Published online Nov 7, 2018. doi: 10.3748/wjg.v24.i41.4622
Peer-review started: July 5, 2018
First decision: August 25, 2018
Revised: October 8, 2018
Accepted: October 16, 2018
Article in press: October 16, 2018
Published online: November 7, 2018
Processing time: 125 Days and 3.7 Hours
The chronic inflammatory process underlying inflammatory bowel disease (IBD), comprising Crohn’s disease and ulcerative colitis, derives from the interplay of several components in a genetically susceptible host. These components include environmental elements and gut microbiota a dysbiosis. For decades, immune abnormalities have been investigated as critically important in IBD pathogenesis, and attempts to develop effective therapies have predominantly targeted the immune system. Nevertheless, immune events represent only one of the constituents contributing to IBD pathogenesis within the context of the complex cellular and molecular network underlying chronic intestinal inflammation. These factors need to be appreciated within the milieu of non-immune components. Damage-associated molecular patterns (DAMPs), which are essentially endogenous stress proteins expressed or released as a result of cell or tissue damage, have been shown to act as direct pro-inflammatory mediators. Excessive or persistent signalling mediated by such molecules can underlie several chronic inflammatory disorders, including IBD. The release of endogenous DAMPs amplifies the inflammatory response driven by immune and non-immune cells and promotes epigenetic reprogramming in IBD. The effects determine pathologic changes, which may sustain chronic intestinal inflammation and also underlie specific disease phenotypes. In addition to highlighting the potential use of DAMPs such as calprotectin as biomarkers, research on DAMPs may reveal novel mechanistic associations in IBD pathogenesis and is expected to uncover putative therapeutic targets.
Core tip: Damage-associated molecular patterns (DAMPs) are basically endogenous stress molecules expressed or released as a consequence of cell or tissue damage. The release of endogenous DAMPs precipitates a secondary inflammatory response in inflammatory bowel disease (IBD), which may determine a self-sustaining chronic inflammatory process. DAMPs amplify the inflammatory response driven by immune and non-immune cells and promote several pathologic changes, which may be associated with specific disease phenotypes. Excessive or persistent DAMP-mediated signalling can result in epigenetic modifications, which may sustain chronic inflammation and also characterize IBD phenotypes. Preliminary studies targeting DAMPs have shown promising beneficial therapeutic effects both in human and experimental IBD.
- Citation: Nanini HF, Bernardazzi C, Castro F, de Souza HSP. Damage-associated molecular patterns in inflammatory bowel disease: From biomarkers to therapeutic targets. World J Gastroenterol 2018; 24(41): 4622-4634
- URL: https://www.wjgnet.com/1007-9327/full/v24/i41/4622.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i41.4622
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), constitutes a chronic inflammatory condition that primarily affects the gastrointestinal tract. Although the aetiology of IBD remains largely unclear, evidence to date supports a multifactorial background[1,2]. From a clinical perspective, IBD has been considered to be a heterogeneous condition, with a wide range of clinical manifestations that usually change throughout the course of the disease. Despite the remarkable accumulation of knowledge regarding disease mechanisms in the last decades, therapeutic options are still relatively scarce. Moreover, defining the best treatment for individual patients remains a challenge.
Within the context of chronic inflammation, particularly when severe injury ensues, the tissue damage occurring in cell death results in the release of a multitude of potentially pro-inflammatory endogenous molecules. Damage-associated molecular patterns (DAMPs) are such endogenous molecules released from cells in response to endogenous or exogenous stimuli. DAMPs can function as signalling mediators of stress responses and the immune response via specific membrane or intracellular receptors or after endocytic uptake[3,4]. DAMPs may originate from diverse cellular compartments, including the cytosol, nucleus, and mitochondria, and also from tissue components such as the extracellular matrix[5].
Evidence accumulated in the last decade indicates that abnormal signalling through receptors associated with DAMPs occurs in several diseases[6-8]. Such findings have attracted attention regarding the potential role of DAMPs in both IBD pathogenesis and clinical practice[9-12].
Here, we review mechanisms involving DAMPs in chronic intestinal inflammation and the potential use of DAMPs as biomarkers. Promising novel therapeutic targets for IBD are also discussed.
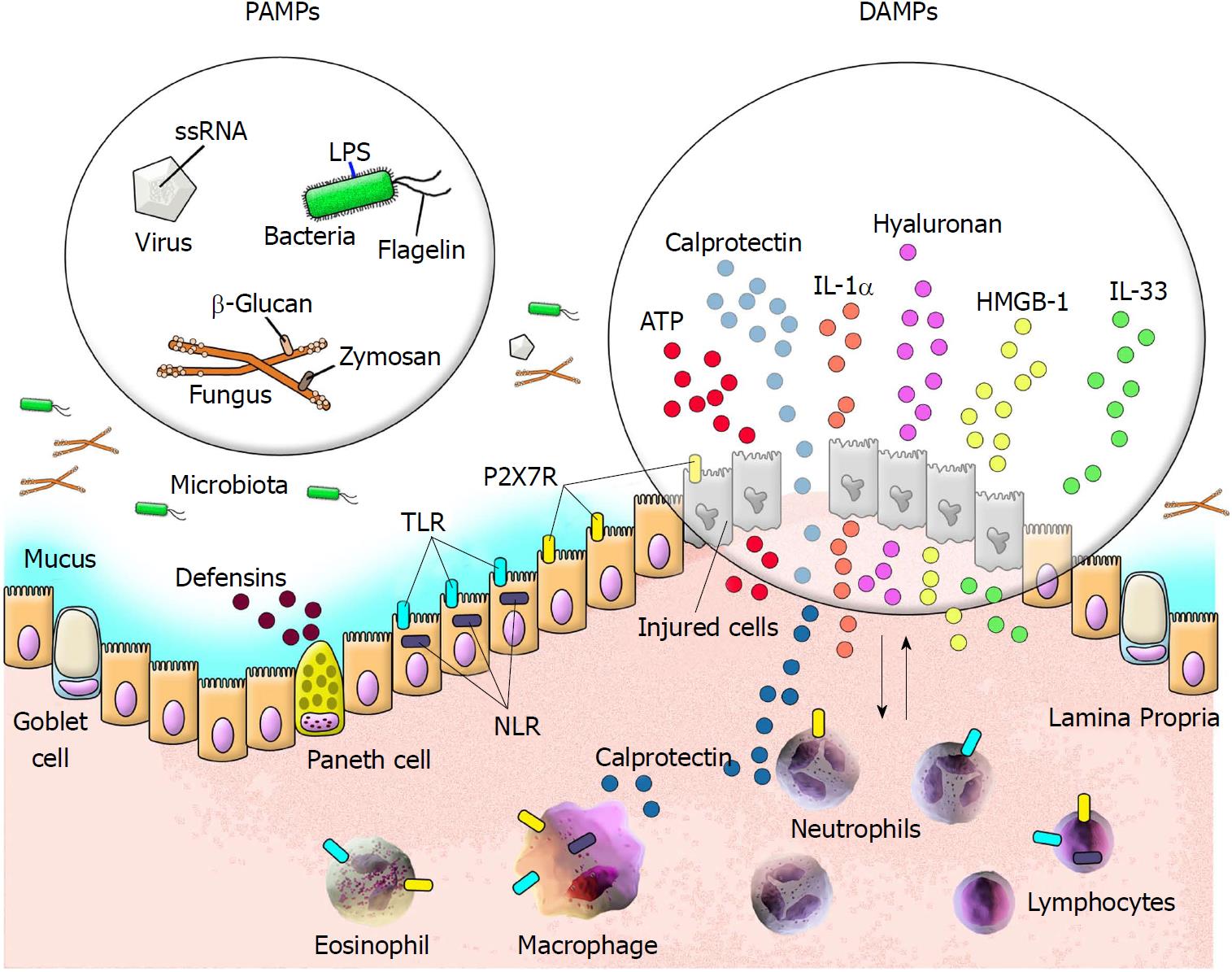
The human body harbours an efficient defence system against potentially harmful elements in the environment. This protective mechanism is composed of several components, including cells programmed to combat exogenous elements utilizing a complex immunological system that consists of innate and adaptive responses. Cells of the innate immune system respond to a variety of molecules from different microorganisms known as pathogen-associated molecular patterns (PAMPs). Nevertheless, infectious and non-infectious challenges invariably result in host tissue damage, which directs the release of components normally found in intracellular compartments. Several molecules released into the extracellular milieu by damaged cells have been termed DAMPs[13,14].
DAMPs comprise various endogenous molecules that are capable of activating pattern recognition receptors (PRRs). DAMPs may be released after plasma membrane disruption secondary to several forms of cell death or may be actively secreted via non-classical pathways by cells under stress[15]. In addition to the ubiquitous origin of DAMPs, such as intracellular proteins and purinergic molecules in distinct sub-cellular compartments, DAMPs may also be derived from the extracellular matrix[5]. Although DAMPs are not recognized by the innate immune system under physiological conditions, extracellularly released DAMPs signal danger upon tissue damage and induce both inflammatory and repair processes[14]. However, within the context of significant tissue injury, the persistent release of DAMPs may fuel a stress-inflammation amplification loop that underlies the pathogenesis of several chronic inflammatory disorders.
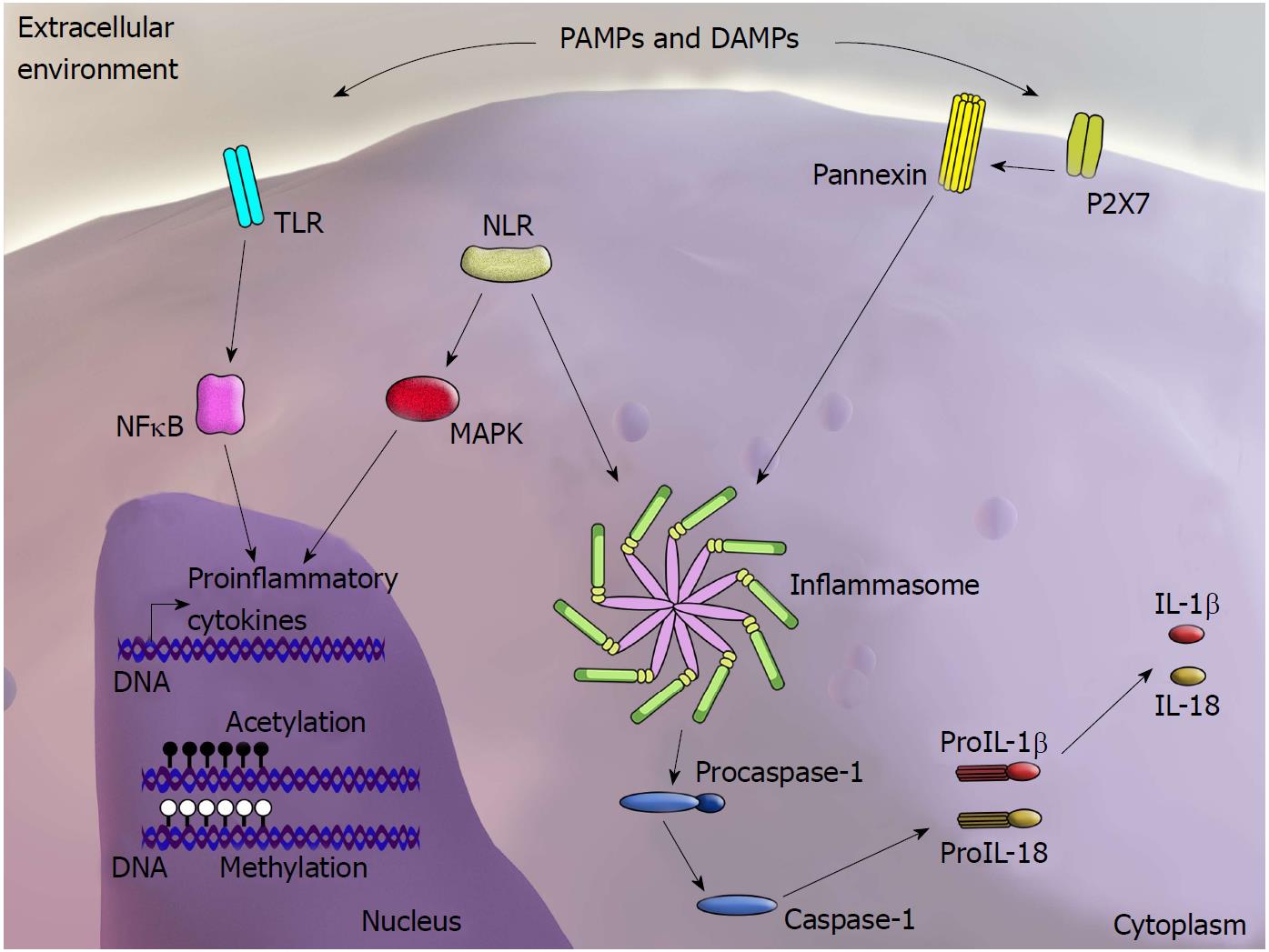
PRRs can be activated by DAMPs within the scenario of “sterile inflammation”, in which tissue damage occurs in the absence of invasive microorganisms[16,17]. PRRs comprise several cell surface or endosomal receptors of four major types: Toll-like receptors (TLRs); cytoplasmic nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) and inflammasomes; RIG-like receptors (RLRs); and C-type lectin receptors (CLRs)[18]. Although the precise mechanisms underlying the interaction between DAMPs and PRRs have yet to be clarified, it is interesting to note that regardless of their structural diversity, DAMPs and PAMPs are frequently recognized by the same receptors. After detecting PAMPs or DAMPs, PRRs activate intracellular signalling pathways, resulting in upregulation of pro-inflammatory genes and stimulation of mechanisms involved in the inflammatory response as well as antimicrobial actions[19] (Figure 1).
The pathways associated with NLR activation are poorly understood and remain under investigation. Nevertheless, two distinct mechanisms have been proposed: direct binding and indirect binding of PAMPs and DAMPs to receptors. These mechanisms are based on three models. The most studied model involves activation of the NLRP3 inflammasome, whereby the purinergic P2X7 receptor is stimulated by adenosine triphosphate (ATP), which triggers K+ efflux and opening of the pannexin-1 pore. This allows passage of the NLRP3 agonist into the cytosol, leading to the direct activation of NLRP3[20] (Figure 2). The second model relates to the observation that crystalline and particulate structures can be phagocytosed and released into the cytosol following damage to the phagolysosome, thus directly activating NLRP3. The third model proposes that DAMPs and PAMPs induce production of reactive oxygen species (ROS), indirectly activating the inflammasome[21].
Overexpression of interleukin (IL)-1β and IL-18, as well as IL-18 and NLRP3 polymorphisms described in patients with CD, also supports the involvement of inflammasomes in IBD[22-24]. Studies using experimental models typically corroborate these findings. For instance, NLRP6-deficient mice develop spontaneous intestinal hyperplasia and show inflammatory cell recruitment and exacerbation of chemically induced colitis[25]. NLRP6 is highly expressed in the intestine; by preserving the integrity of the intestinal epithelial barrier, NLRP6 exhibits protective effects against the development of intestinal inflammation[26,27]. In accordance with these findings, most studies have reported that NLRP3-deficient mice are more likely to develop colitis[28,29]. However, another independent study has shown opposing results, suggesting a protective effect of NLRP3 deficiency against chemically induced colitis[30]. Independent of the exact role of the NLRP3 inflammasome in experimental colitis, the fact that NLRP3-, ASC-, and caspase-1-deficient mice do not develop colitis in the absence of chemical stimuli indicates that inflammasome impairment alone does not lead to intestinal inflammation[31]. Regardless, these data support the importance of PRR function in maintaining intestinal homeostasis and highlight the role of intracellular signalling via DAMPs in the pathogenesis of intestinal inflammation.
Several studies have contributed to our understanding of the role of DAMPs in IBD. DAMPs are currently thought to contribute to the development of intestinal inflammation, particularly via activation of lamina propria cells, which are directly involved in innate immunity[32]. In fact, several types of molecules identified as being involved during the course of IBD, such as calprotectin, lactoferrin, calreticulin, high-mobility group box 1 (HMGB1), ATP, IL-1α, IL-33, and fragments of the extracellular matrix, are considered DAMPs[5] (Figure 1). Below, we attempt to delineate the role of DAMPs in the pathogenesis of IBD by highlighting certain molecules and their potential importance as biomarkers of inflammatory activity and as therapeutic targets.
Calprotectin, a calcium-binding protein belonging to the S100 family, is basically composed of S100A8 and S100A9 heterodimers. The S100 family comprises more than 20 members with multiple functions, with calprotectin typically being associated with intestinal inflammation. Calprotectin, which is also known as Mrp8/14, calgranulin A/B, and cystic fibrosis antigen, is commonly found in cells of the immune system, mainly in neutrophils but also in reactive monocytes and macrophages. This protein potentially functions as an antimicrobial agent[33-35]. During the inflammatory response, cells of the innate immune system release their intracellular contents into the extracellular milieu in a degranulation process; this results in increased concentrations of calprotectin at various body sites, including the intestinal lumen, and in faeces[36]. As a non-invasive tool to aid in the detection of intestinal inflammation, faecal calprotectin (FC) levels can be measured using enzyme-linked immunosorbent assays (ELISAs) or, more recently, a home-use kit associated with a smartphone application[37]. Such measurement has a relatively good correlation with clinical and endoscopic results in patients with UC[38] and in those with CD, for which FC has also been used to monitor the risk of disease relapse[39].
Lactoferrin, which binds iron, is an indicator of neutrophil degranulation and acts as an alarmin[40]. Because lactoferrin is relatively resistant to degradation and proteolysis, it can be measured in stool and serve as a biomarker of intestinal inflammation. Thus, lactoferrin has been utilised to differentiate functional diseases from IBD. However, similar to calprotectin, lactoferrin has been most highly correlated with colonic inflammation, as opposed to ileal activity[41].
Calreticulin (CRT) is a calcium-binding protein and an endoplasmic reticulum (ER)-resident lectin-like chaperone that is induced by ER stress[42]. In addition, CRT has been shown to induce ER stress accompanied by a significant increase in proteasome activity[43]. Recently, CRT has been recognized as a potent DAMP capable of influencing homeostasis through immune regulation. In this regard, new evidence has indicated that CRT can translocate to the cell surface and serve as a signal for immune-mediated cell death[44]. Moreover, a significant decrease in the basal transcriptional activity of nuclear factor kappa B (NF-κB) has been observed in CRT-deficient cells. In an experimental model of inflammation, the tubular epithelial cells of rats subjected to unilateral ureteric obstruction showed an upregulation of CRT[45].
In contrast to cellular components and endogenous DAMPs such as DNA, RNA, and ATP, another subset of intracellular proteins released from necrotic cells also appears to participate in sterile inflammatory processes. These proteins, including members of the IL-1 family such as IL-1α, IL-33, and HMGB1, are characteristically bifunctional, acting as cytokines and performing yet-unclear nuclear functions[46]. In contrast to the signalling mediated by DAMPs, which are usually recognized by PAMP receptors such as TLRs, activation of the HMGB1 signalling pathway occurs through interaction with several cell surface receptors. As a result, HMGB1 exerts effects on a multitude of processes, including cell proliferation, survival and death, as well as inflammation[47,48].
HMGB1 is a DNA-binding protein that may be translocated to the cytoplasm when cellular stress occurs. During chronic inflammatory processes, high rates of cellular necrosis result in the abundant release of HMGB1 into the extracellular milieu. As a consequence, extracellular HMGB1 participates in the induction of intestinal epithelial cell autophagy[49], increased expression of adhesion molecules, and secretion of proinflammatory cytokines and chemokines[50,51].
HMGB1 levels have been shown to be elevated in the dextran sodium sulphate (DSS)-induced colitis model[52]. In addition, genetically modified HMGB1-deficient mice (Vil-Cre Hmgb1fl/fl) had more apoptosis of intestinal cells following induction of colitis with DSS[53].
IL-1α
IL-1α is an IL-1 family member synthesized as a precursor protein (pIL-1α) with a molecular weight of approximately 31 kDa that may be cleaved into mature 17-kDa forms. The two forms are biologically active and serve as ligands for the receptor IL-1R1[54]. These proteins are constitutively expressed in different immune cells as well as in intestinal epithelial cells[55]. IL-1α expression is upregulated in response to growth factors or to pro-inflammatory or stressful stimuli; the molecule then is translocated from the cytosol to the nucleus, where it acts as a pro-inflammatory transcription factor[54]. For example, upon stimulation with lipopolysaccharide (LPS) or tumor necrosis factor alpha (TNFα), IL-1α translocates to the nucleus to promote expression of inflammatory genes, including IL-8 and IL-6[56]. However, cells undergoing necrosis can release pIL-1α, which results in cell chemotaxis and inflammation[57]; therefore, pIL-1α functions as a DAMP. In fact, in the extracellular milieu, IL-1α appears to induce a pro-inflammatory response via binding with IL-1R1[58].
With regard to chronic intestinal inflammation, high levels of IL-1α have been detected in lamina propria mononuclear cells from patients with IBD[59] and in supernatants of colonic explant cultures from CD or UC patients[11]. In experimental colitis, release of IL-1α from damaged intestinal epithelial cells has been associated with the initiation and propagation of colonic inflammation[60]. IL-1α has also been shown to amplify gut inflammation in experimental colitis by inducing cytokine production in mesenchymal cells[61].
IL-33 is a member of the IL-1 cytokine family that is predominantly expressed in stromal cells and in the epithelium lining surfaces in contact with the environment[62]. Primarily is described as a proinflammatory cytokine that induces the Th2 immune response and is involved in defence against parasitic infections. IL-33 has also been proposed as an inducer of Th1 cells, group 2 innate lymphoid cells, regulatory T (Treg) cells, and CD8+ T cells[63]. In addition, IL-33 may act as a signalling molecule that alerts the immune system to danger or tissue damage[64]. IL-33 localises to the nucleus; however, once released into the extracellular milieu upon membrane disruption, it may act as a dual-function alarmin, similar to HMGB1 and IL-1 alpha[65].
IL-33/ST2 signalling in the gut has been implicated in the pathogenesis of inflammatory processes. In fact, abnormal expression of IL-33/ST2 has been detected in the inflamed mucosa of patients with IBD, as well as in experimental models of chemically induced colitis[66]. Because a predominant Th2 immune response underlies UC pathogenesis, several studies have attempted to investigate the role of IL-33 in this specific condition. For example, investigators found a significant increase in mucosal IL-33 mRNA expression in patients with active UC compared to healthy controls. Moreover, a significant reduction in IL-33 was detected after anti-TNF therapy, thus supporting the notion that enterocyte-derived IL-33 is induced and maintained by the inflammatory milieu[67].
Under normal conditions, nucleotides such as ATP are present in high concentrations intracellularly. However, upon stimulation by different stresses such as necrosis, apoptosis, hypoxia, or pathogen invasion, cells may release nucleotides into the extracellular milieu. ATP in the extracellular environment is thought to act as a messenger and behave as a danger signal capable of modulating immunity and inflammation[68] via activation of transmembrane receptors known as P2 receptors. The family of P2 receptors comprises P2Y (G-coupled proteins) and P2X (ionic channels)[69]. Among all P2 family members, P2X7 receptors, which are expressed on different cell types such as monocytes, macrophages, dendritic cells, lymphocytes, neurons, fibroblasts, and epithelial cells, have been studied the most[70].
Upon activation, ATP-P2X7 signalling promotes the release of pro-inflammatory cytokines such as IL-1β and IL-18[71], stimulates free radical production, and participates in cell cycle regulation and apoptosis induction[69]. We previously showed that the P2X7 receptor is positively modulated by IFN-gamma in intestinal epithelial cells[72] and that its activation induces apoptosis and autophagy via ROS production[73]. With regard to human IBD, we showed that P2X7 receptors are overexpressed in inflamed colonic mucosa, particularly in CD patients[74]. Moreover, we demonstrated that P2X7 receptors promote intestinal inflammation by triggering the death of mucosal regulatory T cells[75]. In addition, we found that systemic blockade of P2X7 receptors prevents the development of chemically induced colitis in rats[76], whereas P2X7-deficient mice essentially do not develop intestinal inflammation[74]. Taken together, these findings strongly support a role for ATP-P2X7 signalling in the pathogenesis of IBD and may offer avenues for the development of inflammatory biomarkers and new therapeutic options.
The extracellular matrix (ECM) comprises a complex and dynamic non-cellular network that is present within all tissues. The ECM provides the architectural structure for cellular components and a microenvironment for the chemical and mechanical interactions necessary for tissue homeostasis. Although the ECM basically consists of water, proteins and polysaccharides, its composition is tissue specific[77]. Proteoglycans permeate most of the interstitial space within a tissue[78], and in the gastrointestinal tract, hyaluronan is a highly prevalent proteoglycan component of the ECM[79].
Hyaluronan, a non-sulphated glycosaminoglycan that interacts with different proteins, including ECM components and membrane receptors[80], has been shown to induce leukocyte recruitment in the extravascular space within the context of intestinal injury[81]. In fact, hyaluronan accumulates in the vicinity of infiltrating leukocytes in the colon, both in human IBD[82] and in experimental colitis tissues[83]. Under normal conditions, hyaluronan exists as a high molecular weight molecule that may function as an anti-angiogenic factor[84], prevent immune cell recognition, and block phagocytosis by macrophages[85,86]. In addition, high molecular weight hyaluronan prevents T cell-mediated liver injury[87] and promotes the persistence of tolerogenic regulatory T cells[88] in experimental models.
Conversely, hyaluronan displays an altered distribution in inflammatory settings and consists of a variety of polymers with different lengths and functions[89,90]. Small fragments resulting from hyaluronan degradation have been implicated in activation of the innate immune response via TLR2, whereas the intact hyaluronan molecule is capable of inhibiting activation of the same receptor[91]. In another study, investigators observed that fibroblasts from CD patients produce high levels of KIAA1199, a protein responsible for excessive hyaluronan degradation, which leads to the generation of pro-inflammatory fragments, potentially enhancing inflammation[92].
Table 1 summarises information on specific disease phenotypes and also presents details on human and experimental studies.
| DAMP | Human IBD | Experimental IBD |
| Calprotectin | Increased levels in the intestinal lumen and stools in both UC and CD[36,38,39] | - |
| Lactoferrin | Mostly correlates with colonic inflammation[41] | Beneficial therapeutic effects in colitis models[93,94] |
| Calreticulin | Related to inflammatory activity[44] | - |
| HMGB1 | Increased levels in the stools of both adult and paediatric IBD patients[113] | Increased levels in DSS-induced colitis mice[52] |
| IL-1 alpha | Increased levels in the lamina propria of both UC and CD[59] | Associated with colonic inflammation initiation and amplification[60,61] |
| IL-33 | Increased levels in the inflamed intestinal mucosa of IBD patients, especially in UC[66,67] | Increased levels in chemically induced colitis[66]; beneficial effects upon ST2 blockage[121] |
| ATP-P2X7 | Overexpressed in IBD patients, particularly in CD[74] | Increases intestinal inflammation in chemically induced colitis[75]; P2X7-deficient mice essentially do not develop intestinal inflammation[74] |
| S 100 proteins | Increased faecal[95-98], mucosal[99], and serum[99-101] levels | |
| HSPs | Increased levels[102-105] | Beneficial therapeutic effects in colitis models[106] |
| Galectins | Increased serum levels in UC and CD[107] | Galectins 1 and 2 show anti-inflammatory action[108,109] |
| Galectin 4: Antibody blockage reduces inflammation[110] | ||
| Hyaluronan | ECM components accumulate in the colon of IBD patients[82], particularly in UC[115] | ECM components accumulate in experimental colitis tissues[83] |
Recent progress in epigenetics has suggested that genome modifications may be more dynamic than previously thought. For instance, immune cells, including monocytes and macrophages, and epithelial cells are known to promote an inflammatory response upon LPS stimulation. This phenomenon involves the reprogramming of cell-specific gene expression, which can occur through different mechanisms, including epigenetic modifications[111,112]. Nevertheless, in the case of LPS, epigenetic modifications are likely not exclusively associated with the acute response but also may be associated with the establishment of epigenetic memory, thus impacting the future response mediated by exposure to new microorganisms[113]. In parallel, tissue damage per se is known to induce a local inflammatory response, which may be followed by subsequent regenerative processes involving macrophages and other immune cells as well as non-immune cells[114]. In such circumstances, similar to the events that follow microbial stimulation[115], cells of the innate immune system develop immunological memory via epigenetic reprogramming after exposure to non-microbial ligands[116]. This functional adaptation of the immune system may direct exacerbated inflammatory responses upon subsequent challenges and may explain the long-term reprogramming of inflammatory genes induced by endogenous DAMPs[117] (Figure 2).
Atherosclerosis, a fundamental mechanism underlying most cardiovascular diseases and progressively recognized as an inflammatory disorder, is one ubiquitous example of epigenetic reprogramming. Indeed, the inflammatory nature of atheromatous plaques comprises interaction between elements such as modified low-density oxidized lipoproteins functioning as DAMPs and macrophage foam cells filled with cholesterol droplets; these foam cells produce chemokines that attract additional circulating leucocytes to atherosclerotic plaques[118]. Evidence for an epigenetic background underlying the atherogenic phenotype has been demonstrated by the observation that macrophages trained by exposure to beta-glucan display transcriptional activation at several loci encoding both inflammatory mediators and genes directly associated with basic metabolic processes in the development of atherogenesis[119]. In fact, the hypothesis that trained monocytes/macrophages may become pro-atherogenic has been further confirmed by the demonstration that oxidized LDL can train primary human monocytes to upregulate expression of proinflammatory cytokines, PRRs and LDL receptors[120].
Due to their wide range of participation in several disorders that directly or indirectly involve the immune system, regulatory T cells (Tregs), a subset of CD4+ T cells that play a fundamental role in peripheral immune tolerance, continue to attract attention. New progress in this field points to potential Treg immune plasticity and regulation by receptors for PAMPs and DAMPs[121-123] as well as to the epigenetic regulation of Treg phenotypes and functions[124]. In light of these relatively novel findings, Yang et al[125] proposed an innovative concept in which Tregs might be subjected to re-shaping from a physiological phenotype into a pathological phenotype within the setting of diverse pathological conditions. Based on a similar line of evidence, macrophages are known to polarize into distinct phenotypes in vitro upon exposure to different stimuli; in vivo, these cells respond to signals, including PAMPs and DAMPs, that control their homeostatic functions[126,127]. Recently, polarization of macrophages in response to complex tissue damage and wound repair signals has been associated with expression of Rev-erb nuclear receptors. Interestingly, Rev-erbs repress subsets of genes activated by TLR ligands, IL-4, TGF beta, and DAMPs. Thus, Rev-erbs have been postulated to function as key molecules integrating signalling pathways involved in tissue injury to promote a wound repair phenotype[128].
The recent discovery that CRT possesses transacetylase activity, which is involved in a critical post-translational modification capable of shaping epigenetic regulation and signal transduction, suggests additional roles for CRT in diseases involving immune regulation. In this sense, CRT can also be considered a potential target for the development of anti-inflammatory therapies based on semi-synthetic acetyl donors such as polyphenolic acetates and related agents[129].
The above considerations represent a first attempt to relate the ability of endogenous signals such as DAMPs to promote trained immunity to IBD, offering a new principle for understanding the chronic and persistent nature of the inflammatory process that occurs in IBD. In the near future, the detailed epigenetic scenario in each IBD phenotype may become even more relevant, thus allowing for new therapeutic approaches directed towards the mediators or enzymes involved in the induction of relevant epigenetic modifications.
In light of the inconsistency among the currently available tests and the cost and potential risks of invasive procedures, contributing to a scenario of remarkable clinical variability, biomarkers of gut inflammation in IBD have been persistently investigated in recent decades. In addition, the fluctuating course of IBD creates a demand for more precise predictors of clinical outcomes to inform therapeutic decisions. In particular, the quantification of inflammatory activity, identification of specific disease behaviours, and prediction of responses and adverse effects due to a certain medication appear critical for the appropriate management of IBD.
Currently, FC and lactoferrin have been utilized as indicators of intestinal mucosal inflammation; together with other clinical and imaging approaches, these indicators, despite their limitations, contribute to the diagnosis and follow-up of patients with IBD[130]. Nonetheless, several other DAMPs have been proposed as promising biomarkers for IBD[5].
Among IL-1 family proteins, HMGB1 released following cellular necrosis has been detected in chronically inflamed intestinal tissues and found abundantly in the stool of both adult and paediatric patients[131]. Notably, faecal HMGB1 has been supported as a reliable biomarker of intestinal inflammation; it significantly correlates with FC and may identify histological inflammation in IBD patients in clinical and endoscopic remission[132].
Another IL-1 family member, IL-1α, has been detected in the supernatants of intestinal explant cultures from patients with IBD[11]. IL-33, another member of the IL-1 family, is also released in the extracellular milieu upon cell or tissue damage, and it has been detected in the inflamed mucosa of IBD patients[66].
Because CRT is involved in processes related to inflammatory activity and translocates to the cell surface and signals immune-mediated cell death, CRT is both a DAMP[44] and a potential biomarker. In another category of DAMPs, a positive correlation between high concentrations of serum-derived hyaluronan-associated protein and intestinal inflammation has been found in intestinal samples and serum from experimental colitis models and patients with IBD, particularly those with UC[133]. Therefore, hyaluronan and possibly other ECM components are emerging as relevant DAMPs in intestinal inflammation and potential new biomarkers for IBD.
Considering the relatively disappointing results of current IBD therapies, one of the limitations of orthodox drug development is the lack of consideration of crucial aspects already known about IBD pathogenesis[134,135]. In this regard, DAMPs constitute interesting, underexplored factors, even though they are not the primary causative agents of IBD. Regardless, targeting DAMPs as a novel therapeutic approach for IBD appears to be an arduous but fascinating task. As such, current strategies propose to block the release of DAMPs, to inhibit their downstream signalling pathways, or to interfere with factors that may modulate the pathogenicity of the molecules involved[5].
Data regarding strategies targeting DAMPs for the treatment of inflammatory disorders are fundamentally based on results from in vitro studies and those involving experimental models. For example, tubular epithelial cells have been shown to overexpress CRT in a model of ureteral obstruction[45], and the association of CRT with renal fibrosis progression based on in vitro and in vivo approaches appears to implicate CRT in the molecular mechanisms that drive renal fibrosis progression[136]. Together, these studies suggest that CRT may become a new therapeutic target for fibrosis in chronic inflammatory disorders.
In chemically induced experimental colitis, HMGB1 targeting via either neutralizing antibodies or small molecules has been successful[137,138]. In addition, blockade of receptor for advanced glycation end products (RAGE), which is a receptor for multiple DAMPs, virtually suppresses inflammation in genetically predisposed IL-10-deficient mice, i.e., a model of colitis[139]. Mitochondrial DNA (mtDNA), which shares many similarities with immunogenic bacterial DNA and is also recognized as a DAMP, is increased in the plasma of patients with UC and CD, and levels were significantly correlated with inflammatory mediators and endoscopic evidence of inflammation. Therefore, the investigators proposed that mtDNA may become a new biomarker for disease activity and that mtDNA-TLR9 may be a new therapeutic target in IBD[140].
With regard to IL-33, blockade of ST2 is reportedly beneficial in experimental models of chemically induced colitis[141]. From a clinical perspective, while the therapeutic success observed in animal studies targeting IL-33 ST2 may foster future trials directed towards IBD, specifically for patients with UC, and human studies have shown that loss of IL-33 expression in colonic crypts may be a useful marker of disease remission in UC[67]. Although the exact pathophysiologic importance of these findings has yet to be established, evidence supports dichotomous functions for the IL-33/ST2 pathway in IBD: The ability to enhance Th2 and Th17 responses in gut-associated lymphoid tissues while also stimulating mucosal healing following inflammatory tissue damage.
Recently, in the first phase IIa study designed to assess the efficacy and safety of AZD9056, a selective orally active inhibitor of the purinergic receptor P2X7, for CD, investigators showed a beneficial risk profile with improvement of symptoms in patients with moderate-to-severe disease. However, changes in inflammatory biomarkers among patients with CD were not detected[142]. Although the beneficial effects observed in that study will likely prompt the development of new trials for CD, some specific points concerning the therapeutic use of P2X7 antagonists are noteworthy. Based on our previous experience with P2X7 blockade in experimental colitis, purinergic activation induces the death of Tregs[75], and the beneficial therapeutic effect is characteristically associated with prophylactic treatment, particularly when administered systemically[76]. Such discrepancies might be related to the specific actions of the ATP-P2X7 pathway during the course of the inflammatory process and also to the effects of purinergic signalling in epithelial versus immune cells of the intestinal mucosa.
Hyaluronan accumulation in the intestine of patients undergoing IBD flares[82] and excessive production of ECM fragments, especially smaller polymers, are likely to fuel chronic inflammatory conditions such as IBD[92]. Nevertheless, it is interesting to note that some DAMPs may have a dual role in innate immune defence. In the case of hyaluronan, it has been demonstrated that large molecules may provide protective effects mediated by CD44 and TLR4 in experimental IBD[143]. Furthermore, these molecules may act in host defence at the epithelial cell surface, thus promoting antimicrobial peptide production and improving regulation of the tight junction barrier in the gut[144].
In recent years, considerable advances have been achieved with regard to the pathogenic mechanisms in IBD. However, a complete understanding of IBD pathogenesis will likely depend on more precise recognition and assimilation of the molecular and environmental constituents and the mechanisms by which they interact. In addition to potential use as practical biomarkers, proinflammatory activities and emerging roles in chronic inflammatory processes, including the ability to induce epigenetic modifications, DAMPs remain interesting targets for new discoveries about and innovative therapies for IBD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiba T, Day AS, De Ponti F, De Silva AP, Gazouli M S- Editor: Ma RY L- Editor: A E- Editor: Yin SY
| 1. | Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1233] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 2. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1114] [Article Influence: 123.8] [Reference Citation Analysis (1)] |
| 3. | Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1972] [Cited by in RCA: 2058] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 4. | Zhang Q, Kang R, Zeh HJ 3rd, Lotze MT, Tang D. DAMPs and autophagy: cellular adaptation to injury and unscheduled cell death. Autophagy. 2013;9:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Boyapati RK, Rossi AG, Satsangi J, Ho GT. Gut mucosal DAMPs in IBD: from mechanisms to therapeutic implications. Mucosal Immunol. 2016;9:567-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 640] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 7. | Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 559] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 8. | Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 9. | Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Palone F, Vitali R, Cucchiara S, Pierdomenico M, Negroni A, Aloi M, Nuti F, Felice C, Armuzzi A, Stronati L. Role of HMGB1 as a suitable biomarker of subclinical intestinal inflammation and mucosal healing in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Ludwiczek O, Vannier E, Borggraefe I, Kaser A, Siegmund B, Dinarello CA, Tilg H. Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease. Clin Exp Immunol. 2004;138:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Sponheim J, Pollheimer J, Olsen T, Balogh J, Hammarström C, Loos T, Kasprzycka M, Sørensen DR, Nilsen HR, Küchler AM. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol. 2010;177:2804-2815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 458] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 14. | Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237-35245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 454] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 15. | Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 765] [Cited by in RCA: 687] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 16. | Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3013] [Cited by in RCA: 2710] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 17. | Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2408] [Cited by in RCA: 2255] [Article Influence: 150.3] [Reference Citation Analysis (0)] |
| 18. | Blander JM, Sander LE. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat Rev Immunol. 2012;12:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6:451-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 786] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 21. | Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3880] [Cited by in RCA: 4539] [Article Influence: 302.6] [Reference Citation Analysis (0)] |
| 22. | Tamura K, Fukuda Y, Sashio H, Takeda N, Bamba H, Kosaka T, Fukui S, Sawada K, Tamura K, Satomi M. IL18 polymorphism is associated with an increased risk of Crohn’s disease. J Gastroenterol. 2002;37 Suppl 14:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Li J, Moran T, Swanson E, Julian C, Harris J, Bonen DK, Hedl M, Nicolae DL, Abraham C, Cho JH. Regulation of IL-8 and IL-1beta expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche J, Bitton A. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2009;41:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 25. | Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1659] [Cited by in RCA: 1576] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 26. | Chen GY, Liu M, Wang F, Bertin J, Núñez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186:7187-7194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 27. | Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci USA. 2011;108:9601-9606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 28. | Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 819] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 29. | Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V, Potentier MS, Ng K, Bawa M. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 360] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 30. | Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 714] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 31. | Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 427] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 32. | Mueller C. Danger-associated molecular patterns and inflammatory bowel disease: is there a connection? Dig Dis. 2012;30 Suppl 3:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 492] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 34. | Foell D, Frosch M, Sorg C, Roth J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 35. | Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1107] [Cited by in RCA: 1289] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 36. | Lehmann FS, Burri E, Beglinger C. The role and utility of faecal markers in inflammatory bowel disease. Therap Adv Gastroenterol. 2015;8:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Vinding KK, Elsberg H, Thorkilgaard T, Belard E, Pedersen N, Elkjaer M, Marker D, Carlsen K, Burisch J, Munkholm P. Fecal Calprotectin Measured By Patients at Home Using Smartphones--A New Clinical Tool in Monitoring Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Burri E, Beglinger C. Faecal calprotectin -- a useful tool in the management of inflammatory bowel disease. Swiss Med Wkly. 2012;142:w13557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Turvill J, Rook L, Rawle M, Robins G, Smale S, Kant P, Phillips A. Validation of a care pathway for the use of faecal calprotectin in monitoring patients with Crohn’s disease. Frontline Gastroenterol. 2017;8:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | de la Rosa G, Yang D, Tewary P, Varadhachary A, Oppenheim JJ. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol. 2008;180:6868-6876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817-1826.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 42. | Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci. 2006;119:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 356] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 43. | Uvarov AV, Mesaeli N. Enhanced ubiquitin-proteasome activity in calreticulin deficient cells: a compensatory mechanism for cell survival. Biochim Biophys Acta. 2008;1783:1237-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Chen X, Fosco D, Kline DE, Kline J. Calreticulin promotes immunity and type I interferon-dependent survival in mice with acute myeloid leukemia. Oncoimmunology. 2017;6:e1278332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Prakoura N, Politis PK, Ihara Y, Michalak M, Charonis AS. Epithelial calreticulin up-regulation promotes profibrotic responses and tubulointerstitial fibrosis development. Am J Pathol. 2013;183:1474-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: Feel the Stress. J Immunol. 2017;198:1395-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 47. | Agresti A, Bianchi ME. HMGB proteins and gene expression. Curr Opin Genet Dev. 2003;13:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 303] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 48. | Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta. 2010;1799:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 465] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 49. | Gordy C, He YW. Endocytosis by target cells: an essential means for perforin- and granzyme-mediated killing. Cell Mol Immunol. 2012;9:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 410] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 51. | Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 594] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 52. | Zheng X, Lv Y, Li S, Zhang Q, Zhang X, Hao Z. Adeno-associated virus-mediated colonic secretory expression of HMGB1 A box attenuates experimental colitis in mice. J Gene Med. 2016;18:261-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Zhu X, Messer JS, Wang Y, Lin F, Cham CM, Chang J, Billiar TR, Lotze MT, Boone DL, Chang EB. Cytosolic HMGB1 controls the cellular autophagy/apoptosis checkpoint during inflammation. J Clin Invest. 2015;125:1098-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 54. | Di Paolo NC, Shayakhmetov DM. Interleukin 1α and the inflammatory process. Nat Immunol. 2016;17:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 416] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 55. | Stadnyk AW, Sisson GR, Waterhouse CC. IL-1 alpha is constitutively expressed in the rat intestinal epithelial cell line IEC-6. Exp Cell Res. 1995;220:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, Voronov E, Dinarello CA, Apte RN. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci U S A. 2004;101:2434-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 57. | Cohen I, Rider P, Carmi Y, Braiman A, Dotan S, White MR, Voronov E, Martin MU, Dinarello CA, Apte RN. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107:2574-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 58. | Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1237] [Article Influence: 176.7] [Reference Citation Analysis (0)] |
| 59. | Youngman KR, Simon PL, West GA, Cominelli F, Rachmilewitz D, Klein JS, Fiocchi C. Localization of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology. 1993;104:749-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Bersudsky M, Luski L, Fishman D, White RM, Ziv-Sokolovskaya N, Dotan S, Rider P, Kaplanov I, Aychek T, Dinarello CA. Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut. 2014;63:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 61. | Scarpa M, Kessler S, Sadler T, West G, Homer C, McDonald C, de la Motte C, Fiocchi C, Stylianou E. The epithelial danger signal IL-1α is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am J Pathol. 2015;185:1624-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 62. | Bertheloot D, Latz E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 2017;14:43-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 63. | Hodzic Z, Schill EM, Bolock AM, Good M. IL-33 and the intestine: The good, the bad, and the inflammatory. Cytokine. 2017;100:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 64. | Nunes T, Bernardazzi C, de Souza HS. Interleukin-33 and inflammatory bowel diseases: lessons from human studies. Mediators Inflamm. 2014;2014:423957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 940] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 66. | Sun M, He C, Wu W, Zhou G, Liu F, Cong Y, Liu Z. Hypoxia inducible factor-1α-induced interleukin-33 expression in intestinal epithelia contributes to mucosal homeostasis in inflammatory bowel disease. Clin Exp Immunol. 2017;187:428-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Gundersen MD, Goll R, Hol J, Olsen T, Rismo R, Sørbye SW, Sundnes O, Haraldsen G, Florholmen J. Loss of interleukin 33 expression in colonic crypts - a potential marker for disease remission in ulcerative colitis. Sci Rep. 2016;6:35403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 69. | Savio LEB, de Andrade Mello P, da Silva CG, Coutinho-Silva R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front Pharmacol. 2018;9:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 316] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 70. | Bartlett R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66:638-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 71. | Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451-1458. [PubMed] |
| 72. | Welter-Stahl L, da Silva CM, Schachter J, Persechini PM, Souza HS, Ojcius DM, Coutinho-Silva R. Expression of purinergic receptors and modulation of P2X7 function by the inflammatory cytokine IFNgamma in human epithelial cells. Biochim Biophys Acta. 2009;1788:1176-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Souza CO, Santoro GF, Figliuolo VR, Nanini HF, de Souza HS, Castelo-Branco MT, Abalo AA, Paiva MM, Coutinho CM, Coutinho-Silva R. Extracellular ATP induces cell death in human intestinal epithelial cells. Biochim Biophys Acta. 2012;1820:1867-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Neves AR, Castelo-Branco MT, Figliuolo VR, Bernardazzi C, Buongusto F, Yoshimoto A, Nanini HF, Coutinho CM, Carneiro AJ, Coutinho-Silva R. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2014;20:444-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Figliuolo VR, Savio LEB, Safya H, Nanini H, Bernardazzi C, Abalo A, de Souza HSP, Kanellopoulos J, Bobé P, Coutinho CMLM. P2X7 receptor promotes intestinal inflammation in chemically induced colitis and triggers death of mucosal regulatory T cells. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Marques CC, Castelo-Branco MT, Pacheco RG, Buongusto F, do Rosário A Jr, Schanaider A, Coutinho-Silva R, de Souza HS. Prophylactic systemic P2X7 receptor blockade prevents experimental colitis. Biochim Biophys Acta. 2014;1842:65-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2776] [Article Influence: 198.3] [Reference Citation Analysis (0)] |
| 78. | Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 366] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 79. | de la Motte CA, Kessler SP. The role of hyaluronan in innate defense responses of the intestine. Int J Cell Biol. 2015;2015:481301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Vigetti D, Viola M, Karousou E, De Luca G, Passi A. Metabolic control of hyaluronan synthases. Matrix Biol. 2014;35:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 81. | Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest. 1998;101:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 226] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 82. | de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-alpha-trypsin inhibitor is crucial to structure and function. Am J Pathol. 2003;163:121-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 264] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 83. | Bandyopadhyay SK, de la Motte CA, Kessler SP, Hascall VC, Hill DR, Strong SA. Hyaluronan-mediated leukocyte adhesion and dextran sulfate sodium-induced colitis are attenuated in the absence of signal transducer and activator of transcription 1. Am J Pathol. 2008;173:1361-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer. 1997;71:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 85. | McBride WH, Bard JB. Hyaluronidase-sensitive halos around adherent cells. Their role in blocking lymphocyte-mediated cytolysis. J Exp Med. 1979;149:507-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Forrester JV, Balazs EA. Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology. 1980;40:435-446. [PubMed] |
| 87. | Nakamura K, Yokohama S, Yoneda M, Okamoto S, Tamaki Y, Ito T, Okada M, Aso K, Makino I. High, but not low, molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J Gastroenterol. 2004;39:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol. 2009;86:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 89. | Dahl IM, Husby G. Hyaluronic acid production in vitro by synovial lining cells from normal and rheumatoid joints. Ann Rheum Dis. 1985;44:647-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Miossec P, Dinarello CA, Ziff M. Interleukin-1 lymphocyte chemotactic activity in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1986;29:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 538] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 92. | Soroosh A, Albeiroti S, West GA, Willard B, Fiocchi C, de la Motte CA. Crohn’s Disease Fibroblasts Overproduce the Novel Protein KIAA1199 to Create Proinflammatory Hyaluronan Fragments. Cell Mol Gastroenterol Hepatol. 2016;2:358-368.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 93. | Togawa J, Nagase H, Tanaka K, Inamori M, Nakajima A, Ueno N, Saito T, Sekihara H. Oral administration of lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. J Gastroenterol Hepatol. 2002;17:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | MacManus CF, Collins CB, Nguyen TT, Alfano RW, Jedlicka P, de Zoeten EF. VEN-120, a Recombinant Human Lactoferrin, Promotes a Regulatory T Cell [Treg] Phenotype and Drives Resolution of Inflammation in Distinct Murine Models of Inflammatory Bowel Disease. J Crohns Colitis. 2017;11:1101-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 95. | de Jong NS, Leach ST, Day AS. Fecal S100A12: a novel noninvasive marker in children with Crohn’s disease. Inflamm Bowel Dis. 2006;12:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Kaiser T, Langhorst J, Wittkowski H, Becker K, Friedrich AW, Rueffer A, Dobos GJ, Roth J, Foell D. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 97. | Sidler MA, Leach ST, Day AS. Fecal S100A12 and fecal calprotectin as noninvasive markers for inflammatory bowel disease in children. Inflamm Bowel Dis. 2008;14:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 98. | Däbritz J, Langhorst J, Lügering A, Heidemann J, Mohr M, Wittkowski H, Krummenerl T, Foell D. Improving relapse prediction in inflammatory bowel disease by neutrophil-derived S100A12. Inflamm Bowel Dis. 2013;19:1130-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 99. | Leach ST, Yang Z, Messina I, Song C, Geczy CL, Cunningham AM, Day AS. Serum and mucosal S100 proteins, calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis in children with inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 100. | Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 101. | Manolakis AC, Kapsoritakis AN, Georgoulias P, Tzavara C, Valotassiou V, Kapsoritaki A, Potamianos SP. Moderate performance of serum S100A12, in distinguishing inflammatory bowel disease from irritable bowel syndrome. BMC Gastroenterol. 2010;10:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Peetermans WE, D’Haens GR, Ceuppens JL, Rutgeerts P, Geboes K. Mucosal expression by B7-positive cells of the 60-kilodalton heat-shock protein in inflammatory bowel disease. Gastroenterology. 1995;108:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Tomasello G, Sciumé C, Rappa F, Rodolico V, Zerilli M, Martorana A, Cicero G, De Luca R, Damiani P, Accardo FM. Hsp10, Hsp70, and Hsp90 immunohistochemical levels change in ulcerative colitis after therapy. Eur J Histochem. 2011;55:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 104. | Ludwig D, Stahl M, Ibrahim ET, Wenzel BE, Drabicki D, Wecke A, Fellermann K, Stange EF. Enhanced intestinal expression of heat shock protein 70 in patients with inflammatory bowel diseases. Dig Dis Sci. 1999;44:1440-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Rodolico V, Tomasello G, Zerilli M, Martorana A, Pitruzzella A, Gammazza AM, David S, Zummo G, Damiani P, Accomando S. Hsp60 and Hsp10 increase in colon mucosa of Crohn’s disease and ulcerative colitis. Cell Stress Chaperones. 2010;15:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 106. | Collins CB, Strassheim D, Aherne CM, Yeckes AR, Jedlicka P, de Zoeten EF. Targeted inhibition of heat shock protein 90 suppresses tumor necrosis factor-α and ameliorates murine intestinal inflammation. Inflamm Bowel Dis. 2014;20:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | Frol’ová L, Smetana K Jr, Borovská D, Kitanovicová A, Klimesová K, Janatková I, Malícková K, Lukás M, Drastich P, Benes Z, Tucková L, Manning JC, André S, Gabius HJ, Tlaskalová-Hogenová H. Detection of galectin-3 in patients with inflammatory bowel diseases: new serum marker of active forms of IBD? Inflamm Res. 2009;58:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Santucci L, Fiorucci S, Rubinstein N, Mencarelli A, Palazzetti B, Federici B, Rabinovich GA, Morelli A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;124:1381-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 109. | Paclik D, Berndt U, Guzy C, Dankof A, Danese S, Holzloehner P, Rosewicz S, Wiedenmann B, Wittig BM, Dignass AU. Galectin-2 induces apoptosis of lamina propria T lymphocytes and ameliorates acute and chronic experimental colitis in mice. J Mol Med (Berl). 2008;86:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Hokama A, Mizoguchi E, Sugimoto K, Shimomura Y, Tanaka Y, Yoshida M, Rietdijk ST, de Jong YP, Snapper SB, Terhorst C. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity. 2004;20:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 111. | Aung HT, Schroder K, Himes SR, Brion K, van Zuylen W, Trieu A, Suzuki H, Hayashizaki Y, Hume DA, Sweet MJ. LPS regulates proinflammatory gene expression in macrophages by altering histone deacetylase expression. FASEB J. 2006;20:1315-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 112. | McClure R, Massari P. TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Front Immunol. 2014;5:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 113. | Chiariotti L, Coretti L, Pero R, Lembo F. Epigenetic Alterations Induced by Bacterial Lipopolysaccharides. Adv Exp Med Biol. 2016;879:91-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183:1352-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 115. | Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 1087] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 116. | Netea MG, Latz E, Mills KH, O’Neill LA. Innate immune memory: a paradigm shift in understanding host defense. Nat Immunol. 2015;16:675-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 117. | Crisan TO, Netea MG, Joosten LA. Innate immune memory: Implications for host responses to damage-associated molecular patterns. Eur J Immunol. 2016;46:817-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 118. | Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1679] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 119. | Bekkering S, Joosten LA, van der Meer JW, Netea MG, Riksen NP. Trained innate immunity and atherosclerosis. Curr Opin Lipidol. 2013;24:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 120. | Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, Riksen NP. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol. 2014;34:1731-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 492] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 121. | Yang XF, Yin Y, Wang H. Vascular inflammation and atherogenesis are activated via receptors for PAMPs and suppressed by regulatory T cells. Drug Discov Today Ther Strateg. 2008;5:125-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 122. | Oberg HH, Juricke M, Kabelitz D, Wesch D. Regulation of T cell activation by TLR ligands. Eur J Cell Biol. 2011;90:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 123. | Cordiglieri C, Marolda R, Franzi S, Cappelletti C, Giardina C, Motta T, Baggi F, Bernasconi P, Mantegazza R, Cavalcante P. Innate immunity in myasthenia gravis thymus: pathogenic effects of Toll-like receptor 4 signaling on autoimmunity. J Autoimmun. 2014;52:74-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 124. | Pastrana JL, Sha X, Virtue A, Mai J, Cueto R, Lee IA, Wang H, Yang XF. Regulatory T cells and Atherosclerosis. J Clin Exp Cardiolog. 2012;2012:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 125. | Yang WY, Shao Y, Lopez-Pastrana J, Mai J, Wang H, Yang XF. Pathological conditions re-shape physiological Tregs into pathological Tregs. Burns Trauma. 2015;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 126. | Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1363] [Cited by in RCA: 1709] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 127. | Ostuni R, Piccolo V, Barozzi I, Polletti S, Termanini A, Bonifacio S, Curina A, Prosperini E, Ghisletti S, Natoli G. Latent enhancers activated by stimulation in differentiated cells. Cell. 2013;152:157-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 622] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 128. | Eichenfield DZ, Troutman TD, Link VM, Lam MT, Cho H, Gosselin D, Spann NJ, Lesch HP, Tao J, Muto J. Tissue damage drives co-localization of NF-κB, Smad3, and Nrf2 to direct Rev-erb sensitive wound repair in mouse macrophages. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 129. | Venkateswaran K, Verma A, Bhatt AN, Shrivastava A, Manda K, Raj HG, Prasad A, Len C, Parmar VS, Dwarakanath BS. Emerging Roles of Calreticulin in Cancer: Implications for Therapy. Curr Protein Pept Sci. 2018;19:344-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 130. | Lamb CA, Mansfield JC. Measurement of faecal calprotectin and lactoferrin in inflammatory bowel disease. Frontline Gastroenterol. 2011;2:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Vitali R, Stronati L, Negroni A, Di Nardo G, Pierdomenico M, del Giudice E, Rossi P, Cucchiara S. Fecal HMGB1 is a novel marker of intestinal mucosal inflammation in pediatric inflammatory bowel disease. Am J Gastroenterol. 2011;106:2029-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 132. | Palone F, Vitali R, Cucchiara S, Mennini M, Armuzzi A, Pugliese D, D’Incà R, Barberio B, Stronati L. Fecal HMGB1 Reveals Microscopic Inflammation in Adult and Pediatric Patients with Inflammatory Bowel Disease in Clinical and Endoscopic Remission. Inflamm Bowel Dis. 2016;22:2886-2893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 133. | Yamaguchi Y, Noda H, Okaniwa N, Adachi K, Shinmura T, Nakagawa S, Ebi M, Ogasawara N, Funaki Y, Zhuo L. Serum-Derived Hyaluronan-Associated Protein Is a Novel Biomarker for Inflammatory Bowel Diseases. Digestion. 2017;95:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Danese S, Fiocchi C, Panés J. Drug development in IBD: from novel target identification to early clinical trials. Gut. 2016;65:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 135. | Chan AC, Behrens TW. Personalizing medicine for autoimmune and inflammatory diseases. Nat Immunol. 2013;14:106-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Massaeli H, Jalali S, Viswanathan D, Mesaeli N. Loss of calreticulin function decreases NFκB activity by stabilizing IκB protein. Biochim Biophys Acta. 2014;1843:2385-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 137. | Maeda S, Hikiba Y, Shibata W, Ohmae T, Yanai A, Ogura K, Yamada S, Omata M. Essential roles of high-mobility group box 1 in the development of murine colitis and colitis-associated cancer. Biochem Biophys Res Commun. 2007;360:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 138. | Davé SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D, Sepulveda AR, Fink MP, Lotze MT, Plevy SE. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol. 2009;86:633-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 139. | Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1392] [Cited by in RCA: 1483] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 140. | Boyapati RK, Dorward DA, Tamborska A, Kalla R, Ventham NT, Doherty MK, Whitfield PD, Gray M, Loane J, Rossi AG. Mitochondrial DNA Is a Pro-Inflammatory Damage-Associated Molecular Pattern Released During Active IBD. Inflamm Bowel Dis. 2018;24:2113-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 141. | Sedhom MA, Pichery M, Murdoch JR, Foligné B, Ortega N, Normand S, Mertz K, Sanmugalingam D, Brault L, Grandjean T. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 142. | Eser A, Colombel JF, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M, Persson T, Reinisch W. Safety and Efficacy of an Oral Inhibitor of the Purinergic Receptor P2X7 in Adult Patients with Moderately to Severely Active Crohn’s Disease: A Randomized Placebo-controlled, Double-blind, Phase IIa Study. Inflamm Bowel Dis. 2015;21:2247-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 143. | Riehl TE, Santhanam S, Foster L, Ciorba M, Stenson WF. CD44 and TLR4 mediate hyaluronic acid regulation of Lgr5+ stem cell proliferation, crypt fission, and intestinal growth in postnatal and adult mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G874-G887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 144. | Kessler SP, Obery DR, Nickerson KP, Petrey AC, McDonald C, de la Motte CA. Multifunctional Role of 35 Kilodalton Hyaluronan in Promoting Defense of the Intestinal Epithelium. J Histochem Cytochem. 2018;66:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |