Published online Oct 14, 2018. doi: 10.3748/wjg.v24.i38.4393
Peer-review started: August 1, 2018
First decision: August 24, 2018
Revised: September 20, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 14, 2018
Processing time: 72 Days and 8.2 Hours
To study liver stiffness (LS) during pregnancy and its association with complications during pregnancy.
In this observational, diagnostic study, 537 pregnant women were prospectively enrolled at the Department of Obstetrics and Gynecology, University hospital Heidelberg and Salem Medical Center. LS was measured using the Fibroscan device (Echosens, Paris) in all women and in 41 cases 24 h after delivery. Clinical and morphological data were recorded and abdominal ultrasound and standard laboratory tests were performed. No complications were observed in 475 women (controls) while preeclampsia and intrahepatic cholestasis of pregnancy (ICP) developed in 22 and 40 women, respectively.
In controls, LS increased significantly from initially 4.5 ± 1.2 kPa in the second trimester to 6.0 ± 2.3 kPa (P < 0.001) in the third trimester. In the third trimester, 41% of women had a LS higher than 6 kPa. Elevated LS in controls was significantly correlated with alkaline phosphatase, leukocytes, gestational age and an increase in body weight and body mass index (BMI). In women with pregnancy complications, LS was significantly higher as compared to controls (P < 0.0001). Moreover, in multivariate analysis, LS was an independent predictor for preeclampsia with an odds ratio of 2.05 (1.27-3.31) and a cut-off value of 7.6 kPa. In contrast, ICP could not be predicted by LS. Finally, LS rapidly decreased in all women within 24 h after delivery from 7.2 ± 3.3 kPa down to 4.9 ± 2.2 kPa (P < 0.001).
During pregnancy, LS significantly and reversibly increases in the final trimester of pregnant women without complications. In women with preeclampsia, LS is significantly elevated and an independent non-invasive predictor.
Core tip: Liver stiffness (LS) was measured by transient elastography during pregnancy in 537 healthy pregnant women without complications and in 62 women with pregnancy complications such as preeclampsia or intrahepatic cholestasis of pregnancy. Our results show that LS increases during pregnancy to levels above 6 kPa even in women without pregnancy complications and rapidly normalizes within 24 h after delivery. LS was significantly elevated in women with preeclampsia and, moreover, an independent predictor for preeclampsia in multivariate analysis. In conclusion, LS could be a novel non-invasive screening parameter to identify pregnant women at risk for complications of pregnancy.
- Citation: Ammon FJ, Kohlhaas A, Elshaarawy O, Mueller J, Bruckner T, Sohn C, Fluhr G, Fluhr H, Mueller S. Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia. World J Gastroenterol 2018; 24(38): 4393-4402
- URL: https://www.wjgnet.com/1007-9327/full/v24/i38/4393.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i38.4393
Over the last decades, maternal mortality has been drastically lowered in developed countries remaining now stable at a level of approximately 1 death per 4000 deliveries[1]. However, apart from postpartal bleeding and embolic complications, hypertension and preeclampsia remain the leading causes of maternal morbidity and mortality[2,3]. In total, up to 3 % of all pregnancies are complicated by liver disorders which can be related or unrelated to pregnancy and can have a high maternal and perinatal mortality[4-6]. Despite enormous research activities in the last decades, still little is known about the pathogenesis and efficient treatment options of pregnancy- related liver diseases[7]. Except hyperemesis gravidarum, most of the hepatic complications such as hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome and intrahepatic cholestasis of pregnancy (ICP) typically occur in the third trimester[8,9].
So far, rapid delivery is often the only choice to prevent life-threatening complications in mother and child[10]. At present, obstetricians routinely monitor laboratory markers such as aspartate transaminase (AST), alanine transaminase (ALT) and gamma-glutamyltransferase (GGT). Liver sonography is only performed if abnormalities are found. However, these routine tests provide limited information in predicting severe complications during pregnancy[11]. Moreover, no established screening tests exist for an early risk assessment of most of these pregnancy complications[11,12].
Over the last decade, liver stiffness (LS) as measured by transient elastography (TE) has become the preferred parameter to non-invasively assess liver fibrosis[13-15]. TE does not require dedicated ultrasound knowledge and has a fast learning curve[16]. In contrast to liver biopsy, TE is not only non-invasive but has a 10 times smaller sampling error (3% vs 30%) and a better inter-observer reliability rendering it ideal for follow-up studies[15,17]. While a physiological low LS below 6 kPa has an excellent predictive power to rule out fibrosis, several studies in adults have demonstrated an elevated LS in the absence of fibrosis[15,18-21] by conditions such as inflammation, cholestasis, congestion, elevated arterial pressure e.g. during exercise, rapid changes of the portal flow and food and alcohol intake[22-26]. Taken these observations together, LS seems to represent the sum of fibrosis and pressure-related factors being determined by the hepatic inflow and outflow balance[15,27]. In a preliminary study, we had recently demonstrated a significant elevation of LS during the third trimester and a rapid normalization in four cases after delivery[28].
We here present the final prospective data of LS in a large cohort of pregnant women using Fibroscan with the aim to investigate evolution of LS during pregnancy in healthy pregnant women and the association of LS to pregnancy complications. In addition, the performance of Fibroscan and its potential association with complications of pregnancy such as preeclampsia and ICP was studied. Our extended study confirms that LS is significantly elevated during the final trimester in women with uncomplicated pregnancy and rapidly normalizes after delivery. We further demonstrate, however, that LS is significantly elevated in patients with preeclampsia being an independent predictor of this complication.
The study protocol (435/2006 and S201/2015) was reviewed and approved by the local Ethics Committee and all patients gave written informed consent prior to inclusion. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
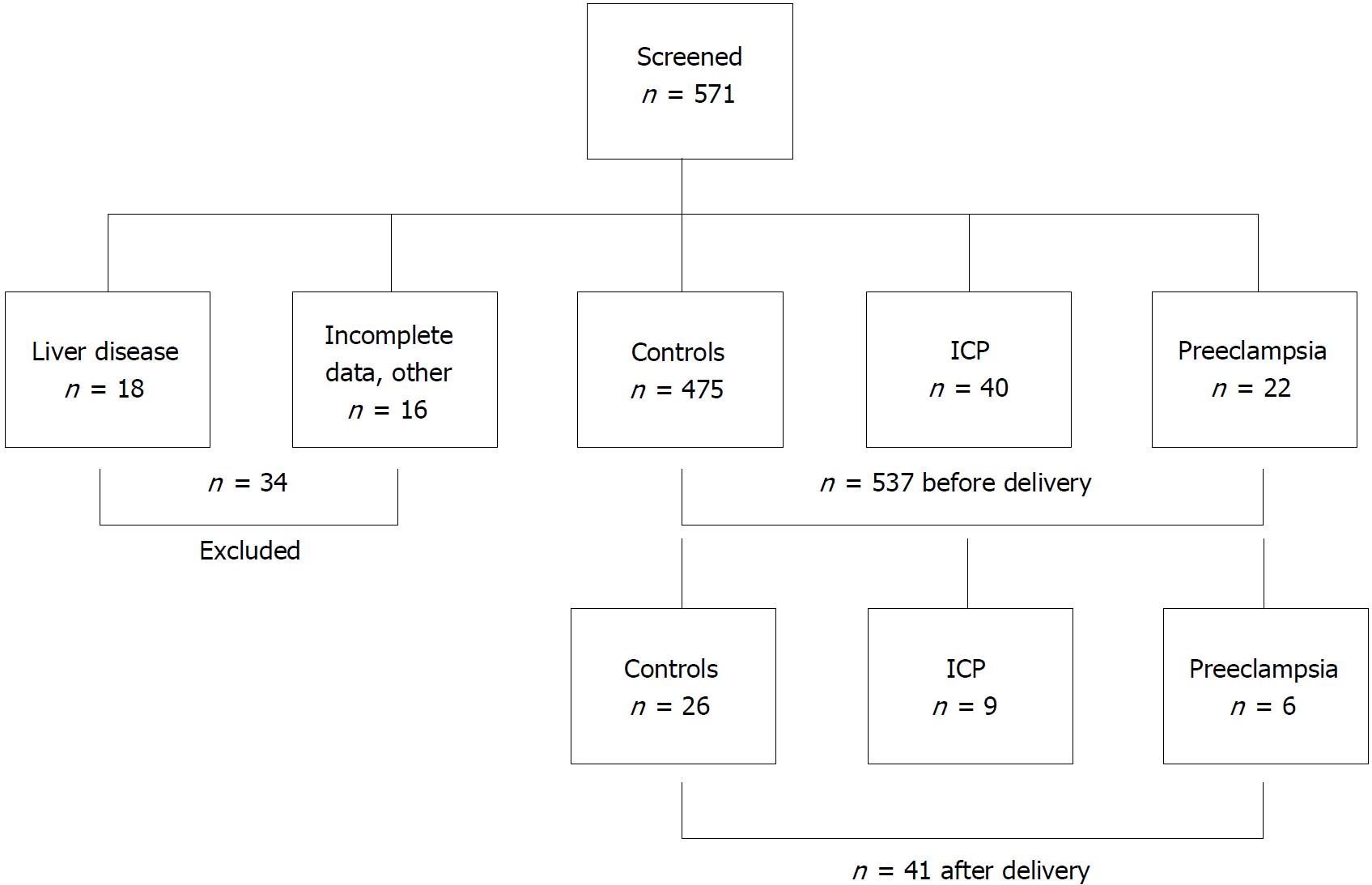
Figure 1 shows the study design. Three study aims were pursued: The primary aim was to measure LS in pregnant women with normal pregnancy and without preexisting liver disease. Here, we also studied the performance of both M and XL probes in order to obtain valid LS measurements and potential confounders that are correlated with an elevated LS. The second aim was to check whether LS is elevated in women with pregnancy-related complications such as preeclampsia or ICP and to analyze the associated conditions. Third, we studied the response of elevated LS directly 24 h after delivery.
Women were either recruited while undergoing prenatal ultrasound screening during gestational week 12 to 33 or presented to the prenatal outpatient’s department with prenatal complications or abnormalities from gestational week 16 to 42.
Postpartum examination (n = 41) took place 24 h after delivery. For every participant of the study general data i.e. weight and age as well as obstetric and hepatic medical history were obtained in form of a personal interview. The 571 participants were recruited at the Department of Gynecology at the University of Heidelberg (n = 468) and at Salem Medical Center in Heidelberg (n = 103). Inclusion criteria were age ≥ 18 years, informed and written consent by the participant and an intact pregnancy at week 9 to 42 or status postpartum (see Figure 1). Exclusion criteria were presence of chronic liver disease either by the patient`s history and in addition by ruling out serum markers with evidence for viral hepatitis, autoimmune hepatitis, hemochromatosis or Wilson’s disease. In addition, a normal routine laboratory tests before pregnancy and alcohol consumption less than 20 g/d were required. Fatty liver in the ultrasound was no exclusion criterium per se. Gynecological exclusion criteria included fatal abnormalities of the fetus or patients being in labor and maternal drug abuse. Patients were also excluded if data were not complete. Altogether, 34 women were excluded with evidence for preexisting liver disease in the majority of cases (see Figure 1).
Patients were considered as control group if no complications occurred and delivery was normal during follow-up. Preeclampsia (hypertonia during pregnancy and proteinuria > 300 mg per day) and ICP (elevated bile acids > 10 μmol/L and pruritus or bile acids > 40 μmol/L) according to standard guidelines[6] were considered as complications of pregnancy. Very rare complications such as Acute Fatty Liver of Pregnancy were not diagnosed during the inclusion period. Two cases of HELLP syndrome were excluded since LS could not be determined reliably due to intrahepatic hematoma.
Blood tests on liver enzymes, bile acids, common electrolytes, coagulation and blood count were performed by the general laboratory of the University Hospital Heidelberg at the same day of TE measurement.
LS and controlled attenuation parameter (CAP) were measured by transient elastography (Fibroscan, Echosens, Paris, France) using both the M and XL probes according to the manufacturer’s specifications in fasting conditions for no less than two hours. Briefly, TE was performed by physicians with at least 12 mo of experience in abdominal ultrasound and TE on the right lobe of the liver in intercostal position according to established protocols[26]. The LS values represent the median values of at least 10 consecutive measurements together with the corresponding IQR. LS data obtained by the XL probe was adjusted and standardized to M probe data according to previous findings[29]. In addition, liver size, spleen size, degree of hepatic steatosis (0-3), diameter of the vena cava inferior (VCI) and skin-to-liver capsule distance (SCD) were assessed by abdominal ultrasound.
The statistical analysis was performed using SPSS Statistics version 23.0 (IBM, United States) and Excel 2016 (Microsoft, United States). For group comparisons mean and standard deviations where calculated and to compare means, the t-test and non-parametric Mann-Whitney-U-test were used where appropriate. For conduction of a correlation analysis, the Spearman rank-order correlation coefficient was calculated. A nonlinear curve fit on data with a Boltzmann function was performed using OriginPro 8 (OriginLab Cooperation). Receiver operating curve (ROC) analysis was performed and to identify cutoff values the maximum of the Youden index was calculated. Multivariate binary logistic regression analysis was used to identify independent predictors for pregnancy complications. The statistical methods of this study were reviewed by Thomas Bruckner from Institute of Medical Biometry und Informatics, University of Heidelberg.
Out of 571 screened pregnant women, 34 were excluded either due to preexisting liver disease, insufficient information prior to pregnancy or other more rare exclusion criteria (Figure 1). Upon delivery and complete follow-up, the remaining 537 patients were stratified into a control group with uncomplicated pregnancies (n = 475, 88%), preeclampsia (n = 22, 4.5%) and ICP (n = 40, 7.4%). Table 1 shows the patient characteristics of the three different groups. Mean duration of pregnancy at date of LS measurement was 192 ± 67 d, 169 ± 59 d and 237 ± 18 d in controls, preeclampsia and ICP, respectively. Transaminase levels and LS values were significantly higher in both preeclampsia and ICP groups. Both groups also had a higher degree of steatosis as measured by ultrasound which could not be confirmed using the CAP parameter. Of note, women with preeclampsia had a significantly enlarged spleen and a higher mean arterial pressure (MAP) while body mass index (BMI) and levels of arterial pressure (AP) were higher in the ICP group. Taken together, important differences were observed with regard to LS between all three groups.
| Parameter | Controls (n = 475) | Preeclampsia (n = 22) | aP value | ICP (n = 40) | aP value |
| Age (yr) | 32.3 ± 5.0 | 33.4 ± 2.9 | 0.3069 | 27.8 ± 4.1 | < 0.0001 |
| Gestational age (d) | 192 ± 67 | 169 ± 59 | 0.1330 | 237 ± 18 | 0.0007 |
| Current BMI (kg/m²) | 28.0 ± 6.1 | 29.0 ± 8.6 | 0.4601 | 32.0 ± 8.3 | 0.0001 |
| BMI before pregnancy (kg/m²) | 24.7 ± 5.8 | 25.9 ± 7.8 | 0.3569 | 28.4 ± 9.1 | 0.0002 |
| MAP (kPa) | 11.0 ± 1.6 | 12.1 ± 1.4 | 0.0279 | 10.4 ± 2.0 | 0.2747 |
| Pruritus (present) | 2% | 0% | 0.4948 | 53% | < 0.0001 |
| Nulliparous | 45% | 50% | 0.6345 | 37% | 0.1883 |
| AST (U/L) | 24 ± 23 | 88 ± 122 | < 0.0001 | 105 ± 122 | < 0.0001 |
| ALT (U/L) | 15 ± 15 | 39 ± 35 | < 0.0001 | 174 ± 174 | < 0.0001 |
| AP (U/L) | 134 ± 126 | 115 ± 43 | 0.5955 | 179 ± 69 | 0.0382 |
| GGT (U/L) | 24 ± 48 | 35 ± 52 | 0.4439 | 32 ± 34 | 0.3863 |
| Bilirubin total (mg/dL) | 0.5 ± 0.3 | 0.5 ± 0.2 | 0.5374 | 0.5 ± 0.3 | 0.2561 |
| Creatinine (mg/dL) | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.1308 | 0.8 ± 0.4 | < 0.0001 |
| Urea (mg/dL) | 18 ± 5 | 23 ± 7 | 0.0006 | 22 ± 7 | 0.0001 |
| Uric acid (mg/dL) | 3.8 ± 1.1 | 5.0 ± 1.4 | 0.0019 | 5.8 ± 2.5 | < 0.0001 |
| Total protein (g/L) | 66.1 ± 5.7 | 63.3 ± 4.3 | 0.1114 | 66.9 ± 5.2 | 0.5143 |
| Albumin (g/L) | 36.0 ± 2.2 | 25.5 ± 15.3 | 0.0046 | 36.4 ± 5.5 | 0.7729 |
| Leukocytes (1/nL) | 11.9 ± 4.2 | 9.6 ± 4.6 | 0.0599 | 10.1 ± 3.5 | 0.0158 |
| Erythrocytes (1/pL) | 3.9 ± 0.4 | 4.2 ± 0.4 | 0.0390 | 4.0 ± 0.5 | 0.4560 |
| Hemoglobin (g/dL) | 11.7 ± 1.3 | 12.4 ± 0.9 | 0.0632 | 11.2 ± 1.4 | 0.0313 |
| Platelets (1/nL) | 231 ± 63 | 173 ± 85 | 0.0022 | 231 ± 55 | 0.9790 |
| INR | 0.91 ± 0.03 | 1.01 ± 0.16 | < 0.0001 | 0.91 ± 0.04 | 0.6616 |
| Liver size (cm) | 13.9 ± 1.8 | 13.2 ± 1.5 | 0.0786 | 14.4 ± 1.1 | 0.0812 |
| Spleen size (cm) | 10.9 ± 1.5 | 12.4 ± 2.8 | < 0.0001 | 11.4 ± 1.8 | 0.0954 |
| Diameter VCI (cm) | 1.6 ± 0.3 | 1.8 ± 0.6 | 0.0075 | 1.5 ± 0.3 | 0.1713 |
| Hepatic steatosis in sonography (0-3) | 0.2 ± 0.4 | 0.2 ± 0.5 | 0.7334 | 0.4 ± 0.5 | 0.0174 |
| Liver stiffness (kPa) | 5.3 ± 2.0 | 17.9 ± 20.7 | < 0.0001 | 6.9 ± 2.1 | < 0.0001 |
| > 6 kPa | 120 (25%) | 14 (63%) | 23 (58%) | ||
| > 8 kPa | 30 (6%) | 10 (45%) | 8 (20%) | ||
| > 12.5 kPa | 7 (1.5%) | 9 (40%) | 1 (2.5%) | ||
| CAP (dB/m) | 226 ± 43 | 228 ± 47 | 0.7843 | 215 ± 40 | 0.1322 |
In all women, 10 successful measurements could be obtained. While 90.9% of women could be measured with the M-probe, all women were measurable with the XL-probe. Failure to measure LS with the M probe was related to a skin capsule difference > 2.4 cm or a BMI > 33.5 kg/m2 (data not shown) while skin liver capsule distance (SCD) was more accurate for predicting the need for using the XL probe (Supplementary Table 1).
According to recently published criteria by Boursier et al[30], 16.7% of all measurements had an IQR to Median LS ratio (IQR/M) less or equal 10% (very reliable measurement), in 78.3%, IQR/M was between 10 and 30% (reliable measurement) and in 5.1% of cases, IQR/M was greater than 30% (poorly reliable). However and in line with previous findings[31], all data were used for further analysis. In summary, the Fibroscan device performs well in most pregnant women using the M probe and in all with the XL probe.
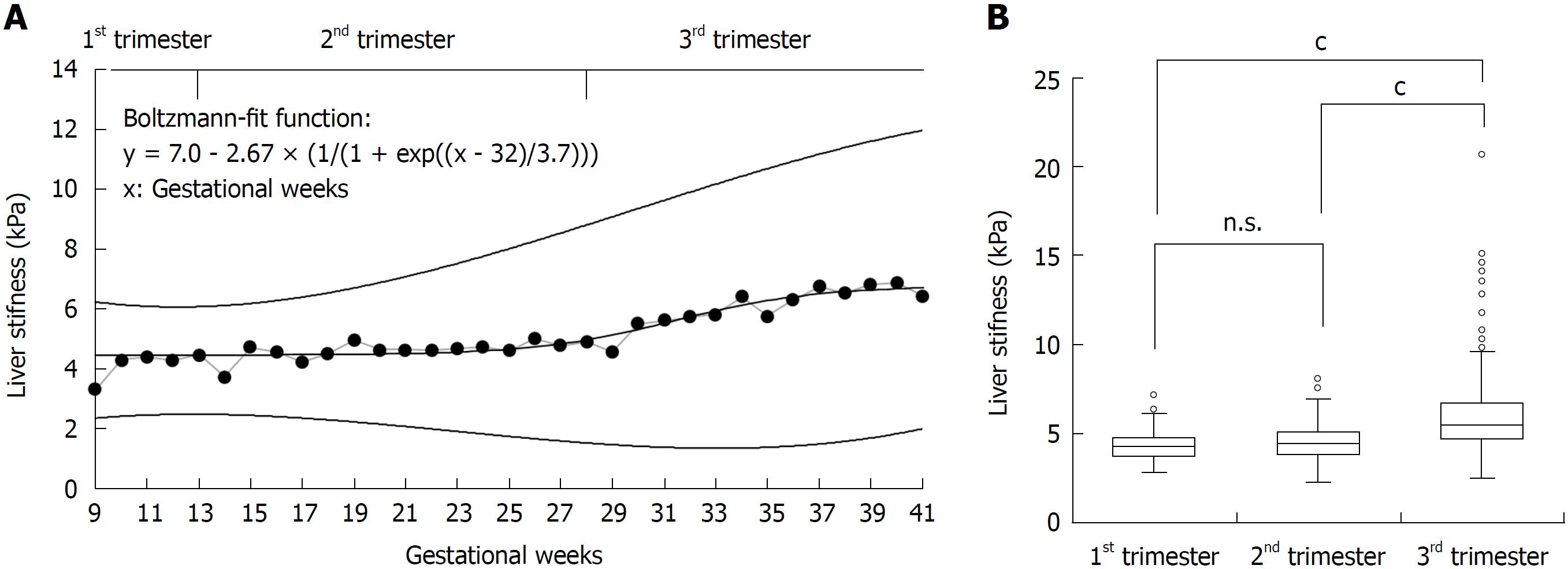
An elevated LS > 6 kPa was seen in 25% of healthy pregnant woman despite uncomplicated pregnancy. LS was higher than 8 and 12.5 kPa in 6% and 1.5%, respectively, the latter being typically regarded as cut-off value for cirrhosis[15,32]. LS increased almost exclusively in the third trimester (Figure 2). Here, 105 of 256 women (41%) showed an elevated LS > 6 kPa. Figure 2A shows LS averaged per week over the course of pregnancy and percentiles 5% and 95% calculated from the standard deviation per week. LS stayed at mean values of 4.5 kPa until week 26 and increased thereafter. The values reached a plateau by gestational week 39 at a mean value of 6.7 kPa. To represent those changes, we calculated the mean values at each week using a non-linear regression fitting (modified Boltzmann fit). The Boltzmann fit showed a good R2 of 0.86.
Figure 2B demonstrates the distribution of LS values for all three trimesters as box plot. LS was significantly elevated in the third trimester with a mean of 6.0 ± 2.3 kPa (P < 0.0001) in comparison to women in the first or second trimester with mean LS values of 4.3 ± 1.0 kPa and 4.5 ± 1.2 kPa, respectively. Taken together, our study demonstrates a significant increase of LS during uncomplicated pregnancy almost exclusively in the third trimester.
Focusing on the control group, we analyzed morphological, laboratory and sonographic parameters to identify potential confounders of elevated LS. Correlation analysis (Table 2) showed that LS is not only positively correlated with gestational age (0.50, P < 0.0001), but also with related parameters such as increase in body weight (0.28, P < 0.0001), change of BMI (0.33, P < 0.0001) and current BMI (0.31, P < 0.0001). In addition, LS was also positively correlated with AP (0.31, P < 0.001) and negatively with total protein (r = -0.35, P < 0.001). A weak association was seen with spleen size and hepatic steatosis in ultrasound which could not be confirmed based on the non- invasive CAP parameter. In multivariate logistic regression analysis (Supplementary Table 2), using the binary state LS > 6 kPa as dependent variable, cumulative estimated fetus weight and gestational age were independent predictors for LS > 6 kPa [odds ratios exp(B) = 1.003, P = 0.012 and exp(B) = 1.016, P = 0.027, respectively], while nulliparous state, current body weight and increase in body weight were not significant.
| Spearman correlations | All | Control | Preeclampsia | ICP |
| General | ||||
| Gestational age (d) | 0.462c | 0.499c | -0.372 | 0.017 |
| Age (yr) | -0.071 | -0.035 | 0.198 | -0.249 |
| Current body weight (kg) | 0.296c | 0.287c | 0.195 | 0.193 |
| Body weight before pregnancy (kg) | 0.182c | 0.161c | 0.315 | 0.175 |
| Increase in body weight (kg) | 0.314c | 0.325c | -0.184 | 0.233 |
| Current BMI (kg/m²) | 0.316c | 0.313c | 0.313 | 0.169 |
| BMI before pregnancy (kg/m²) | 0.188c | 0.169c | 0.686c | 0.175 |
| Increase in BMI (kg/m²) | 0.322c | 0.328c | -0.118 | 0.269 |
| MAP (kPa) | 0.154c | 0.03 | 0.082 | 0.464 |
| pruritus | 0.194c | -0.034 | 0.471b | |
| Nulliparous state | 0.131b | 0.129b | 0.287 | 0.383b |
| Laboratory | ||||
| Creatinine (mg/dL) | 0.138 | 0.045 | -0.478 | 0.048 |
| Urea (mg/dL) | 0.064 | -0.068 | -0.459 | 0.063 |
| AST (U/L) | 0.267c | 0.04 | 0.588a | 0.325a |
| ALT (U/L) | 0.286c | 0.103 | 0.354 | 0.28 |
| AP (U/L) | 0.297c | 0.313c | -0.091 | 0.128 |
| GGT (U/L) | 0.228b | 0.164 | 0.717b | -0.038 |
| Bilirubin total (mg/dL) | 0.027 | -0.128 | 0.754b | 0.257 |
| Uric acid (mg/dL) | 0.283c | 0.152 | -0.157 | 0.059 |
| Total protein (g/L) | -0.201a | -0.350c | -0.018 | -0.018 |
| Albumin (g/L) | -0.199 | -0.195 | -0.5 | -0.21 |
| Leukocytes (1/nL) | -0.346c | -0.205a | -0.834c | -0.595c |
| Erythrocytes (1/pL) | 0.169a | 0.133 | -0.473 | 0.181 |
| Hemoglobin (g/dL) | 0.024 | 0.073 | -0.767b | 0.064 |
| Platelets (1/nL) | -0.132 | 0.025 | -0.790b | -0.480b |
| Quick-test (%) | -0.029 | 0.086 | -0.814b | 0.017 |
| INR | -0.013 | -0.132 | 0.802b | -0.038 |
| Bile acid (µmol/L) | 0.698c | 0.393 | 0.698c | |
| Sonography | ||||
| Liver size (cm) | 0.031 | 0.027 | 0.357 | -0.247 |
| Spleen size (cm) | 0.167b | 0.112a | 0.587b | -0.071 |
| Diameter VCI (cm) | -0.009 | -0.035 | 0.387 | 0.144 |
| Level of hepatic steatosis (0-3) | 0.211c | 0.186c | 0.343 | 0.309 |
| CAP (dB/m) | 0.075 | 0.081 | 0.775c | 0.02 |
| Cumulative estimated fetus weight (g) | 0.244a | 0.223 |
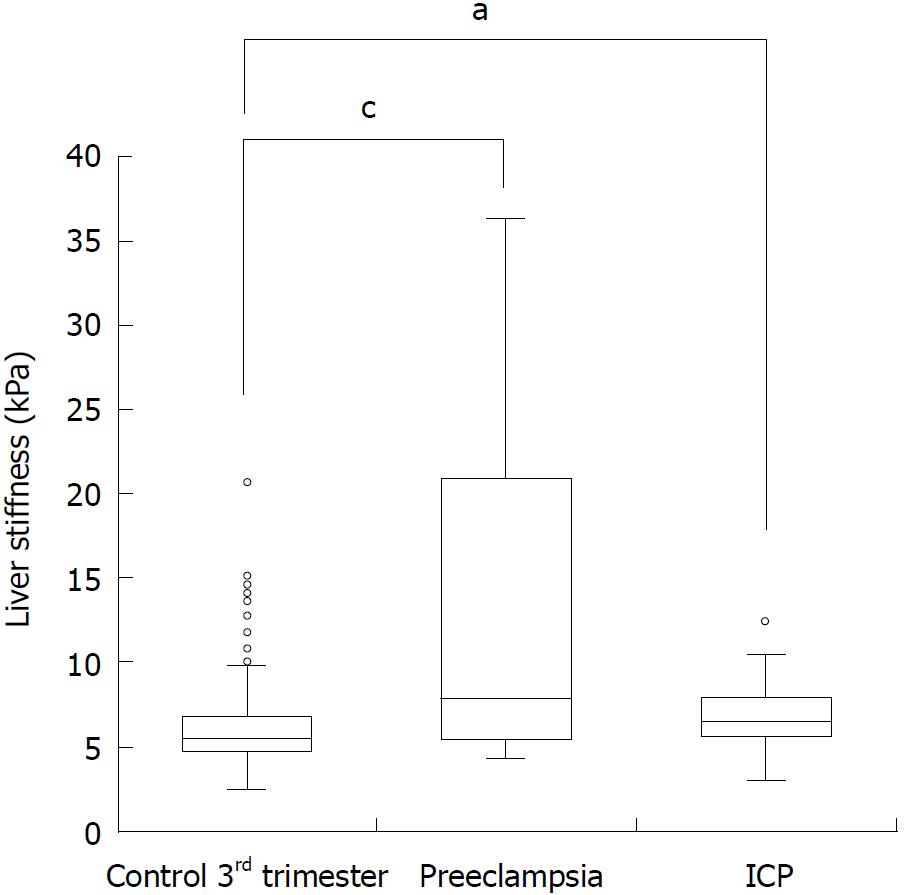
Women with preeclampsia and ICP had significantly higher LS as compared to controls (5.3 ± 1.9 kPa) with mean LS values of 17.9 ± 20.7 kPa (P < 0.0001) and 6.9 ± 2.1 kPa (P < 0.0001), respectively. This was also the case when only focusing on women in the third trimester (Figure 3). In multivariate logistic regression analysis, when comparing with important laboratory screening markers during pregnancy (Supplementary Figure 1A), LS and leukocyte count were independent predictors for preeclampsia with an odds ratio of 2.05 (1.27-3.31) and 1.39 (1.03-1.88), respectively. In contrast, the presence of pruritus and elevated ALT levels were highly predictive for ICP while no additional independent information could be obtained from LS. LS predicted preeclampsia with a fairly good area under the curve (AUROC) of 0.815 (0.722-0.907) and an optimal cut-off value of 7.6 kPa (Supplementary Figure 2). Using these values, preeclampsia was detected with a sensitivity of 0.55 (0.35-0.73) and specificity of 0.92 (0.89-0.94). In summary, an elevated LS seems to be an independent predictor of preeclampsia that is superior to established criteria such as arterial hypertension.
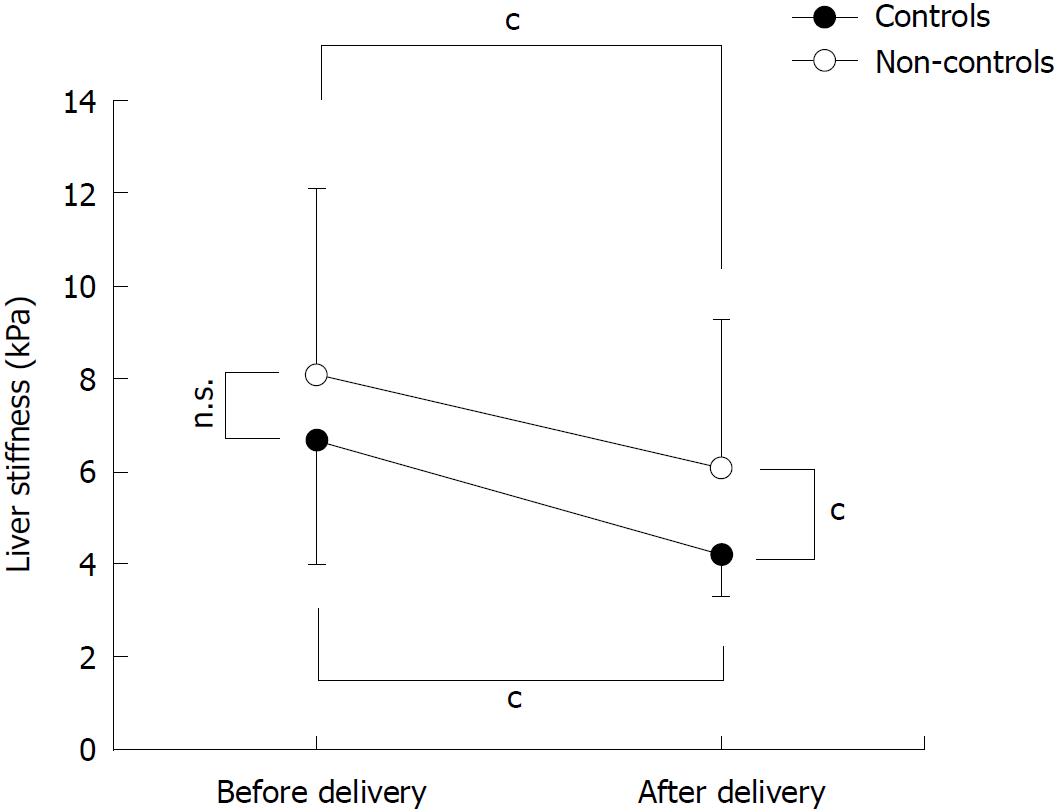
In a subgroup of 41 women, we were able to measure LS in the third trimester of pregnancy and 24 h after delivery (Figure 4). 26 women had uncomplicated pregnancy while ICP and preeclampsia were seen in 9 and 6 cases, respectively (Figure 1). Due to the small sample size, cases with preeclampsia and ICP are combined and depicted as non-controls in Figure 4. LS decreased significantly in all women after delivery from 7.2 ± 3.3 kPa to 4.9 ± 2.2 kPa (P < 0.001, Figure 3). Significant differences were observed between controls and non-controls after delivery but not before. Mean LS before delivery was 6.7 ± 2.7 kPa and 8.1 ± 4.0 kPa in controls and non-controls, respectively (P = 0.18). After delivery, mean LS in controls was significantly lower as compared to non-controls (4.2 ± 1.0 kPa vs 5.6 ± 1.3 kPa; P < 0.001). Of note, LS normalized in all controls to values below 6 kPa while LS remained > 6 kPa in three non-controls with 6.1, 8.1 and 16.8 kPa, respectively. In summary, LS rapidly decreases after delivery. It even normalized in all women without pregnancy complications.
We here show that LS can be easily measured in all women during pregnancy. We demonstrate, first, that LS significantly increases above 6 kPa in a large fraction of pregnant women without complications almost exclusively in the third trimester. Second, women with preeclampsia show even higher LS values and an elevated LS is an independent predictor of this complication. Finally, elevated pregnancy- associated LS rapidly decreased in all women after delivery.
Our data indicate that LS elevation during pregnancy should be interpreted with caution and not be mistaken for a manifest liver disease since it will most likely be reversible after delivery. Second, the significant elevation of a fundamental liver parameter such as LS in such a large fraction of pregnant women suggests that LS itself or its underlying causes could contribute to some of the rare but often fatal complications during late pregnancy[27]. Indeed, elevated LS was significantly higher in women with complications such as preeclampsia or ICP. Moreover, an elevated LS > 7.6 kPa was identified as independent predictor of preeclampsia with a fairly good AUROC of 0.815 being superior to other laboratory markers and arterial pressure. Thus, our data suggest that LS could be a valuable non-invasive tool to screen pregnant women for potential complications.
What are the mechanisms that underlie elevated LS during pregnancy? The rapid normalization of LS after delivery is suggestive of e.g. mechanic, pressure-related conditions of pregnancy in contrast to other e.g. inflammatory or apoptotic conditions. Thus, liver inflammation or apoptosis typically do not resolve within 24 h[33]. Indeed, in healthy women, we could not see any association of LS with laboratory parameters such as transaminases as observed in liver disease[31,33]. The rapid decrease of LS rather suggests potential mechanic factors such as an elevated intraabdominal pressure and its hemodynamic consequences that would rapidly normalize after deliver. Such an explanation would also be in line with the recently proposed inflow/outflow balance model of LS based on liver perfusion through the incoming portal vein and hepatic artery and the outflowing hepatic veins[21-24,27]. The significant association of LS with spleen size could be a further indirect link between portal hypertension and perfusion-associated LS elevation. Unfortunately, our study does not provide detailed insights into the hemodynamics since no information on blood flow and resistance was obtained.
Second, it is known for a long time that pregnancy causes water retention that can contribute to a large extent to weight gain in pregnant women. The underlying mechanisms involve the RAAS system and are comparable to heart failure and liver cirrhosis[34]. In addition, LS can be drastically elevated by water retention e.g. during heart failure[22,27]. Indirect evidence can be derived from the negative correlation between LS and serum protein levels. Unfortunately, however, we could not clearly discriminate between weight gain from water retention or obesity in our study.
Moreover, parameters of steatosis such as ultrasound (US) and CAP did not correlate significantly with LS. It also remains unclear while indirect markers of water retention such as an enlarged caval vein did not show a significant association with LS although it was related with complications. One explanation could be the drastically elevated intraabdominal pressure in pregnant women preventing an unfolding of the lower caval vein[35].
Third, an increased cardiac output of pregnant women may be also responsible for the elevated LS since exercise and arterial pressure have been recently identified as important determinants[23,35]. Notably, in our large study, no significant association could be observed between MAP and LS although a recent small study identified a relation between postpartum LS and arterial pressure in women with eclampsia[36]. Here, larger more detailed studies are required to shed light on the role of arterial pressure on LS during pregnancy.
Fourth, the strong association of elevated LS with parameters such as weight and BMI point towards an important role of elevated intraabdominal pressure. This is further underlined by the strong association with child-related parameters such as fetus weight. However, we have recently shown that an elevated intraabdominal pressure per se does not increase LS in the absence of liver congestion[37]. Thus, it is known that the uterus can compress e.g. the inferior caval vein during the continued growth of the fetus[38]. Again, our study was not able to provide further detailed insights regarding liver congestion or impaired hepatic vein outflow. Another fascinating argument for a mechanic explanation of LS elevation during pregnancy is the weak positive correlation of LS with the nulliparous state. It is well established in the literature that previous uncomplicated deliveries lower the risk for birth complications. The fact that pregnant women with their first pregnancy have a significantly higher LS could point towards a mechanic training or mechanic adaption that could be potentially obtained during the first pregnancies.
In conclusion, we here show that LS can be obtained easily in pregnant women. LS significantly increases in the third trimester despite normal pregnancy and rapidly normalizes after delivery. However, LS is significantly increased in women with preeclampsia and an independent predictor of this complication. Some findings and especially the rapid normalization of LS after delivery is highly suggestive for mechanic e.g. hemodynamic reasons as underlying mechanism while direct liver injury is less likely. More detailed prospective studies are needed to clarify LS elevation during pregnancy. Nevertheless, our data are highly encouraging to more frequently use non-invasive LS measurements in pregnant women with suspicion of pregnancy-related complications.
Hypertension and preeclampsia remain the leading causes of maternal morbidity and mortality. Moreover, up to 3 % of all pregnancies are accompanied by liver complications which can have a high maternal and perinatal mortality. So far, no detailed studies existed on pregnancy and liver stiffness, a novel parameter and gold standard to early screen for liver fibrosis and other pathologies. We here present the first large cohort to study liver stiffness during pregnancy and its association with complications during pregnancy. Our data show that liver stiffness significantly and reversibly increases in the final trimester in pregnant women without complications. In women with preeclampsia, liver stiffness is significantly elevated and an independent non-invasive predictor. In conclusion, we think that liver stiffness could be an easy to use and fast non-invasive tool for the prediction and diagnosis of pregnancy complications.
Many liver-related complications during pregnancy are still poorly understood, life threatening and difficult to diagnose. The main motivation for this study, therefore, was to investigate the novel parameter liver stiffness for the first time in a large cohort of pregnant women and its relation to pregnancy complications. Based on first preliminary observations, it was our further goal to provide the normal range of liver stiffness values during pregnancy and its response to delivery.
The first aim was to test the performance and validity of liver stiffness measurements using FibroScan in pregnant women with and without pregnancy complications. The second aim was to investigate liver stiffness in women with normal pregnancies at different gestational ages and its response to delivery. We eventually demonstrate that liver stiffness can be measured in pregnant women with sufficient accuracy using the XL probe. We further demonstrate an elevated liver stiffness in the final trimester of pregnant women without complications. Interestingly, liver stiffness can reach levels that could suggest the presence of advanced liver fibrosis. Finally, we show that liver stiffness is higher in patients with pregnancy complications such as preeclampsia or intrahepatic cholestasis of pregnancy and can even predict these complications. Our data pave the way for more detailed studies of liver stiffness in pregnant women in the future e.g. with the aim to investigate associations with more rare complications such as the hemolysis, elevated liver enzymes and low platelets (HELLP)-syndrome or acute fatty liver of pregnancy.
We prospectively recruited 537 pregnant women including 22 with preeclampsia and 40 with intrahepatic cholestasis. Liver stiffness was measured by transient elastography (FibroScan, Paris) with the M and XL-probe. Additionally, transabdominal ultrasound and standard laboratory tests were performed. In some patients, liver stiffness was also measured 24 h after delivery. This study design allowed us to demonstrate elevated liver stiffness in the third trimester during uncomplicated pregnancy and its normalization after delivery. In addition, however, elevated liver stiffness was identified as sensitive and early prognostic parameter of pregnancy complications such as preeclampsia and intrahepatic cholestasis of pregnancy.
We first demonstrate that valid liver stiffness measurements can be obtained in most women (> 95%) using the XL-probe. Second, liver stiffness increases from ca. 4.5 kPa to 6 kPa (P < 0.001) in the third trimester of women with normal pregnancy. Importantly, 41% of women in the third trimester showed a liver stiffness above 6 kPa; in rare cases even higher than 20 kPa. Of note, elevated liver stiffness here was significantly correlated with alkaline phosphatase, leukocytes, gestational age and an increase in body weight and body mass index (BMI). Women with pregnancy complications such as preeclampsia or intrahepatic cholestasis had significantly higher liver stiffness measurements than women with uncomplicated pregnancy (P < 0.0001). Results even stayed significant when compared only to women in the third trimester (P < 0.01). Moreover, in multivariate analysis, liver stiffness could be identified as an independent predictor for preeclampsia with an odds ratio of 2.05 (1.27-3.31). Finally, liver stiffness rapidly decreased in all women within 24 h after delivery from 7.2 ± 3.3 kPa down to 4.9 ± 2.2 kPa (P < 0.001).
For the first time, this study investigates liver stiffness in a large prospective cohort of pregnant women prior and after delivery. It shows that liver stiffness can be accurately measured in pregnant women. Surprisingly, liver stiffness significantly increased in the final trimester but normalized after delivery. Thus, an elevated liver stiffness during pregnancy should not be mistaken for a liver disease and e.g. prompt to further invasive diagnostic measures. Furthermore, liver stiffness is significantly higher in women with pregnancy complications and it can independently predict complications such as preeclampsia. Although an exact molecular cause of the liver stiffness elevation could not be identified, its rapid normalization after delivery in the absence of inflammation highly suggests mechanical factors e.g. hemodynamic changes as underlying cause. The findings are also in line with our recently introduced sinusoidal pressure hypothesis that highlights the role of pressure in modulating liver stiffness and eventually causing liver complications. Based on our findings we also suggest to routinely assess liver stiffness during pregnancy in order to early identify women at risk of preeclampsia.
Transient elastography is a promising non-invasive, easy to use method to study liver stiffness during pregnancy. In future research, broader and more detailed studies are needed to investigate the cause for liver stiffness elevation in the third trimester and in complicated pregnancies and to investigate other aspects such as HELLP-syndrome or acute fatty liver of pregnancy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
STROBE Statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
P- Reviewer: Kamimura K S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
| 1. | Hill K, AbouZhar C, Wardlaw T. Estimates of maternal mortality for 1995. Bull World Health Organ. 2001;79:182-193. [PubMed] |
| 2. | Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2100] [Cited by in RCA: 2256] [Article Influence: 150.4] [Reference Citation Analysis (0)] |
| 3. | Clark SL, Belfort MA, Dildy GA, Herbst MA, Meyers JA, Hankins GD. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol. 2008;199:36.e1-5; discussion 91-92.e7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 361] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 4. | Hay JE. Liver disease in pregnancy. Hepatology. 2008;47:1067-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Westbrook RH, Dusheiko G, Williamson C. Pregnancy and liver disease. J Hepatol. 2016;64:933-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Joshi D, James A, Quaglia A, Westbrook RH, Heneghan MA. Liver disease in pregnancy. Lancet. 2010;375:594-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Mihu D, Costin N, Mihu CM, Seicean A, Ciortea R. HELLP syndrome - a multisystemic disorder. J Gastrointestin Liver Dis. 2007;16:419-424. [PubMed] |
| 8. | Kelly C, Pericleous M. Pregnancy-associated liver disease: a curriculum-based review. Frontline Gastroenterol. 2018;9:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Kamimura K, Abe H, Kawai H, Kamimura H, Kobayashi Y, Nomoto M, Aoyagi Y, Terai S. Advances in understanding and treating liver diseases during pregnancy: A review. World J Gastroenterol. 2015;21:5183-5190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 10. | Kondrackiene J, Kupcinskas L. Liver diseases unique to pregnancy. Medicina (Kaunas). 2008;44:337-345. [PubMed] |
| 11. | Suresh I, Tr V, Hp N. Predictors of Fetal and Maternal Outcome in the Crucible of Hepatic Dysfunction During Pregnancy. Gastroenterology Res. 2017;10:21-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 12. | Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J Hum Hypertens. 2010;24:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] |
| 14. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 955] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 15. | Mueller S, Sandrin L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepat Med. 2010;2:49-67. [PubMed] |
| 16. | Nahon P, Thabut G, Ziol M, Htar MT, Cesaro F, Barget N, Grando-Lemaire V, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement versus clinicians’ prediction or both for the assessment of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2006;101:2744-2751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, Pirisi M. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838-845. [PubMed] |
| 18. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 19. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [PubMed] |
| 20. | Dechêne A, Sowa JP, Gieseler RK, Jochum C, Bechmann LP, El Fouly A, Schlattjan M, Saner F, Baba HA, Paul A. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology. 2010;52:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 463] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 22. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 23. | Piecha F, Peccerella T, Bruckner T, Seitz HK, Rausch V, Mueller S. Arterial pressure suffices to increase liver stiffness. Am J Physiol Gastrointest Liver Physiol. 2016;311:G945-G953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Piecha F, Paech D, Sollors J, Seitz HK, Rössle M, Rausch V, Mueller S. Rapid change of liver stiffness after variceal ligation and TIPS implantation. Am J Physiol Gastrointest Liver Physiol. 2018;314:G179-G187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009;29:1500-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 26. | Mueller S, Seitz HK, Rausch V. Non-invasive diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:14626-14641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 27. | Mueller S. Does pressure cause liver cirrhosis? The sinusoidal pressure hypothesis. World J Gastroenterol. 2016;22:10482-10501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Kohlhaas A, Millonig G, Schütz F, Seitz HK, Mueller S. Liver stiffness during pregnancy. J Hepatol. 2011;54:134-135. [DOI] [Full Text] |
| 29. | Durango E, Dietrich C, Seitz HK, Kunz CU, Pomier-Layrargues GT, Duarte-Rojo A, Beaton M, Elkhashab M, Myers RP, Mueller S. Direct comparison of the FibroScan XL and M probes for assessment of liver fibrosis in obese and nonobese patients. Hepat Med. 2013;5:43-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Jérôme B, Jean-Pierre Z, Victor dL, Marie-Christine R, Nathalie S, Brigitte L, Isabelle F-H, Yves G, Frédéric O, Sandrine B, Paul C. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 496] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 31. | Mueller S, Englert S, Seitz HK, Badea RI, Erhardt A, Bozaari B, Beaugrand M, Lupsor-Platon M. Inflammation-adapted liver stiffness values for improved fibrosis staging in patients with hepatitis C virus and alcoholic liver disease. Liver Int. 2015;35:2514-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 33. | Mueller S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, Eisele S, Stickel F, Longerich T, Schirmacher P. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16:966-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Schrier RW, Cadnapaphornchai MA, Ohara M. Water retention and aquaporins in heart failure, liver disease and pregnancy. J R Soc Med. 2001;94:265-269. [PubMed] |
| 35. | Moll W. [Physiological cardiovascular adaptation in pregnancy--its significance for cardiac diseases]. Z Kardiol. 2001;90 Suppl 4:2-9. [PubMed] |
| 36. | Frank Wolf M, Peleg D, Kariv Silberstein N, Assy N, Djibre A, Ben-Shachar I. Correlation between changes in liver stiffness and preeclampsia as shown by transient elastography. Hypertens Pregnancy. 2016;35:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Kohlhaas A, Durango E, Millonig G, Bastard C, Sandrin L, Golriz M, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Transient elastography with the XL probe rapidly identifies patients with non-hepatic ascites. Hepat Med. 2012;4:11-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Scott DB. Inferior vena caval occlusion in late pregnancy and its importance in anaesthesia. Br J Anaesth. 1968;40:120-128. [PubMed] |