascular area | Tubular | a | a | a | a | a | a | |
| Papillary | c | c | c | b | c | c | ||
| Vessel meandering | Tubular | c | c | c | c | c | c | |
| Papillary | a | b | c | c | a | a | ||
Of the vascular pattern categories, the presence/absence and extent of the “avascular area” was found to associate with the degree of degeneration/prolapse of the lining epithelium, regularity of glandular duct arrangement, degree of superficial differentiation, presence/absence of a superficial desmoplastic reaction, degree of loss of the muscularis mucosae, and presence/absence of granuloma formation only in tubular-type lesions. The presence or absence of “disrupted vessels” was found to associate with the degree of degeneration or prolapse of the lining epithelium, regularity of glandular duct arrangement, degree of superficial desmoplastic reaction, degree of loss of the muscularis mucosae, and presence/absence of granuloma formation in both tubular- and papillary-type lesions. The “thick vessels” pattern was found to associate with the degree of degeneration or prolapse of lining epithelium in both tubular- and papillary-type lesions and with the regularity of glandular duct arrangement, presence/absence of superficial desmoplastic reaction, and degree of loss of the muscularis mucosae only in tubular-type lesions.
Table 4 presents the relationships of the tumor grade and depth of invasion with the tissue architecture. The histological findings of degeneration or prolapse of the lining epithelium (P < 0.001) and irregular glandular duct size/arrangement (P < 0.0001) were significantly associated with a higher tumor grade.
| Tumor grade | Invasion depth | ||||||
| Variable | Adenoma(n = 81) | Cancer1(n = 49) | P value | SM invasion depth ≥ 1000 μm (n = 49) | SM invasion depth < 1000 μm (n = 31) | P value | |
| Lining epithelium degeneration/prolapse | Absent | 69 (85.2) | 20 (40.8) | 20 (40.8) | 1 (3.2) | ||
| Present | 12 (14.8) | 29 (59.2) | < 0.0001 | 29 (59.2) | 30 (96.8) | 0.001 | |
| Irregularity in glandular duct size/arrangement | Absent | 54 (66.7) | 10 (20.4) | 10 (20.4) | 1 (3.2) | ||
| Present | 27 (33.3) | 39 (79.6) | < 0.0001 | 39 (79.6) | 25 (80.6) | 0.113 | |
| Superficial differentiation | Well-diff. | 42 (85.7) | 42 (85.7) | 20 (64.5) | |||
| Moderately diff. | 7 (14.3) | 7 (14.3) | 10 (32.3) | ||||
| Poorly diff. | 0 (0.0) | 0 (0.0) | 1 (3.2) | 0.027 | |||
| Superficial desmoplastic reaction | Absent | 81 (100) | 47 (95.9) | 47 (95.9) | 17 (54.8) | ||
| Present | 0 (0.0) | 2 (4.1) | 0.273 | 2 (4.1) | 14 (45.2) | < 0.0001 | |
| Loss of muscularis mucosae | Absent | 0 (0.0) | 3 (6.1) | 3 (6.1) | 29 (93.5) | ||
| Present | 81 (100) | 46 (93.9) | 0.099 | 46 (93.9) | 2 (6.5) | < 0.0001 | |
| Granuloma formation | Absent | 80 (98.8) | 44 (89.8) | 44 (89.8) | 19 (61.3) | ||
| Present | 1 (1.2) | 5 (10.2) | 0.054 | 5 (10.2) | 2 (38.7) | 0.002 | |
Furthermore, the histological findings of degeneration or prolapse of the lining epithelium (P = 0.001), moderate or poor superficial differentiation (P = 0.027), superficial desmoplastic reaction (P < 0.0001), loss of the muscularis mucosae (P < 0.0001), and granuloma formation (P = 0.002) were significantly associated with a deeper invasion (≥ 1000 μm), relative to a lesser depth of invasion (< 1000 μm).
In this study, we were the first to assign scores to the NBI findings of a series of colorectal lesions with the intent to correlate the tumor grade and invasion depth with the surface and vascular microarchitectures of polyps imaged using NBI. Based on the estimated statistical values and a multivariate analysis (logistic regression), we developed a model for predicting the tumor grade and depth of invasion from NBI findings and demonstrated the sensitivity and specificity of this model for differentiating non-neoplastic colorectal lesions, benign adenomas, and malignant neoplasms.
The NBI findings of an “irregular” surface pattern and “avascular area” vascular pattern were identified as important predictors of the tumor grade. A recent report described the problems and limitations of predicting the tumor grade based solely on the vascular pattern as revealed through NBI observations[26]. Indeed, we determined the NBI surface pattern to be superior to the vascular pattern intensity, a measure of microvascular density, for differentiating hyperplastic from other non-neoplastic polyps. We expect that the accuracy of tumor grading based on NBI findings will improve if evaluations are based on both the vascular and surface patterns.
The NBI findings of the “disrupted vessels” and “thick vessels” vascular patterns were found to be useful for diagnosing the depth of tumor invasion, consistent with previous studies[31]. However, the surface pattern was not shown to be useful for predicting the depth of tumor invasion into the SM in the present study. This finding is likely attributable to the following factors: (1) in elevated lesions, tumors may exhibit deep invasion of the SM layer while maintaining a surface pattern characteristic of an intramucosal lesion; and (2) in superficial lesions (particularly depressed lesions), classification of the surface pattern into a regular or irregular type is more difficult than in elevated lesions. Our findings also agree with those of previous studies. Misawa et al[32] analyzed the depth of the surface pattern relative to the macroscopic type and reported that the rate of accurate diagnosis was lower for depressed lesions. The vascular pattern exhibits a high level of contrast, enabling relatively uniform images to be obtained in any setting. In contrast, the surface pattern can undergo changes depending on the angle or magnification scale of observation. Further study is warranted to determine the parameters that would facilitate a precise evaluation of surface patterns.
The clinical usefulness, diagnostic accuracy, sensitivity, and superiority of NBI relative to chromoendoscopy have been well-established[18-21]. However, many of the existing reports have merely observed many types of different endoscopic findings and classified these into categories according to their surface and/or vascular patterns[22-25]. In routine clinical practice, a precise endoscopic diagnosis cannot be based easily or reliably on such reports because the diagnostic accuracy may be compromised by subjective interpretations and inter-observer variability. Indeed, multiple expert groups have advocated for a simple classification system and universal standardized diagnostic criteria[27-29]. NBI-based classification systems have also been proposed. The JNET classification comprises four vessel and surface pattern categories. Specifically, Types 1, 2A, 2B, and 3 correlate with the histopathological findings of hyperplastic polyp/sessile serrated polyp, low-grade intramucosal neoplasia, high-grade intramucosal neoplasia/shallow SM invasive cancer, and deep SM invasive cancer, respectively[29]. The NICE classification is based on lesion color, microvascular architecture, and surface pattern and comprises three types. Types 1, 2, and 3 are indices for hyperplastic lesions, adenoma or mucosal carcinoma, and deep SM invasive carcinoma, respectively[33]. In the present study, we used statistical methods to independently score the surface and vascular patterns visualized by NBI. Subsequently, we developed a statistical model to predict the tumor grade and depth of invasion from these parameters. The sensitivity and specificity of our model for a precise diagnosis were demonstrated in patients with a range of colorectal lesions. Importantly, the sensitivity and specificity of our prediction model can be adjusted by changing the cut-off values for the total score, which enables tailoring to the objectives of the NBI examination in clinical practice. Thus, by using a ROC curve analysis, endoscopists can ascertain whether changing the cut-off values would improve diagnostic accuracy.
Based on the results obtained to date, we propose an efficient and highly precise endoscopic prediction model for diagnosing malignant colorectal tumors and differentiating these lesions from pre-malignant adenomas. This diagnostic tool will enable clinicians to determine the most appropriate therapeutic strategy and individualize treatment. We propose the following diagnostic strategy, which should be evaluated in future prospective studies. First, clinicians should screen patients suspected of harboring colorectal cancer/lesions using magnifying endoscopy with NBI, and then use our highly sensitive model to analyze the NBI findings and the predict tumor grade. A lesion diagnosed as adenoma would be subjected to endoscopic treatment, whereas a lesion diagnosed as cancer should be subjected to a surgical operation. Following resection, the residual lesions should be examined by chromoendoscopy for a pit pattern diagnosis to determine the real extent of cancer invasion[34]. Here, endoscopists should use our model to predict the depth of tumor invasion to enhance the specificity of diagnosis and minimize unnecessary surgery.
Additionally, this study evaluated NBI findings in relation to the tumor tissue architecture in colorectal cancers. To the best of our knowledge, no previous study has conducted a direct comparison of this type. Our results suggest that an “irregular” surface pattern, which is associated with a higher tumor grade, may reflect irregularity in the size and arrangement of the glandular ducts.
Our finding that the “avascular area” vascular pattern correlated with a high tumor grade may seem counterintuitive, given that tumor progression is typically accompanied by an increase in the blood vessel density and thickness[26]. However, as we observed an association of the “avascular area” vascular pattern with the incidence of a desmoplastic reaction, we speculate that this pattern may reflect an amorphous region arising from the resorption of glandular ducts and blood vessels consequent to a desmoplastic reaction at the tumor invasive edge. “Disrupted vessels,” an NBI finding that predicts the depth of tumor invasion, would seem to indirectly reflect degeneration or prolapse of the lining epithelium induced by deep SM invasion of the tumor, with a concomitant loss of the muscularis mucosae. These phenomena may increase the fragility of this type of tumor, leading to the risks of rupture and spillage during surgical resection. Furthermore, “thick vessels” may reflect vascular congestion in the SM layer, which arises as a result of massive tumor invasion and, presumably, neoangiogenesis.
Although our model is both novel and promising, this retrospective study is limited by its nature as a single-center analysis of exclusively Japanese patients. Therefore, the results cannot be generalized directly to other institutes or ethnic groups. Subsequent studies should evaluate this new technology and scoring model in diverse populations and ethnic groups, determine inter- and intra-observer variability, and conduct prospective studies in patients who have not yet been diagnosed. Additionally, the model should also be validated in other cohorts of patients with colorectal polyps.
In conclusion, the NBI finding-based scoring system proposed in this study demonstrated good specificity and sensitivity for predicting the tumor grade and depth of invasion and, thereby, for determining appropriate therapeutic strategies in patients with colorectal tumors.
Colonoscopy is currently the most useful examination method for finding, diagnosing, and treating colorectal lesions. When a lesion is found by colonoscopy, the surgeon must first determine whether it is nontumorous or tumorous. If it is tumorous, it must be assessed as adenoma or cancer. If it is cancer, it is essential to diagnose its depth. Intramucosal carcinomas are unlikely to metastasize, while carcinomas that invade into the submucosa (SM) are more likely to metastasize to the lymph nodes. Carcinomas with a depth of SM invasion < 1000 μm almost never metastasize, while those with a depth of SM invasion ≥ 1000 μm are more likely to metastasize. Accordingly, distinguishing which lesions have a depth of SM invasion < 1000 μm (indicated for endoscopic therapy) is important when selecting a treatment.
Narrow-band imaging (NBI) was recently developed as a novel form of image-enhanced endoscopy that has several advantages over chromoendoscopy. Not only is pigment not used, NBI allows for the acquisition of endoscopic images with a uniform mucosal pattern across images. Further, vascular findings in the mucosal surface, which are difficult to observe with chromoendoscopy, can be enhanced and observed in more detail with NBI. Several studies have reported that using NBI to observe the fine architecture of a lesion’s surface (surface pattern) and microvessels (vascular pattern) is useful for diagnosing the grade and depth of colorectal tumors. However, all of these studies placed endoscopic findings into categories expressed as numbers or letters. These can be difficult to use in actual clinical practice, particularly for inexperienced endoscopists. If the important findings observed using NBI can be appropriately selected and quantified, it would enable more precise predictions of colorectal tumor grade and depth, which would be extremely useful in deciding treatment strategies, including for endoscopic resection.
Several NBI-based classification systems have been proposed earlier. However, these systems are difficult to rely upon due to subjectivity and inter-observer variability. There is therefore a need to identify a more objective classification that can be easily interpreted by endoscopists during routine clinical practice.
We therefore wanted to determine a model for predicating tumor grade using NBI scores. We developed a multivariate statistical model for predicting tumor grades and invasion depths from NBI-finding scores. We determined the utility of this model in patients with a range of colorectal lesions and propose an efficient prediction model for diagnosing colorectal lesions and differentiating malignant lesions from pre-malignant adenomas. Such a model would then help clinicians determine the most appropriate therapeutic strategies.
This was a retrospective examination of the correlations between NBI findings and histopathological findings at the site of colorectal tumorous lesions that were endoscopically or surgically resected after regular observation with colonoscopy and observation with NBI magnifying endoscopy. NBI findings and the tumor grade and depth of invasion at the site examined with NBI were analyzed using multivariate analysis based on a logistic regression model. The results were used to create a model for predicting tumor grade and depth. In addition, we performed the first-ever examination of correlations between colorectal tumor NBI findings and histopathological architecture.
This study demonstrated the usefulness of using statistical methods to score NBI findings to determine tumor grade. Using findings of both the surface pattern and vascular pattern, we created a model for predicting the grade and depth of colorectal tumors. Another novel finding of this study came from the comparison of colorectal tumor NBI findings and tissue architecture. Because clear contrast of vascular patterns can be obtained using NBI, it is possible to obtain relatively uniform images under any conditions. However, when observing surface patterns, the findings that are evaluated changed depending on the angle and rate of magnification. Therefore, further research is required to overcome these issues. This study was limited by only including Japanese subjects and because it was a single-center, retrospective study. Going forward, a prospective study that includes other institutions should be conducted.
The NBI finding scoring system proposed in this study could be a useful marker for predicting tumor grade and depth of invasion in colorectal tumors, which could also be applied in deciding treatment plans. The sensitivity and specificity of this predictive model can be adjusted by changing the total score to suit the goal of the NBI examination. Previous results have indicated that to efficiently and precisely carry out endoscopic diagnostics and decide treatment plans for colorectal tumors, NBI should be performed first, then the sensitivity of the tumor grade predictive model should be increased to pick up as many carcinomas as possible. Next, the specificity of the depth predictive model should be increased to reduce over-surgery. Finally, chromoendoscopy should be used to observe the remaining lesions to diagnose their pit patterns. The results of this study indicate that “irregularity,” an NBI finding useful for diagnosing colorectal tumor grade, may reflect the presence of size disparities and irregular pathways in glandular ducts. An “avascular area” could reflect an amorphous region arising from reduced glandular duct density caused by exposure to a desmoplastic reaction. Further, “disrupted vessels,” an NBI finding useful in diagnosing the depth of tumor invasion, may indirectly reflect degeneration or prolapse of the lining epithelium caused by deep invasion of the tumor and consequent loss of the muscularis mucosae, which leads to fragility of the tumor surface. “Thick vessels” may reflect vascular congestion in the SM layer caused by massive tumor invasion.
It would be helpful to conduct a prospective study that includes more cases using the predictive model proposed in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Fiori E, Kato J, Matsuda A S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
| 1. | Brenner H, Jansen L, Ulrich A, Chang-Claude J, Hoffmeister M. Survival of patients with symptom- and screening-detected colorectal cancer. Oncotarget. 2016;7:44695-44704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Moore JS, Aulet TH. Colorectal Cancer Screening. Surg Clin North Am. 2017;97:487-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Bresalier RS. Management of high-risk colonoscopy patients. Gastrointest Endosc Clin N Am. 2010;20:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 5. | Kaimakliotis PZ, Chandrasekhara V. Endoscopic mucosal resection and endoscopic submucosal dissection of epithelial neoplasia of the colon. Expert Rev Gastroenterol Hepatol. 2014;8:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985;89:328-336. [PubMed] |
| 7. | Nivatvongs S, Rojanasakul A, Reiman HM, Dozois RR, Wolff BG, Pemberton JH, Beart RW Jr, Jacques LF. The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum. 1991;34:323-328. [PubMed] |
| 8. | Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 556] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 486] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 10. | Yamamoto S, Watanabe M, Hasegawa H, Baba H, Yoshinare K, Shiraishi J, Kitajima M. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004;51:998-1000. [PubMed] |
| 11. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 12. | Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880-885. [PubMed] |
| 13. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [PubMed] |
| 14. | Saito S, Tajiri H, Ikegami M. Endoscopic features of submucosal deeply invasive colorectal cancer with NBI characteristics : S Saito et al. Endoscopic images of early colorectal cancer. Clin J Gastroenterol. 2015;8:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Tobaru T, Mitsuyama K, Tsuruta O, Kawano H, Sata M. Sub-classification of type VI pit patterns in colorectal tumors: relation to the depth of tumor invasion. Int J Oncol. 2008;33:503-508. [PubMed] |
| 16. | Kanao H, Tanaka S, Oka S, Kaneko I, Yoshida S, Arihiro K, Yoshihara M, Chayama K. Clinical significance of type V(I) pit pattern subclassification in determining the depth of invasion of colorectal neoplasms. World J Gastroenterol. 2008;14:211-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 611] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 18. | Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007;39:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Katagiri A, Fu KI, Sano Y, Ikematsu H, Horimatsu T, Kaneko K, Muto M, Yoshida S. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther. 2008;27:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, Kaneko K, Soetikno R, Yoshida S. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 21. | Wada Y, Kudo SE, Misawa M, Ikehara N, Hamatani S. Vascular pattern classification of colorectal lesions with narrow band imaging magnifying endoscopy. Dig Endosc. 2011;23 Suppl 1:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Kanao H, Tanaka S, Oka S, Hirata M, Yoshida S, Chayama K. Narrow-band imaging magnification predicts the histology and invasion depth of colorectal tumors. Gastrointest Endosc. 2009;69:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Oba S, Tanaka S, Oka S, Kanao H, Yoshida S, Shimamoto F, Chayama K. Characterization of colorectal tumors using narrow-band imaging magnification: combined diagnosis with both pit pattern and microvessel features. Scand J Gastroenterol. 2010;45:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Oka S, Tanaka S, Takata S, Kanao H, Chayama K. Clinical usefulness of narrow band imaging magnifying classification for colorectal tumors based on both surface pattern and microvessel features. Dig Endosc. 2011;23 Suppl 1:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Hayashi N, Tanaka S, Kanao H, Oka S, Yoshida S, Chayama K. Relationship between narrow-band imaging magnifying observation and pit pattern diagnosis in colorectal tumors. Digestion. 2013;87:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | East JE, Suzuki N, Bassett P, Stavrinidis M, Thomas HJ, Guenther T, Tekkis PP, Saunders BP. Narrow band imaging with magnification for the characterization of small and diminutive colonic polyps: pit pattern and vascular pattern intensity. Endoscopy. 2008;40:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Oba S, Tanaka S, Sano Y, Oka S, Chayama K. Current status of narrow-band imaging magnifying colonoscopy for colorectal neoplasia in Japan. Digestion. 2011;83:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Tanaka S, Sano Y. Aim to unify the narrow band imaging (NBI) magnifying classification for colorectal tumors: current status in Japan from a summary of the consensus symposium in the 79th Annual Meeting of the Japan Gastroenterological Endoscopy Society. Dig Endosc. 2011;23 Suppl 1:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 30. | R Core Team. R: A language and environment for statistical computing. (2014) R Foundation for Statistical Computing, Vienna, Austria. Available from: http://www.R-project.org/. |
| 31. | Hirata M, Tanaka S, Oka S, Kaneko I, Yoshida S, Yoshihara M, Chayama K. Evaluation of microvessels in colorectal tumors by narrow band imaging magnification. Gastrointest Endosc. 2007;66:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Misawa M, Kudo SE, Wada Y, Nakamura H, Toyoshima N, Hayashi S, Mori Y, Kudo T, Hayashi T, Wakamura K. Magnifying narrow-band imaging of surface patterns for diagnosing colorectal cancer. Oncol Rep. 2013;30:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Tanaka S, Hayashi N, Oka S, Chayama K. Endoscopic assessment of colorectal cancer with superficial or deep submucosal invasion using magnifying colonoscopy. Clin Endosc. 2013;46:138-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Wada Y, Kashida H, Kudo SE, Misawa M, Ikehara N, Hamatani S. Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig Endosc. 2010;22:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |