Published online Nov 7, 2018. doi: 10.3748/wjg.v24.i41.4716
Peer-review started: July 25, 2018
First decision: August 27, 2018
Revised: August 31, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: November 7, 2018
Processing time: 105 Days and 19 Hours
Progressive familial intrahepatic cholestasis type 3 is caused by a mutation in the ATP-binding cassette, subfamily B, member 4 (ABCB4) gene encoding multidrug resistance protein 3. A 32-year-old woman with a history of acute hepatitis at age 9 years was found to have jaundice during pregnancy in 2008, and was diagnosed as having intrahepatic cholestasis of pregnancy. In 2009, she underwent cholecystectomy for gallstones and chronic cholecystitis. However, itching and jaundice did not resolve postoperatively. She was admitted to our hospital with fatigue, jaundice, and a recently elevated γ-glutamyl transpeptidase level. Liver biopsy led to the diagnosis of biliary cirrhosis with ductopenia. Genetic testing revealed a pathogenic heterozygous mutation, ex13 c.1531G > A (p.A511T), in the ABCB4 gene. Her father did not carry the mutation, but her mother’s brother carried the heterozygous mutation. We made a definitive diagnosis of familial intrahepatic cholestasis type 3. Her symptoms and liver function improved after 3 mo of treatment with ursodeoxycholic acid.
Core tip: A 32-year-old woman with a history of acute hepatitis at age 9 years was diagnosed as having intrahepatic cholestasis of pregnancy in 2008. In 2009, she underwent cholecystectomy for gallstones and chronic cholecystitis. However, itching and jaundice did not resolve postoperatively. She was admitted with fatigue, jaundice, and a recently elevated γ-glutamyl transpeptidase level. Liver biopsy led to the diagnosis of biliary cirrhosis with ductopenia. Genetic testing revealed a pathogenic heterozygous mutation, ex13 c.1531G > A (p.A511T), in the ATP-binding cassette, subfamily B, member 4 gene. We made a definitive diagnosis of familial intrahepatic cholestasis type 3.
- Citation: Tan YW, Ji HL, Lu ZH, Ge GH, Sun L, Zhou XB, Sheng JH, Gong YH. Ductopenia and cirrhosis in a 32-year-old woman with progressive familial intrahepatic cholestasis type 3: A case report and review of the literature. World J Gastroenterol 2018; 24(41): 4716-4720
- URL: https://www.wjgnet.com/1007-9327/full/v24/i41/4716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i41.4716
Progressive familial intrahepatic cholestasis type 3 (PFIC3) is caused by a mutation in the ATP-binding cassette, subfamily B, member 4 (ABCB4) gene encoding multidrug resistance protein 3 (MDR3)[1,2]. PFIC3 is characterized by recurrent itching, jaundice, white clay-like stool, hepatosplenomegaly, and gastrointestinal bleeding, often progressing to cirrhosis and liver failure before adulthood[3,4]. PFIC3 is a subtype of progressive familial intrahepatic cholestasis that differs from PFIC1 and PFIC2 in that serum γ-glutamyl transpeptidase (GGT) is elevated[5]. The pathological manifestations of liver tissue include obvious ductopenia or interlobular bile duct hyperplasia and terminal bile duct biliary sludge formation rather than abnormal synthesis of bile[6-8]. The incidence is 1/500000. However, reports of PFIC3 with high GGT are rare. We present the case of a patient with a mutant gene fragment in PFIC3, which has not been previously reported in the East Asian population.
The study was approved by the Medical Ethics Committee of the Third Hospital of Zhenjiang Affiliated Jiangsu University, and written informed consent was obtained from the patient. The study was conducted in accordance with the Declaration of Helsinki.
A 32-year-old woman with a history of acute hepatitis at age 9 years was admitted to a local hospital for an unknown disease process. She was discharged after 10 d of treatment and recovered from her condition. During pregnancy in 2008, she developed jaundice and was diagnosed as having intrahepatic cholestasis of pregnancy (ICP). In the 2 years after delivery, she experienced recurrent itching and jaundice. She underwent cholecystectomy for gallstones and chronic cholecystitis detected on ultrasound. However, her itching and jaundice did not resolve postoperatively, and high serum total bilirubin (TBIL) was repeatedly observed, with the highest level of > 100 μmol/L. Alkaline phosphatase (ALP) and GGT were also repeatedly increased and remained at 5-10 times the upper limit of normal.
She was admitted to our hospital with fatigue, jaundice, and decreased food intake during the prior week. Liver function tests showed the following results: TBIL 59.8 μmol/L, direct bilirubin 34.6 μmol/L, serum total protein 64.5 g/L, albumin 37.5 g/L, alanine aminotransferase (ALT) 123 U/L, aspartate aminotransferase 117 U/L, ALP 371 U/L, and GGT 252 U/L. Coagulation tests showed prothrombin time of 16.3 s, an international normalized ratio of 1.43, and activated partial thromboplastin time of 44.1 s. Routine blood tests showed the following results: White blood cells 2.71 × 109/L, red blood cells 3.77 × 1012/L, hemoglobin 120 g/L, platelets 67 × 109/L, and neutrophils 64.3%. Antinuclear antibody, anti-smooth muscle antibody, anti-liver/kidney microsomal antibody type 1, anti-nuclear glycoprotein antibody, anti-soluble acid nucleoprotein antibody, anti-hepatocyte cytoplasmic antigen type 1 antibody, anti-soluble liver antigen/hepatopancreatic antigen antibody, and other tests were negative, whereas the immunoglobulin G and immunoglobulin M levels were 11.4 g/L (< 17.1 g/L) and 1.2 g/L (< 4 g/L), respectively. Viral hepatitis (A-E), Epstein-Barr virus, and cytomegalovirus infections were ruled out. Computed tomography of the upper abdomen revealed cirrhosis, splenomegaly, ascites, and intrahepatic bile duct stones.
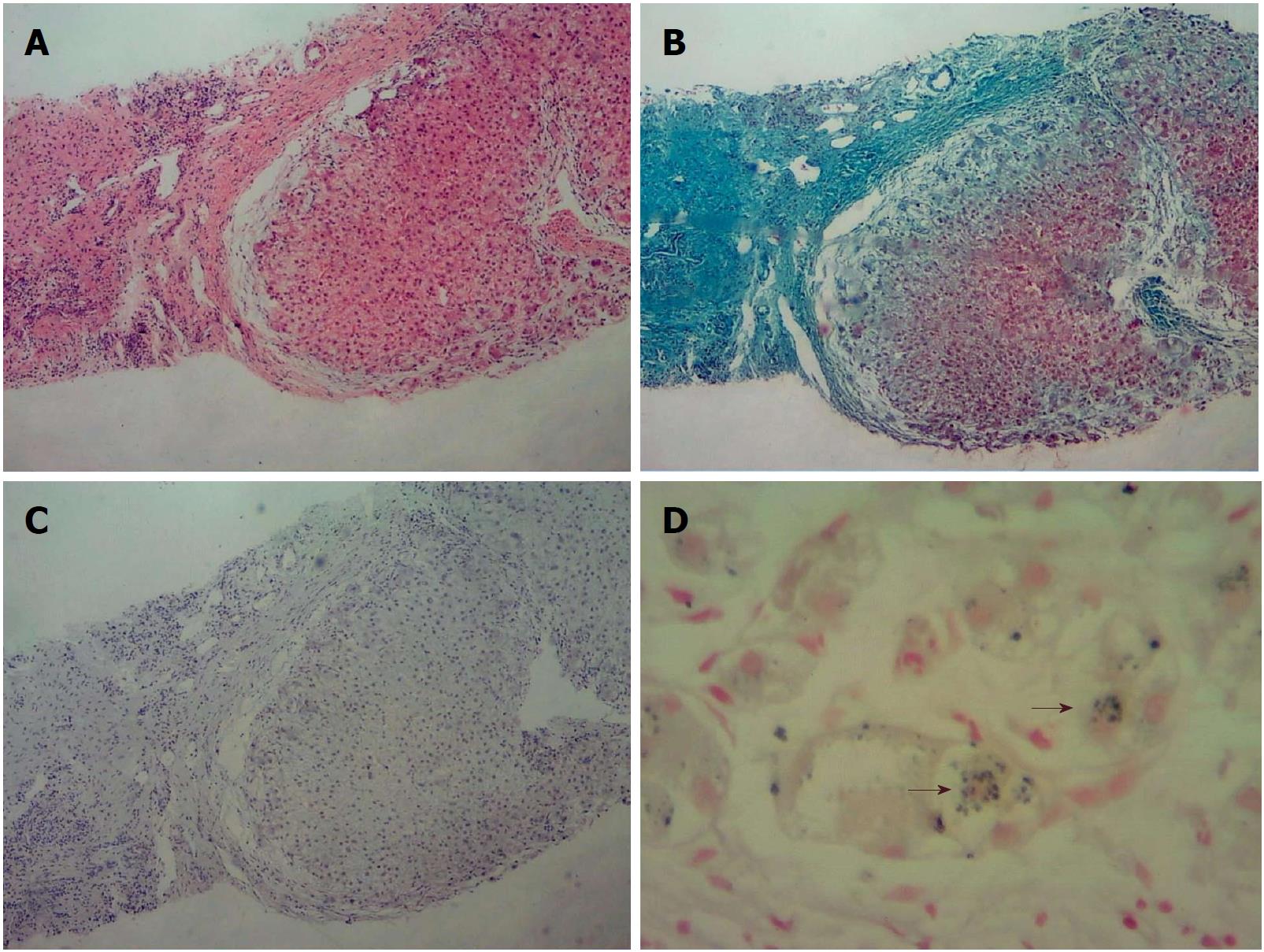
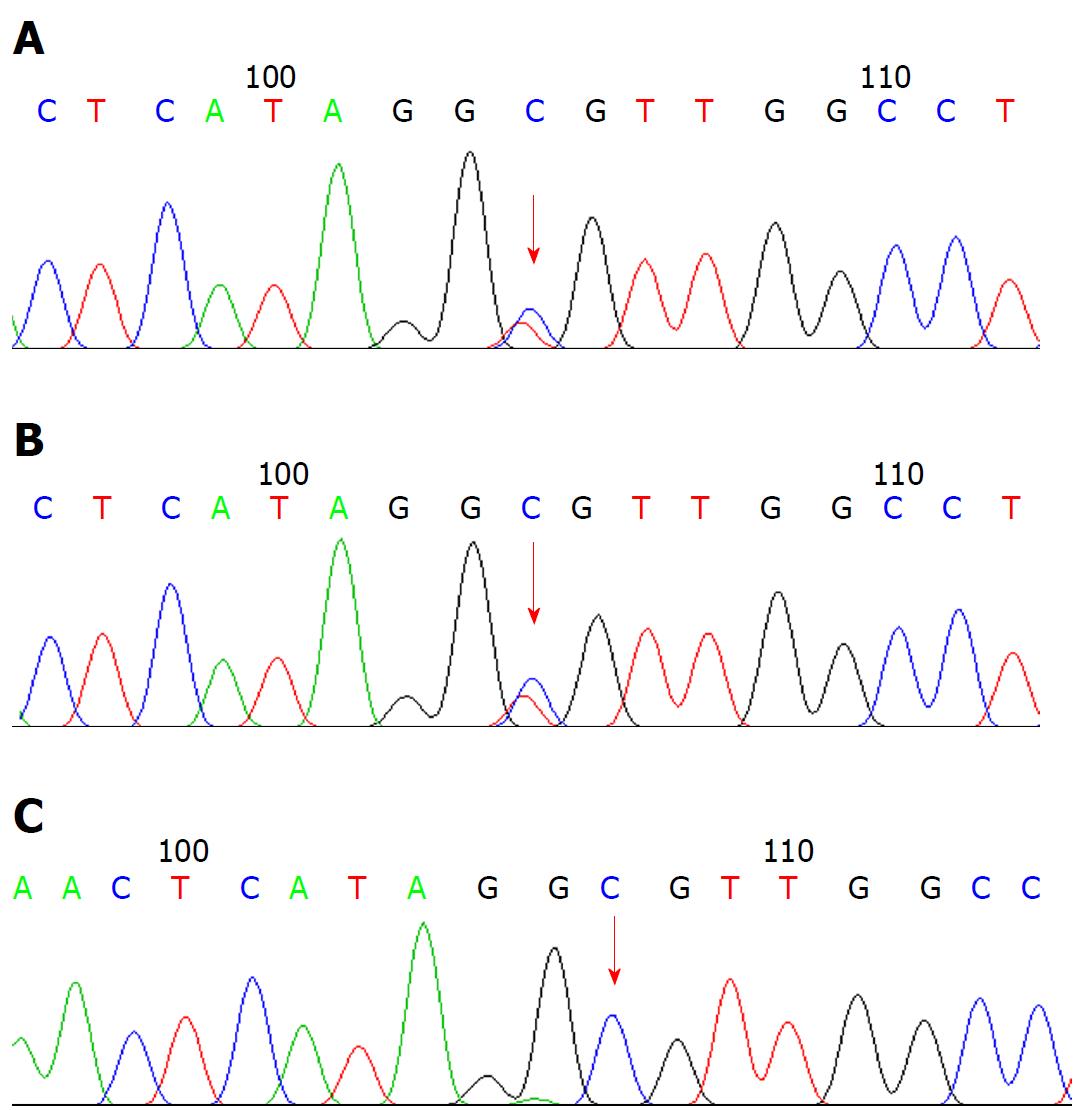
Liver biopsy led to the diagnosis of biliary cirrhosis with ductopenia (Figure 1). Genetic testing was performed for all 254 exons of liver disease-related genes, and adjacent ± 10 bp introns were sequenced. The results revealed a pathogenic heterozygous mutation, ex13 c.1531G > A (p.A511T), in the ABCB4 gene (Figure 2).
Her father did not carry the mutation, but her mother’s brother carried the heterozygous mutation and her mother died of cirrhosis at age 62 years. We made a definitive diagnosis of PFIC3. Her symptoms and liver function tests improved after 3 mo of treatment with ursodeoxycholic acid. The results of serum tests after treatment were as follows: Albumin 37.1 g/L, TBIL 21.9 μmol/L, ALT 58 U/L, ALP 127 U/L, and GGT 127 U/L.
PFIC is a group of autosomal recessive genetic diseases that cause bile excretion disorders due to mutations. The clinical manifestations of intrahepatic cholestasis usually present in infancy or childhood, eventually leading to liver failure. PFIC is mainly divided into three types: PFIC1, which is caused by an ATP8B1 gene mutation, resulting in a P-type ATPase-FICl defect encoded by the gene; PFIC2, which is derived from a mutation in the ABCBI1 gene encoding for bile salt export pump protein; and PFIC3, which is caused by a mutation in the ABCB4 gene encoding MDR3.
PFIC3 is a subtype of progressive familial intrahepatic cholestasis, and its main difference from PFIC1 and PFIC2 is the presence of elevated GGT[9,10]. PFIC3 is characterized by recurrent itching, jaundice, white clay-like stools, hepatosplenomegaly, and gastrointestinal bleeding, often progressing to cirrhosis and liver failure before adulthood[11].
PFIC3 is a rare disease, mostly sporadic, and the incidence rate has not been accurately reported. According to a survey, the estimated incidence is 1/500000 (http://demo.istat.it/).
The MDR3 protein encoded by the ABCB4 gene is located on the hepatic capillary bile duct membrane. It is a phospholipid export pump and phospholipid flipping enzyme, and transports phospholipid from liver cells into the bile duct, which is the rate-limiting step of phospholipid secretion. Under normal circumstances, phospholipids synthesized by hepatocytes are transported into bile by MDR3. Together with bile salts, the phospholipids form microparticles, which increase the hydrophilicity and reduce the descaling effect of bile salts, and protect bile duct cells from toxic damage caused by bile salts. Mutation in the ABCB4 gene results in the loss of expression of the MDR3 protein associated with phospholipid secretion in the bile duct. With the lack of phospholipids in bile, the formation of mixed particles of bile salts and phospholipids is reduced, resulting in the release of bile salts and causing toxic and detergent effects on the capillary bile duct membrane. When the biliary cells are damaged, cholestasis, small bile duct hyperplasia, inflammatory infiltration, and gradual progression to portal fibrosis, cirrhosis, and portal hypertension occur. Patients often die from liver failure.
More than 40 different ABCB4 gene mutations have been reported[12], including missense mutations, nonsense mutations, deletion mutations, and insertion of small fragment bases. The severity of the disease is related to the type of gene mutation. Nonsense mutations and deletion mutations are more likely to have serious clinical symptoms. When family members are homozygous for a mutation, they present with progressive intrahepatic cholestasis, often requiring liver transplantation in early childhood or leading to death due to liver failure[13,14].
When family members are heterozygous, other diseases associated with mutations in the ABCB4 gene[8,15-18], such as ICP, cholelithiasis, drug-induced intrahepatic cholestasis, and primary biliary cirrhosis, often emerge. The most prominent of these presentations are associated with the heterozygous ABCB4 gene, and can recur multiple times in ICP. The incidence of ICP in patients with PFIC3 is unknown. Gotthardt et al[17] found that all pregnant women in a family with the ABCB4 gene mutation had ICP, suggesting that the ABCB4 gene mutation is closely related to ICP. Our patient also developed ICP.
Ex13 c.1531G > A (p.A511T) describes a c.1531G > A (p.A511T) heterozygous missense mutation in exon 13 of the ABCB4 gene, which is included in the Human Gene Mutation Database and has been detected in patients with PFIC3[12]. The frequency of this mutation in the normal East Asian population is 0, and its protein function can be predicted using SIFT (http://sift.jcvi.org/) and Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/) software. As the results are all harmful, this mutation is suspected to be disease causing.
The clinical features of PFIC3 may overlap with many other forms of liver disease. In the present case, liver histology showed copper-associated protein sinking in hepatocyte cytoplasm (Figure 1C). Wilson disease (WD) may mimic non-Wilsonian liver disease. The diagnosis of WD should be based on clinical and pathologic parameters as well as genetic testing. Liver biopsy often demonstrates glycogenated nuclei and hepatic steatosis. Typically, there is little or no accumulation of copper. In this patient, WD was finally ruled out because the serum copper level was 90 μg/dL (80-155 μg/dL), the serum ceruloplasmin level was 210 mg/L (200-500 mg/L), no Kayser-Fleischer (K-F) rings were observed, and genetic testing revealed no ATP7B mutation.
Copper accumulation may also be seen in primary biliary cirrhosis, primary sclerosing cholangitis, secondary sclerosing cholangitis, biliary atresia, prolonged extrahepatic biliary obstruction, and in the livers of some normal neonates. The differential diagnosis of these cholestatic liver diseases depends on the corresponding immunological antibody detection and imaging examination.
In conclusion, we made a definitive diagnosis of PFIC3 in a 32-year-woman based on clinical symptoms, pathological findings, and gene mutation detection. She was treated with ursodeoxycholic acid, and her symptoms and liver function improved after 3 mo. We reported a new mutant gene fragment of PFIC-3, which has not been previously reported in the East Asian population.
The female patient was admitted to our hospital with fatigue, jaundice, and a recently elevated γ-glutamyl transpeptidase (GGT) level.
The patient had a history of acute hepatitis at age 9 years and was diagnosed as having intrahepatic cholestasis of pregnancy in 2008.
Viral hepatitis (A-E), Epstein-Barr virus, cytomegalovirus infections, and other chronic cholestatic diseases were ruled out.
High serum total bilirubin level was repeatedly observed, whereas alkaline phosphatase and GGT were also repeatedly increased and remained at 5-10 times the upper limit of normal.
Genetic testing revealed a pathogenic heterozygous mutation, ex13 c.1531G > A (p.A511T), in the ATP-binding cassette, subfamily B, member 4 (ABCB4) gene.
Liver biopsy led to the diagnosis of biliary cirrhosis with ductopenia.
The patient’s symptoms and liver function improved after 3 mo of treatment with ursodeoxycholic acid.
Reports on progressive familial intrahepatic cholestasis type 3 (PFIC3) with high GGT are rare. We identified a mutant gene fragment in PFIC3, which has not been previously reported in the East Asian population.
PFIC3 is caused by a mutation in the ABCB4 gene encoding multidrug resistance protein 3.
PFIC3 is a subtype of progressive familial intrahepatic cholestasis that differs from PFIC1 and PFIC2 in that serum GGT is elevated.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
CARE Checklist (2013) statement: This case report was written in accordance with the CARE (2013) guidelines.
P- Reviewer: Dourakis SP, Goto Y, Karagiannakis D, Kim IH S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Jansen PL, Müller MM. Progressive familial intrahepatic cholestasis types 1, 2, and 3. Gut. 1998;42:766-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Deleuze JF, Jacquemin E, Dubuisson C, Cresteil D, Dumont M, Erlinger S, Bernard O, Hadchouel M. Defect of multidrug-resistance 3 gene expression in a subtype of progressive familial intrahepatic cholestasis. Hepatology. 1996;23:904-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 171] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Crupi I. Progressive familial intrahepatic cholestasis (PFIC) type 3 in two Sicilian siblings of nonconsanguineous parents. ScientificWorldJournal. 2010;10:1065-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Delaunay JL, Durand-Schneider AM, Dossier C, Falguières T, Gautherot J, Davit-Spraul A, Aït-Slimane T, Housset C, Jacquemin E, Maurice M. A functional classification of ABCB4 variations causing progressive familial intrahepatic cholestasis type 3. Hepatology. 2016;63:1620-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Gaur K, Sakhuja P. Progressive familial intrahepatic cholestasis: A comprehensive review of a challenging liver disease. Indian J Pathol Microbiol. 2017;60:2-7. [PubMed] |
| 6. | Folmer DE, van der Mark VA, Ho-Mok KS, Oude Elferink RP, Paulusma CC. Differential effects of progressive familial intrahepatic cholestasis type 1 and benign recurrent intrahepatic cholestasis type 1 mutations on canalicular localization of ATP8B1. Hepatology. 2009;50:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Chen HL, Chang PS, Hsu HC, Lee JH, Ni YH, Hsu HY, Jeng YM, Chang MH. Progressive familial intrahepatic cholestasis with high gamma-glutamyltranspeptidase levels in Taiwanese infants: role of MDR3 gene defect? Pediatr Res. 2001;50:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Sundaram SS, Sokol RJ. The Multiple Facets of ABCB4 (MDR3) Deficiency. Curr Treat Options Gastroenterol. 2007;10:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Maisonnette F, Abita T, Barriere E, Pichon N, Vincensini JF, Descottes B. [The MDR3 gene mutation: a rare cause of progressive familial intrahepatic cholestasis (PFIC)]. Ann Chir. 2005;130:581-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 456] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 11. | Oude Elferink RP, Paulusma CC. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch. 2007;453:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Degiorgio D, Colombo C, Seia M, Porcaro L, Costantino L, Zazzeron L, Bordo D, Coviello DA. Molecular characterization and structural implications of 25 new ABCB4 mutations in progressive familial intrahepatic cholestasis type 3 (PFIC3). Eur J Hum Genet. 2007;15:1230-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Englert C, Grabhorn E, Richter A, Rogiers X, Burdelski M, Ganschow R. Liver transplantation in children with progressive familial intrahepatic cholestasis. Transplantation. 2007;84:1361-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Mehl A, Bohorquez H, Serrano MS, Galliano G, Reichman TW. Liver transplantation and the management of progressive familial intrahepatic cholestasis in children. World J Transplant. 2016;6:278-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Jacquemin E. Role of multidrug resistance 3 deficiency in pediatric and adult liver disease: one gene for three diseases. Semin Liver Dis. 2001;21:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Gotthardt D, Runz H, Keitel V, Fischer C, Flechtenmacher C, Wirtenberger M, Weiss KH, Imparato S, Braun A, Hemminki K. A mutation in the canalicular phospholipid transporter gene, ABCB4, is associated with cholestasis, ductopenia, and cirrhosis in adults. Hepatology. 2008;48:1157-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Floreani A, Carderi I, Paternoster D, Soardo G, Azzaroli F, Esposito W, Montagnani M, Marchesoni D, Variola A, Rosa Rizzotto E. Hepatobiliary phospholipid transporter ABCB4, MDR3 gene variants in a large cohort of Italian women with intrahepatic cholestasis of pregnancy. Dig Liver Dis. 2008;40:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |