Published online Oct 28, 2018. doi: 10.3748/wjg.v24.i40.4554
Peer-review started: July 27, 2018
First decision: August 27, 2018
Revised: September 2, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 28, 2018
Processing time: 91 Days and 10.1 Hours
To investigate how natural killer (NK) cells are affected in the elimination of hepatitis C virus (HCV) by sofosbuvir/ledipasvir, two highly effective direct-acting antivirals (DAAs).
Thirteen treatment-naïve and treatment-experienced chronic hepatitis C (CHC) patients were treated with sofosbuvir/ledipasvir, and NK cells were detected at baseline, weeks 2, 4, 8 and 12 during therapy, and week post of treatment (Pt)-12 and 24 after the end of therapy by multicolor flow cytometry and compared with those from 13 healthy controls.
All patients achieved sustained virological response. There was a significant decline in CD56bright NK cell frequencies at week 8 (P = 0.002) and week 12 (P = 0.003), which were altered to the level comparable to healthy controls at week Pt-12, but no difference was observed in the frequency of CD56dim NK cells. Compared with healthy controls, the expression levels of NKG2A, NKp30 and CD94 on NK cells from CHC patients at baseline were higher. NKG2A, NKp30 and CD94 started to recover at week 12 and reached the levels similar to those of healthy controls at week Pt-12 or Pt-24. Before treatment, patients have higher interferon (IFN)-γ and perforin levels than healthy controls, and IFN-γ started to recover at week 8 and reached the normalized level at week Pt-12.
NK cells of CHC patients can be affected by DAAs, and phenotypes and function of NK cells recover not at early stage but mainly after the end of sofosbuvir/ledipasvir treatment.
Core tip: In our study, we observed the dynamic changes of natural killer (NK) cell subsets, phenotypes and functional parameters during and after direct-acting antivirals (DAAs) treatment and investigated the effect of sofosbuvir/ledipasvir therapy on innate immunity in genotype 1b hepatitis C virus (HCV)-infected patients. We illustrated that NK cells of chronic hepatitis C patients can be affected by DAAs and phenotypes and function of NK cells recovered not at early stage but mainly after the end of sofosbuvir/ledipasvir treatment. These findings may provide an explanation for HCV reinfection or liver carcinogenesis after HCV elimination.
- Citation: Wang XX, Luo BF, Jiang HJ, Cong X, Jin Q, Ma DL, Wei L, Feng B. Recovery of natural killer cells is mainly in post-treatment period in chronic hepatitis C patients treated with sofosbuvir plus ledipasvir. World J Gastroenterol 2018; 24(40): 4554-4564
- URL: https://www.wjgnet.com/1007-9327/full/v24/i40/4554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i40.4554
Chronic hepatitis C virus (HCV) infection is a disease that affects about 71 million people worldwide[1]. It can lead to mortality from hepatic as well as extra-hepatic causes[2]. In the era of HCV treatment with pegylated-interferon (PEG-IFN)/ribavirin (RBV), the sustained virological response (SVR) rate is different according to HCV genotypes: Approximately 40% to 50% in patients with genotype 1 and 80% in patients with genotype 2 or 3[3-5]. However, because of their higher SVR rate (> 95%) and less toxicity[6], IFN-free direct antiviral agents (DAAs) have replaced PEG-IFN/RBV as a first-line treatment option recommended by international guidelines[7,8].
IFN-α can induce immunomodulatory effects by acting the innate and adaptive immune systems of various cells[9,10]. Since IFN-free DAAs take the virus life cycle as a target, they can inhibit NS3 protease, NS5A replication complex or NS5B polymerase activity specifically[11]. These regimens can help us clarify the interaction between HCV clearance and the innate immune response, regardless of the IFNα induced immune modulation[12]. This provides a unique opportunity to analyze whether DAAs can change natural killer (NK) cell activation when HCV replication is inhibited. Previous studies have explored the effect of IFN therapy on the NK cells. It has been shown that chronic hepatitis C (CHC) patients with SVR to IFN therapy exhibited greater levels of NK cell degranulation and enhanced NK cytotoxicity[13,14]. Combination therapy of PEG-IFN-α/RBV reversed NK subtype distribution and function in HCV-eliminated patients[15]. Until now, only few studies have reported the effect of DAAs on NK cells[16-18], in which the results and conclusions were controversial.
NK cells are enriched among lymphocytes in the blood (5%-20%), and their percentage increases further in viral hepatitis[19]. NK cells play an important role in the antiviral immune defense and undergo great changes in subsets, phenotypes and function during persistent viral infection[20]. NK cells can be divided into three subgroups according to the expression levels of CD56 and CD16, including CD56bright NK cells, CD56dim NK cells and CD56neg (CD16positive) NK cells[21]. CD56bright NK cells, which can produce IFN-γ mainly and inhibit viral replication, are the less-mature subset that can differentiate into CD56dim NK cells[22]. CD56dim NK cells are cytotoxic NK cell subset expressing higher levels of killer immunoglobulin-like receptors (KIR), CD16 and perforin[21]. CD56neg NK cells express lower perforin and exhibit lower cytotoxicity[23].
The function of NK cells is regulated by interaction of NK cell receptors (NKRs), which can be divided into activating and inhibitory NKRs, and their respective ligands[24]. Activating NKRs include NKG2C, NKG2D and the “natural cytotoxicity receptors”, for example, NKp30, NKp44 and NKp46. Inhibitory receptors comprise NKG2A and the KIR family members[25]. During viral infection, the balance shifts from inhibition to activation because the threshold value of activation receptors exceeds that of inhibition[26]. For example, the integration of all signals results in activation of blood and liver NK cells in HCV infection[27] and altered functional phenotypes with increased cytotoxicity and decreased antiviral cytokine production[27,28].
Up to now, more and more DAAs have been approved for clinical practice. In China, sofosbuvir/ledipasvir have been increasingly used among CHC patients, especially those with genotype 1 HCV infection. Sofosbuvir is an NS5B polymerase inhibitor and ledipasvir is an NS5A replication complex inhibitor. In the current study, we aimed to observe the dynamic changes of NK cell subsets, phenotypes and functional parameters during and after DAAs treatment, and to investigate the effect of DAAs (sofosbuvir/ledipasvir) treatment on innate immunity in genotype 1b HCV-infected patients.
NK cells were studied in 13 genotype 1b HCV infected patients at baseline, weeks 2, 4, 8 and 12 in a 12-wk treatment course with DAAs (90 mg ledipasvir once daily and 400 mg sofosbuvir once daily), and then at week post of treatment (Pt)-12 and 24 after the end of therapy. There are six treatment-experienced patients who relapsed after treatment with PEG-IFN/RBV and seven treatment-naïve patients in our study. Thirteen age- and sex-matched uninfected subjects were enrolled for comparison. Informed consent was obtained from all participants. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Peking University People’s Hospital. Patients had no signs or evidence of coinfection with hepatitis A virus, hepatitis B virus, hepatitis D virus, hepatitis E virus and human immunodeficiency virus. Patients with evidence of hepatocellular carcinoma or cirrhosis were excluded. Besides, pregnant patients or patients with psychiatric disorders were also excluded.
Serum HCV RNA level was quantitated using the Cobas TaqMan automated real-time PCR platform reaction (Roche Molecular System, Pleasanton, CA, United States) with a lowest limit of detection of 15 IU/mL and a lower limit of quantification of 43 IU/mL.
Peripheral blood mononuclear cells (PBMCs) were separated from EDTA- anticoagulated blood on Ficoll Histopaque (GE Healthcare Bio-Science AB, Germany) density gradients, washed three times with phosphate-buffered saline (BD, Bioscience, Franklin Lakes, NJ, United States)[17], cryopreserved at -80 °C and transferred to the liquid nitrogen after 24 h.
For each patient, cryopreserved PBMCs from week 0 to week Pt-24 were thawed and tested. PBMCs of healthy donors were included in this experiment. Thawed PBMCs were stained with anti-CD45-APC-H7, anti-CD3-PerCP-Cy5.5, anti-CD56-APC, anti-CD16-BV510, anti-CD94-PE, anti-CD335 (NKp46) -PE-Cy7, anti-CD336 (NKp44) -BB515, anti-CD337 (NKp30) - BV421 (BD Bioscience), anti-CD314 (NKG2D)-PE-Cy7 (Biolegend), anti-CD159a (NKG2A)-PE (R and D Systems) and anti-CD159C (NKG2C)-VioBright™FITC (Miltenyi Biotech, Bergisch Gladbach, Germany) for 15 min and with 7-AAD (BD Bioscience) for 10 min before being detected by flow cytometry (BD FACSAria II, BD Bioscience). Data were analyzed with BD FACSDiva Software v7.0.
Thawed PBMCs were incubated with Leukocyte Activation Cocktail with BD GolgiPlug (BD Bioscience) 2 μL/1 × 106 cells for 4 h. Cells were washed, fixed, and permeabilized with the BD IntraSure™ Kit and stained with anti-IFN-γ-FITC, anti-Perforin-BV421 and anti-Granzyme B-PE-CF594 (all from BD Biosciences) for 30 min before being detected by flow cytometry (BD FACSAria II, BD Bioscience). Data were analyzed with BD FACSDiva Software v7.0.
Statistical analyses were performed using Graphpad Prism Version 5.0a (Graphpad Software Inc., San Diego, CA, United States) and SPSS 16.0 (SPSS, Chicago, IL, United States). Normal distribution was tested by the Kolmogorov-Smirnov test. Values in our study were not normally distributed, and comparisons of expression levels among different time points in CHC patients were performed by the Wilcoxon matched pairs test. Comparisons of expression levels between the CHC patients and healthy controls were performed by the Mann-Whitney test. Two-sided P-values less than 0.05 were considered significant.
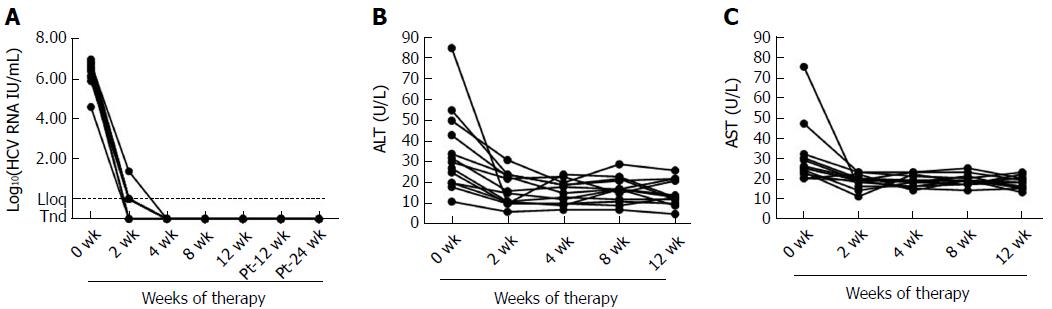
Table 1 describes the main demographical and clinical characteristics of the CHC patients at baseline. The median log HCV RNA level was 6.39 (range, 4.60-6.98). The median alanine aminotransferase (ALT) level was 34 U/L (range, 11-55 U/L). All 13 patients were treated with sofosbuvir/ledipasvir within 12 wk and reached SVR24. Treatment with DAAs induced rapid and early clearance of serum HCV RNA within the first 2 wk, accompanied by a significant reduction in liver inflammation as demonstrated by a decrease in ALT (P = 0.007) and AST levels (P = 0.015) (Figure 1).
| Sex (F/M) | Age (yr) | BMI (kg/m2) | Fibroscan index | Treatment-naïve /experienced | ALT/AST(U/L)-baseline | ALT/AST(U/L)-week 2 | Log HCV RNA | Response to sofosbuvir/ledipasvir |
| F | 27 | 18.71 | 3.8 | N | 25/25 | 10/19 | 6.16 | SVR24 |
| M | 62 | 15.85 | 6.5 | N | 55/47 | 24/23 | 6.09 | SVR24 |
| F | 30 | 20.07 | 5.9 | N | 18/20 | 10/19 | 6.38 | SVR24 |
| F | 53 | 20.10 | 3.9 | N | 11/25 | 6/19 | 6.98 | SVR24 |
| M | 25 | 24.39 | 6.1 | N | 43/22 | 23/18 | 4.60 | SVR24 |
| F | 27 | 19.38 | 7.9 | N | 20/20 | 11/22 | 6.87 | SVR24 |
| F | 29 | 26.67 | 6.4 | N | 32/24 | 16/14 | 5.90 | SVR24 |
| M | 24 | 29.33 | 7.6 | E | 85/75 | 10/16 | 6.45 | SVR24 |
| M | 63 | 25.10 | 4.8 | E | 50/32 | 31/23 | 6.51 | SVR24 |
| M | 55 | 23.62 | 4.5 | E | 34/30 | 24/20 | 6.56 | SVR24 |
| F | 57 | 22.31 | 4.7 | E | 20/26 | 15/20 | 6.76 | SVR24 |
| F | 63 | 26.75 | 4.3 | E | 27/29 | 11/19 | 6.76 | SVR24 |
| M | 25 | 26.53 | 6.0 | E | 30/23 | 11/22 | 6.60 | SVR24 |
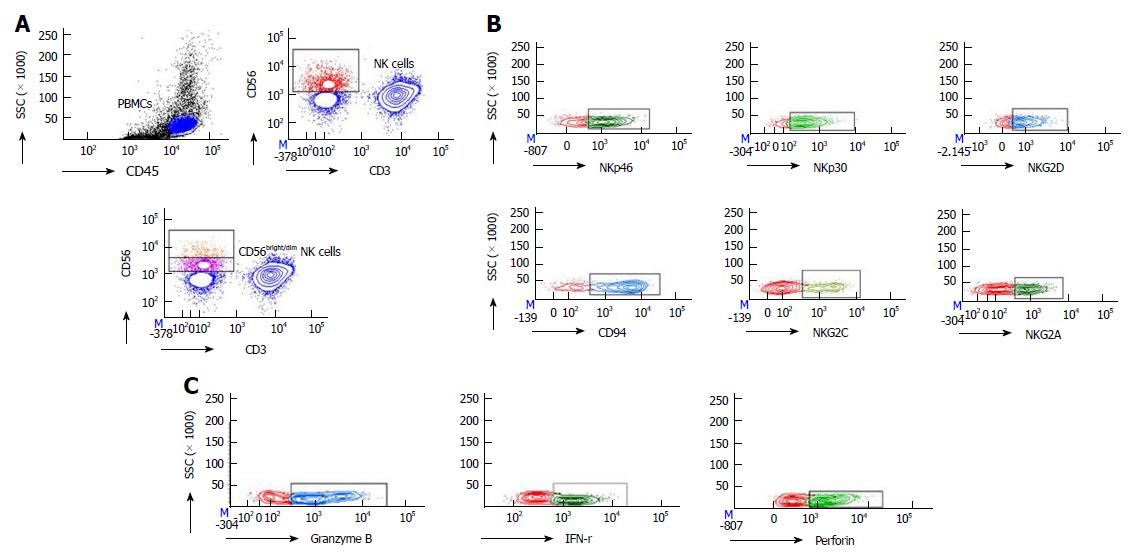
NK cells were identified as CD3-CD56+ cells in the PBMC population, and CD56bright NK cells and CD56dim NK cells were determined by sequential gating on CD3-CD56+ NK cells (Figure 2A).
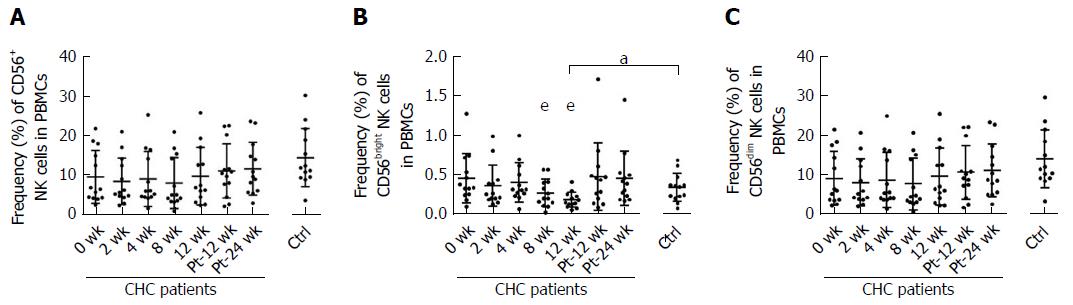
Our study showed that during the 12 wk of IFN-free DAAs therapy, there was a significant decline in CD56bright NK cell frequencies at week 8 (P = 0.002) and week 12 (P = 0.003), which were lower than that of healthy controls at week 12. The frequency of CD56bright NK cells was altered to the level comparable to that of healthy controls at week Pt-12 (Figure 3B). There was no difference in the frequency of CD56+ NK cells or CD56dim NK cells between chronically HCV-infected patients and healthy controls at baseline. No difference was found in the frequency of CD56+ NK cells or CD56dim NK cells among different time points during and after DAAs therapy (Figure 3A and C).
To illustrate the effect of the rapid DAA-mediated decrease in HCV RNA levels on NK cell phenotypes, we detected activating and inhibitory receptors on the surface of NK cells by multicolor flow cytometry (Figure 2B).
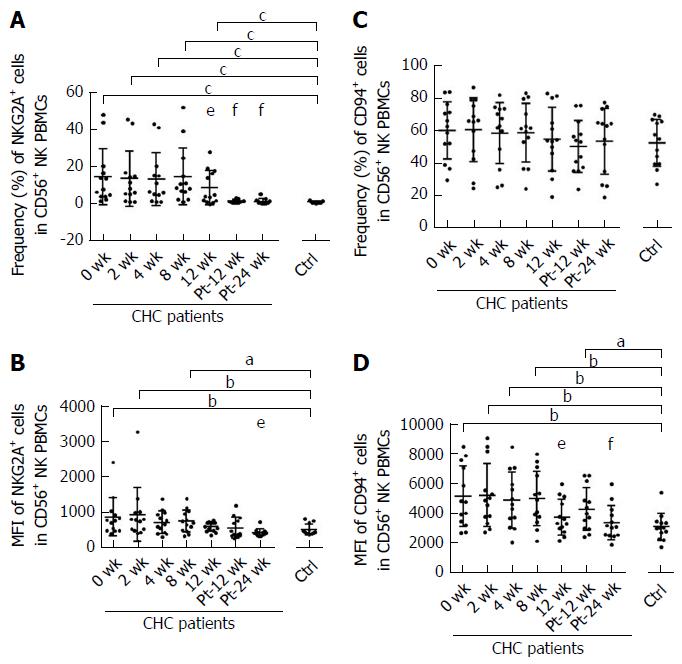
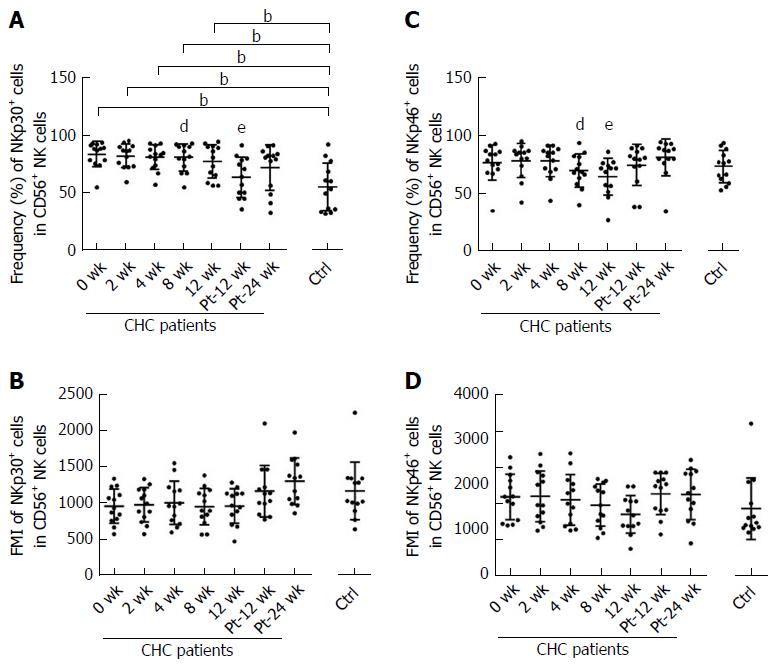
Compared with uninfected healthy controls, the frequencies of the inhibitory NKG2A and activating NKp30 on NK cells from CHC patients were higher at baseline (P < 0.001) (Figures 4A and 5A). The frequency and mean fluorescence intensity (MFI) of NKG2A and the frequency of NKp30 started to decline at week 12 of treatment and reached the levels similar to those of NK cells from healthy controls at week Pt-12 (Figure 4A and B, Figure 5A). However, MFI of NKp30 did not differ on NK cells from CHC patients and healthy controls and did not change during and after the end of sofosbuvir/ledipasvir therapy (Figure 5B).
There was no difference in the frequency of CD94+ NK cells between CHC patients at baseline and healthy controls, and the frequency of CD94+NK cells from CHC patients did not change significantly during and after sofosbuvir/ledipasvir therapy (Figure 4C). The MFI of CD94 on NK cells started to decline in CHC patients at week 12 of treatment and normalized at week Pt-24 (Figure 4D).
There was no difference in the frequency or MFI of NKp46 on NK cells between healthy controls and CHC patients at baseline. During DAAs treatment, there was a significant decline in the frequency of NKp46 on NK cells at week 8 and week 12, but NKp46 expression levels increased to those of uninfected controls at week Pt-12 and week Pt-24 (Figure 5C and D).
Frequencies and MFI of NKp44, NKG2C and NKG2D on NK cells from CHC patients at baseline did not differ from those from healthy controls and did not change during and after sofosbuvir/ledipasvir therapy.
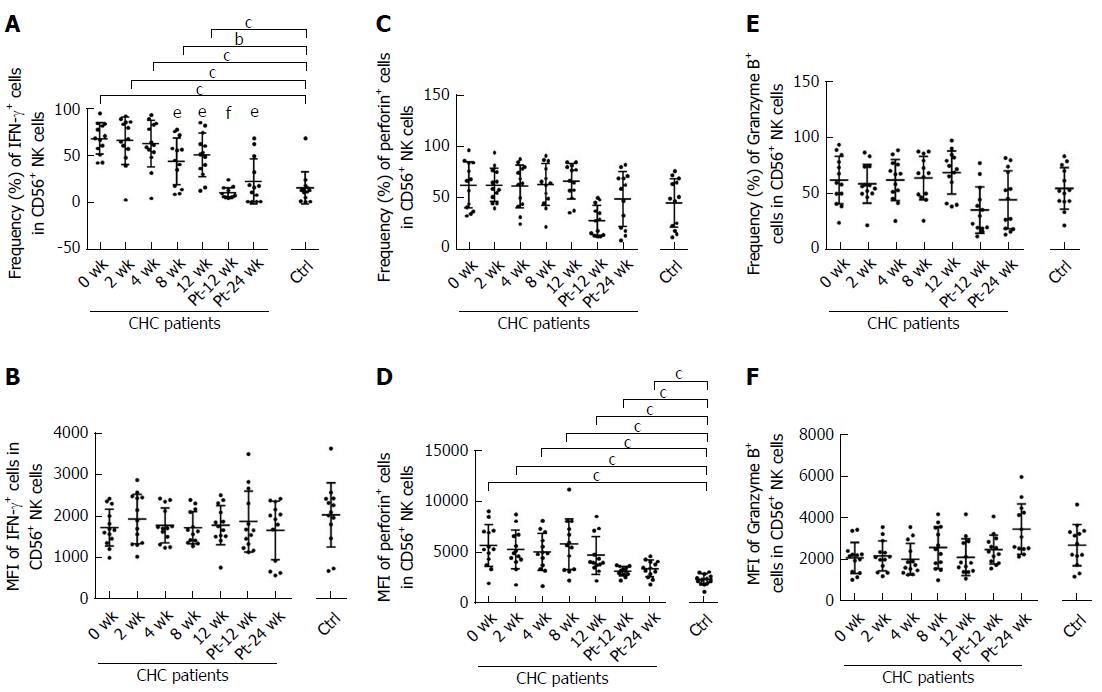
The frequency and MFI of IFN-γ+ NK cells from CHC patients at baseline were significantly higher than those from uninfected subjects (P < 0.001). Frequency of IFN-r+ NK cells started to decline in CHC patients at week 8 of treatment and reached the level similar to that of healthy controls at week Pt-12 (Figure 6A). However, no difference was shown in MFI of IFN-γ+ NK cells from CHC patients during and after DAAs therapy (Figure 6B). There was no difference in the frequency of perforin+ NK cells between healthy controls and CHC patients at each time point (Figure 6C). MFI of perforin+ NK cells from CHC patients was significantly higher than that from uninfected subjects from week 0 to week Pt-24W persistently (Figure 6D). However, there was no difference in the frequency (Figure 6E) or MFI (Figure 6F) of Granzyme B of NK cell groups between CHC patients at baseline and healthy controls, and the expression of NKp46 did not change during and after sofosbuvir/ledipasvir therapy.
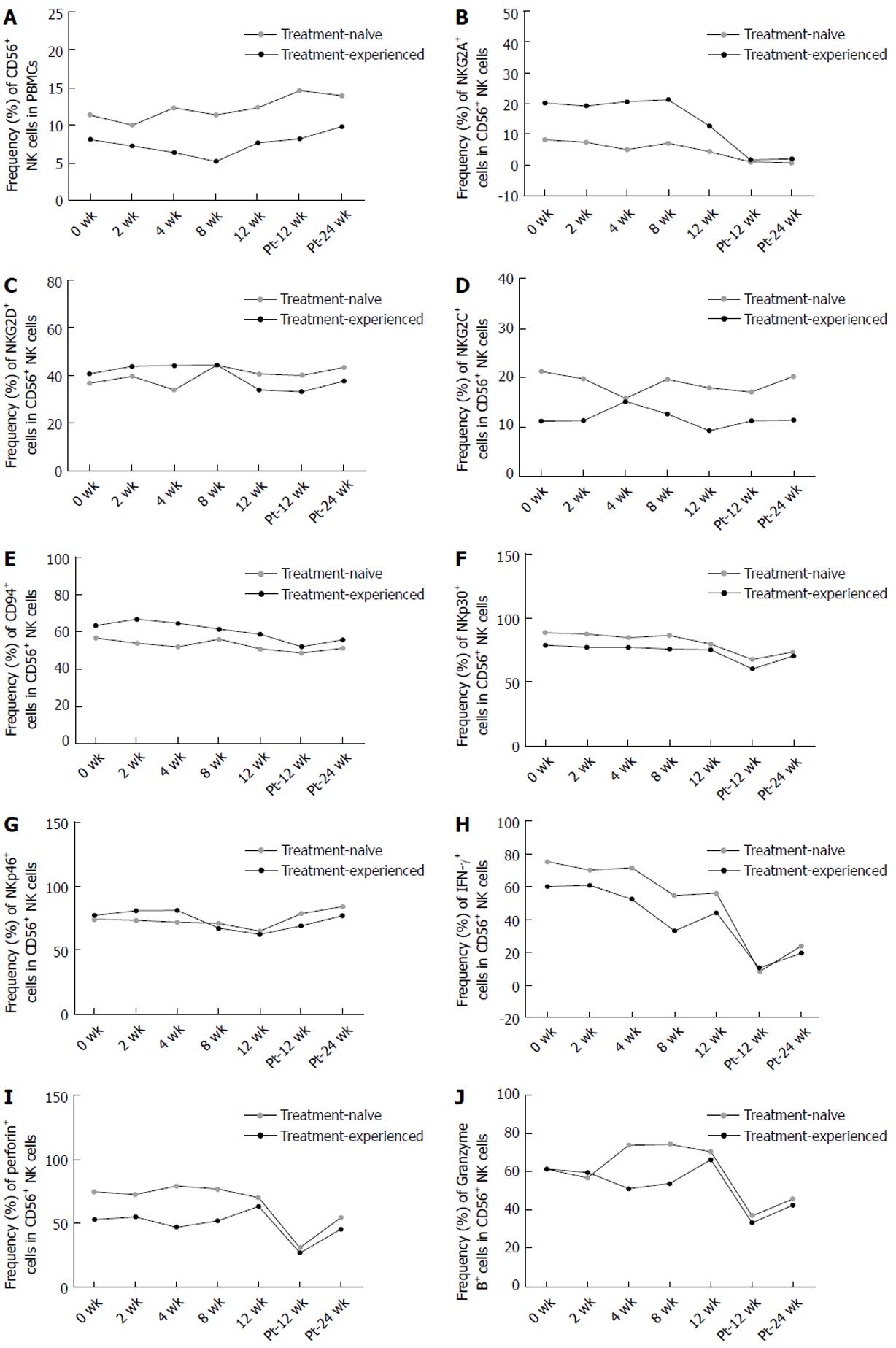
There was no difference in the frequency of CD56+ NK cells or the expression of NKG2A, NKG2D, NKG2C, CD94, NKp30, NKp46, IFN-γ, perforin and Granzyme B between treatment-naïve and treatment-experienced CHC patients during and after DAAs treatment. The changing trends of these phenotypes and cytokines of NK cells between these two groups were similar. (Figure 7A-J)
In our study, we evaluated the innate immune effects in 13 CHC patients successfully treated with sofosbuvir/ledipasvir. All patients achieved SVR12 and SVR24, and no patients had virologic breakthrough.
The development of DAAs has opened a new era of HCV treatment[29]. IFN-free regimens for HCV infection provide a unique opportunity to study the interaction between HCV and the immune system because DAAs rapidly decrease viremia to undetectable levels regardless of the IFNα induced immune modulation[12]. Due to the direct effect of IFN-α on NK cells, the consequence of viral load decline on NK cells could not be examined precisely. Our study examined the innate immune effects of sofosbuvir/ledipasvir therapy induced HCV RNA level decline. There was no difference in the frequency of total NK cells from CHC patients at baseline and healthy controls, which is inconsistent with other two studies assuming that NK cell frequency decreases in the blood in chronic HCV infection[28,30]. We found that only CD56bright NK cells showed a slightly decline at week 8 and week 12 during DAAs treatment and normalized at week Pt-12. There was no change in total NK cells or CD56dim NK cells. However, another study reported that DAA therapy enhances the frequency of CD56dim and decreases CD56bright cells in chronically HCV-infected patients[30].
As for NK cell phenotypes and function of CHC patients, we showed that reduced HCV RNA load altered NK cell phenotype and function, including NKG2A, CD94, NKp30, NKp46, IFN-γ and perforin. Most of these phenotypes and cytokines became to change at week 12 approximately and normalized to the levels of healthy controls at week Pt-12 or Pt-24, which are different from previous studies[17,30]. Serti et al[17] demonstrated that the expression levels of NKp46 and NKG2A normalized in patients with undetectable viremia by week 8 in daclatasvir (DCV) and asunaprevir (ASV) therapy, and they assumed that the percentage of IFN-γ producing NK cells and the IFN-γ expression level were significantly lower in chronically HCV-infected patients compared with healthy controls and increased within the first 8 wk. Spaan et al[30] illustrated that NK cell phenotype is already normalized at week 12 during DCV/ASV therapy, and they assumed that DAAs treatment did not alter the frequency of NK cells producing perforin. However, we found that the MFI of perforin+ NK cells was higher than that of healthy controls before, during and after DAAs therapy persistently, which has not been found in previous studies. Importantly, researchers in these two studies have not demonstrated the changes of NK cells after the end of DAAs therapy.
Patients in other two studies were treated with DCV/ASV and all patients were HCV-infected non-responders to previous PEG-IFN/RBV therapy[17,30]. However, patients in our study were treatment-naïve CHC patients and treatment-experienced CHC patients who had a relapse after PEG-IFN/RBV therapy. NK cells might be affected by PEG-IFN/RBV therapy[13,14]. Combination therapy of PEG-IFN-α/RBV reversed NK subtype distribution and functions in HCV-eliminated patients[15]. However, we found that there was no difference in the changes of the NK cells during and after DAAs treatment between patients who were treatment-naïve and those who had a relapse after PEG-IFN/RBV therapy. And the effect of non-response to PEG-IFN/RBV treatment on the changes of NK cells during and after DAA treatment should not be excluded. Because it is unclear whether the response pattern to PEG-IFN/RBV treatment can affect the subsequent changes of NK cells induced by DAAs in treatment-experienced patients. Second, HCV genotype may affect dynamic changes of NK cells during DAAs therapy. In a study of Ning et al[31] in which patients with different genotypes were included, they assumed that different HCV genotypes may have an impact on their results. After treatment with sofosbuvir/ledipasvir or sofosbuvir/daclatasvir, the frequency of CD16+CD56+ NK cells gradually increased to normal levels of healthy controls at week 12. Third, different DAAs may have different impact on NK cells. Sofosbuvir is an NS5B polymerase inhibitor, ledipasvir and DCV are NS5A replication complex inhibitor and ASV is an NS3 protease inhibitor. As Ning et al[31] described, we cannot exclude the possibility that different DAA regimens induce different dynamic changes of NK cells. Fourth, the race of CHC patients may be an important factor. Whether in era of PEG-IFN/RBV or DAAs treatment, race is one of the most important factors which can affect SVR[32,33]. Therefore, we cannot rule out the distinction induced by different races between our studies and others. Additionally, we assumed that it may take a long term for NK cell recovery and NK cells cannot recover immediately after the influence from DAAs was relieved.
We confirm earlier studies on increased inhibitory NKG2A expression on NK cells in HCV infection[18,27]. NKG2A is a major and prominent inhibitory NK cell receptor and is known as lectin superfamily group A[34]. DAA-induced NKG2A level reduction might be the consequence of compensatory mechanisms exerted upon declining activating signals. Besides, CD94 is mainly expressed as a heterodimer with NKG2A, NKG2B or NKG2C protein[35]. The inhibitory signals of NK cells are mainly mediated by HLA class I-binding receptors, including KIRs and CD94/NKG2A[36]. Accordingly, there are something in common of the changing trends between NKG2A and CD94 MFI in our study.
Frequency of NKp30+ NK cells at baseline in our cohort was higher than those of healthy controls. During DAAs treatment, the percentage of NKp30+ NK cells was reduced to the level of healthy controls, which is consistent with the studies of Serti et al[17] and Spaan et al[30], but there was no difference in the MFI of NKp30.
In all changed phenotypes, NKp46 is a special one. The changing trend of NKp46 is completely different from that of other receptors during sofosbuvir/ledipasvir treatment. There was a significant decline only at week 8 and week 12. As we all know, NKp46, a member of the natural cytotoxicity receptor family, is a main activating NK-cell receptor[37]. NK cells with high expression of NKp46 were characterized by a high functional capacity (e.g., high cytotoxicity and IFN-γ production) and a high antiviral activity in vitro[38,39]. However, the percentage of IFN-γ+ NK cells was similar to the level of healthy controls at week Pt-12, and this level was maintained until week Pt-24.
The effect of HCV infection on NKG2D was controversial. Oliviero et al[28] reported that CHC patients have increased NKG2D expression, while Dessouki et al[15] demonstrated that the frequency of NKG2D+ NK cells decreased in HCV infection. In our study, the expression of NKp44, NKG2C, NKG2D and Granzyme B did not differ between NK cells from CHC patients at baseline and healthy donors in our study, and there was no change in the expression during and after the end of treatment (EOT) of DAAs, which is consistent with the published articles[19,40].
Our results showed that NK cells phenotypes and function started to change in the later period of sofosbuvir/ledipasvir treatment and reversed to the normalized level of healthy individuals mainly after EOT. What we found in our research is different from previous studies which assumed that HCV clearance induced by DAAs can mediate NK recovery rapidly. Whether dynamic changes of NK cells in DAA-treated patients are related to HCV reinfection or liver carcinogenesis after HCV elimination is a great topic in the future.
Chronic hepatitis C virus (HCV) infection can lead to mortality from hepatic as well as extra-hepatic causes. Until now, direct-acting antivirals (DAAs) have replaced pegylated-interferon (PEG-IFN)/ribavirin as a first-line treatment option. IFN-free DAAs take the virus life cycle as a target and can help us clarify the interaction between HCV clearance and the innate immune response, regardless of the IFN-α induced immune modulation. Previous studies showed that PEG-IFN-α can change natural killer (NK) cell subtype distribution and function in HCV-eliminated patients. However, it is controversial whether DAAs can change the phenotypes and function of NK cells.
More and more DAAs have been approved for clinical practice. In China, sofosbuvir/ledipasvir have been increasingly used among chronic hepatitis C (CHC) patients, especially those with genotype 1 HCV infection. Previous studies illustrated that NK cells play an important role in the antiviral immune defense and undergo great changes in subsets, phenotype and function during persistent viral infections. Therefore, it is meaningful to investigate how NK cells are affected in the elimination of HCV by sofosbuvir/ledipasvir.
The objectives of this study are to observe the dynamic changes of NK cell subsets, phenotypes and functional parameters during and after DAAs treatment, and investigate the effect of DAAs (sofosbuvir/ledipasvir) treatment on innate immunity in genotype 1b HCV-infected patients.
Thirteen treatment-naïve and treatment-experienced CHC patients were treated with sofosbuvir/ledipasvir, and NK cells were detected at baseline, week 2 to 12 during therapy, and week post of treatment (Pt)-12 and 24 after the end of therapy by multicolor flow cytometry and compared with 13 healthy controls.
There was a significant decline in CD56bright NK cell frequencies at week 8 (P = 0.002) and week 12 (P = 0.003), which were altered to the level comparable to that of healthy controls at week Pt-12, but there was no difference in the frequency of CD56dim NK cells. Compared with healthy controls, the expression levels of NKG2A, NKp30 and CD94 on NK cells from CHC patients at baseline were higher. NKG2A, NKp30 and CD94 started to recover at week 12 and reached the levels similar to those of healthy controls at week Pt-12 or Pt-24. Before treatment, patients had higher IFN-γ and perforin levels than healthy controls, and IFN-γ started to recover at week 8 and reached the normalized level at week Pt-12.
NK cells of CHC patients can be affected by DAAs, and NK cell phenotypes and function started to change at the later period of sofosbuvir/ledipasvir treatment and reversed to the normalized level of healthy individuals mainly after end of treatment. What we found in our research is different from previous studies which assumed that HCV clearance induced by DAAs can mediate NK recovery rapidly.
In hepatitis B virus (HBV)/HCV coinfected patients, HBV reactivation often occurred at the later period or even after the end of DAAs treatment. Our study may provide an explanation for this observation. Whether dynamic changes of NK cells in DAA-treated patients are related to HCV reinfection or liver carcinogenesis after HCV elimination is a great topic in the future.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pellicano R, Rezaee-Zavareh MS, Said ZN S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Bian YN
| 1. | World Health Organization. Hepatitis C. Available on October 13, 2017. Updated July. 2017; Available from: URL: http://www.who.int/mediacentre/factsheets/fs164/en/. |
| 2. | Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ; R. E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 3. | Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr, Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM; PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2109] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 4. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 5. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 6. | Chu PS, Nakamoto N, Taniki N, Ojiro K, Amiya T, Makita Y, Murata H, Yamaguchi A, Shiba S, Miyake R. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS One. 2017;12:e0179096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 910] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 8. | Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, Lesmana CR, Sollano J, Kumar M, Jindal A. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016;10:702-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | García-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in détente. Science. 2006;312:879-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 711] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 10. | Markova AA, Mihm U, Schlaphoff V, Lunemann S, Filmann N, Bremer B, Berg T, Sarrazin C, Zeuzem S, Manns MP. PEG-IFN alpha but not ribavirin alters NK cell phenotype and function in patients with chronic hepatitis C. PLoS One. 2014;9:e94512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 12. | Lin JC, Habersetzer F, Rodriguez-Torres M, Afdhal N, Lawitz EJ, Paulson MS, Zhu Y, Subramanian GM, McHutchison JG, Sulkowski M. Interferon γ-induced protein 10 kinetics in treatment-naive versus treatment-experienced patients receiving interferon-free therapy for hepatitis C virus infection: implications for the innate immune response. J Infect Dis. 2014;210:1881-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Edlich B, Ahlenstiel G, Zabaleta Azpiroz A, Stoltzfus J, Noureddin M, Serti E, Feld JJ, Liang TJ, Rotman Y, Rehermann B. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C patients. Hepatology. 2012;55:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Ahlenstiel G, Edlich B, Hogdal LJ, Rotman Y, Noureddin M, Feld JJ, Holz LE, Titerence RH, Liang TJ, Rehermann B. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231-1239, 1239.e1-1239.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Dessouki O, Kamiya Y, Nagahama H, Tanaka M, Suzu S, Sasaki Y, Okada S. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: Reversion by anti-viral treatment. Biochem Biophys Res Commun. 2010;393:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Meissner EG, Kohli A, Virtaneva K, Sturdevant D, Martens C, Porcella SF, McHutchison JG, Masur H, Kottilil S. Achieving sustained virologic response after interferon-free hepatitis C virus treatment correlates with hepatic interferon gene expression changes independent of cirrhosis. J Viral Hepat. 2016;23:496-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, Ghany M, Rehermann B. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology. 2015;149:190-200.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 18. | Serti E, Park H, Keane M, O’Keefe AC, Rivera E, Liang TJ, Ghany M, Rehermann B. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNα. Gut. 2017;66:724-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 284] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Pollmann J, Rölle A, Hofmann M, Cerwenka A. Hepatitis C Virus and Human Cytomegalovirus-Natural Killer Cell Subsets in Persistent Viral Infections. Front Immunol. 2017;8:566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Lunemann S, Schlaphoff V, Cornberg M, Wedemeyer H. NK cells in hepatitis C: role in disease susceptibility and therapy. Dig Dis. 2012;30 Suppl 1:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Saha B, Szabo G. Innate immune cell networking in hepatitis C virus infection. J Leukoc Biol. 2014;96:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Tatsumi T, Takehara T. Impact of natural killer cells on chronic hepatitis C and hepatocellular carcinoma. Hepatol Res. 2016;46:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2049] [Cited by in RCA: 2195] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 25. | Kokordelis P, Krämer B, Körner C, Boesecke C, Voigt E, Ingiliz P, Glässner A, Eisenhardt M, Wolter F, Kaczmarek D. An effective interferon-gamma-mediated inhibition of hepatitis C virus replication by natural killer cells is associated with spontaneous clearance of acute hepatitis C in human immunodeficiency virus-positive patients. Hepatology. 2014;59:814-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: facts and controversies. Eur J Clin Invest. 2010;40:851-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325-335.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151-1160, 1160.e1-1160.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 29. | Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 336] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 30. | Spaan M, van Oord G, Kreefft K, Hou J, Hansen BE, Janssen HL, de Knegt RJ, Boonstra A. Immunological Analysis During Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. J Infect Dis. 2016;213:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Ning G, Li YT, Chen YM, Zhang Y, Zeng YF, Lin CS. Dynamic Changes of the Frequency of Classic and Inflammatory Monocytes Subsets and Natural Killer Cells in Chronic Hepatitis C Patients Treated by Direct-Acting Antiviral Agents. Can J Gastroenterol Hepatol. 2017;2017:3612403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Yan KK, Guirgis M, Dinh T, George J, Dev A, Lee A, Zekry A. Treatment responses in Asians and Caucasians with chronic hepatitis C infection. World J Gastroenterol. 2008;14:3416-3420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Su F, Green PK, Berry K, Ioannou GN. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology. 2017;65:426-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187:813-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 549] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 35. | Borrego F, Masilamani M, Marusina AI, Tang X, Coligan JE. The CD94/NKG2 family of receptors: from molecules and cells to clinical relevance. Immunol Res. 2006;35:263-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Enqvist M, Nilsonne G, Hammarfjord O, Wallin RP, Björkström NK, Björnstedt M, Hjerpe A, Ljunggren HG, Dobra K, Malmberg KJ. Selenite induces posttranscriptional blockade of HLA-E expression and sensitizes tumor cells to CD94/NKG2A-positive NK cells. J Immunol. 2011;187:3546-3554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 38. | Krämer B, Körner C, Kebschull M, Glässner A, Eisenhardt M, Nischalke HD, Alexander M, Sauerbruch T, Spengler U, Nattermann J. Natural killer p46High expression defines a natural killer cell subset that is potentially involved in control of hepatitis C virus replication and modulation of liver fibrosis. Hepatology. 2012;56:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Golden-Mason L, Stone AE, Bambha KM, Cheng L, Rosen HR. Race- and gender-related variation in natural killer p46 expression associated with differential anti-hepatitis C virus immunity. Hepatology. 2012;56:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |