Published online Oct 21, 2018. doi: 10.3748/wjg.v24.i39.4428
Peer-review started: July 10, 2018
First decision: July 18, 2018
Revised: August 24, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 21, 2018
Processing time: 100 Days and 2.9 Hours
Colorectal cancer (CRC) is the third most common cancer of mortality in the world. Chemotherapy based treatment leads to innumerable side effects as it delivers the anticancer drug to both normal cells besides cancer cells. Sonic Hedgehog (SHH), Wnt wingless-type mouse mammary tumor virus/β-catenin, transforming growth factor-β/SMAD, epidermal growth factor receptor and Notch are the main signaling pathways involved in the progression of CRC. Targeted therapies necessitate information regarding the particular aberrant pathways. Advancements in gene therapies have resulted in the recognition of novel therapeutic targets related with these signal-transduction cascades. CRC is a step-wise process where mutations occur over the time and activation of oncogenes and deactivation of tissue suppressor genes takes place. Genetic changes which are responsible for the induction of carcinogenesis include loss of heterozygosity in tumor suppressor genes such as adenomatous polyposis coli, mutation or deletion of genes like p53 and K-ras. Therefore, many gene-therapy approaches like gene correction, virus-directed enzyme-prodrug therapy, immunogenetic manipulation and virotherapy are currently being explored. Development of novel strategies for the safe and effective delivery of drugs to the cancerous site is the need of the hour. This editorial accentuates different novel strategies with emphasis on gene therapy and immunotherapy for the management of CRC.
Core tip: In spite of the advancements in the diagnosis and the treatment approaches for colorectal cancer (CRC), its survival rate is quite low. Therefore, there arises an urge to develop novel targeting strategies for its effective treatment. A meticulous apprehension of the signaling cascade is necessitated for better outcomes. In a nutshell, this editorial highlights various novel targeting approaches like gene therapy and immunotherapy which could usher better targeting of CRC.
- Citation: Tiwari A, Saraf S, Verma A, Panda PK, Jain SK. Novel targeting approaches and signaling pathways of colorectal cancer: An insight. World J Gastroenterol 2018; 24(39): 4428-4435
- URL: https://www.wjgnet.com/1007-9327/full/v24/i39/4428.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i39.4428
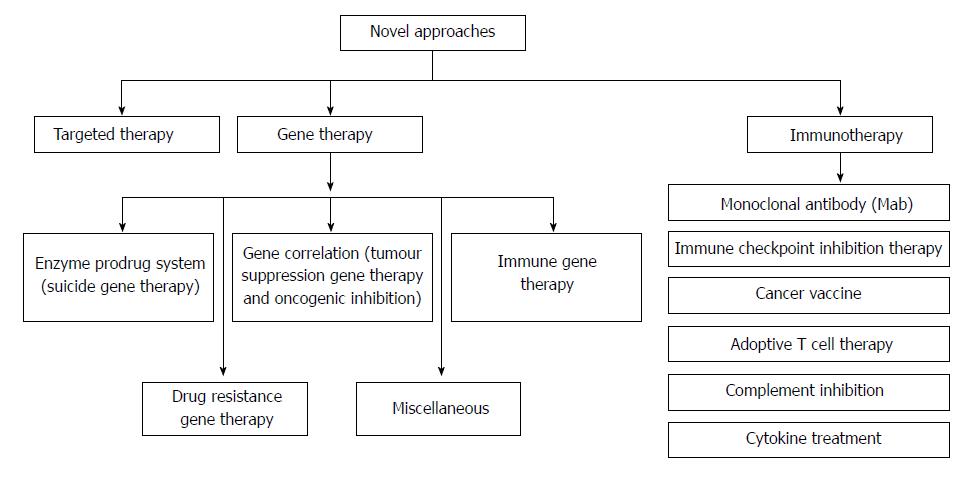
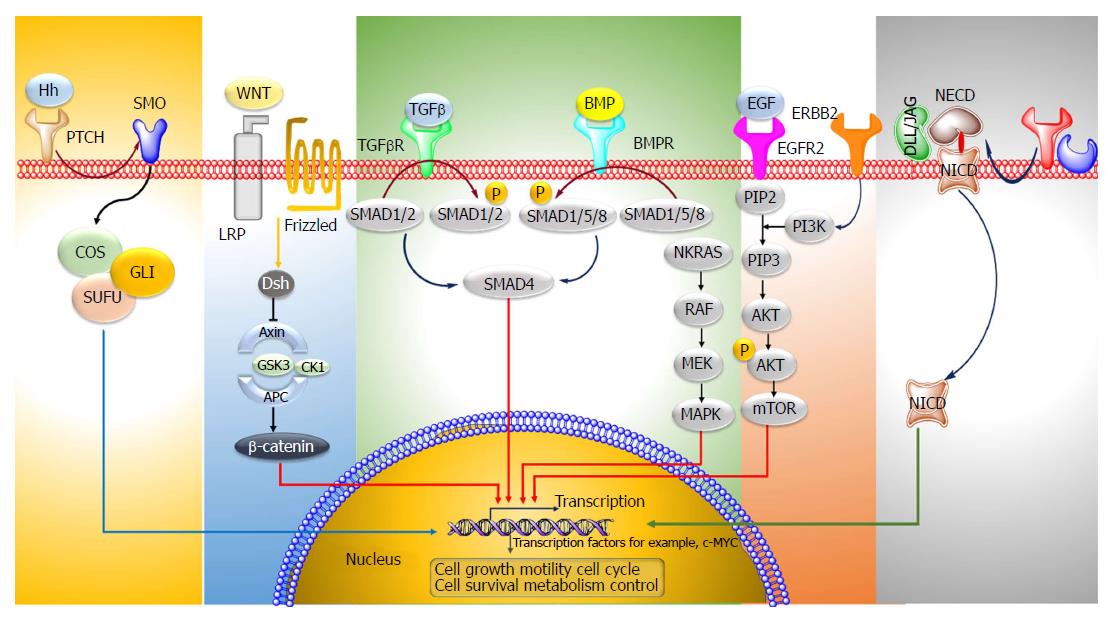
Colorectal cancer (CRC) is the third most predominant cancer amongst the world. In 2017, 97220 and 43030 new patients of colon and rectum cancers were reported in United States, respectively. CRC is manifested by the development of adenomatous polyps and malignant cells in the colon. These abnormal cells producing tumors are characterized by uncontrolled replication and the property of metastasis. The early detection, diagnosis, and the utilization of efficient and safe delivery systems would tremendously enhance the efficacy of therapy. The novel targeting approaches (Figure 1), of raising concern as manifested by cancer drugs in the past years, block transduction pathways leading to the cell death through apoptosis and triggering of the immune system, or deliver anticancer drugs to cancer cells, reducing the side effects. The major pathways which could be targeted for CRC therapy are, Sonic Hedgehog (SHH), Wnt/β-catenin, transforming growth factor-β (TGF-β)/SMAD, EGFR and Notch pathways[1,2] (Figure 2).
The Hh pathway is crucial in the normal development of various organs like gut epithelium. The Hh ligands bind to the Patched protein (Ptch) receptor, which subdues the activity of Smoothened (Smoh) receptor. Binding of the ligands to PTCH1 results in the Smoh-mediated activation of GLI transcription factors, which then modulates the expression of various Hh target genes. The expression of SHH, SMO, GLI1 mRNA in colon cancer tissues is remarkably enhanced as compared to the normal cells[3]. Vismodegib is an Hh inhibitor which acts by targeting Smoothened which is a modulator of the Hh pathway. In order to enquire novel Hh antagonists with apoptosis-triggering activity, a group of ~300 potential smoothened antagonists were screened. In colon cancer cells, Hh003 triggered caspase-dependent apoptosis whereas no apoptotic activity was depicted by vismodegib. In comparison to vismodegib, Hh003 displayed similar suppression on the Hh pathway. Hh003 depicted more suppression of the in vitro tumor forming colonies and colon cancer proliferation in vivo[4].
Frizzled (Fz) receptors and low-density lipoprotein receptor-related protein 5 or 6 (LRP5 or LRP6) are the targets of the Wnt family of proteins. The primary element of the Wnt/β-catenin signaling pathway is the β-catenin destruction complex; which is comprised of a tumor suppressor protein encoded by the antigen-presenting cells (APC) gene, Axin, CKI, and GSK3. When the receptor binding does not occur, this complex undergoes binding with the β-catenin protein (encoded by CTNNB1 gene), which then undergoes degradation through an ubiquitin-proteasome pathway. In contrary, binding of the receptor by Wnt ligands causes the deactivation of the β-catenin destruction complex and accumulation of β-catenin. It is then translocated to the nucleus for complex formation with T-cell factor/lymphoid enhancer factor, a transcription factor, causing the transcriptional actuation of the target genes. In majority of colon cancers (sporadic) mutation of both alleles of APC (a tumor suppressor gene) occurs which leads to stabilization of β-catenin and stimulation of WNT pathway genes, like TCF, which are needed for the maintenance of colon crypt. In few colon cancers identification of point mutation in β-catenin bearing wild-type alleles of APC has been done[5]. Aquaporin5 (AQP5), a water protein channel, has an oncogenic activity in many types of malignant cancers like CRC. The effect of AQP5 silencing on 5-fluorouracil (5-FU) sensitivity was inquired in cancer cells. It was observed that the Wnt/β-catenin pathway mediated the 5-FU chemosensitivity. AQP5 silencing suppressed the Wnt pathway. While, overexpression of the β-catenin (S33Y) mutant (which shows resistance to degradation) reversed the apoptosis process triggered by AQP5 silencing[6]. Berberine, which is an alkaloid derived from plants and its synthetic 13-arylalkyl derivatives have been accounted to possess antitumor potential; they were investigated for their involvement in Wnt/β-catenin signaling cascade. The cellular levels of active β-catenin were found to decrease accompanied by a rise in the expression of E-cadherin. The berberine derivatives depicted a 100-times reduced EC50 values in comparison to berberine for Wnt-repression[7]. Esculetin, (6, 7-dihydroxycoumarin) potentially inhibits the Wnt-β-catenin pathway. It interrupted the β-catenin-Tcf complex formation by binding with the Lys312, Gly307, Lys345, and Asn387 residues of β-catenin in tumor cells. Besides, esculetin efficaciously reduced the viability and suppressed the anchorage-independent proliferation of cancer cells[8]. Novel Wnt signaling inhibitors, isopropyl 9-ethyl-1- (naphthalen-1-yl)-9H-pyrido (3, 4-b) indole-3-carboxylate (Z86) have been recognized. Z86 suppressed the Wnt signaling functions and genes expression in mammalian cells. It suppressed the GSK3β (Ser9) phosphorylation, causing its overactivity and elevating the phosphorylation and β-catenin degradation[9].
TGF-β and BMP signaling pathways are often impaired in CRC. Ligand-induced oligomerization of the TGFBR1 serine/threonine receptor kinases leads to the initiation of the signal cascade succeeded by the phosphorylation of Smad1, Smad2 and Smad3 (signaling molecules). This leads to their association with Smad4 (signaling transducer) and translocation to the nucleus. Triggered Smads modulate various biological effects by binding to transcription factors and leading to the modulation of transcription. Juvenile polyposis is observed in colon cancer due to mutated Smad4 or BMPRI. In most of sporadic colon cancers, the phosphorylation of Smad1, Smad5 and Smad8 does not occur[10]. Genistein (obtained from soybean) is an isoflavone possessing an anticancer potential. A dose-dependent rise in TGF-beta1 mRNA expression was found in MC-26 cells in mouse. It stimulated the generation of Smad-DNA complexes and phosphorylated Smad2 and Smad3, depicting enhanced TGF-beta1 signaling[11].
The binding of epidermal growth factor and TGF to the EGFR, leads to the stimulation of homodimerization/heterodimerization of the receptor and phosphorylation of specific tyrosine residues (P). This in turn stimulates the downstream RAS/RAF/mitogen-activated protein kinase (MAPK) and phosphoinositide 3’-kinase (PI3K) signaling pathways and expression of genes responsible for cell proliferation, angiogenesis and metastasis. KRAS2 and BRAF mutations have been seen in colon cancer. Mutations in PIK3CA which is the p110α catalytic subunit of PI3K have also been observed in few cases of colon cancers[12]. Everolimus (an inhibitor of mTOR) in combination with nilotinib (a platelet-derived growth factor receptors tyrosine kinase inhibitor) suppressed the growth and liver metastasis of colon cancer. The stromal reaction and cancer cell proliferation was reduced and apoptosis was stimulated in tumor cells[13].
The Notch signaling pathway is involved in the growth of intestinal epithelium. Notch ligands i.e., Delta-like (DLL) bind to their transmembrane receptors (Notch 1-4) and induce the proteolytic breakdown of the receptors by the enzymes α-secretase and γ-secretase to release the intracellular domain of the Notch receptor. The cleaved Notch receptors (NICD) are then transferred into the nucleus which forms complexes with RBP-jk (CSL or CBF-1) and lead to the stimulation of Notch-target gene Transforming growth factor-β. An overexpression of ligands namely Jagged1, Jagged2, DLL1, DLL3, DLL4, Notch receptors 1-4 and genes like hairy-enhancer-of-split (Hes-1), Deltex and Notch intracellular domain (NICS) has been observed in colorectal cancer cells[14]. Withaferin-A is a natural compound (source Withania somnifera), which curbs Notch-1 signaling and downregulates various pathways like Akt/NF-kappa B/Bcl-2, in HCT-116, SW-480, and SW-620 cell lines. Besides, Withaferin-A downregulated the expression of mammalian target of rapamycin (mTOR) signaling components, pS6K and p4E-BP1, and stimulated c-Jun-NH (2)-kinase-mediated apoptosis in tumor cells[15].
Nanotechnology is a rising arena in drug delivery which furnishes many advantages over the conventional system. Colon-specific novel delivery systems would allow for the local delivery of a high concentration of drugs in the colon to improve pharmacotherapy and reduce its potential systemic toxicity and side effects. Recently, theranostic nanocarriers are introduced to simultaneously monitor and treat the disease using a single delivery system[16]. Colon targeted nanocarriers have been described in brief in the Table 1[17-26].
| System | Chemotherapeutic agent | Significance | Ref. |
| Nanoparticles | Resveratrol (RSV) | Sustained release of RSV (over 72 h), and drug solubility enhancement | [17] |
| Micellar delivery system | Docetaxel | Enhanced the efficacy of hydrophobic chemotherapy and reduced systemic toxicity | [18] |
| Self-nanoemulsifying drug delivery systems (SNEDDS) | Sunitinib malate | Enhancement of in vitro dissolution rate and anticancer potential of drugs possessing low water solubility such as sunitinib malate | [19] |
| Small molecule-based theranostic system, Gal-Dox | Doxorubicin | Drug localization and site of action can be monitored | [20] |
| Polymeric micelles | Tanshinone IIA (TAN) | Improved efficacy of anticancer drugs and promoted the growth of beneficial commensal flora in the gut | [21] |
| Pressure-sensitive nanogels | 5-Fluorouracil (5-FU) | Higher 5-FU intracellular accumulation and a significant cell death extension by apoptosis | [22] |
| Microspheres | Atorvastatin and celecoxib | Synergistic effect on colon cancer prevention and inhibition | [23] |
| Microbeads | Doxorubicin | Exhibited reduction-responsive character, release the DOX in reducing environments due to cleavage of the disulfide linkers | [24] |
| Carboxymethyl dextran (CMD) chitosan nanoparticles | Small interfering RNA | Significant changes of Epithelial mesenchymal transition genes and apoptosis | [25] |
| Liposomes | Apatinib | cRGD-modified liposomes displayed greater apoptosis | [26] |
It involves introduction of genetic components for treating various diseases including cancer. The genetic component may be the nucleic acid i.e., DNA or RNA which may help to replace or correct the malfunction due to defective genes. Gene therapy can also be utilized to actuate an immune response or itself used as a therapeutic agent.
Progression of colorectal cancer is mediated by mutation and aberration of genes. Modification and correction of these defective genes and prevention of those overexpressed genes can have the capability to prevent CRC. The alteration of multiple genes is involved in the development of colon carcinogenesis. Point mutation, formation of oncogenes, de-regulation or deletion of proto-oncogenes and lack of function of suppressor-oncogenes may lead to cancer.
Till November 2017, near about 2600 clinical trials had been conducted in 38 countries and more than 50% are in phase I clinical trial[27]. While 1309 gene therapy based trials which were performed across the world, merely 45 reached the phase III. Eleven gene therapies for CRC are being subjected for trial in the United Kingdom[28]. There are about 50000 to 100000 genes which exist in the body and a few of them take part in the cell cycle. Defective genes could be most allied factors for CRC and it has been discovered that at least 30% of colon cancers are due to defective genes. Few of them are associated with familial colon cancers. The core benefit of gene therapy is the transfer of the specific genes to the specific tumors cells so that the abnormal function of mutated gene would be suppressed and tumor progression could be inhibited[29-32].
Tumor immunotherapy has seized researchers in this scenario as it depicts remarkable clinical potential in CRC. Presently, there are various immunotherapies which are being subjected to clinical trials in human CRC. Various immunotherapy approaches employed in CRC are monoclonal antibody (mAb) therapy, immune checkpoint inhibitors therapy, cancer vaccines, adoptive cell therapy, complement inhibition and cytokine treatment. Majority of them are in phase I and II clinical trials and some of these trials showed promising results. So far, more than 24 immunotherapy-based clinical trials for human CRC have been completed and more than 40 clinical trials are recruiting or about to recruit patients[33]. Table 2[34-40] depicts various clinical studies of CRC.
| Therapy | Agent | Clinical status | Ref. |
| Five peptides combination with oxaliplatin-based chemotherapy | Oxaliplatin | Phase II | [34] |
| Panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) vs FOLFOX4 alone | Fluorouracil, Oxaliplatin | Phase III trial | [35] |
| Checkpoint inhibitors | Nivolumab and pembrolizumab | Phase 2 study | [36] |
| Combination vaccine treatment of five therapeutic epitope-peptides | Fluorouracil, irinotecan or oxaliplatin | Phase I | [37] |
| Autologous dendritic cell based adoptive immunotherapy | - | Phase I-II | [38] |
| Autologous antigen-activated dendritic cells in the treatment of CRC | - | Phase I-II | [39] |
| Adjuvant chemotherapy (FOLFOX) | 5-fluorouracil (FU)/leucovorin (LV) | Phase III | [40] |
In this therapy, humanized antibodies like Cetuximab and Panitumumab which selectively recognize the epidermal growth factor receptor (EGFR) are employed for the treatment of metastatic CRC. There are some MAbs presently in various phases of clinical trials for CRC such as adecatumumab against EpCAM, labetuzumab against carcinoembryonic antigen (CEA), and pemtumomab against Mucins[41].
T cell activation is down-regulated by CTLA-4 which is an immune checkpoint moiety by binding to CD80/CD86 entities on antigen-presenting cells (APC). T cell function is negatively regulated by programmed death receptor ligand 1/2 (PD-L1/L2) by binding to PD-1 receptor present on T cells usually stimulated by their various ligands which are expressed on either tumor cells (e.g., PDL1/ L2→PD-1) or APCs (e.g., CD80/86→ CTLA-4; PD-L1/L2→PD-1), activated CTLA-4 and PD-1 immune checkpoint signaling pathways efficiently inhibit the tumor-reactive T cell activation and consequent tumor detection[42]. A phase II clinical trial of individual drug Nivolumab and also a combination of dual drugs like Nivolumab plus Ipilimumab is in undergoing process for CRC (ClinicalTrials.gov Identifier: NCT02060188).
They have been designed to induce antigen specific T-cell or B-cell activity against cancer by rendering antigens to APC like dendritic cells (DCs). Besides, vaccines likewise include constituents proposed to activate DCs pulsated with antigens and aim them to move to a local lymph node.eg DC vaccine and OncoVAX.
DC vaccine: Because majority of CRCs express carcinoembryonic antigen (CEA) which is a tumor-associated antigen DCs, can be pulsed with CEA mRNA or CEA peptides. Most of the CRC patients who were administered with DC vaccine evoked CEA-specific T cell immune activities.
Oncovax: It has been developed to use patients’ own cancer cells with an immune-stimulating adjuvant to evoke antitumor immune activities to evade the relapse of colon cancer after surgery. A combination of specific immunotherapy with surgery depicts a remarkable improvement in the survival of the patients[43].
This therapy possesses the potential to raise antitumor immunity and increase vaccine efficacy. Recent researches have riveted on endowing effector T cells with desired antigen receptors, like chimeric antigen receptor T cells. An ex vivo expanded human Vδ1 γδ T cells displayed a remarkable therapeutic activity in human colon cancer xenografted mouse mode[44].
Complement is a key part of immune system and its stimulation has been taken as an essential component of the immune surveillance response against CRC. Complement comprises of more than 30 proteins and fragments, is a part of the innate and adaptive immune system. Various protein inhibitors of complement such as cobra venom factor, humanized cobra venom factor, and recombinant staphylococcus aureus super antigen-like protein 7-have been assessed in murine colon cancer model. Complement depletion presents an efficient type of immunotherapy in CRC by its capability to vitiate tumor progression by raising the host’s immune responses to cancer and reducing the immunosuppressive effect generated by the tumor microenvironment and finally could be employed as a constituent of combination immunotherapy[45].
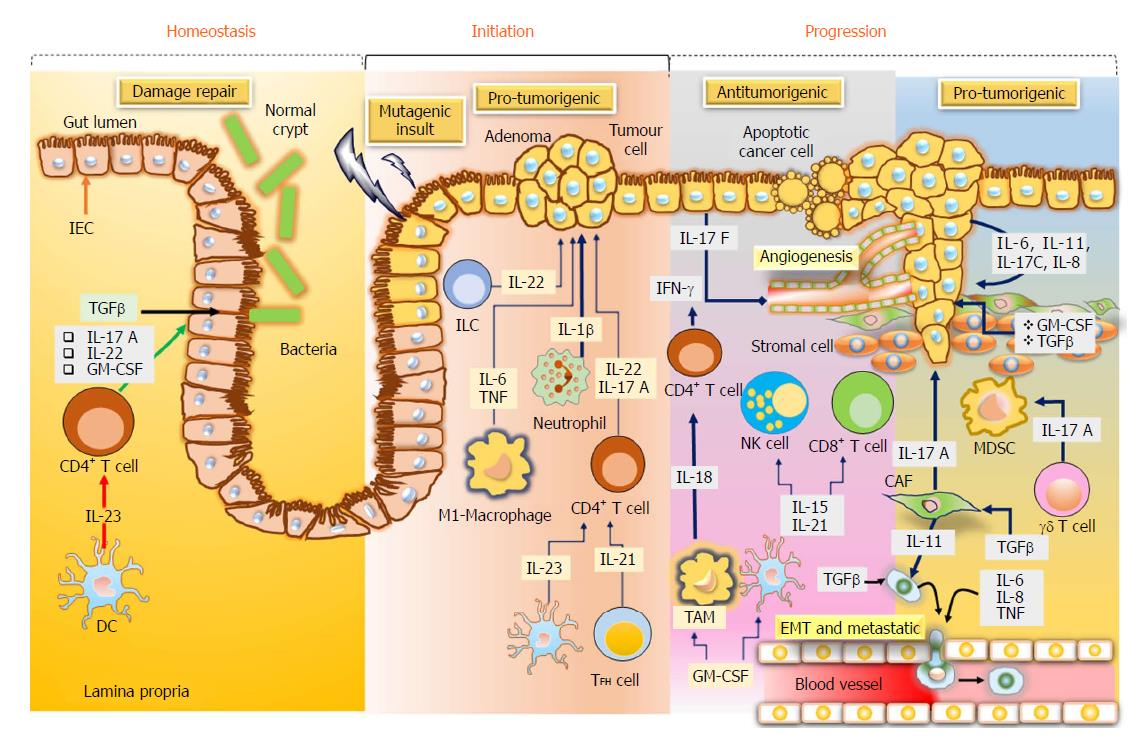
Cytokines are considered as essential aspects of tumour immunology, particularly for CRC, in which the tumor growth is determined by the inflammatory process and immunogenic responses. Cytokines like tumour necrosis factor and interleukin-6 are considered as important factors in CRC, triggering the stimulation of the central oncogenic factors nuclear factor-κB and inducer of transcription 3 (STAT3), respectively, in the intestinal cells to enhance the proliferation and the development of apoptosis resistance[46] (Figure 3).
Increasing evidences show that several signaling pathways play an essential role in the development and progression of CRC. Targeting these signaling cascades using nanocarriers might be advantageous for the treatment of CRC. The identification of various genes and other biomarkers improved the conventional therapy and target the specific tumor cells. The gene therapy and various immunotherapy including cytokine therapy, cancer vaccine, adoptive cell therapy, monoclonal antibody etc. have been recently introduced which may unravel new ways for the treatment of CRC and provide its efficient management in comparison to the conventional therapy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bordonaro M, Caputo D S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 996] [Article Influence: 99.6] [Reference Citation Analysis (2)] |
| 2. | Gulbake A, Jain A, Jain A, Jain A, Jain SK. Insight to drug delivery aspects for colorectal cancer. World J Gastroenterol. 2016;22:582-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 621] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 4. | Chen Q, Zhang H, Wu M, Wang Q, Luo L, Ma H, Zhang X, He S. Discovery of a potent hedgehog pathway inhibitor capable of activating caspase8-dependent apoptosis. J Pharmacol Sci. 2018;137:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Santoyo-Ramos P, Likhatcheva M, García-Zepeda EA, Castañeda-Patlán MC, Robles-Flores M. Hypoxia-inducible factors modulate the stemness and malignancy of colon cancer cells by playing opposite roles in canonical Wnt signaling. PLoS One. 2014;9:e112580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Li Q, Yang T, Li D, Ding F, Bai G, Wang W, Sun H. Knockdown of aquaporin5 sensitizes colorectal cancer cells to 5-fluorouracil via inhibition of the Wnt/β-catenin signaling pathway. Biochem Cell Biol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Albring KF, Weidemüller J, Mittag S, Weiske J, Friedrich K, Geroni MC, Lombardi P, Huber O. Berberine acts as a natural inhibitor of Wnt/β-catenin signaling--identification of more active 13-arylalkyl derivatives. Biofactors. 2013;39:652-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Lee SY, Lim TG, Chen H, Jung SK, Lee HJ, Lee MH, Kim DJ, Shin A, Lee KW, Bode AM. Esculetin suppresses proliferation of human colon cancer cells by directly targeting β-catenin. Cancer Prev Res (Phila). 2013;6:1356-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Li X, Bai B, Liu L, Ma P, Kong L, Yan J, Zhang J, Ye Z, Zhou H, Mao B. Novel β-carbolines against colorectal cancer cell growth via inhibition of Wnt/β-catenin signaling. Cell Death Discov. 2015;1:15033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Biswas S, Chytil A, Washington K, Romero-Gallo J, Gorska AE, Wirth PS, Gautam S, Moses HL, Grady WM. Transforming growth factor beta receptor type II inactivation promotes the establishment and progression of colon cancer. Cancer Res. 2004;64:4687-4692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Yu Z, Tang Y, Hu D, Li J. Inhibitory effect of genistein on mouse colon cancer MC-26 cells involved TGF-beta1/Smad pathway. Biochem Biophys Res Commun. 2005;333:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4468] [Cited by in RCA: 4587] [Article Influence: 199.4] [Reference Citation Analysis (0)] |
| 13. | Yuge R, Kitadai Y, Shinagawa K, Onoyama M, Tanaka S, Yasui W, Chayama K. mTOR and PDGF pathway blockade inhibits liver metastasis of colorectal cancer by modulating the tumor microenvironment. Am J Pathol. 2015;185:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Yin L, Velazquez OC, Liu ZJ. Notch signaling: emerging molecular targets for cancer therapy. Biochem Pharmacol. 2010;80:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C. Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Mol Cancer Ther. 2010;9:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Tiwari A, Jain SK, Jain A, Verma A, Saraf S, Panda PK, Gour G. Application Potential of Polymeric Nanoconstructs for Colon-Specific Drug Delivery. Contemporary Healthcare Applications. 2018;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Suktham K, Koobkokkruad T, Wutikhun T, Surassmo S. Efficiency of resveratrol-loaded sericin nanoparticles: Promising bionanocarriers for drug delivery. Int J Pharm. 2018;537:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Su CY, Liu JJ, Ho YS, Huang YY, Chang VH, Liu DZ, Chen LC, Ho HO, Sheu MT. Development and characterization of docetaxel-loaded lecithin-stabilized micellar drug delivery system for improving the therapeutic efficacy and reducing systemic toxicity. Eur J Pharm Biopharm. 2018;123:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Alshahrani SM, Alshetaili AS, Alalaiwe A, Alsulays BB, Anwer MK, Al-Shdefat R, Imam F, Shakeel F. Anticancer Efficacy of Self-Nanoemulsifying Drug Delivery System of Sunitinib Malate. AAPS PharmSciTech. 2018;19:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Sharma A, Kim EJ, Shi H, Lee JY, Chung BG, Kim JS. Development of a theranostic prodrug for colon cancer therapy by combining ligand-targeted delivery and enzyme-stimulated activation. Biomaterials. 2018;155:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Wang D, Sun F, Lu C, Chen P, Wang Z, Qiu Y, Mu H, Miao Z, Duan J. Inulin based glutathione-responsive delivery system for colon cancer treatment. Int J Biol Macromol. 2018;111:1264-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Hosseinifar T, Sheybani S, Abdouss M, Hassani Najafabadi SA, Shafiee Ardestani M. Pressure responsive nanogel base on Alginate-Cyclodextrin with enhanced apoptosis mechanism for colon cancer delivery. J Biomed Mater Res A. 2018;106:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Li W, Liu D, Zhang H, Correia A, Mäkilä E, Salonen J, Hirvonen J, Santos HA. Microfluidic assembly of a nano-in-micro dual drug delivery platform composed of halloysite nanotubes and a pH-responsive polymer for colon cancer therapy. Acta Biomater. 2017;48:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Cheewatanakornkool K, Niratisai S, Manchun S, Dass CR, Sriamornsak P. Characterization and in vitro release studies of oral microbeads containing thiolated pectin-doxorubicin conjugates for colorectal cancer treatment. Asian J Pharm. 2017;12:509-520. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Song Z, Lin Y, Zhang X, Feng C, Lu Y, Gao Y, Dong C. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: enhanced cellular uptake and improved therapeutic effects. Int J Nanomedicine. 2017;12:1941-1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Sadreddini S, Safaralizadeh R, Baradaran B, Aghebati-Maleki L, Hosseinpour-Feizi MA, Shanehbandi D, Jadidi-Niaragh F, Sadreddini S, Kafil HS, Younesi V. Chitosan nanoparticles as a dual drug/siRNA delivery system for treatment of colorectal cancer. Immunol Lett. 2017;181:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: An update. J Gene Med. 2018;20:e3015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 544] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 28. | Durai R, Yang SY, Seifalian AM, Winslet MC. Principles and applications of gene therapy in colon cancer. J Gastrointestin Liver Dis. 2008;17:59-67. [PubMed] |
| 29. | Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19-27. [PubMed] |
| 30. | Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992;70:1727-1731. [PubMed] |
| 31. | Takami K, Yana I, Kurahashi H, Nishisho I. Multistep carcinogenesis in colorectal cancers. Southeast Asian J Trop Med Public Health. 1995;26 Suppl 1:190-196. [PubMed] |
| 32. | Zhang J, Kale V, Chen M. Gene-directed enzyme prodrug therapy. AAPS J. 2015;17:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Nappi A, Berretta M, Romano C, Tafuto S, Cassata A, Casaretti R, Silvestro L, Divitiis C, Alessandrini L, Fiorica F. Metastatic Colorectal Cancer: Role of Target Therapies and Future Perspectives. Curr Cancer Drug Targets. 2018;18:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Hazama S, Nakamura Y, Tanaka H, Hirakawa K, Tahara K, Shimizu R, Ozasa H, Etoh R, Sugiura F, Okuno K. A phase ΙI study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study). J Transl Med. 2014;12:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1393] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 36. | Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 2083] [Article Influence: 260.4] [Reference Citation Analysis (0)] |
| 37. | Hazama S, Nakamura Y, Takenouchi H, Suzuki N, Tsunedomi R, Inoue Y, Tokuhisa Y, Iizuka N, Yoshino S, Takeda K. A phase I study of combination vaccine treatment of five therapeutic epitope-peptides for metastatic colorectal cancer; safety, immunological response, and clinical outcome. J Transl Med. 2014;12:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Hunyadi J, András C, Szabó I, Szántó J, Szluha K, Sipka S, Kovács P, Kiss A, Szegedi G, Altorjay I. Autologous dendritic cell based adoptive immunotherapy of patients with colorectal cancer-A phase I-II study. Pathol Oncol Res. 2014;20:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 39. | Riley JM, Cross AW, Paulos CM, Rubinstein MP, Wrangle J, Camp ER. The clinical implications of immunogenomics in colorectal cancer: A path for precision medicine. Cancer. 2018;124:1650-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Saleh K, Khalife-Saleh N, Kourie HR, Chahine G. How and when adjuvant treatment should be intensified in stage III colorectal cancers? Future Oncol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3707] [Article Influence: 176.5] [Reference Citation Analysis (1)] |
| 42. | Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1790] [Article Influence: 179.0] [Reference Citation Analysis (0)] |
| 43. | Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809-8814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 393] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 44. | Wu D, Wu P, Wu X, Ye J, Wang Z, Zhao S, Ni C, Hu G, Xu J, Han Y. Ex vivo expanded human circulating Vδ1 γδT cells exhibit favorable therapeutic potential for colon cancer. Oncoimmunology. 2015;4:e992749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 45. | Downs-Canner S, Magge D, Ravindranathan R, O’Malley ME, Francis L, Liu Z, Sheng Guo Z, Obermajer N, Bartlett DL. Complement Inhibition: A Novel Form of Immunotherapy for Colon Cancer. Ann Surg Oncol. 2016;23:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |