Published online Nov 14, 2018. doi: 10.3748/wjg.v24.i42.4773
Peer-review started: August 8, 2018
First decision: August 24, 2018
Revised: September 3, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: November 14, 2018
Processing time: 98 Days and 0.1 Hours
To investigate the function and mechanism of ubiquitin-like modifier activating enzyme 2 (Uba2) in progression of gastric cancer (GC) cells.
Uba2 level in patients with GC was analyzed by Western blotting and immunohistochemistry. MTT and colony formation assays were performed to examine cell proliferation. Flow cytometry was used for cell cycle analysis. Wound healing and Transwell assays were conducted to examine the effects of Uba2 on migration and invasion. Expression levels of cell cycle-related proteins, epithelial-mesenchymal transition (EMT) biomarkers, and involvement of the Wnt/β-catenin pathway was assessed by Western blotting. Activation of the Wnt/β-catenin pathway was confirmed by luciferase assay.
Uba2 expression was higher in GC than in normal tissues. Increased Uba2 expression was correlated with tissue differentiation, Lauren’s classification, vascular invasion, and TNM stage, as determined by the analysis of 100 GC cases (P < 0.05). Knock-down of Uba2 inhibited GC cell proliferation, induced cell cycle arrest, and altered expression of cyclin D1, P21, P27, and Bcl-2, while up-regulation of Uba2 showed the opposite effects. The wound healing and Transwell assays showed that Uba2 promoted GC cell migration and invasion. Western blotting revealed alterations in EMT biomarkers, suggesting the role of Uba2 in EMT. Furthermore, the luciferase reporter assay indicated the involvement of the Wnt/β-catenin signaling pathway as a possible modulator of Uba2 oncogenic functions.
Uba2 plays a vital role in GC cell migration and invasion, possibly by regulating the Wnt/β-catenin signaling pathway and EMT.
Core tip: This study elucidated the function and mechanism of action of ubiquitin-like modifier activating enzyme 2 (Uba2) in gastric cancer (GC) progression. Uba2 was knocked-down or over-expressed to examine its effects on proliferation, migration and invasion of GC cells. The effects of Uba2 on the Wnt/β-catenin pathway was investigated by Western blotting and luciferase reporter assay. Uba2 affected GC cell invasion and migration by modulating the Wnt/β-catenin signaling pathway, as well as epithelial-mesenchymal transition. This study was intended to create a preclinical basis to establish Uba2 as a new molecular drug target for GC.
- Citation: Li J, Sun X, He P, Liu WQ, Zou YB, Wang Q, Meng XW. Ubiquitin-like modifier activating enzyme 2 promotes cell migration and invasion through Wnt/β-catenin signaling in gastric cancer. World J Gastroenterol 2018; 24(42): 4773-4786
- URL: https://www.wjgnet.com/1007-9327/full/v24/i42/4773.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i42.4773
Gastric cancer (GC) is the fourth most common cancer worldwide, with a high incidence rate in East Asia[1,2]. Surgery is the main treatment option for early-stage patients; however, due to high aggressiveness and lack of predictive biomarkers, most patients are diagnosed at late stages, making it the second most lethal cancer, with a 5-year survival rate of 20%-40% for patients with advanced GC[2-4]. Median survival time for patients with unresectable and metastatic disease is around 10-14 mo[5,6]. To date, only a few drugs target GC at a molecular level, with limited survival benefits. Thus, to improve the prognosis of GC, further investigation of molecular biomarkers is required.
As a key post-translational modification pathway, small ubiquitin-like modify-lation (SUMOylation) plays an essential role in a substantial number of cellular processes, such as maintenance of transcriptional factor stability, regulation of enzymatic reactions, protein localization, protein-protein interactions, and DNA damage repair[7-11]. It is facilitated by covalently linked, deconjugated small ubiquitin-like modifiers (SUMOs) following the sequential action of E1, E2, E3 and SUMO proteases. Recent studies have shown that the proteins involved in the SUMO system could regulate carcinogenesis-related processes, such as cell growth, differentiation, senescence, oxidative stress, and apoptosis[12]. As an essential component of SUMO-activating enzyme E1, ubiquitin-like modifier activating enzyme 2 (Uba2) is over-expressed in many malignancies, including liver cancer[13], small cell lung cancer[14], colorectal cancer[15], and GC[16]. Besides, knock-down of Uba2 suppresses certain malignancies. Uba2 level is correlated with the Wnt/β-catenin pathway in colorectal cancer[15]; however, its role in GC remains unclear.
To explore the function of Uba2 and its possible mechanism of action in the progression of GC, Uba2 knock-down and over-expression studies were carried out in GC cell lines. Knock-down of Uba2 suppressed epithelial-mesenchymal transition (EMT), cell migration, and invasion, while over-expression of Uba2 showed the opposite effects. Furthermore, regulation of the Wnt/β-catenin canonical signaling pathway was correlated with Uba2 levels. These data suggest that Uba2 modulates the Wnt/β-catenin pathway and hence might be a new molecular target for the treatment of GC.
All specimens were derived from patients with GC in The First Hospital of Jilin University, Changchun, China from 2013 to 2018. Eight paired fresh GC tissues and corresponding normal gastric tissues were collected for Western blotting. These tissues were immediately frozen in liquid nitrogen and stored at -80 °C until further use. The specimens for immunohistochemistry were fixed in formalin for 24 h and embedded in paraffin until needed. The enrolled patients had not received chemotherapy or radiation before the operation. Clinicopathological characteristics, such as Lauren’s classification, pTNM stage, and grade of differentiation were evaluated by two independent pathologists using the criteria of the American Joint Committee on Cancer Stage. All participating patients gave their written informed consent prior to enrollment. This study was approved by the Ethics Committee of The First Hospital of Jilin University.
All specimens were fixed with formalin, embedded in paraffin, and 3-μm serial sections were prepared. The sections were deparaffinized and activity of endogenous peroxidase was blocked by 30 mL/L hydrogen peroxide, followed by incubation of the sections with non-immune goat serum for 20 min. The sections were subsequently incubated with Uba2 primary antibody (1:100; Abcam, Cambridge, MA, United States) and the corresponding secondary antibody. Antigen-antibody complexes were then washed three times with phosphate-buffered saline (PBS) and incubated with high-sensitivity diaminobenzidine (Sigma-Aldrich, St. Louis, MO, United States) substrate. Counterstaining with hematoxylin was carried out and the sections were dehydrated and mounted.
Uba2 expression levels were observed by light microscopy and categorized into negative/low expression and high expression by two independent, blinded pathologists. Any disagreement was resolved by a third party. The defining criteria were as follows: samples with nuclear staining in < 5% of cells were graded as negative expression; samples with nuclear staining in 5%-25% cells were graded as low expression; and, samples with nuclear staining in > 25% cells were graded as high (moderate and strong) expression.
Three human GC cell lines, SGC-7901, BGC-823 and MGC80-3, and a normal gastric epithelial cell line, GES-1, were obtained from American Type Culture Collection (Manassas, VA, United States). BGC-823 and MGC80-3 cells were poorly differentiated gastric adenocarcinoma cell lines, and SGC-7901 was a well-differentiated gastric adenocarcinoma cell line. BGC-823 and SGC-7901 cells were intestinal-type GC cell lines. MGC80-3 cells were a diffuse-type GC cell line. Cells were routinely cultured in complete RPMI-1640 medium (Gibco, Grand Island, NY, United States) supplemented with 100 mL/L fetal bovine serum (Biological Industries, Beit Haemek, Israel) and 10 mL/L penicillin-streptomycin (Invitrogen, Carlsbad, CA, United States) at 37 °C in a humidified atmosphere with 50 mL/L CO2.
For Uba2 knock-down, a lentiviral vector encoding a short interfering (si)RNA targeted against Uba2, and a negative control lentiviral vector (Control; NC) were constructed (Genechem, Shanghai, China). For over-expression studies, the Uba2 gene was synthesized according to the human UBA2 mRNA sequence and inserted into a lentiviral vector (Genechem). Transfection was carried out as previously described[15]. BGC-823 cells were infected with siUba2 or siNC lentivirus at a multiplicity of infection of 100. SGC-7901 cells were infected with lentivirus-Uba2 (Uba2) or lentiviral empty vector (EV) at a multiplicity of infection of 10. The infected cells were harvested and used for experimentation after 72 h.
The influence of Uba2 on cell viability was evaluated using the MTT assay. In brief, 2000 cells of each group were seeded in 96-well plates in 100 μL complete RPMI-1640 medium, and were incubated for 5 d at 37 °C in 50 mL/L CO2. MTT reagent (20 μL, 5 mg/mL in PBS; Sigma-Aldrich) was added to each well and incubated for 4 h. The medium was removed from each well and formazan crystals were dissolved in 150 μL of dimethyl sulfoxide. The plate was measured at 490 nm. The experiments were carried out in triplicate.
A total of 800 GC cells were suspended in RPMI-1640 containing 100 mL/L fetal bovine serum, and were seeded in 6-well plates. Fresh complete RPMI-1640 medium was changed every 3 d and the cells were cultured for 2 wk. The cells were washed with PBS and fixed with 40 mL/L paraformaldehyde. GIEMSA was used to stain the cells and images were taken with a digital camera. The experiments were carried out in triplicate.
For cell cycle analysis, 1 × 106 cells from each group were harvested and fixed with cold 700 mL/L ethanol at 4 °C overnight. The cells were then washed twice with cold PBS and resuspended with 0.5 mL PI/RNase Staining Solution (Sungene Biotech, Tianjin, China). The cells were incubated for 30 min at room temperature, and protected from light. Cells were subsequently analyzed by flow cytometry. The experiments were carried out in triplicate.
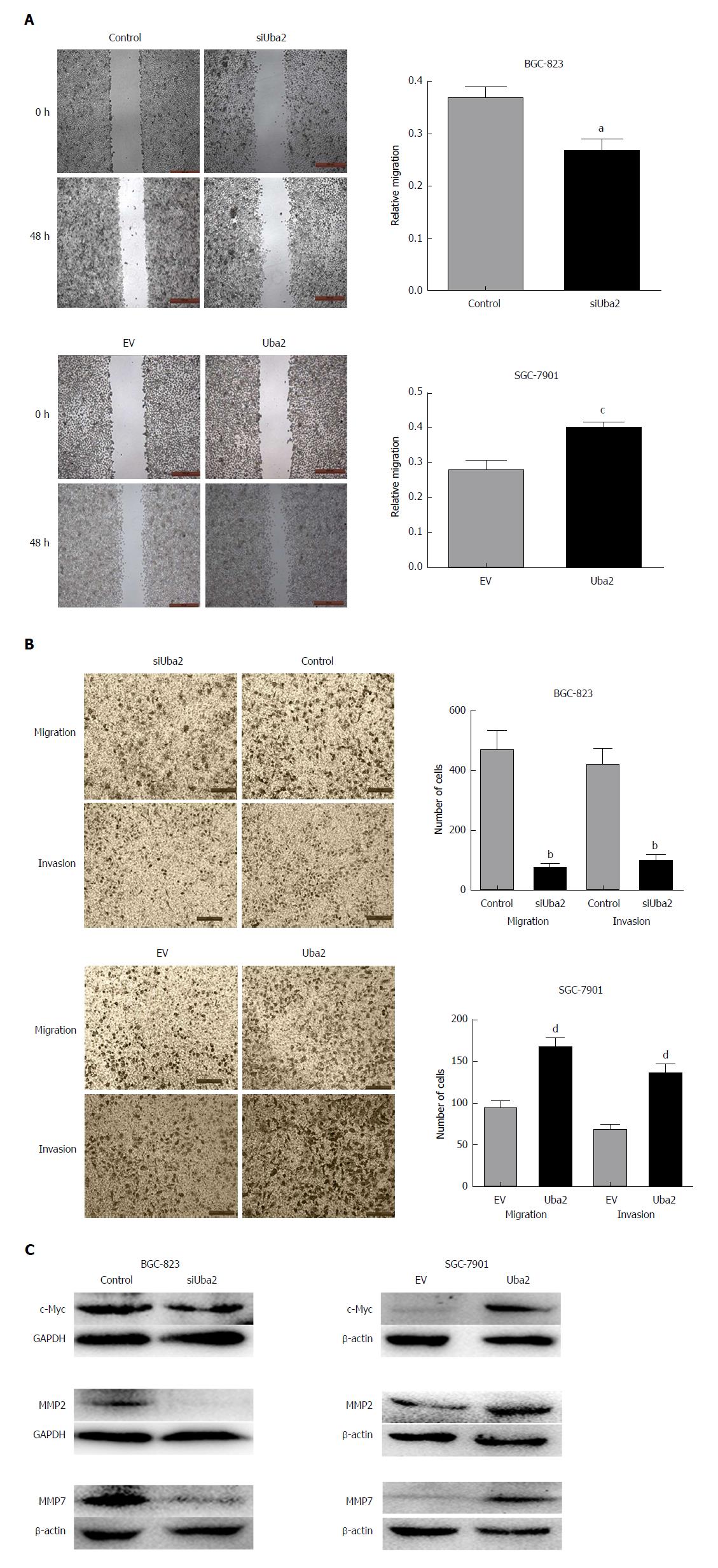
Cells plated in 6-well plates at 90% confluence were wounded with sterile 200-μL pipette tips. After wounding, the cells were washed twice with PBS to remove cell debris and incubated in serum-free medium. The cell-free wound area was photographed every 24 h at 50 × magnification. The motility speed of cells was determined using Image J software (National Institutes of Health, Bethesda, MD, United States) as an average closed area of the wound relative to the initial wound area at 48 h after wounding.
The Transwell assay was carried out to evaluate cell migration and invasion. Cells were resuspended in serum-free medium, and seeded in the upper chamber with an 8-μm pore size filter membrane at 2 × 105 cells/mL (100 μL/chamber), while conditioned medium with 200 mL/L fetal bovine serum was added to the lower chamber (Corning, Inc., Corning, NY, United States). The cell invasion assay was similar to the cell migration assay, except that upper chambers were coated with Matrigel (Corning, Inc.). The cells were incubated for 48 h in a 37 °C atmosphere containing 50 mL/L CO2. Cells in the upper chamber were removed with cotton swabs, whereas migrated/invaded cells on the bottom side of the membrane were fixed in 40 mL/L paraformaldehyde, stained with GIEMSA, and counted in five randomly selected microscopic fields (100 ×) per well. All experiments were carried out in triplicate.
T-cell factor (TCF) luciferase reporter gene lymphoid enhancer factor (LEF)/TCF cognate sequences (TOP Flash), its mutant FOP Flash, and pGMR-TK Renilla (Genomeditech, Shanghai, China) were constructed. BGC-823 (BGC-823 Uba2-siRNA and BGC-823 Control) and SGC-7901 (SGC-7901 Uba2 and SGC-7901 EV) cells were plated in 24-well plates and co-transfected with TOP Flash or FOP Flash with pGMR-TK Renilla. After 48 h, cells were lysed following manufacturer’s instructions (Dual luciferase reporter gene assay kit; Beyotime, Shanghai, China). Luciferase activity was measured using a multifunctional microplate tester (Synergy H1; BioTek, Beijing, China). The firefly luciferase activity was normalized to Renilla luciferase activity and the TOP/FOP ratio reflected the activity of the Wnt/β-catenin signaling pathway. The experiments were performed in triplicate.
Whole cell lysates were extracted using RIPA lysis buffer supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN, United States), and Phosphatase Inhibitor Cocktail (CoWin Biosciences, Beijing, China). Nucleoproteins were extracted using a nuclear and cytoplasmic protein extraction kit (Transgene, Beijing, China) and quantified by the bicinchoninic acid assay. A total of 40 μg protein was separated by 100 mL/L SDS-PAGE and transferred electrophoretically onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, United States). The membranes were blocked in 50 g/L nonfat milk diluted in Tris-buffered saline containing 1 mL/L Tween-20 (referred to as TBST) for 1 h at room temperature, before addition of appropriate primary antibody for overnight incubation at 4 °C. Primary antibodies were as follows: anti-Uba2 rabbit monoclonal antibody (1:2000; Abcam), anti-β-catenin rabbit monoclonal antibody (1:2000; CST, Danvers, MA, United States), anti-phosphorylation β-catenin rabbit monoclonal antibody (1:1000; CST), anti-E-cadherin rabbit monoclonal antibody (1:2000; CST), anti-vimentin rabbit monoclonal antibody (1:2000; CST), anti-MMP7 rabbit monoclonal antibody (1:1000; Abcam), anti-MMP2 rabbit monoclonal antibody (1:1000; CST), anti-c-Myc rabbit monoclonal antibody (1:1000; CST), anti-cyclin D1 rabbit monoclonal antibody (1:1000; CST), anti-Bcl-2 rabbit monoclonal antibody (1:1000; Abcam), anti-P27 rabbit monoclonal antibody (1:2000; CST), anti-CDK4 rabbit monoclonal antibody (1:2000; CST), anti-P21 rabbit monoclonal antibody (1:2000; CST), anti-LaminB1 mouse monoclonal antibody (1:1000; Sigma, Japan), anti-GAPDH mouse monoclonal antibody (1:3000; CST) and anti-β-actin rabbit monoclonal antibody (1:1000; Sigma). The membranes were then washed three times with TBST and incubated with the corresponding horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The target proteins on the membrane were visualized using a chemiluminescence reagent (Proteintech, Chicago, IL, United States) and photographed by Tanon 5200 series chemiluminescence imaging analysis system (Tanon, Shanghai, China).
Statistical analyses were carried out using SPSS version 21.0 (IBM Corp., Armonk, NY, United States). Histograms and related statistical analyses were performed using GraphPad Prism (version 5.0; GraphPad Software, Inc). The association between Uba2 expression and clinicopathological parameters of the patients with GC was analyzed using Pearson’s χ2 test. Paired sample t-test or one-way analysis of variance was used as appropriate. Differences were considered statistically significant at P < 0.05.
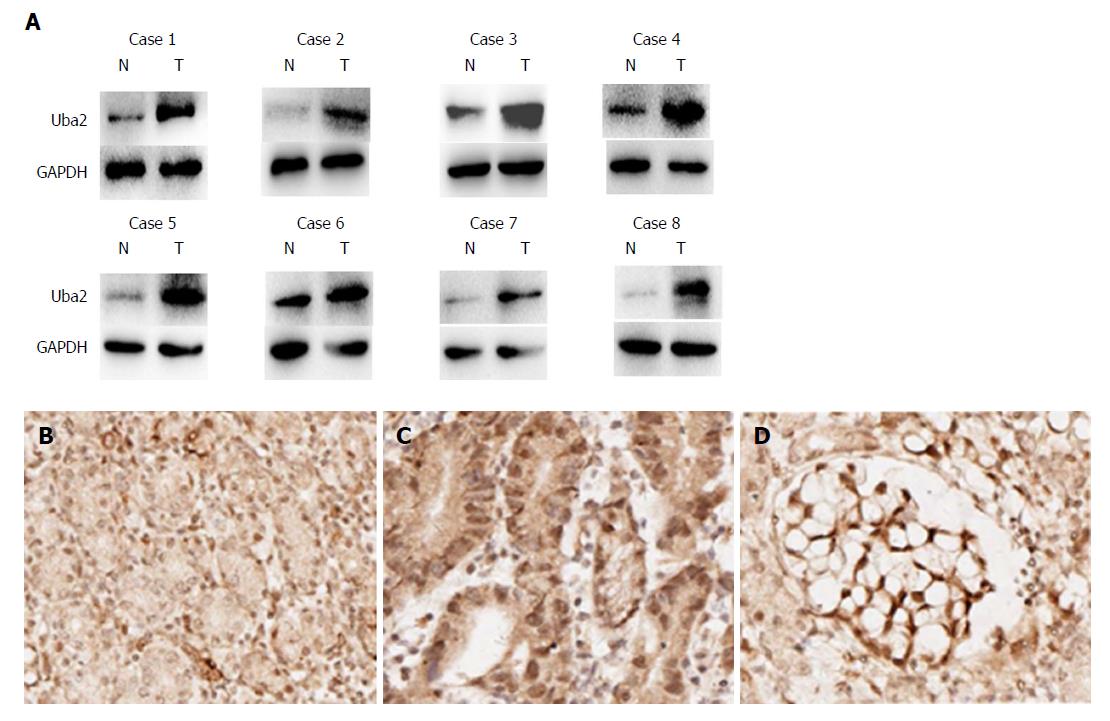
To analyze the levels of Uba2 in GC tissues and paracancerous tissues, eight GC tissues and paired normal gastric tissues were examined by Western blotting (Figure 1A). Compared to normal gastric tissues, the level of Uba2 was higher in GC tissues. Furthermore, Uba2 protein level was evaluated by immunohistochemistry in 100 GC specimens and 22 normal gastric tissues. Uba2 protein was mainly localized in the nucleus, and a higher level of Uba2 expression was detected in GC tissues than in normal gastric tissues (Figure 1B-D). In all, 24 and 76 of 100 GC tissues were divided into negative/low staining and high staining respectively, based on Uba2 protein levels. Correlations between Uba2 expression and clinicopathological factors were analyzed subsequently. In the 100 tumor samples, Uba2 expression was associated with differentiation, Lauren’s classification, vascular invasion, TNM stage, p-T category, and p-N category (Table 1). No significant differences were seen in age, gender, tumor size, and perineural invasion. These results indicated that Uba2 might be associated with GC development, invasion and metastasis.
| Characteristic | Cases | Uba2 expression | |
| Low, negative and low | High, moderate and strong | ||
| Age in yr | |||
| < 60 | 39 | 8 | 31 |
| ≥ 60 | 61 | 16 | 45 |
| Gender | |||
| Male | 74 | 19 | 55 |
| Female | 26 | 5 | 21 |
| Tumor size in cm | |||
| < 5 | 59 | 16 | 43 |
| ≥ 5 | 41 | 8 | 33 |
| Lauren classification | |||
| Intestinal | 24 | 11 | 13b |
| Diffuse | 44 | 4 | 40 |
| Mixed | 32 | 9 | 23 |
| Differentiation | |||
| Moderate or well | 43 | 18 | 25c |
| Poor | 57 | 6 | 51 |
| TNM stage | |||
| I/II | 41 | 14 | 27a |
| III/IV | 59 | 10 | 49 |
| pT-category | |||
| T1,T2 | 33 | 12 | 21a |
| T3,T4 | 67 | 12 | 55 |
| pN-category | |||
| N0 | 28 | 11 | 17a |
| N1,N2,N3 | 72 | 13 | 59 |
| Vascular invasion | |||
| Yes | 74 | 14 | 60a |
| No | 26 | 10 | 16 |
| Perineural invasion | |||
| Yes | 62 | 11 | 51 |
| No | 38 | 13 | 25 |
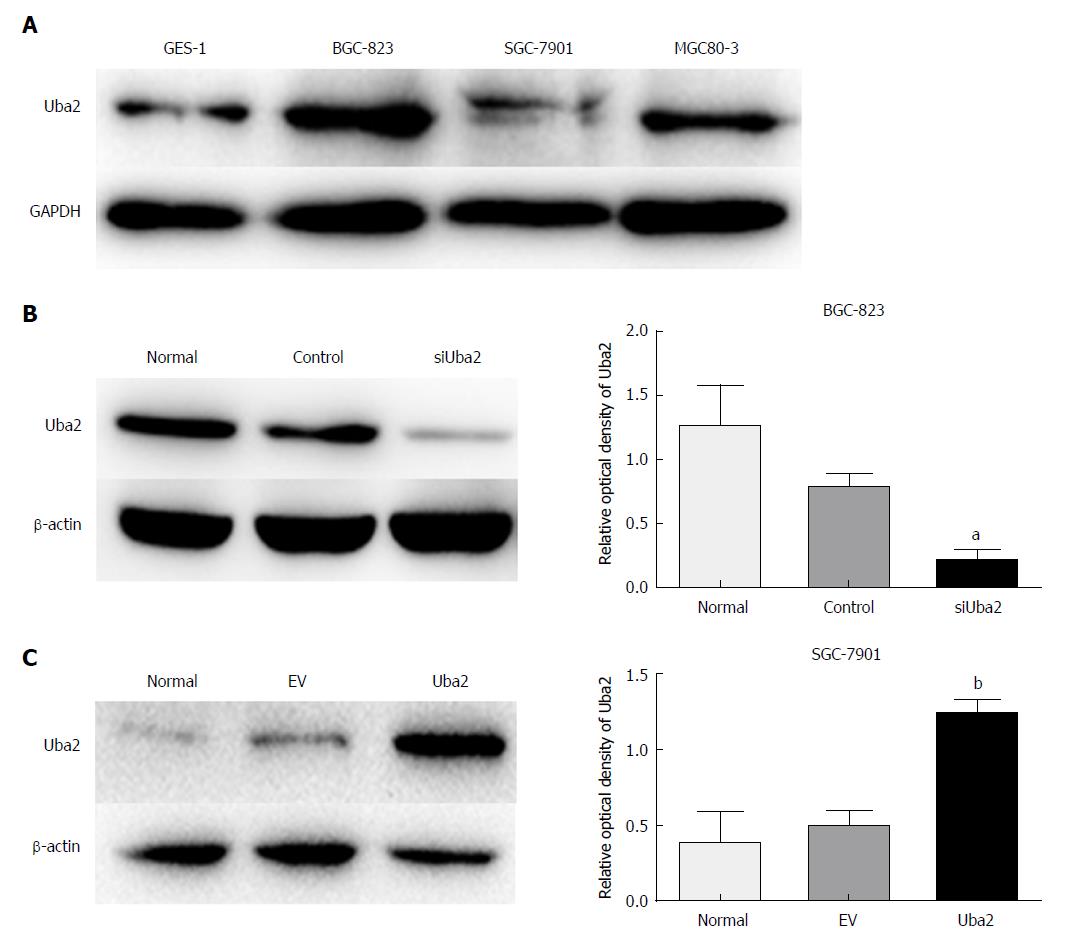
To investigate the biological function of Uba2 in GC, expression of Uba2 was analyzed in three GC cell lines and a normal gastric epithelial cell line. Uba2 was expressed in all three GC cell lines, and expression in BGC-823 and MGC80-3 cells was higher than in GES-1 cells (Figure 2A). The BGC-823 cell line had the highest level of Uba2 expression and was infected with siRNA (siUba2). Similarly, the SGC-7901 cell line, which had the lowest level of Uba2 expression, was infected with Uba2 plasmid for subsequent experiments (Figure 2B and C).
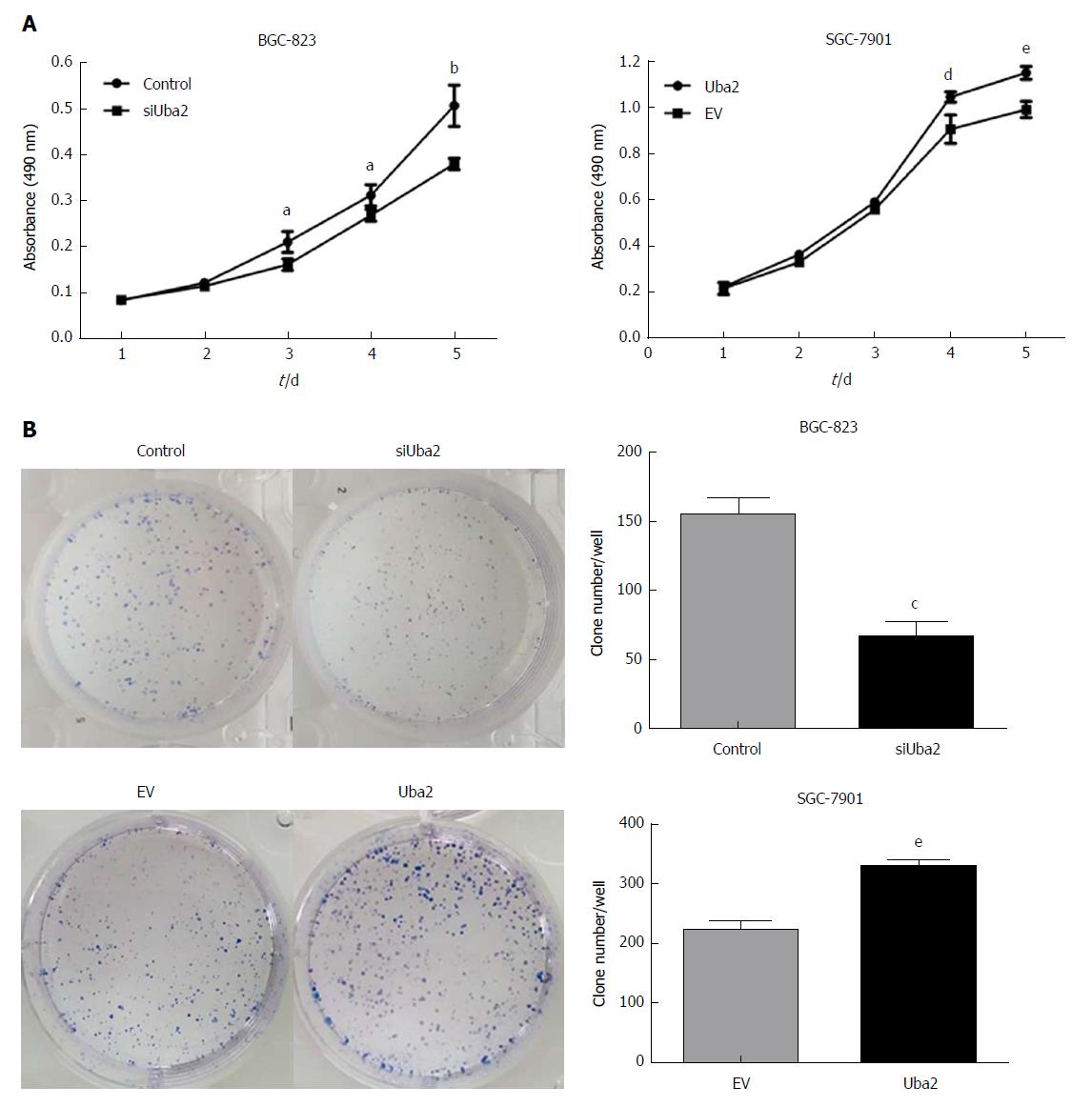
MTT and colony formation assays were performed to explore the effects of Uba2 on proliferation in GC. Compared to that in control cells, knock-down of Uba2 inhibited cell growth and decreased colony formation, while in SGC-7901 cells with Uba2 over-expression, cell growth and colony formation were increased (Figure 3).
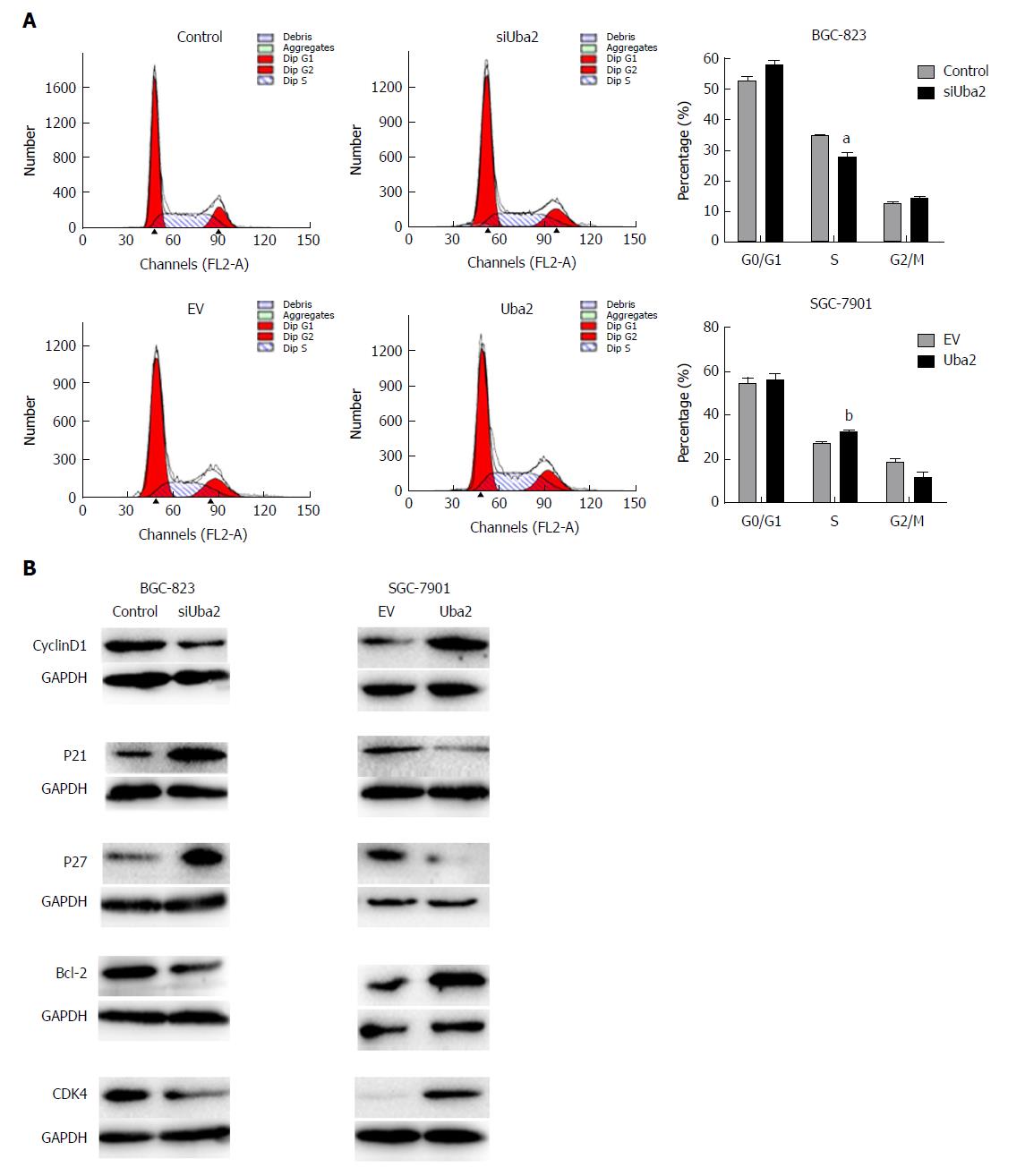
Flow cytometry was used to examine the effects of Uba2 on the cell cycle. Compared to control cells, S phase cells were reduced in BGC-823 siUba2 cells, while S phase cells were significantly increased in SGC-7901 cells with Uba2 over-expression (Figure 4A). Knock-down of Uba2 in BGC-823 cells decreased expression of cyclin D1 and Bcl-2, but increased expression of P21 and P27. The opposite results were found in SGC-7901 cells with Uba2 over-expression (Figure 4B). These findings indicated that Uba2 promoted GC cell proliferation and cell cycle regulation.
To explore the role of Uba2 in GC invasion and migration, wound healing and Transwell assays were performed. The wound healing assay showed that compared to control cells, closure of wound gaps was delayed in BGC-823 siUba2 cells and the opposite effects were seen in SGC-7901 cells with over-expression of Uba2 (Figure 5A). The Transwell assay showed that cell migration and invasion were significantly reduced after down-regulation of Uba2, while the number of cells was increased after Uba2 over-expression (Figure 5B). We analyzed expression of matrix metalloproteinase (MMP)2, MMP7, and c-Myc, which are related to cancer metastasis. In support of our findings, MMP2, MMP7, and c-Myc were reduced after Uba2 knock-down, while their expression was increased with Uba2 over-expression (Figure 5C). These results suggest that Uba2 may promote invasion and migration of cancer cells by up-regulating the expression of MMP2, MMP7, and c-Myc.
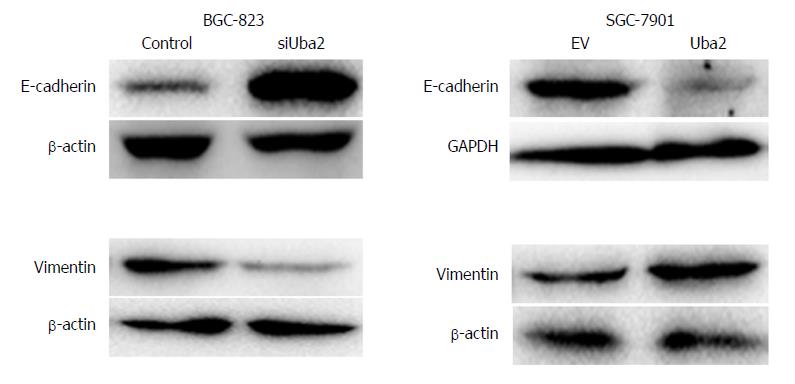
Recent studies have suggested that EMT results in the acquisition of characteristics required for carcinoma progression, such as migration and invasion. Because Uba2 promoted migration and invasion of GC cells, we speculated that it might also be involved in EMT. EMT is often associated with a decrease or loss of epithelial markers and a gain of mesenchymal markers. Consistent with our speculation, compared to control cells, increased expression of E-cadherin and decreased expression of vimentin was found in BGC-823 cells with Uba2 knock-down, whereas over-expression of Uba2 in SGC-7901 cells had the opposite effects (Figure 6). These data suggested that Uba2 might induce EMT in GC cells.
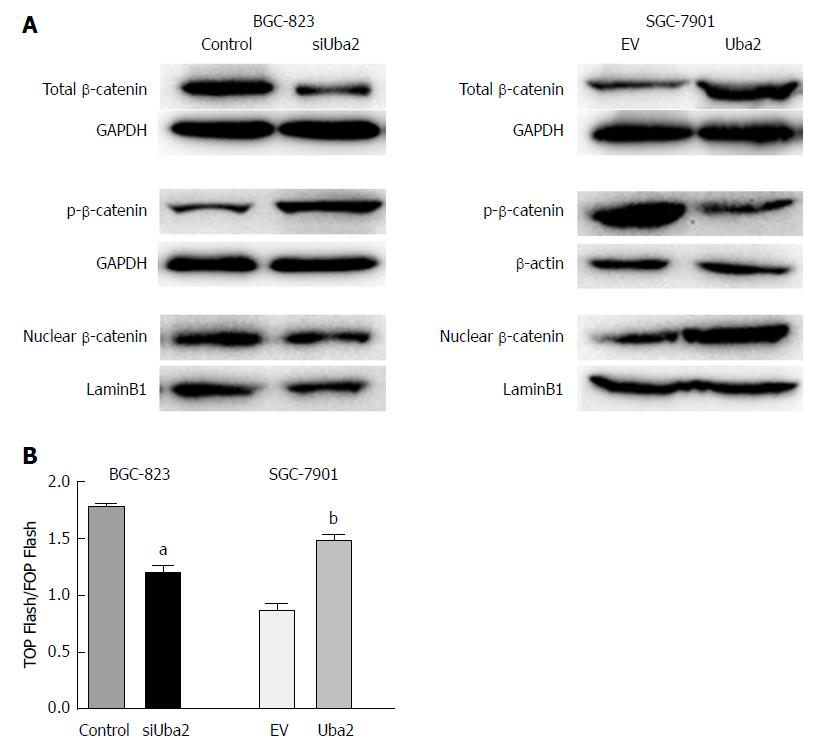
Involvement of the Wnt/β-catenin signaling pathway in the Uba2-mediated modulation of EMT and cell migration and invasion was further examined by Western blotting and luciferase reporter assay. As the key molecule of the Wnt/β-catenin pathway, expression of total and nuclear β-catenin was significantly reduced, while that of phosphorylated β-catenin increased after knock-down of Uba2. The opposite effects were observed in SGC-7901 cells with over-expression of Uba2 (Figure 7A). Luciferase reporter assay is a commonly used method to evaluate β-catenin-dependent activity driving the expression of TCF. Our results showed that knock-down of Uba2 resulted in a 1.5-fold decrease, while over-expression of Uba2 resulted in a 1.7-fold increase in the ratio of TOP Flash to FOP Flash activity (Figure 7B). These data implied that Uba2 was involved in regulating the Wnt/β-catenin signaling pathway in GC.
Despite the declining incidence and mortality rate in GC, there are still 1 million new cases and > 700000 fatal cases annually, accounting for 10% of cancer-related deaths globally[2]. The poor outcomes imply a critical need for the detection of molecular targets that may confer better survival benefits.
Uba2 is an essential component of SUMO-activating enzyme E1, which is correlated with the development and progression of several cancers, including GC[16]. However, the role of Uba2 in EMT and its underlying mechanism in GC remain unknown. In our study, expression of Uba2 in GC and normal gastric tissues was compared by Western blotting and immunohistochemistry. The results showed higher expression of Uba2 in GC than in normal gastric tissues. Uba2 expression was also higher in GC cells than in normal gastric epithelial cells. Uba2 expression was associated with differentiation. BGC-823 and MGC80-3 cells were poorly differentiated and SGC-7901 cells were well differentiated. The former two had worse differentiation than SGC-7901, which might be the reason why Uba2 expression was lower in SGC-7901 than BGC-823 and MGC80-3 cells. Uba2 expression was also associated with Lauren’s classification, vascular invasion and TNM stage, which suggests that Uba2 is involved in GC development and progression.
MTT and colony formation assays showed that Uba2 promoted GC cell proliferation and cell cycle regulation. Cyclin D1 could bind and activate G1-phase-specific cyclin-dependent kinase 4 (CDK4), which promotes transition of the cell cycle from G1 to S phase[17,18]. P27 and P21 serve as cell cycle inhibitors by regulating cell cycle progression at the G1 and S phases. Moreover, P27 level negatively correlates with cell growth rate[19,20]. The changes in expression of cell cycle-related proteins were in accordance with our results of cell cycle analysis. Bcl-2 is an important member of the Bcl-2 family of proteins that regulate apoptosis, and changes in its expression suggest involvement of apoptosis. Subsequently, wound healing and Transwell assays were performed with knock-down or over-expression of Uba2. Knock-down of Uba2 suppressed cell migration and invasion, which was consistent with a previous report[16]. On the contrary, Uba2 over-expression promoted cancer-related activities. These findings demonstrated that Uba2 was associated with GC invasion and metastasis in vitro.
Further investigation of the potential Uba2 mechanisms was carried out. The Wnt/β-catenin canonical pathway is a crucial therapeutic target in a variety of cancers[21]. The pathway is initiated by secreted glycoproteins, such as Wnt1 and Wnt3a, which bind to cell surface receptors, such as Frizzled and low-density lipoprotein receptor-related protein 6, leading to degradation of the complex containing adenomatous polyposis coli, axis inhibition protein, and glycogen synthase kinase 3 beta and stabilization of β-catenin. β-Catenin then translocates to the nucleus to bind to TCF or LEF, followed by transcriptional activation of the Wnt target genes involved in tumor migration, invasion and EMT[22-24]. Accumulating evidence indicates that activation of Wnt/β-catenin signaling is one of the direct causes of gastric and intestinal tumor development[25-27]. It has also been shown that aberrantly activated Wnt/β-catenin pathway is involved in the initiation and progression of GC in both humans and mice[28,29]. These results suggest that the Wnt/β-catenin signaling pathway plays an important role in gastrointestinal carcinogenesis. Furthermore, Choi et al[30] found that transducing β-like protein 1 (TBL1) -TBL1-related protein 1 (TBLR1) can activate the key molecule β-catenin upon SUMOylation, which activates expression of Wnt/β-catenin downstream target genes. Another study showed that SUMOylation positively regulates the Wnt signaling pathway, while deSUMOylation has the opposite effect[31].
As an important SUMOylation component, it is possible that Uba2 is involved in modulating the Wnt signaling pathway. However, until now, the role of Uba2 in modulating the Wnt signaling pathway in GC was largely unknown. In the present study, Uba2 over-expression increased expression of β-catenin and induced nuclear accumulation, while opposite results were found upon Uba2 knock-down. These findings showed that Uba2 might induce nuclear translocation of β-catenin. Luciferase reporter assay also showed increased activity of the Wnt/β-catenin pathway in SGC-7901 cells with over-expression of Uba2, while the activity was decreased when Uba2 was knocked down in BGC-823 cells. Our study demonstrates that Uba2 regulates the malignant behavior of GC possibly by facilitation of the Wnt/β-catenin signaling pathway.
EMT is an essential initial step in the acquisition of migratory and invasive capabilities, by which epithelial cells lose cell-cell junctions and polarity, leading to a mesenchymal cell phenotype[32]. The process is characterized by the production of MMPs to degrade the extracellular matrix, while cell adhesion weakens and metastasis continues[14]. E-cadherin is a calcium-dependent transmembrane glycoprotein expressed in most epithelial cells; the loss of which is a biomarker of EMT[33]. The increased expression of MMP2 and MMP7 is involved in promoting cancer metastasis[34,35]. Previous studies have also concluded that c-Myc expression might represent an aggressive phenotype of GC[36,37]. Our results were in accordance with these findings. In our study, decreased expression of E-cadherin, up-regulation of mesenchymal marker vimentin, and increased levels of MMP2, MMP7 and c-Myc confirmed the importance of Uba2 in tumor progression, invasion and metastasis through EMT in GC.
In summary, our study provides evidence of an association between Uba2 expression and GC migration and invasion via modulation of the Wnt/β-catenin signaling pathway and enhancement of EMT. Our results suggest that Uba2 might serve as a potential therapeutic target in GC.
Gastric cancer (GC) is the fourth most common malignancy and the second leading cause of cancer-related death globally. Although decreasing trends have been observed in GC incidence and mortality rates due to recent advances in diagnosis and treatment, the burden remains the highest in East Asia (especially in China). However, many GC patients are already at an inoperable advanced stage at diagnosis, which results in poor outcome. Thus, new therapeutic targets that may confer survival benefit are urgently needed in GC.
There are insufficient reports about the function and mechanism of ubiquitin-like modifier activating enzyme 2 (Uba2) in GC migration, epithelial-mesenchymal transition, and invasion.
The aim of this study is to investigate the role of Uba2 in GC migration and invasion, and its underlying mechanism.
Uba2 expression was examined at the protein level by immunohistochemistry or Western blotting in tissues and cells. Correlations of Uba2 expression and clinicopathological characteristics were also analyzed. Transwell and wound healing assays were used to examine the effects of Uba2 on GC migration and invasion. Western blotting and luciferase reporter assay were used to investigate the underlying mechanism.
The up-regulation of Uba2 expression was found in both GC tissues and cells. Knock-down of Uba2 inhibited cell proliferation, migration and invasion, and over-expression of Uba2 showed the opposite effects. Wnt/β-catenin signaling might be involved in the oncogenic function of Uba2.
The study shows that Uba2 is an enhancer of cell migration and invasion in GC, and the possible mechanism is via regulating the canonical Wnt signaling pathway and enhancing epithelial-mesenchymal transition. Thus, Uba2 is expected to be an important oncoprotein and potential therapeutic target in GC.
The function and mechanism of Uba2 in GC development has been confirmed, and the significance of Uba2 as a promising therapeutic target for GC is highlighted.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gassler N, Huang CM S- Editor: Ma RY L- Editor: Filipodia E- Editor: Huang Y
| 1. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1468] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 3. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1327] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 4. | Zhao Z, Han F, Yang S, Wu J, Zhan W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt-mTOR signaling pathway. Cancer Lett. 2015;358:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 461] [Cited by in RCA: 483] [Article Influence: 43.9] [Reference Citation Analysis (5)] |
| 6. | Yang W, Raufi A, Klempner SJ. Targeted therapy for gastric cancer: molecular pathways and ongoing investigations. Biochim Biophys Acta. 2014;1846:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Gill G. Post-translational modification by the small ubiquitin-related modifier SUMO has big effects on transcription factor activity. Curr Opin Genet Dev. 2003;13:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Deyrieux AF, Rosas-Acosta G, Ozbun MA, Wilson VG. Sumoylation dynamics during keratinocyte differentiation. J Cell Sci. 2007;120:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Gill G. SUMO changes Sox for developmental diversity. Mol Cell. 2005;20:495-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ihara M, Koyama H, Uchimura Y, Saitoh H, Kikuchi A. Noncovalent binding of small ubiquitin-related modifier (SUMO) protease to SUMO is necessary for enzymatic activities and cell growth. J Biol Chem. 2007;282:16465-16475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | He X, Riceberg J, Pulukuri SM, Grossman S, Shinde V, Shah P, Brownell JE, Dick L, Newcomb J, Bence N. Characterization of the loss of SUMO pathway function on cancer cells and tumor proliferation. PLoS One. 2015;10:e0123882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Mattoscio D, Chiocca S. SUMO pathway components as possible cancer biomarkers. Future Oncol. 2015;11:1599-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Tu J, Chen Y, Cai L, Xu C, Zhang Y, Chen Y, Zhang C, Zhao J, Cheng J, Xie H. Functional Proteomics Study Reveals SUMOylation of TFII-I is Involved in Liver Cancer Cell Proliferation. J Proteome Res. 2015;14:2385-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Liu X, Xu Y, Pang Z, Guo F, Qin Q, Yin T, Sang Y, Feng C, Li X, Jiang L. Knockdown of SUMO-activating enzyme subunit 2 (SAE2) suppresses cancer malignancy and enhances chemotherapy sensitivity in small cell lung cancer. J Hematol Oncol. 2015;8:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Cheng H, Sun X, Li J, He P, Liu W, Meng X. Knockdown of Uba2 inhibits colorectal cancer cell invasion and migration through downregulation of the Wnt/β-catenin signaling pathway. J Cell Biochem. 2018;119:6914-6925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Shao DF, Wang XH, Li ZY, Xing XF, Cheng XJ, Guo T, Du H, Hu Y, Dong B, Ding N. High-level SAE2 promotes malignant phenotype and predicts outcome in gastric cancer. Am J Cancer Res. 2014;5:140-154. [PubMed] |
| 17. | Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol. 2009;220:292-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 18. | Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene. 2006;25:1620-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4008] [Cited by in RCA: 4202] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 20. | Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1466] [Cited by in RCA: 1554] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 21. | Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, Huang H, Xue J, Liu M, Wang Y. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 485] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 22. | Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 757] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 23. | Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, Hu CJ, Dong H, Yang SM. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016;374:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Cai J, Feng D, Hu L, Chen H, Yang G, Cai Q, Gao C, Wei D. FAT4 functions as a tumour suppressor in gastric cancer by modulating Wnt/β-catenin signalling. Br J Cancer. 2015;113:1720-1729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503-3506. [PubMed] |
| 26. | Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 670] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 27. | Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Zhang H, Xue Y. Wnt pathway is involved in advanced gastric carcinoma. Hepatogastroenterology. 2008;55:1126-1130. [PubMed] |
| 29. | Du YC, Oshima H, Oguma K, Kitamura T, Itadani H, Fujimura T, Piao YS, Yoshimoto T, Minamoto T, Kotani H. Induction and down-regulation of Sox17 and its possible roles during the course of gastrointestinal tumorigenesis. Gastroenterology. 2009;137:1346-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Choi HK, Choi KC, Yoo JY, Song M, Ko SJ, Kim CH, Ahn JH, Chun KH, Yook JI, Yoon HG. Reversible SUMOylation of TBL1-TBLR1 regulates β-catenin-mediated Wnt signaling. Mol Cell. 2011;43:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Yamamoto H, Ihara M, Matsuura Y, Kikuchi A. Sumoylation is involved in beta-catenin-dependent activation of Tcf-4. EMBO J. 2003;22:2047-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7749] [Article Influence: 484.3] [Reference Citation Analysis (0)] |
| 33. | Bolós V, Peinado H, Pérez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 902] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 34. | Kubben FJ, Sier CF, van Duijn W, Griffioen G, Hanemaaijer R, van de Velde CJ, van Krieken JH, Lamers CB, Verspaget HW. Matrix metalloproteinase-2 is a consistent prognostic factor in gastric cancer. Br J Cancer. 2006;94:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Liu XP, Kawauchi S, Oga A, Tsushimi K, Tsushimi M, Furuya T, Sasaki K. Prognostic significance of matrix metalloproteinase-7 (MMP-7) expression at the invasive front in gastric carcinoma. Jpn J Cancer Res. 2002;93:291-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Yang GF, Deng CS, Xiong YY, Gong LL, Wang BC, Luo J. Expression of nuclear factor-kappa B and target genes in gastric precancerous lesions and adenocarcinoma: association with Helicobactor pylori cagA (+) infection. World J Gastroenterol. 2004;10:491-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Zhang L, Hou Y, Ashktorab H, Gao L, Xu Y, Wu K, Zhai J, Zhang L. The impact of C-MYC gene expression on gastric cancer cell. Mol Cell Biochem. 2010;344:125-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |