Published online Oct 28, 2018. doi: 10.3748/wjg.v24.i40.4527
Peer-review started: August 22, 2018
First decision: August 30, 2018
Revised: September 11, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 28, 2018
Processing time: 66 Days and 24 Hours
Hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT) is a disease that is not uncommon, but the treatments vary drastically between Eastern and Western countries. In Europe and America, the first line of treatment is systemic therapy such as sorafenib and the surgical treatment is not a recommend option. While an increasing number of studies from China and Japan have suggested that surgical treatment results in better outcomes when compared to transcatheter arterial chemoembolization (TACE), sorafenib, or other nonsurgical treatments, and two classification systems, Japanese Vp classification and Chinese Cheng’s classification, were very useful to guide the surgical treatment. We have also found that surgical treatment may be more effective, as we have performed surgical treatment for HCC-PVTT patients over a period of approximately 15 years and achieved good results with the longest surviving time being 13 years and onward. In this study, we review the efficacy and principles of current surgical treatments and introduce our new, more effective surgical technique named “thrombectomy first”, which means the tumor thrombus in the main portal vein, the bifurcation or the contralateral portal vein should be removed prior to liver resection. Thus, compression and crushing of PVTT during the operation could be avoided and new intrahepatic metastases caused by tumor thrombus to the remnant liver minimized. The new technique is even beneficial to the prognosis of Cheng’s classification Types III and IV PVTT. The vital tips and tricks for the surgical approach are described.
Core tip: The treatments for hepatocellular carcinoma with portal vein tumor thrombus between Eastern and Western countries vary drastically. In Western countries, the first-line treatment is systemic therapy such as sorafenib, while studies from China and Japan suggest that surgical treatment results in better outcomes. We review the efficacy and principles of current surgical treatments and introduce our new, more effective surgical technique named “thrombectomy first”, which means the tumor thrombus would be removed prior to liver resection. We have performed this technique over approximately 15 years and achieved good results with the longest surviving time being 13 years and onward.
- Citation: Peng SY, Wang XA, Huang CY, Li JT, Hong DF, Wang YF, Xu B. Better surgical treatment method for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol 2018; 24(40): 4527-4535
- URL: https://www.wjgnet.com/1007-9327/full/v24/i40/4527.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i40.4527
Portal vein tumor thrombus (PVTT) is not rare, but it is correlated with an extremely poor prognosis, with approximately 40% of patients with hepatocellular carcinoma (HCC) having PVTT when diagnosed[1]. HCC with PVTT is recognized as an advanced stage disease (BCLC-C) according to the Barcelona Clinic for Liver Cancer (BCLC) staging system[2]. Surgical resection is not a recommended treatment option; the only proposed treatment option is systemic therapy. The tyrosine kinase inhibitors sorafenib and regorafenib have been reported to prolong survival and have been selected as treatment options[3]. Both the American Association for the Study of the Liver Disease (AASLD) and the European Association for the Study of the Liver (EASL) accepted the BCLC treatment algorithm[4,5].
Eastern countries have totally opposite views. Experts from mainland China[6] and Japan[7] as well as other eastern experts[8] have recognized that HCC with PVTT is not a contradiction of surgical approach. In selected HCC-PVTT patients, surgical treatment could not only improve the life quality but also prolong the survival time, even though a R0 resection for this malignant disease could not be achieved. The criteria for surgical resection are defined as follows: (1) PVTT type I/II or type III/IV (but these classifications remain controversial); (2) Good general medical conditions with Eastern Cooperative Oncology Group (ECOG) scores of 0-2; (3) Liver function was Child-Pugh class A or selected class B; (4) Resectable primary tumor, without metastasis; and (5) Enough future liver remnant.
We have performed surgical treatment for HCC-PVTT patients over a period of approximately 15 years and achieved good results, with the longest surviving time being 13 years and onward. In this study, we review the efficacy and principles of current surgical treatments and introduce our new, more effective surgical technique named “thrombectomy first”.
A Japanese nationwide survey[9] of 6474 patients from 645 registered institutions showed that the median survival time (MST) of the operation group was prolonged by 1.77 years compared to the nonsurgical group (2.87 years vs 1.10 years; P < 0.001) for the overall cohort and by 0.88 years (2.45 years vs 1.57 years; P < 0.001) for the propensity score-matched cohort. Furthermore, the survival rates at 1, 3, and 5 years after diagnosis for the operation group were significantly higher compared to the conventional treatment group, with the survival rates being 70.9%, 43.5%, and 32.9% compared to 62.9%, 31.6%, and 20.1%, respectively. Moreover, subgroup analysis after propensity score matching revealed that surgical treatment provided a survival benefit regardless of age, etiology, serum α-fetoprotein elevation, tumor number, or hepatitis B or C infection; however, a previous study[7] showed that the survival benefits of surgical treatment in Vp4 patient groups were not statistically significant, and most of them could not achieve R0 or R1 resection. Thus, the authors only recommended liver resection as a first line of treatment when PVTT was limited to the first-order branch of the portal vein.
Another large cohort study from China[10] analyzed 1580 patients diagnosed with HCC with PVTT from four of the largest tertiary hospitals. The MSTs of the surgical treatment group for types I and II patients were 15.9 and 12.5 mo, respectively, and these MSTs were much better compared to their nonsurgical counterparts. The authors concluded that surgical approaches are the best treatment for type I/II PVTT patients with good liver function. Likewise, several other studies from Eastern countries also suggested the potential survival benefits of surgical treatment in HCC-PVTT patients[11-14]; however, most of these studies suggested that operation is not useful for type IV PVTT patients who had a poor prognosis.
Transcatheter arterial chemoembolization (TACE) is commonly used to help patients with advanced HCC including PVTT. Recently, many studies reported that surgical treatment had a better prognosis compared to those undergoing TACE, especially for Cheng’s types I and II PVTT. Peng et al[15] compared 201 HCC patients with PVTT who received surgical treatment and 402 matched cases treated with TACE. The median survival of the surgical treatment and TACE groups was 20.0 mo ± 1.8 mo and 13.1 mo ± 0.6 mo, respectively. The 1-, 3-, and 5-year overall survival rates for the surgical and TACE groups were 42.0%, 14.1%, and 11.1% and 37.8%, 7.3%, and 0.5%, respectively (P < 0.001). The survival curve for the operation group was better than that for the TACE group. Subgroup analyses showed that the overall survival for the operation group was better than that of the TACE group for type I/II PVTT but not for type III/IV PVTT. This result was concordant with a stratified meta-analysis[16]. Other typical studies[10,17] also favor the surgical treatment, which showed that the survival rates were better for the surgical resection group than the TACE group for Cheng’s type I/II PVTT.
Combining preoperative TACE with surgical resection alone was also compared by Yamamoto[18] and Ban[19]; neither were able to find a survival benefit with preoperative TACE for HCC-PVTT patients, especially for Cheng’s types III and IV PVTT.
In Western countries, the first-line treatment option for HCC-PVTT is sorafenib, but the MST was short at 6.5 mo in the Eastern countries[20] and 10.7 mo in Western areas[3]. In a study by Jeong et al[21], 30 patients with HCC-PVTT (Vp3 or Vp4) treated with sorafenib monotherapy had a median survival period of only 3.1 mo. Another typical study by Nakazawa et al[22] also revealed that the MST(4.3 mo) was similar for PVTT in the main trunk or in the first branch after treated with sorafenib alone. These data demonstrated that the MST was quite shorter for HCC-PVTT treated with surgical method.
The tumor extent and the range and position of PVTT affect surgical planning and correlate with overall survival after surgical resection. Thus, the surgical method should aim to achieve a complete resection of the primary tumor and removal of PVTT with a sufficient future remnant liver. Therefore, classification of PVTT is necessary to determine the surgical process.
Currently, there are two classifications that are well accepted and widely used in the Asia-Pacific: the Japanese Vp classification[23,24] and the classification suggested by Cheng, who is a Chinese professor[12,25].
The Liver Cancer Study Group of Japan proposed a helpful macroscopic classification divided into five grades (Vp0-Vp4)[23,25] for HCC with PVTT. Each one is defined as follows: (1) no tumor thrombus in the portal vein, Vp0; (2) existence of a tumor thrombus distal to, but not in, the 2nd-order branches of the portal vein, Vp1; (3) presence of a tumor thrombus in the 2nd-order branches of the portal vein, Vp2; (4) existence of a tumor thrombus in the 1st-order branches of the portal vein, Vp3; and (5) presence of a tumor thrombus in the main trunk of the portal vein or a portal vein branch contralateral to the mainly involved lobe (or both), Vp4.
Cheng’s new classification system comprises five grades: (1) Type I0: tumor thrombus formation found under a microscope; (2) Type I: tumor thrombus involving segmental branches of the portal vein or above; (3) Type II: tumor thrombus involving the right/left portal vein; (4) Type III: tumor thrombus involving the main portal vein trunk; and (5) Type IV: tumor thrombus involving the superior mesenteric vein or the inferior vein cava. Furthermore, two subgroups were classified for each type: (1) For Type I: Ia, tumor thrombus involving segmental branches of the portal vein or above, and Ib, tumor thrombus involving segmental branches of the portal vein extending to sectoral branch; (2) For Type II: IIa, tumor thrombus involving the right/left portal vein, and IIb, tumor thrombus involving both the left and right portal veins; (3) For Type III: IIIa, tumor thrombus involving the main portal vein trunk for no more than 2 cm below the confluence of the left and right portal veins, and IIIb, tumor thrombus involving the main portal vein trunk for more than 2 cm below the confluence of the left and right portal veins; and (4) For Type IV: IVa, tumor thrombus involving the superior mesenteric vein (SMV), and IVb, tumor thrombus involving the inferior vein cava (IVC).
Compared to the Cheng’s classification, the Vp classification does not mention the extension of PVTT involving the SMV or IVC. Vp0 is equal to Type 0, Vp1 is equal to Type Ia, Vp2 is equal to Type Ib, Vp3 is equal to Type IIa, and Vp4 is equal to Type III and Type IIb. Most Japanese studies concluded that surgical treatment did not achieve a better survival outcome compared to nonsurgical treatments in Vp4 PVTT. There are, however, disadvantages of the two classifications: (1) both of them do not consider liver function or tumor-correlated factors, such as tumor number and size; and (2) no pathological information of PVTT is described, such as necrotic degree, proliferative type, necrotic type and organized type. Several studies recognized that the Cheng’s classification was more applicable than the Vp classification for disease assessment and treatment.
Based on the two classifications, several surgical resection methods and techniques were introduced.
En bloc resection of PVTT with the tumor could be achieved: (1) Segmental hepatectomy is suitable for Japanese classification Vp1-2 or Cheng’s classification Type I; (2) Hemihepatectomy is suitable for Japanese classification Vp3 or Cheng’s classification Type IIa, and the primary tumor should be located in the same liver without extending beyond the hemiliver; and (3) Extended hemihepatectomy is suitable for Cheng’s classification Type IIb and Japanese classification Vp4 (tumor thrombus in the bifurcation of the portal vein).
Hepatectomy plus thrombectomy or en bloc resection (with or without reconstruction of the main portal vein) can be selected in this kind of HCC-PVTT, which includes Japanese classification Vp4 and Cheng’s classification Types III and IV. The PVTT is typically extracted through an incision in the portal vein after resection to the liver.
Combined with the main portal resection, a direct end-to-end reconstruction is usually needed after the hepatectomy when the wall of the main portal vein is invaded by the tumor thrombus or if the removal is difficult. However, this concept is not recommended by the study based on the findings of the Japanese registry, and the result showed that when the tumor thrombus lies beyond the liver resection line or invades the main portal vein wall (type III/IV PVTT), there was no significant improvement in survival after surgical treatment.
Several studies compared the surgical approaches, complications, survival benefit, and recurrence between hepatectomy with thrombectomy and hepatectomy with en bloc resection. Most of reports showed that hepatectomy plus thrombectomy or en bloc resection was not significantly different no matter for patients with Vp4 or Vp3 PVTT when compared for overall survival and disease-free survival at 1, 3, and 5 years, but hepatectomy had a lower mortality and morbidity[26,27]. While some other studies suggested that thrombectomy had a risk of residual tumor and hepatectomy plus en bloc resection with or without reconstruction of the main portal vein would theoretically improve the oncological outcomes. Another typical study[28] also revealed that the 5-year overall survival and disease-free survival rates were not significantly decreased in HCC patients with PVTT extended to the bifurcation of the portal vein if en bloc resection was selected. However, the decision of such surgical manipulations could be based on various factors including the surgeon’s expertise in portal vein resection and reconstruction as well as the nature of the thrombus and no randomized controlled trial had been done to compare these techniques.
Most of the studies report that the prognosis is poor for Vp4 or Types III and IV patients with HCC and PVTT. Shi et al[25] compared the surgical effects of Type I/II and Type III/IV PVTT and found that the overall survival and disease-free survival rates of patients with type III or IV PVTT were significantly worse than those of type I or II. The 1- and 3-year overall survival and disease-free survival rates of 78 Type III patients were 24.7% and 3.6% and 3.0% and 0%, respectively. The 1- and 3-year overall survival and disease-free survival rates of 20 Type IV patients were 18.0% and 0% and 0% and 0%, respectively. In Li’s report[27], the 1-, 3-, and 5-year OS and DFS rates for the patients whose PVTT extended beyond the resection line were 42.6%, 11.4%, and 5.7% and 15.4%, 5.1%, and 5.1%, respectively.
We analyzed these reports and found that almost all of them performed the thrombectomy after the liver resection. Only Daisuke[19] performed the removal of PVTT prior to the mobilization and transection of the liver, and the MST and OS were much higher than those of the other studies. The MST of 19 Vp4 patients was 28 mo and the 3- and 5-year overall survival rates were 41.8% and 20.9%, respectively. The MST of 26 Vp3 patients was 18 mo and the 3- and 5-year overall survival rates were 25.2% and 21.2%, respectively.
We have performed surgical treatments for Types III and IV PVTT since 2003, and we proposed a “thrombectomy first” surgical concept to provide superior surgical therapeutic effects and simplify the management approach. The tumor thrombus in the main portal vein, the bifurcation or the contralateral portal vein would be removed prior to liver resection. The main advantages of this concept are as follows: (1) Compression and crushing of PVTT during the operative process could be avoided; thus, new intrahepatic metastases caused by tumor scattering from the PVTT to the remnant liver were minimized; (2) The contralateral portal venous flow could be recovered at an early stage of the operation, which is a beneficial measure for the preservation of remnant liver function; and (3) The portal venous pressure decreases as a result of complete removal of tumor thrombus.
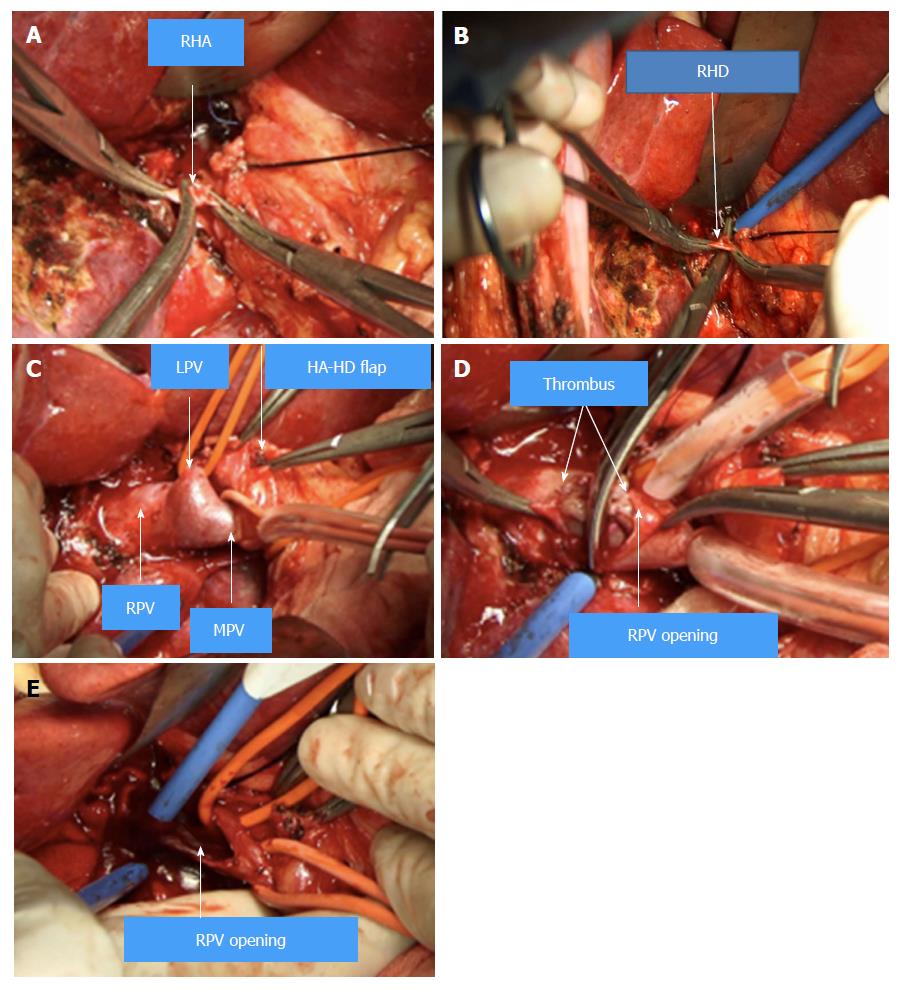
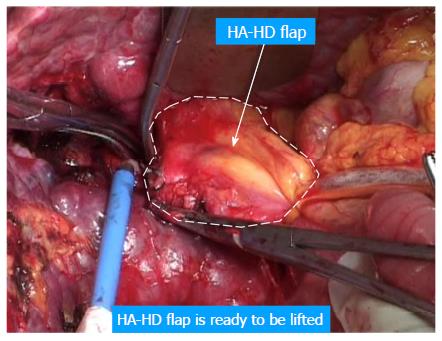
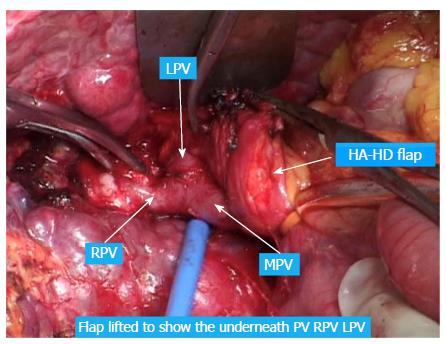
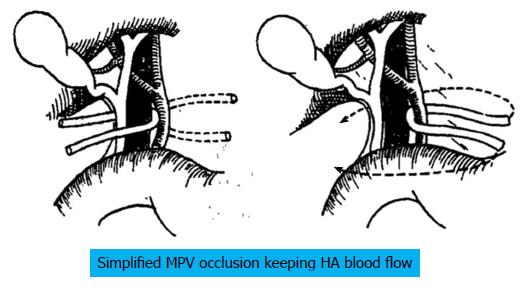
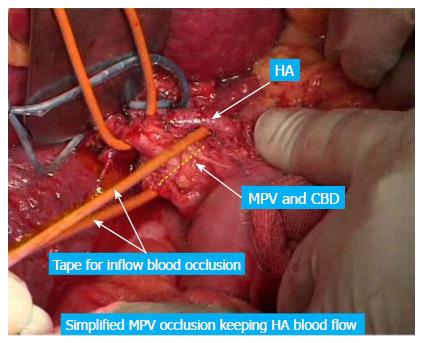
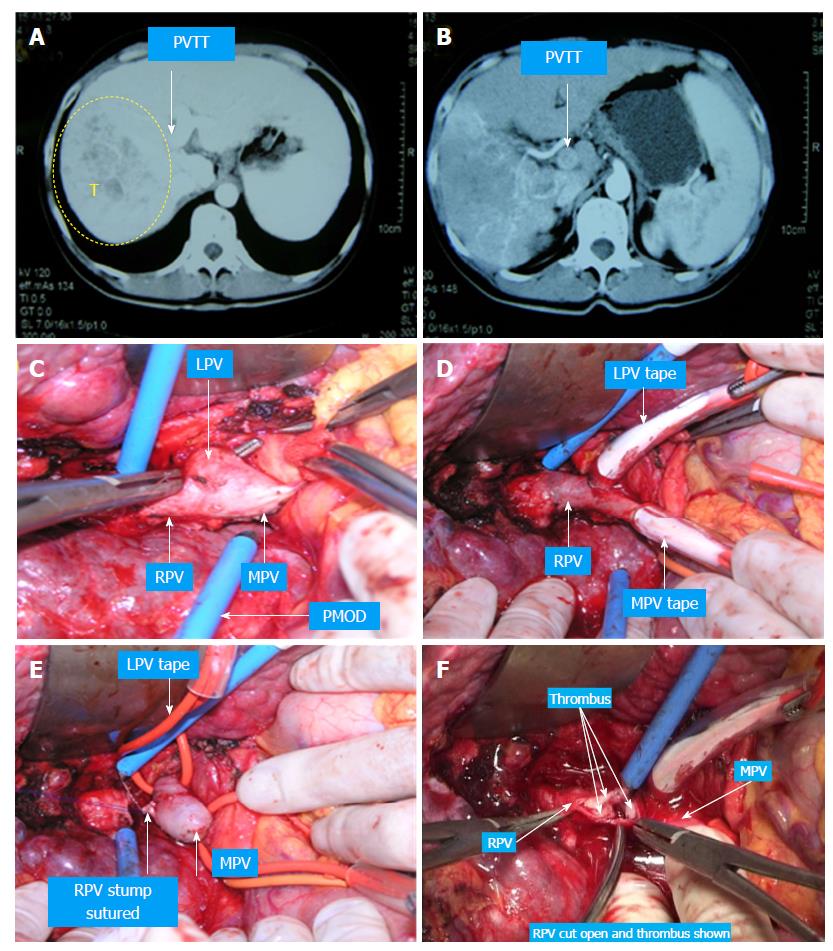
The following considers a right hemihepatectomy as an example to explain the detailed surgical process. The main portal vein (MPV) and the right portal vein (RPV) were clearly revealed by dissecting the fascia and Glisson sheath along the right edge of the hepatoduodenal ligament until the point where it entered the right liver. The tissues in the front were then pushed forward to fully expose the anterior wall of the RPV. Both the right hepatic artery (HA) and the right hepatic duct (HD) were then cut and ligated separately with a 1 cm distance from the entry point of the right liver (Figure 1A and B). The right HA and right HD along with the anterior tissues of the Glisson sheath was called the hepatic artery-duct flap (HA-HD flap) (Figures 2 and 3). The left hepatic portal vein was revealed by pulling up and dissecting the flap, and a small branch of the portal vein that entered the caudate lobe was cut and ligated in this process. The dissection and taping of the RPV, the left portal vein (LPV) and MPV by vascular slings continued prior to ligation of the RPV distant to the bifurcation, followed by clamping of the LPV and MPV distal to the PVTT (Figure 1C). The anterior wall of the RPV was opened to a 1 cm distance to the confluence (the incision could be extended to the portal trunk longitudinally if necessary). The PVTT in the RPV and MPV was then extracted from the opening (Figure 1D), followed by flushing the potential residual tumor thrombus by the portal vein blood flow by releasing the vascular occlusion band of the MPV. The MPV was occluded again, and the PVTT in the LPV was extracted. The left remnant tumor thrombus was flushed by the reverse portal vein blood through releasing the vascular occlusion band of LPV (the left hepatic artery was not occluded), and a catheter was used to help flushing when necessary. After flushing and confirming that no PVTT remained (Figure 1E), the stump was closed by a continuous Prolene suture, and the vascular occlusion was released. If the tumor thrombus strongly adhered to the portal vein wall, resection of the portal vein would be required. Direct end-to-end anastomosis was conducted to reconstruct the portal vein with the use of a 6/0 Prolene continuous suture. After suturing, the right liver was mobilized and transected via standard procedure, or the anterior approach was performed using PMOD (Peng’s Multiple Operative Dissector). To achieve long-term survival, the thrombectomy must be completely cleared. A key element to successfully performing the operation is doing so with no time pressure to prevent sloppiness. When blocking the MPV, the HA blood flow should be not occluded to protect remnant liver function. If the MPV was difficult to dissect, the selected portal vein occlusion method was used by isolating the common hepatic artery and occluding the portal trunk and common hepatic duct together (Figures 4 and 5).
We have performed the “thrombectomy first” approach at two campuses of the Second Hospital affiliated with Zhejiang University School of Medicine, two campuses of the Sir Run Run Shaw Hospital affiliated with Zhejiang University School of Medicine and Yuebei People’s Hospital affiliated with Shantou University School of Medicine for roughly 15 years. Detailed data is still being collected and analyzed (unpublished), but we would like to share three typical Cheng’s Type III/IV (Vp4) cases that had long-term survival. The disease-free survival time was 13, 9 and 4.6 years, respectively (Table 1).
| Gender | Age | HBV | Tumor location | Tumor size | PVTT classification | Operation | Pre-optreatment | Post-optreatment | Tumor recurrence | Survival time (mo) |
| M | 40 | + | Right liver | 8 cm × 6 cm | Type IV or Vp4 (right branch extending to the main PV) | Thrombectomy and right hemihepatectomy | TACE (once) | TACE (4 times) | No | 162 (still alive) |
| M | 52 | + | Right liver | 6 cm × 6 cm | Type III or Vp4 (left and right branch) | Thrombectomy and right hemihepatectomy | TACE (once) | TACE (twice) | No | 108 (still alive) |
| M | 50 | + | Left liver | 20 cm × 16 cm | Type IV or Vp4 (left branch and main PV) | Thrombectomy and left hemihepatectomy | No | TACE (once) | No | 55 (still alive) |
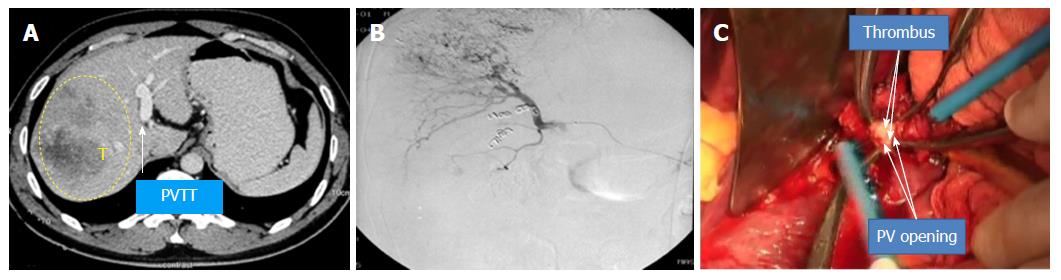
Case 1 (Figure 6, the pictures were taken by camera 13 years ago and had poor quality): A 40-year-old man had HBV infection for more than 10 years. The CT scan showed an 8 cm × 6 cm mass with satellite lesions in the right liver, and tumor thrombus was found in the right branch of the portal vein and extended to the MPV, and the liver function was Class A according to Child-Pugh classification. The patient was diagnosed with HCC-PVTT and received TACE once before surgery. The “thrombectomy first” approach plus right hemi–hepatectomy was performed in February 2005. The patient recovered smoothly after surgery and continued with TACE treatment four times after the operation. The patient is still alive and working normally without recurrence.
Case 2 (Figure 7): A 52-year-old man had HBV infection for more than 15 years. The CT scan showed a 6 cm × 6 cm mass in the right liver, a 3 cm × 3 cm tumor thrombus in the right branch of the portal vein, and two tumors approximately 0.9 cm × 1.0 cm and thrombus in the left branch. The liver function was Class A according to Child-Pugh classification. The “thrombectomy first” approach plus right hemihepatectomy was performed in August 2009. The patient recovered without any complications and was treated with TACE twice postoperatively. The patient is still alive and working normally without recurrence.
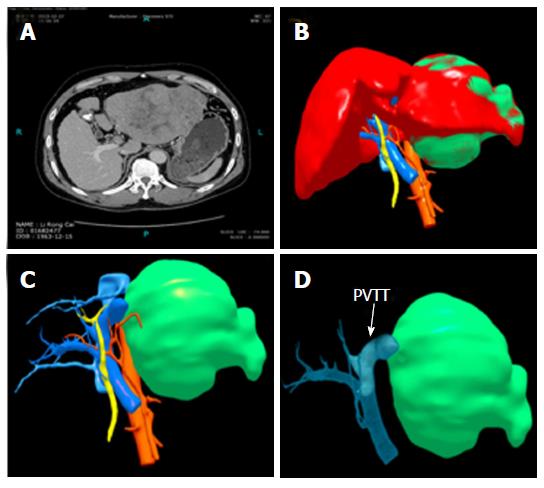
Case 3 (Figure 8): A 50-year-old man had HBV infection for more than 10 years. The CT scan showed a huge mass in the left liver larger than 20 cm × 16 cm, and tumor thrombus was found in the left branch of the portal vein and the main portal vein. The liver function was Child-Pugh Class A. The “thrombectomy first” approach plus left hemihepatectomy was performed in December 2013. The patient received anti-HBV treatment and TACE once after the operation. He is still alive without recurrence.
We usually classify PVTT as follows: (1) Segmental PVTT (thrombus confined in a segment); (2) Lobar PVTT (thrombus confined in the right/left lobe), which is then subdivided into R lobar and L lobar; (3) Main PVTT (thrombus extending to main trunk portal vein), which is then subdivided into R Main PVTT and L Main PVTT; (4) R + M PVTT (Right PVTT extending to both main trunk and left portal vein); and (5) L + M PVTT (Left PVTT extending to both main trunk and right portal vein). Accordingly, case one belongs to R Main PVTT; case two belongs to R + Main PVTT; case three belongs to L Main PVTT. Our classification seems more helpful for determining the method of surgical intervention when compared with other classifications. It is simpler and easier to remember, and thus, more practical for clinical use.
HCC with PVTT is not a surgical contraindication, and the accumulation of experience and development of technology favor surgical treatments. Treatment guidelines led by China and Japan have confirmed the therapeutic effects of surgical treatment in Type I/II, but the benefit of surgical treatment for Type III/IV is still controversial. Through our experience, we have found that the appropriate surgical approach, especially the new “thrombectomy first” method, would remarkably improve the survival outcomes in patients with Cheng’s III/IV PVTT.
Many thanks to Yuan-Quan Yu, Yun Jin, Guo-Jun Chen and Xiao-Long Liu for their help collecting the data for the three cases.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tomizawa M S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Yin SY
| 1. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 907] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3595] [Article Influence: 276.5] [Reference Citation Analysis (4)] |
| 3. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10261] [Article Influence: 603.6] [Reference Citation Analysis (2)] |
| 4. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 5. | European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4519] [Article Influence: 347.6] [Reference Citation Analysis (2)] |
| 6. | Cheng S, Chen M, Cai J; National Research Cooperative Group for Diagnosis and Treatment of Hepatocellular Carcinoma with Tumor Thrombus. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. Oncotarget. 2017;8:8867-8876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 489] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 8. | Ho MC, Hasegawa K, Chen XP, Nagano H, Lee YJ, Chau GY, Zhou J, Wang CC, Choi YR, Poon RT. Surgery for Intermediate and Advanced Hepatocellular Carcinoma: A Consensus Report from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014). Liver Cancer. 2016;5:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima O. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 10. | Wang K, Guo WX, Chen MS, Mao YL, Sun BC, Shi J, Zhang YJ, Meng Y, Yang YF, Cong WM. Multimodality Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Large-Scale, Multicenter, Propensity Mathching Score Analysis. Medicine (Baltimore). 2016;95:e3015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Kojima H, Hatano E, Taura K, Seo S, Yasuchika K, Uemoto S. Hepatic Resection for Hepatocellular Carcinoma with Tumor Thrombus in the Major Portal Vein. Dig Surg. 2015;32:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, Zhang BX, He SQ, Zhang WG. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561-7567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 209] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 15. | Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118:4725-4736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Liu PH, Lee YH, Hsia CY, Hsu CY, Huang YH, Chiou YY, Lin HC, Huo TI. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014;21:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Yamamoto Y, Ikoma H, Morimura R, Shoda K, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T. Post-hepatectomy survival in advanced hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2015;21:246-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Ban D, Shimada K, Yamamoto Y, Nara S, Esaki M, Sakamoto Y, Kosuge T. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg. 2009;13:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4648] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 21. | Jeong SW, Jang JY, Shim KY, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS. Practical effect of sorafenib monotherapy on advanced hepatocellular carcinoma and portal vein tumor thrombosis. Gut Liver. 2013;7:696-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Nakazawa T, Hidaka H, Shibuya A, Okuwaki Y, Tanaka Y, Takada J, Minamino T, Watanabe M, Kokubu S, Koizumi W. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Ikai I, Yamamoto Y, Yamamoto N, Terajima H, Hatano E, Shimahara Y, Yamaoka Y. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am. 2003;12:65-75, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M; HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 25. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 26. | Chok KS, Cheung TT, Chan SC, Poon RT, Fan ST, Lo CM. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg. 2014;38:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Shaohua L, Qiaoxuan W, Peng S, Qing L, Zhongyuan Y, Ming S, Wei W, Rongping G. Surgical Strategy for Hepatocellular Carcinoma Patients with Portal/Hepatic Vein Tumor Thrombosis. PLoS One. 2015;10:e0130021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Wu CC, Hsieh SR, Chen JT, Ho WL, Lin MC, Yeh DC, Liu TJ, P’eng FK. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg. 2000;135:1273-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |