Published online Nov 14, 2018. doi: 10.3748/wjg.v24.i42.4787
Peer-review started: July 23, 2018
First decision: August 25, 2018
Revised: August 29, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: November 14, 2018
Processing time: 115 Days and 13.5 Hours
To understand the effects of delivery mode on the immune cells frequency and function in cord blood and placenta.
We evaluated immunological differences in cord blood and placental tissues for a case of twins one of which delivered vaginally while the other delivered by caesarian section (C-section). Cord blood mononuclear cells were isolated and placenta tissues were processed for cell isolation. Immune phenotyping was performed by flow cytometry methods following staining for T cells, natural killer (NK) cells, monocytes, neutrophils and CD71+ erythroid cells in both cord blood and placenta tissues. In addition, fetal calprotectin of twins was measured 12 wk after birth.
We found lower percentages of immune cells (e.g. T cells, monocytes and neutrophils) in the cord blood of C-section delivered compared to vaginally delivered newborn. In contrast, percentages of monocytes and neutrophils were > 2 folds higher in the placental tissues of C-section delivered newborn. More importantly, we observed lower percentages of CD71+ erythroid cells in both cord blood and placental tissues of C-section delivered case. Lower CD71+ erythroid cells were associated with a more pro-inflammatory milieu at the fetomaternal interface reflected by higher expression of inhibitory receptors on CD4+ T cells, higher frequency of monocytes and neutrophils. Furthermore, type of delivery impacted the gene expression profile in CD71+ erythroid cells. Finally, we found that C-section delivered child had > 20-fold higher FCP in his fecal sample at 12 wk of age.
Mode of delivery impacted immune cells profile in cord blood/placenta. In particular frequency of immunosuppressive CD71+ erythroid cells was reduced in C-section delivered newborn.
Core tip: Mode of delivery may influence the immune system of offspring with possible long-term consequences. We report a case of twins one of which delivered vaginally while the other delivered by caesarian section (C-section). We found lower frequency of immune cells in the cord blood and placenta of C-section delivered compared to vaginally delivered newborn. However, higher percentage of neutrophils was observed in the placenta of C-section delivered newborn. Interestingly, for the very first time we found lower percentages of immunosuppressive CD71+ erythroid cells in both cord blood and placenta tissues of C-section delivered offspring. Thus, mode of delivery can modulate the immune system of newborn. In particular, lower frequency of CD71+ erythroid cells and its potential impact on adaptation to microbiome merits further investigations.
- Citation: Dunsmore G, Koleva P, Sutton RT, Ambrosio L, Huang V, Elahi S. Mode of delivery by an ulcerative colitis mother in a case of twins: Immunological differences in cord blood and placenta. World J Gastroenterol 2018; 24(42): 4787-4797
- URL: https://www.wjgnet.com/1007-9327/full/v24/i42/4787.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i42.4787
Pregnancy is a fascinating biological and immunological phenomenon that not only impacts the health of the growing infant but also influences its future wellbeing. A significant body of literature has suggested that the method of delivery at the end of this journey can influence the future health of the infant[1]. Vaginal delivery is commonly performed but in complicated or high-risk pregnancies delivery is performed by caesarian sections (C-sections). According to a report by World Health Organization (WHO) in 2013, 32.7% of births were achieved by C-section in the United States of America, which demonstrates a significant demand for this procedure[2]. This trend is reflected in many parts of the world such as China (approximately 50%), in some parts of Brazil (80%)[1] and appears to be on the rise globally[3,4]. Despite the fact that C-section is integral for the safety of the mother and the newborn in some cases, maternal demand or recommendation by physicians for this procedure has increased world-wide without the consideration of potential long-term impacts on the growing newborn. Many studies suggest that C-section may come at a price for the offspring. The initial establishment of the neonatal microbiome is mainly determined by maternal-newborn exchanges of microbiota. During the normal vaginal delivery, the infant is exposed to a myriad of commensal bacteria, which colonize the urogenital tract of the mother[5]. C-section delivery appears to be the greatest insult to the natural congregation of the neonatal microbiome[6]. Upon exposure to these commensal bacteria, the infant’s immune system becomes educated, and through these interactions gets trained to tolerate non-harmful and non-self-antigens[7]. Thus, vaginal delivery and exposure to maternal microbiota via breastfeeding are evolutionary important to the newborn’s development and health. Furthermore, continued exposure to environmental microbiota is considered to be essential for the development of the microbiome and subsequently the immune system of the offspring as suggested by the hygiene hypothesis[8]. Together these studies elude to the growing body of work that describes how clinically intervened delivery methods may impact the immune system and overall health of the newborn.
The mechanisms which contribute to immune system training are diverse and can be complicated; however, disrupting the mother-to-child transmission of microbiota by C-sections may result in increased risk of asthma, celiac disease, obesity and autoimmune diseases such as type 1 diabetics[9-11].
For instance, a study conducted on monozygotic (MZ) twins at different ages demonstrated that MZ twins’ immune systems became increasingly divergent at later ages suggesting immunological variations stem primarily from the environmental and non-heritable factors[12]. Although initial microbial interactions and mother-to-offspring microbiota exchanges are crucial in the development of neonatal microbiome and immune system education, immediate effects of delivery methods on the neonatal immune system is not very well studied.
Several studies have reported differences in cord blood biomarkers in C-section vs vaginal deliveries[11-13]. However, we are unaware of any study showing the possible impact of delivery mode on cord blood and placenta immunological biomarkers in twins born to an inflammatory bowel disease (IBD) mothers.
Recently, we have reported that CD71+ erythroid cells co-expressing CD71 (transferrin receptor) and CD235 (erythroid lineage marker) are physiologically abundant in human cord blood and placenta tissues[13,14]. These cells have distinctive immunosuppressive properties and quench excessive inflammation induced by abrupt commensal colonization in the newborn[14]. In addition, we have shown that CD71+ erythroid cells expand during pregnancy and play an important role in feto-maternal tolerance[13]. A more recent study reported lower frequency of CD71+ erythroid cells in pre-term deliveries[15] however their frequency and function in vaginal vs C-section deliveries of full-term pregnancies in particular in IBD patients’ needs to be determined.
Here a delivery of twins by a mother with ulcerative colitis is reported. In this study, we analyzed the delivery effects on immune biomarkers in cord blood, placental tissues and fecal samples 12 wk postpartum. Particular attention was made on the frequency of CD71+ erythroid cells with immunomodulatory activities[13,14,16-18].
Twin A was born by naturally induced vaginal delivery, the other twin by urgent C-section which is commonly practiced in order to reduce stress for the second twins[19] or due to delivery associated complications. In this case, the head of baby B was high and variable uncomplicated fetal heart rate decelerations was noted. As the head was descending the cervix did clamp down, at that point urgent C-section was recommended. Child A was born at 21:45 and child B by C-section at 22:22 pm, 37 min apart.
Cord blood and placental tissues were collected at the time of delivery from an ulcerative colitis patient participating in an IBD related study. Fecal samples from twins were collected 12 wk later. The patient was human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) seronegative.
Immune cells from cord blood mononuclear cells (CBMCs) were isolated by Ficoll-paque gradient separation on premium Ficoll-paque (GE). Placental immune cells were isolated from the extravillous placental tissues followed by Ficoll-paque gradient separation according to our previous report[13]. CD71+ erythroid cells were isolated and enriched as we have previously reported elsewhere[14,16]. Purity of enriched CD71+ erythroid cells was ≥ 96% for subsequent experiments.
Antibodies used for this study were purchased from BD bioscience or eBioscience: anti-CD3 (SK7), anti-CD4 (RPA-T4), anti-CD8 (SK1), anti-CD14 (M5E2), anti-CD16 (3G8), anti-CD71 (HB15e), anti-CD235a (GA-R2), anti-program death-1 (PD-1) (EH12.1), anti-lymphocyte-activation gene 3 (LAG-3) (3DS223H), and anti-T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) (F38-2E2). Cell viability was measured using LIVE/DEAD Aqua (Life Technologies).
Fecal samples were collected from newborns at 12 wk of age and kept frozen until use. The frozen fecal samples were thawed and a CALEX Cap Stool Extraction Device (Bühlmann Laboratories, AG) was used to dilute the samples to a working concentration. The fecal calprotectin (FCP) was measured using a fCAL ELISA Calprotectin kit (Bühlmann Laboratories, AG).
Total RNA was isolated from enriched CD71+ erythroid cells in TRIzol (Sigma) using the RNeasy Mini Kit (Qiagen). The purified RNA was quantified on NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies) and 1 μg RNA of each sample was reverse-transcribed using QuantiTect Reverse Transcription kit (Qiagen). The analysis of mRNA expression level was performed on CFX96 TouchTM Real-Time PCR Detection System (BioRad) using TaqMan Fast Advanced Master Mix (Applied Biosystems) with TaqMan probes for arginase-2 (Hs00982833-m1), transforming growth factor beta-1 (TGF-β1) (Hs00998133-m1), vascular endothelial growth factor A (VEGFα) (Hs00900055-m1) and the phagocyte NADPH oxidase (NOX2) (QT00029533). Actin (QT00088935) was used as a reference gene, and the gene expression of the targeted genes was calculated by the 2-ΔΔCt method.
All patient samples and information were collected according to the Institutional Review Boards at the University of Alberta. The study participant provided written consent to participate in this study.
The mother had ulcerative colitis and on Sulfasalazine during pregnancy with clinical history outlined in Table 1. The twins were born at gestational age of 37 wk with different APGAR scores and weight at birth (Table 2). Follow ups indicated that both twins were healthy, gained weight in similar trend and breastfed until 12 wk of age.
| Age (yr) | Diagnosis | Disease phenotype | Daily medications | FCP Score at third trimester | Objective CRP | pMayo Score | Clinical remission |
| 34 | Ulcerative colitis | E3 pancolitis | Sulfasalazine (2 g) | 176.41 μg/mL | 11.5 | 1 | Yes |
| Omeprazole (20 mg) | |||||||
| Prenatal vitamin (1) |
| Vaginally delivered twin | Urgent C-section | |
| Gestational age (wk) | 37 | 37 |
| Sex of the baby | Female | Male |
| Birthweight (g) | 2720 | 3150 |
| APGAR Score | 9, 9, 9, 9 | 1, 8, 9 |
| FCP (12 wk of age) | 40.89 | 982 |
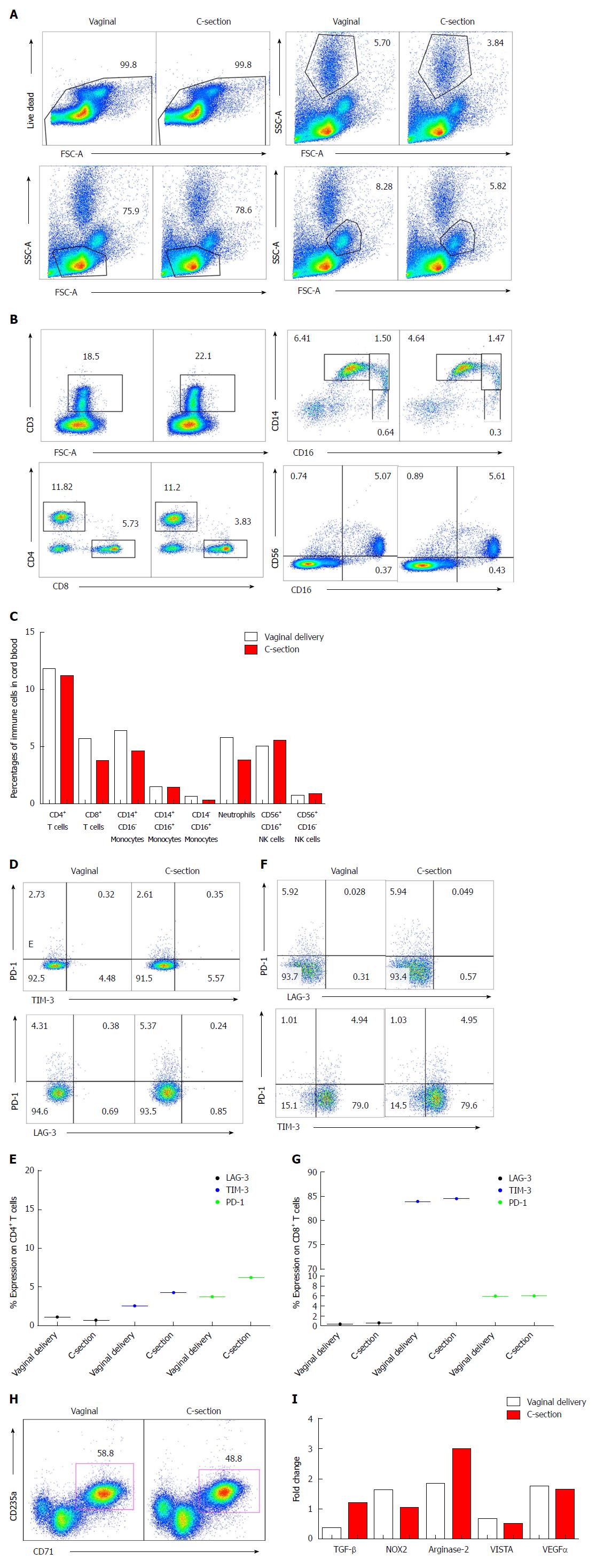
Immune phenotyping was performed for the frequency of different immune cells such as T cells, natural killer (NK) cells, monocytes, CD71+ erythroid cells and neutrophils. Cell populations were gated according to the gating strategies (Figure 1A). We observed a trend in higher abundance of immune cells (e.g. T cells, monocytes and neutrophils) in the vaginally delivered newborn in comparison to the C-section delivered (Figure 1B and C), which in consistent with other reports[20,21]. Following these observations, the expression of immune checkpoint molecules (PD-1, LAG-3 and TIM-3) were measured on the surface of CD4+ and CD8+ T cells. A slight increase in (PD-1/Tim-3) expression was noted on the CD4+ T cells from the infant delivered by C-section compared to the naturally delivered newborn, while the expression of other inhibitory molecules remained constant (Figure 1D and E). Interestingly, the expression of inhibitory molecules TIM-3 and PD-1 were much higher on CD8+ T cells compared to CD4+ T cells but no difference was observed between the twins (Figure 1F and G). Although the functionality of T cells was not investigated in this study, these findings suggest that CD4+ and CD8+ T cells may function differently in cord blood due to different expression pattern of inhibitory receptors.
CD71+ erythroid suppressor cells have been shown to play an integral role in feto-maternal tolerance and neonatal immunity[13,14,16-18]. Thus, we measured their frequency in cord blood and interestingly observed higher frequency of CD71+ erythroid cell in the cord blood of the offspring born by vaginal route compared to C-section delivered newborn (Figure 1H). Higher frequency of CD71+ erythroid cells due to their immunosuppressive nature may create a more immunosuppressive milieu in the cord blood of vaginally delivered newborn. This is reflected by a trend in higher expression of inhibitory receptors on CD4+ T cells in C-section delivered cord blood. Inhibitory receptors can be considered as activation markers in an acute setting but in chronic conditions these receptors are associated with T cell exhaustion[22,23]. Furthermore, we analyzed the expression of some genes [TGF-β, NOX2, arginase-2, V-domain Ig suppressor of T cell activation (VISTA) and VEGFα] associated with immunological properties of CD71+ erythroid cells as we have described elsewhere[13,14,16]. Interestingly, we observed higher expression of TGF-β and arginase-2 mRNA however lower expression of NOX2 mRNA in CD71+ erythroid cells isolated from the cord blood of C-section compared to the vaginally delivered newborn (Figure 1I). However, the mRNA expression for VISTA and VEGFα remained similar in CD71+ erythroid cells obtained from the cord blood of both newborns (Figure 1I).
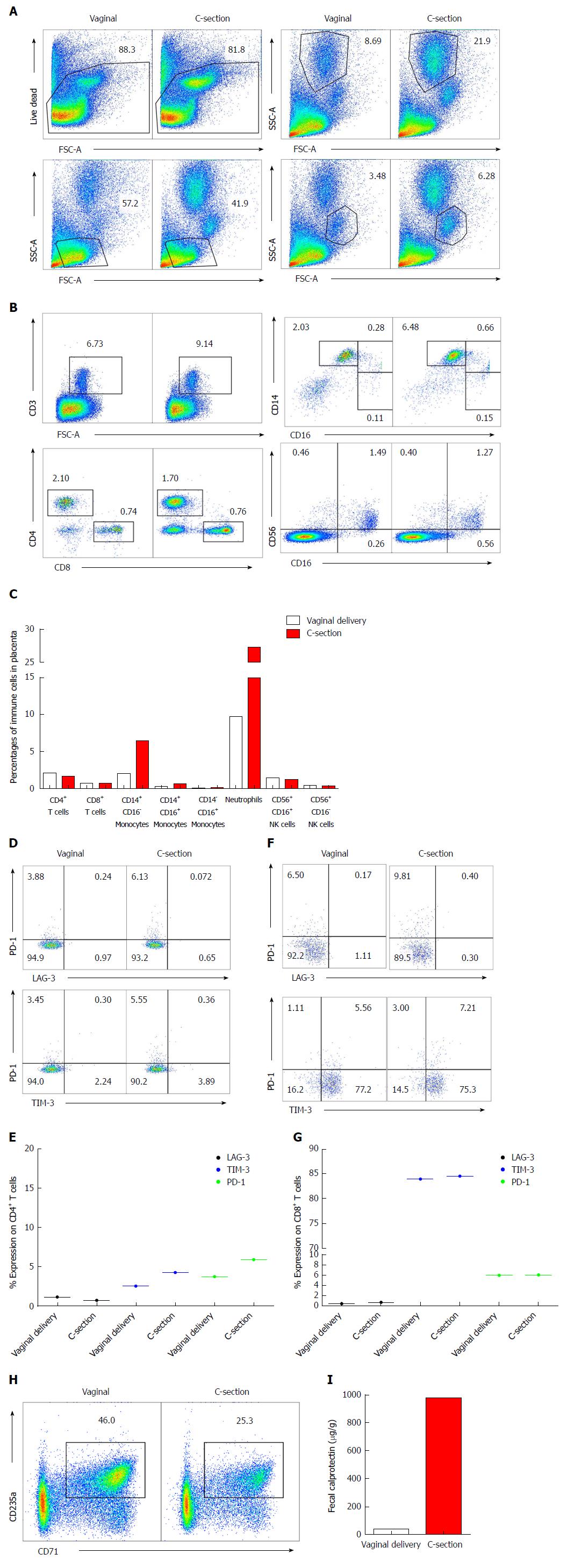
Placental immune cells were analyzed using flow cytometry by the gating strategies detailed in Figure 2A for different immune cells (e.g., T cells, NK cells, neutrophils, monocytes and CD71+ erythroid cells). No difference in the frequency of T cells and NK cells was observed (Figure 2B and C) however we found a substantial increase in CD14+ CD16- monocytes (6.28% compared to 2%) and neutrophils (21.9% compared to 8.69%) in the placental tissues of the infant born by C-section compared to the vaginally delivered newborn (Figure 2A-C). Upon further analysis, we found that CD4+ T cells from the placenta of the C-section delivered child had higher expression of TIM-3 and PD-1 (Figure 2D and E). In consistent with cord blood data, higher expression of TIM-3 on placental CD8+vs CD4+ T cells was observed without any difference between the twins (Figure 2F and G).
More interestingly, we observed almost 50% reduction in the abundance of CD71+ erythroid cells in the placental tissues of the infant born by C-section compared to her naturally delivered brother (Figure 2H). Therefore, lower frequency of CD71+ erythroid cells may explain more inflammatory response in C-section child reflected by higher monocytes and neutrophils presence (Figure 2A and C).
To investigate the potential impact of C-section on the newborns’ intestinal health, we analyzed the abundance of FCP in the fecal samples of both newborns at 12 wk of age. We found that the amount of FCP was substantially elevated in the fecal sample of the infant born by C-section, indicating increased intestinal inflammation (Figure 2I).
Mode of delivery is critical in shaping the immune system of the offspring. Existing evidence support immunological differences in newborns delivered by C-section vs vaginal deliveries[21,24]. In this report, we have investigated immunological differences in cord blood and placenta of twins one delivered by vaginal and the other by urgent C-section. A trend in reduction of T cells, monocytes and neutrophils was observed in the cord blood of C-section delivered vs the vaginally delivered twin. This is in line with other reports indicating that C-section newborns have lower leukocytes count in their cord blood[20]. Similarly, lower percentages of neutrophils, NK cells and monocytes in the cord blood are reported for C-section deliveries[25,26] however we did not observe any difference in frequency of NK cells in this report. There is no report about the possible changes in the frequency of CD71+ erythroid cells in cord blood of C-section vs vaginal deliveries. Of note, we found that the C-section twin had 10% lower CD71+ erythroid cells in cord blood vs his sister who was delivered by vaginal route. Strikingly, we observed 3-fold higher percentages of monocytes (2.03% to 6.48%) and neutrophils (9% to 22%) in C-section delivered vs vaginal delivered placental tissues. In contrast, we found 50% lower percentages of CD71+ erythroid cells in placental tissues of C-section delivered twin. CD71+ erythroid cells have distinctive immunosuppressive properties as we have reported elsewhere[13,14,16]. Therefore, higher expression of inhibitory receptors (PD-1 and TIM-3) on CD4+ T cells in cord blood and placental tissues of C-section delivered newborn suggest the presence of a pro-inflammatory milieu. This is reflected by the presence of more neutrophils and monocytes in the placental tissues of the C-section delivered twin. The most striking observation was that the C-section delivered newborn had > 20-fold FCP in his fecal sample compared to the vaginally delivered sister at 12 wk of age. Of note, FCP can be normally higher in healthy breast-fed compared to formula-fed infants[27], however both twins in this study were breastfed until the time of sample collection.
Of note, we have shown that CD71+ erythroid cells prevent improper immune activation against commensal microbial colonization in neonates[14], therefore lower frequency of CD71+ erythroid cells in C-section deliveries may impair swift adaptation to microbiota and predisposes these newborns to a more pro-inflammatory response in their gut.
Although, existing data suggest that C-section delivery alters bacterial exchange between mother-newborn with long term immunological subsequences, our observations indicate immediate immunological differences at the feto-maternal interface in twins delivered by different delivery modes. It was very interesting to see that CD71+ erythroid cells had different profiles in terms of mRNA expression for genes associated with their immunomodulatory properties. Therefore, our observations suggest that the stress associated with the mode of delivery or any complications during delivery can have substantial effects on both the mother’s and the newborn’s immune systems. In our case the child B was delivered 37 min later than child A and therefore, delay in delivery as a confounding factor should be taken in consideration.
As for the clinical significance of our finding, mode of delivery not only can have long-term consequences (microbiome development) for the child but also short-term immunological effects should be taken in consideration. Although, we are unable to dissociate the possible impact of delay in the delivery of the second twin’s due to urgent C-section, our observations merit further examinations.
In IBD patients, decision-making around delivery is more complex than the general population because of medications (e.g. immunosuppressive use), wound healing, anatomical considerations such as previous surgeries, rectal and perianal disease[28]. In the meantime, decision making between the providers and IBD patients is crucial especially considering the potential long-term impact of delivery mode in the newborn’s future wellbeing.
A higher awareness among childbearing mothers and professional about the association of elective C-section and adverse immunological effects is warranted. Thus, both short- and long-term consequences of delivery modes appear to be greater than what is considered nowadays.
In conclusion, our study provides another piece of evidence on how route of delivery can influence the newborn’s immune system. In particular, for the very first time we have shown that mode of delivery impacts the frequency and functionality of CD71+ erythroid cells in cord blood and placenta tissues. Our findings suggest mode of delivery directs immunological changes which may have potential long-term effects on the offspring. Future research should focus on different modes of delivery in twins especially longitudinal aspects targeting immunological functions.
The delivery mode may impact newborn’s immune system with possible short-term and long-term consequences. Several studies suggest that caesarian section (C-section) may come at a price for the newborn. C-section delivery prevents the exposure of newborn to the mother’s microbiome in the birth canal. Whereas vaginal delivery and exposure to maternal microbiota via breastfeeding are evolutionary important to the newborn’s development and health. However, the impacts of mode of delivery in twins is not fully understood.
Disrupting the mother-to-child transmission of microbiota by C-sections may result in increased risk of asthma, celiac disease, obesity and autoimmune diseases. Thus, investigating the impact of delivery mode on newborns immune system development is crucial for protecting this highly fragile population.
We investigated whether delivery mode by C-section compared to vaginal delivery in twins can influence the immunological components of their cord blood and placenta. In addition, our aim was to determine if C-section delivery impacts the frequency of CD71+ erythroid cells in cord blood and placenta tissues.
We performed immune cells isolation from fresh cord blood and placenta tissues. Then, immunophenotyping was performed for different immune cell subsets in cord blood and placenta of twins. Finally, CD71+ erythroid cells were isolated for RNA isolation and quantitative polymerase chain reaction PCR (qPCR) analysis.
Our results indicated lower frequency of immune cells in cord blood of C-section delivered newborn compared to the vaginally delivered newborn. In contrast, we observed higher percentages of monocytes and neutrophils in placenta tissues of C-section delivered newborns and higher expression of inhibitory receptors on T cells. More importantly, we found lower frequency of CD71+ erythroid cells in both cord blood and placenta of C-section delivered newborn compared to the vaginally delivered newborn. Interestingly, CD71+ erythroid cells from C-section delivered newborn had a different gene profile compared to the vaginally delivered one.
Our study provides another piece of evidence that delivery mode can influence the newborn’s immune system. In particular, we have investigated this in a case of twins one delivered by C-section while the other by vaginal route. We have shown for the very first time that mode of delivery impacts the frequency and functionality of CD71+ erythroid cells in cord blood and placenta tissues. Our findings suggest mode of delivery may result in immunological changes with possible long-term effects on the offspring.
C-section delivery may negatively impact neonatal immune system. Our study is a clear example indicating immunological differences in cord blood and placenta tissues of twins. Further longitudinal studies are required to investigate potential short-term and long-term impacts of C-section vs vaginally delivery in twins.
We thank our study volunteer for providing samples and supporting this work.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
STROBE Statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
P- Reviewer: Serban DE, Sergi CM S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
| 1. | Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38:321-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Patah LE, Malik AM. Models of childbirth care and cesarean rates in different countries. Rev Saude Publica. 2011;45:185-194. [PubMed] |
| 3. | Arabin B, Kyvernitakis I, Liao A, Zugaib M. Trends in cesarean delivery for twin births in the United States: 1995-2008. Obstet Gynecol. 2012;119:657-658; author reply 658-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Lee HC, Gould JB, Boscardin WJ, El-Sayed YY, Blumenfeld YJ. Trends in cesarean delivery for twin births in the United States: 1995-2008. Obstet Gynecol. 2011;118:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1965] [Cited by in RCA: 1974] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 6. | Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971-11975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2922] [Cited by in RCA: 3156] [Article Influence: 210.4] [Reference Citation Analysis (0)] |
| 7. | Kelly D, King T, Aminov R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat Res. 2007;622:58-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Guarner F, Bourdet-Sicard R, Brandtzaeg P, Gill HS, McGuirk P, van Eden W, Versalovic J, Weinstock JV, Rook GA. Mechanisms of disease: the hygiene hypothesis revisited. Nat Clin Pract Gastroenterol Hepatol. 2006;3:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 636] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 10. | Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedón JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135:e92-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 331] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 12. | Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 761] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 13. | Delyea C, Bozorgmehr N, Koleva P, Dunsmore G, Shahbaz S, Huang V, Elahi S. CD71+ Erythroid Suppressor Cells Promote Fetomaternal Tolerance through Arginase-2 and PDL-1. J Immunol. 2018;200:4044-4058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 15. | Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, C MacKenzie T, Frascoli M, Hassan SS. Umbilical cord CD71+ erythroid cells are reduced in neonates born to women in spontaneous preterm labor. Am J Reprod Immunol. 2016;76:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Dunsmore G, Bozorgmehr N, Delyea C, Koleva P, Namdar A, Elahi S. Erythroid Suppressor Cells Compromise Neonatal Immune Response against Bordetella pertussis. J Immunol. 2017;199:2081-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Elahi S. New insight into an old concept: role of immature erythroid cells in immune pathogenesis of neonatal infection. Front Immunol. 2014;5:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Namdar A, Koleva P, Shahbaz S, Strom S, Gerdts V, Elahi S. CD71+ erythroid suppressor cells impair adaptive immunity against Bordetella pertussis. Sci Rep. 2017;7:7728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Bogner G, Wallner V, Fazelnia C, Strobl M, Volgger B, Fischer T, Jacobs VR. Delivery of the second twin: influence of presentation on neonatal outcome, a case controlled study. BMC Pregnancy Childbirth. 2018;18:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Nikischin W, Peter M, Oldigs HD. The influence of mode of delivery on hematologic values in the umbilical vein. Gynecol Obstet Invest. 1997;43:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Schlinzig T, Johansson S, Stephansson O, Hammarström L, Zetterström RH, von Döbeln U, Cnattingius S, Norman M. Surge of immune cell formation at birth differs by mode of delivery and infant characteristics-A population-based cohort study. PLoS One. 2017;12:e0184748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Okoye I, Namdar A, Xu L, Crux N, Elahi S. Atorvastatin downregulates co-inhibitory receptor expression by targeting Ras-activated mTOR signalling. Oncotarget. 2017;8:98215-98232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Okoye IS, Houghton M, Tyrrell L, Barakat K, Elahi S. Coinhibitory Receptor Expression and Immune Checkpoint Blockade: Maintaining a Balance in CD8+ T Cell Responses to Chronic Viral Infections and Cancer. Front Immunol. 2017;8:1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 25. | Thilaganathan B, Meher-Homji N, Nicolaides KH. Labor: an immunologically beneficial process for the neonate. Am J Obstet Gynecol. 1994;171:1271-1272. [PubMed] |
| 26. | Grönlund MM, Nuutila J, Pelto L, Lilius EM, Isolauri E, Salminen S, Kero P, Lehtonen OP. Mode of delivery directs the phagocyte functions of infants for the first 6 months of life. Clin Exp Immunol. 1999;116:521-526. [PubMed] |
| 27. | Savino F, Castagno E, Calabrese R, Viola S, Oggero R, Miniero R. High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology. 2010;97:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Cohan J, Finlayson E. Delivery Mode in Pregnant Patients with IBD: Uncertainty Remains. Inflamm Bowel Dis. 2017;23:727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |