Published online Oct 14, 2018. doi: 10.3748/wjg.v24.i38.4369
Peer-review started: June 13, 2018
First decision: July 4, 2018
Revised: July 11, 2018
Accepted: August 1, 2018
Article in press: August 1, 2018
Published online: October 14, 2018
Processing time: 121 Days and 8.1 Hours
To evaluate the sex-specific effects of a hydroalcoholic extract from Eugenia punicifolia (HEEP) leaves on gastric ulcer healing.
In this rat study involving males, intact (cycling) females, and ovariectomized females, gastric ulcers were induced using acetic acid. A vehicle, lansoprazole, or HEEP was administered for 14 d after ulcer induction. Body weight was monitored throughout the treatment period. At the end of treatment, the rats were euthanized and the following in vivo and in vitro investigations were performed: macroscopic examination of the lesion area and organ weights, biochemical analysis, zymography, and evaluation of protein expression levels. Additionally, the concentration-dependent effect of HEEP was evaluated in terms of subacute toxicity and cytotoxicity.
Compared to the vehicle, HEEP demonstrated a great healing capacity by substantially reducing the ulcerative lesion area in males (52.44%), intact females (85.22%), and ovariectomized females (65.47%), confirming that HEEP accelerates the healing of acetic acid-induced gastric lesions and suggesting that this effect is modulated by female sex hormones. The antiulcer effect of HEEP was mediated by prostaglandin E2 only in male rats. Overall, the beneficial effect of HEEP was the highest in intact females. Notably, HEEP promoted the expression of vascular endothelial growth factor (intact vs ovariectomized females) and decreased the expression of Caspase-8 and Bcl-2 (intact female vs male or ovariectomized female). Additionally, HEEP enhanced fibroblast proliferation and migration into a wounded area in vitro, confirming its healing effect. Finally, no sign of subacute toxicity or cytotoxicity of HEEP was observed.
In gastric ulcers, HEEP-induced healing (modulated by female sex hormones; in males, mediated by prostaglandin) involves extracellular matrix remodeling, with gastric mucosa cell proliferation and migration.
Core tip: Gastric ulcers, which occur due to an imbalance between protective and aggressive agents at the gastric mucosa surface, is a chronic disease that affects millions worldwide and has high rates of relapse. The conventional treatment for gastric ulcers is associated with several side effects and poor healing of the gastric mucosa. Eugenia punicifolia is a medicinal plant used to treat inflammation and wounds. The present study in rats with gastric ulcers confirms the healing effect of Eugenia punicifolia extract and clarifies its differential effect in males and females. These findings are useful for developing novel and safe therapies for gastric ulcers.
- Citation: Périco LL, Rodrigues VP, Ohara R, Bueno G, Nunes VVA, dos Santos RC, Camargo ACL, Júnior LAJ, de Andrade SF, Steimbach VMB, da Silva LM, da Rocha LRM, Vilegas W, dos Santos C, Hiruma-Lima CA. Sex-specific effects of Eugenia punicifolia extract on gastric ulcer healing in rats. World J Gastroenterol 2018; 24(38): 4369-4383
- URL: https://www.wjgnet.com/1007-9327/full/v24/i38/4369.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i38.4369
Gastric ulcers, which result from an imbalance between the protective and aggressive agents at the surface of the gastric mucosa, is a chronic disease that affects millions around the world and has high relapse rates[1,2]. Alcohol consumption, prolonged treatment with non-steroidal anti-inflammatory drugs, stress, and Helicobacter pylori infection favor such an imbalance and represent key etiological factors of gastric ulcers[3]. Current treatment strategies for gastric ulcers involve the use of anti-secretory drugs, including antagonists of histamine receptor type 2 (e.g., ranitidine) and proton pump inhibitors [e.g., lansoprazole (LZ)], as well as antibiotics for the eradication of Helicobacter pylori. While such treatments are effective, prolonged use of anti-secretory drugs, especially ranitidine and proton pump inhibitors, is associated with several side effects[4] and poor healing of the gastric mucosa[5].
In 1991, Tarnawski et al[6] proposed the concept of quality of ulcer healing, which takes into consideration the fact that tissue regeneration within the ulcer scar is often incomplete. Within the quality of ulcer healing concept, the evaluation of gastric ulcer healing is focused on whether the structure and function of the mucosal and submucosal tissue have recovered completely, in addition to endoscopic examination and evaluation of ulcer size. It has been shown that ulcer recurrence is closely related to the quality of ulcer healing[7].
Ulcers frequently recur following treatment with anti-secretory drugs[7,8]. Therefore, alternative therapies for gastric ulcers are desirable[9]. Herbal combination preparations are popular among traditional herbal medicine practitioners. The rationale behind such combinations is frequently questioned, and it remains challenging to assess the individual contribution of each component to the overall activity of the herbal combination preparation. This holds especially true when the preparation is used in the treatment of a chronic multifactorial disease such as gastric ulcers[10].
Eugenia punicifolia (Kunth) DC (Myrtaceae), popularly known as pedra-ume-caá, pedra-ume, murta, or muta, is a shrub found mainly in the Savanna biome and in the Amazon region. The leaves of E. punicifolia are popularly used as a natural remedy for inflammation[11], wounds, infections[12], diabetes[13], fever, and flu[14,15]. The gastroprotective activity of the hydroalcoholic extract of E. punicifolia (Kunth) DC leaves (HEEP) against ethanol- or non-steroidal anti-inflammatory drug-induced ulcers in rodents has been reported[16]. Nevertheless, it remains unclear whether HEEP has any beneficial effects in the healing of installed gastric ulcers, since the gastroprotective activities of an extract do not ensure their gastric healing effects in installed gastric ulcers[17]. Therefore, the present study aimed to evaluate the sex-specific effects of HEEP in the healing of gastric ulcers in a rat model. For this purpose, we employed a rat model of acetic acid-induced gastric ulcers and analyzed the curative action of HEEP in males, intact females, and ovariectomized females.
The following chemicals and reagents were used: acetic acid, methanol (Dinamica Contemporary Chemicals™, Diadema, São Paulo, Brazil), LZ (Cruz Vermelha Pharmacy of Manipulation, Botucatu, São Paulo, Brazil), RIPA buffer, protease inhibitor cocktail, Ethylenediaminetetraacetic acid (EDTA), N,N,N′,N′-Tetramethylethylenediamine (TEMED), ammonium persulfate (APS), sodium chloride, 400 g/L acrylamide/bis-acrylamide solution, Triton X-100, Tris-HCl, calcium chloride, zinc chloride, coomassie brilliant blue, tris base, glycine, sodium dodecyl sulfate (SDS), glycerol, Tween 20, β-mercaptoethanol, bromophenol blue, Ponceau (Sigma Chemical Co., St. Louis, MO, United States) gelatin (Merck KGaA, Darmstadt, Germany). Saline solution (9 g/L NaCl) was used to dilute the hydroalcoholic extract from the leaves of E. punicifolia. The same saline solution used as a vehicle served as a negative control.
In December 2009, Dr. Catarina dos Santos collected the leaves of E. punicifolia from a site in Assis State Forest (latitude, 22°33’ to 22°37’ S; longitude, 50°21’ to 50°24’ W) located near one of the experimental stations of the Forestry Institute, Assis, state of São Paulo, Brazil. Dr. Antônio CG Melo identified the species and a voucher (No. 43322) was deposited in the Herbarium D Bento Pickel, available at the Forestry Institute in Assis, São Paulo, Brazil.
The dried and crushed leaves (10 g of plant material) were dissolved in 100 mL of solvent consisting of a 70:30 mix of ethanol and water (v/v). Dynamic maceration of the solution was performed for 2 h at room temperature (25 °C ± 2 °C). Thereafter, the solution was filtered, and the residue was extracted twice more. The solution was dried using a rotary evaporator (40 °C). The extract yield was 45% (4.49 g) of the original plant material.
The animal protocol was designed to minimize pain or discomfort to the animals. The in vivo experiments used male (280 g) and female (220 g) Wistar albino rats obtained from the breeding facility of the State University of Campinas (Multidisciplinary Center for Biological Research). The HEEP dose (125 mg/kg) for the in vivo experiments was determined based on a dose-response curve previously obtained in a gastric injury induction test[16]. Male and female rats were kept in separate rooms, allocated into five animals per cage, fed with Presence® (Paulínia, SP, Brazil) rodents diet, and allowed free access to filtered water. The animals were kept in cages with raised, wide-mesh floors to prevent coprophagy. The cages were kept under controlled conditions of illumination (12 h/12 h light/dark cycle) and temperature (22 °C ± 2 °C).
The estrous cycle was verified through a vaginal smear performed daily starting on postnatal day 60. The material was observed under an optical microscope, and the estrous cycle phase was determined by cytology[18-20]. The duration of the estrous cycle was calculated as the number of days between one estrus phase and the next. Only female rats that showed two consecutive regular cycles of 4-5 d were included in the experiment. Additionally, ovariectomized (OVZ) females were included. We performed bilateral ovariectomies in ten-week-old female rats and we verified the absence of estrous cycle through a vaginal smear after 2 wk. The experiments were performed in 90 d old males as well as in intact and OVZ female rats.
The 84 animals included in the study (30 males, 30 intact females, 24 ovariectomized females) were divided into nine groups with 7-10 individuals each. Group allocation was performed according to sex (male vs female), hormonal status in females (intact vs OVZ), and treatment (negative control vs positive control vs HEEP). All subsequent analyses (except for the cell viability and migration assays) were performed in each of the nine groups. The nine groups were compared to assess the sex-specific effects of HEEP vs vehicle and vs LZ.
After being fasted for 16 h, the animals were anesthetized with a solution of ketamine (80 mg/kg) and xylazine (8 mg/kg) injected intraperitoneally and received laparotomy through a midline epigastric incision. A plastic tube with an internal diameter of 4.2 mm was firmly applied to the serosal surface of the stomach wall, and 70 μL of concentrated acetic acid solution (80%) were delivered to the gastric mucosa through the tube. The acetic acid was left to act for 20 s and then completely removed. The stomach was bathed with saline to avoid adherence to the external surface of the ulcerated region, and the abdomen was closed. Subsequently, all animals were fed normally.
In order to determine the healing effect of HEEP, three 14-d treatment protocols were evaluated in this study. The three groups of negative controls (males, intact females, and OVZ females) received 0.9% NaCl solution dosed at 10 mL/kg. The three groups of positive controls (males, intact females, and OVZ females) received LZ dosed at 30 mg/kg. The remaining three groups (males, intact females, and OVZ females) received HEEP dosed at 125 mg/kg. All treatments were delivered orally once daily beginning one day after surgery and continuing for 14 d. One day after the last drug administration, the rats were euthanized through decapitation and the stomachs were removed to evaluate the lesions. The ulcer tissue was also removed and analyzed. The affected area, expressed in mm2, was determined using the AvSoft Bioview™ Spectra 4.0 software, according to a previously established protocol[21,22]. The organs were removed and weighed. Additionally, analysis of blood collected upon euthanasia was performed. The animals were not anesthetized prior to decapitation, since anesthetics may interfere with the results of the biochemical parameters evaluated.
L929 cells (1 × 105) were cultured in 96-well plates (in triplicate) in the presence of 10% DMSO (control), a vehicle (culture medium with 0.1% DMSO), or HEEP (0.3-30 μg/mL) at 37 °C for 24 h. MTT (0.5 mg/mL) was added to the cultures, the cells were incubated for 3 h at 37 °C, and the absorbance at 570 nm was analyzed after solubilization of reduced formazan crystals with pure DMSO[23]. The percentage of viable cells was calculated as (Abs.sample - Abs.blank/ Abs.vehicle × 100), where “Abs.” represents the absorbance.
The proliferation ability of fibroblasts exposed to HEEP was assessed using the scratch-wound assay, according to a protocol previously described by Balekar et al[24], with a few modifications. The scratch test measures the expansion of a cell population on surfaces. Monolayers of L929 cells were allowed to form in a 24-well plate containing enriched DMEM supplemented with 10% FBS. When nearly confluent, the plate was taken out and artificial wounds were created in the monolayers by making a linear scratch in the center of each well using the tip of a sterile 200 μL plastic pipette. Any cellular debris created while making the scratch was removed by gently washing the wells with PBS. The wells with scratch wounds were divided into three wells per group and treated with HEEP at low (3 μg/mL), intermediate (10 μg/mL), or high (30 μg/mL) concentration. DMEM supplemented with 5% FBS was used as a negative control. The plates were then incubated at 37 °C in a humidified incubator with 5% CO2 atmosphere. The plates were evaluated after 24 h and 48 h of incubation to assess the closure of the scratch wounds. Micrographs were used to record the wound closure activity, which was captured using an inverted microscope (Olympus CK40; Olympus, Melville, NY, United States) with 100 × magnification. All experiments were performed in triplicate.
Serum samples were obtained from all animals at the end of the 14-d treatment with either vehicle, LZ, or HEEP. The serum prostaglandin E2 (PGE2) levels were quantified using a suitable enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, United States).
Tissue containing the gastric ulcer was used to extract total protein. The extraction was carried out by crushing the tissue using a homogenizer (Polytron Benchtop Homogenizers, Daigger Scientific, Vernon Hills, IL, United States) in a RIPA buffer and protease inhibitor cocktail (1:5 ratio). The homogenate was centrifuged at 18620 g for 45 min at 4 °C. The supernatant was collected and then centrifuged again at 13680 g for 15 min at 4 °C. Finally, the protein content was quantified using the biuret method[25,26].
The complete protocol for gelatin zymography was previously described by Justulin et al[27]. Briefly, samples containing extracted proteins (35 μg) obtained from the gastric ulcer tissue were subjected to non-reducing electrophoresis on 8% polyacrylamide gel copolymerized with 0.1% purified gelatin. After electrophoresis, the gels were subjected to two 15 min washes in a solution of 2.5% Triton X-100 to remove SDS, and to two 5 min washes in 50 mmol/L Tris-HCl buffer (pH 8.0). Subsequently, the gels were incubated for 20 h at 37 °C in 50 mmol/L Tris-HCl buffer (pH 8.0) containing 5 mmol/L CaCl2 and 1 μmol/L ZnCl2. Finally, the gels were stained with Coomassie Brilliant Blue R-250. The relative molecular weight of the bands was determined according to the molecular weight standard (Precision Plus Protein™; Bio-Rad Laboratories, Hercules, CA, United States) used in electrophoresis. The bands obtained through zymography were scanned and analyzed by densitometry. The bands representative of the gelatinase activity of matrix metalloproteinase (MMP)-2 and MMP-9 were analyzed in terms of the integrated optical density (IOD) of the bands. Specifically, the band IODs were obtained using the Image J software (National Institutes of Health, Bethesda, MA, United States). The IOD values were then plotted in a histogram showing the IOD of the treated groups (LZ, HEEP) and control group (vehicle).
For this test, the protein extracts were obtained as described above for zymography. After extraction, the samples were treated with Laemmili buffer (0.5 mol/L PB buffer; pH 6.8; glycerol, 10% SDS, 0.1% bromophenol, β-mercaptoethanol) in a ratio of 1:1. Equal amounts of protein (70 μg) were separated on 10% acrylamide gel using SDS-PAGE. In the next step, the proteins were electrophoretically transferred onto a nitrocellulose membrane or PVDF membrane (for epidermal growth factor (EGF)), blocked with 5% non-fat milk diluted in PBS, and incubated with the following primary antibodies: anti-cyclooxygenase (COX)-1 (1:10.000, ab133319); anti-COX-2 (1:1000, ab52237); anti-vascular endothelial growth factor (VEGF) (1:1.000, ab46154); anti-EGF (1:800, ab77851), anti-Bcl-2 (1:800, ab59348), anti-caspase-3 (1:10.000, ab32499), anti-caspase-8 (1:2.000, ab25901), and anti-caspase-9 (1:5.000, ab32539) (all from Abcam, Cambridge, MA, United States). The membranes were washed with PBS and incubated with a specific secondary antibody for 2 h (horseradish peroxidase, 1:10.000, ab 97051; Abcam). After washing, the reactions were detected using an enhanced chemiluminescence kit (Amersham Biosciences, Westborough, MA, United States) and the signals were captured using a G:BOX system (Syngene, Cambridge, England). The IODs of the targeted protein bands were measured using Image J. The expression levels were normalized to that of actin (1:10000; ab179467; Abcam).
Body weight was recorded daily throughout the experimental period. Macroscopic analyses and weighing of the vital organs (liver, kidneys, heart, spleen, and lungs) were performed at the end of the treatment. Additionally, analysis of blood collected upon euthanasia was performed. The blood samples were centrifuged (at 3000 g for 15 min), and the serum obtained was frozen at -80 °C until biochemical analysis. Serum biochemical parameters including glucose, urea, creatinine, γ-glutamyl transpeptidase (γ-GT), aspartate aminotransferase (AST), and alanine amino transferase (ALT) were measured using an automated biochemical analyzer.
The statistical methods of this study were reviewed by Dr. Clélia Akiko Hiruma-Lima from the Institute of Biosciences, UNESP. The results were expressed as the mean ± standard error of the mean. Two-group comparisons were performed using Student’s t-test, while comparisons of three or more groups were performed using one-way analysis of variance followed by Dunnett’s or Tukey’s test, or using two-way analysis of variance followed by Bonferroni’s test. The minimal significance level considered was P < 0.05.
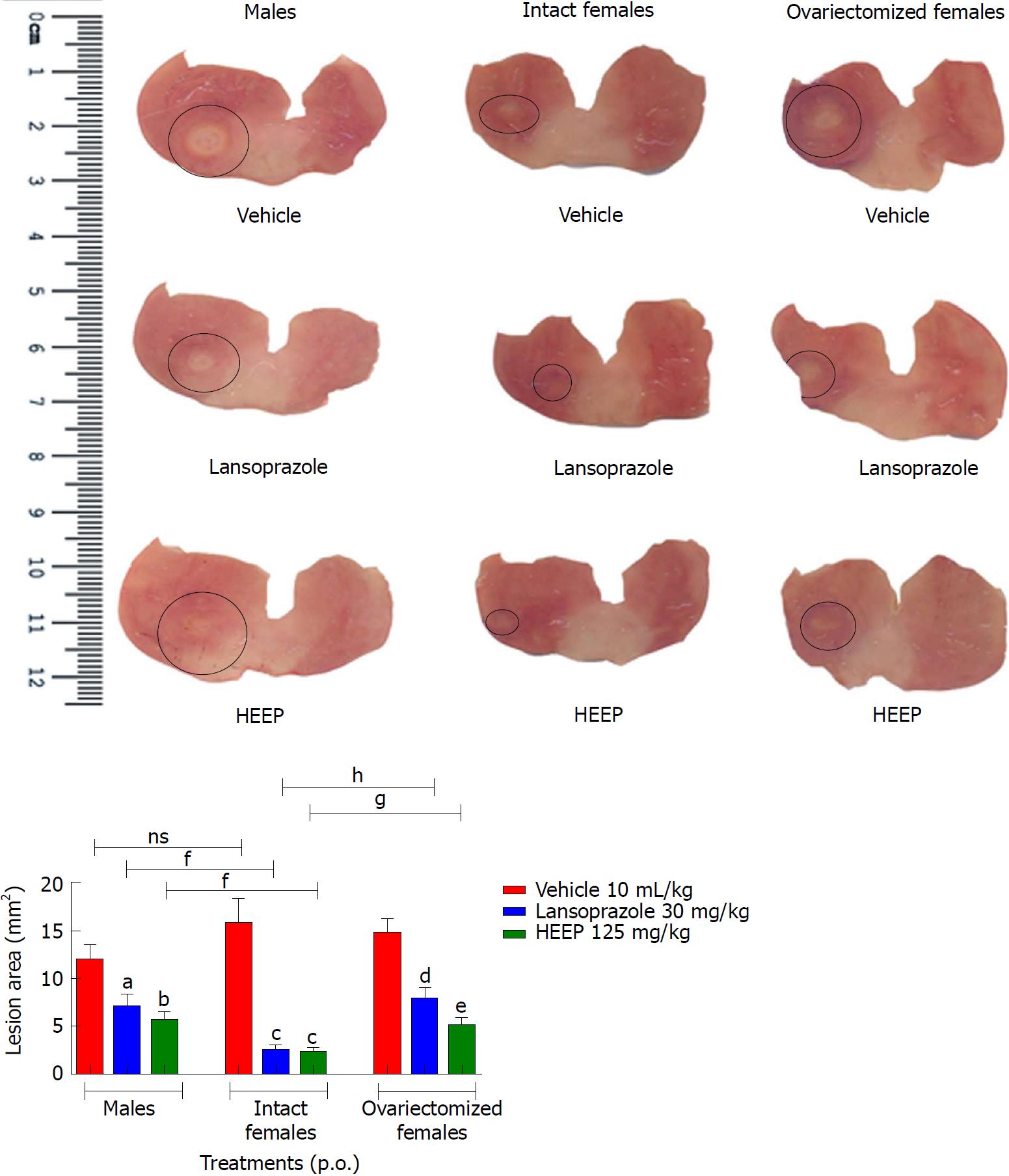
To evaluate the HEEP-mediated healing effect of gastric ulcers, we measured the gastric lesion area at the end of treatment. In male rats, 14-d treatment with HEEP was associated with significantly decreased lesion area (52.44%, P < 0.01 vs vehicle); the same effect, though less pronounced, was noted for LZ treatment (40.81%, P < 0.05 vs vehicle) (Figure 1). Among females, HEEP was associated with a very significant reduction in lesion area, both in cycling rats (85.22%, P < 0.0001 vs vehicle) and in OVZ rats (65.47%, P < 0.001 vs vehicle); the same effect was noted for LZ treatment (P-value vs vehicle: 84.21%, < 0.0001 and 49.40%, < 0.01, respectively) (Figure 1).
Compared to males, intact females showed higher benefit in terms of gastric lesion area reduction following treatment with HEEP or LZ (P < 0.01 for both comparisons), whereas no significant difference in lesion area reduction was noted between males and OVZ females treated with HEEP or LZ (P > 0.05). Compared to OVZ females, intact females had greater reduction in the lesion area (P < 0.01 for HEEP; P < 0.001 for LZ) (Figure 1).
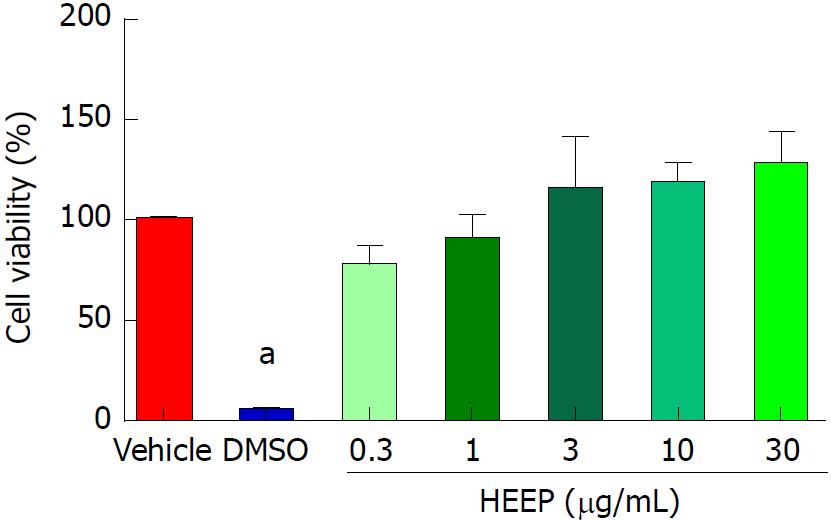
The cytotoxic potential of HEEP was evaluated in an MTT assay of L929 fibroblasts. No cytotoxicity signal was found after 24 h of incubation with HEEP, regardless of concentration (0.3-30 μg/mL) (P > 0.05 vs vehicle) (Figure 2).
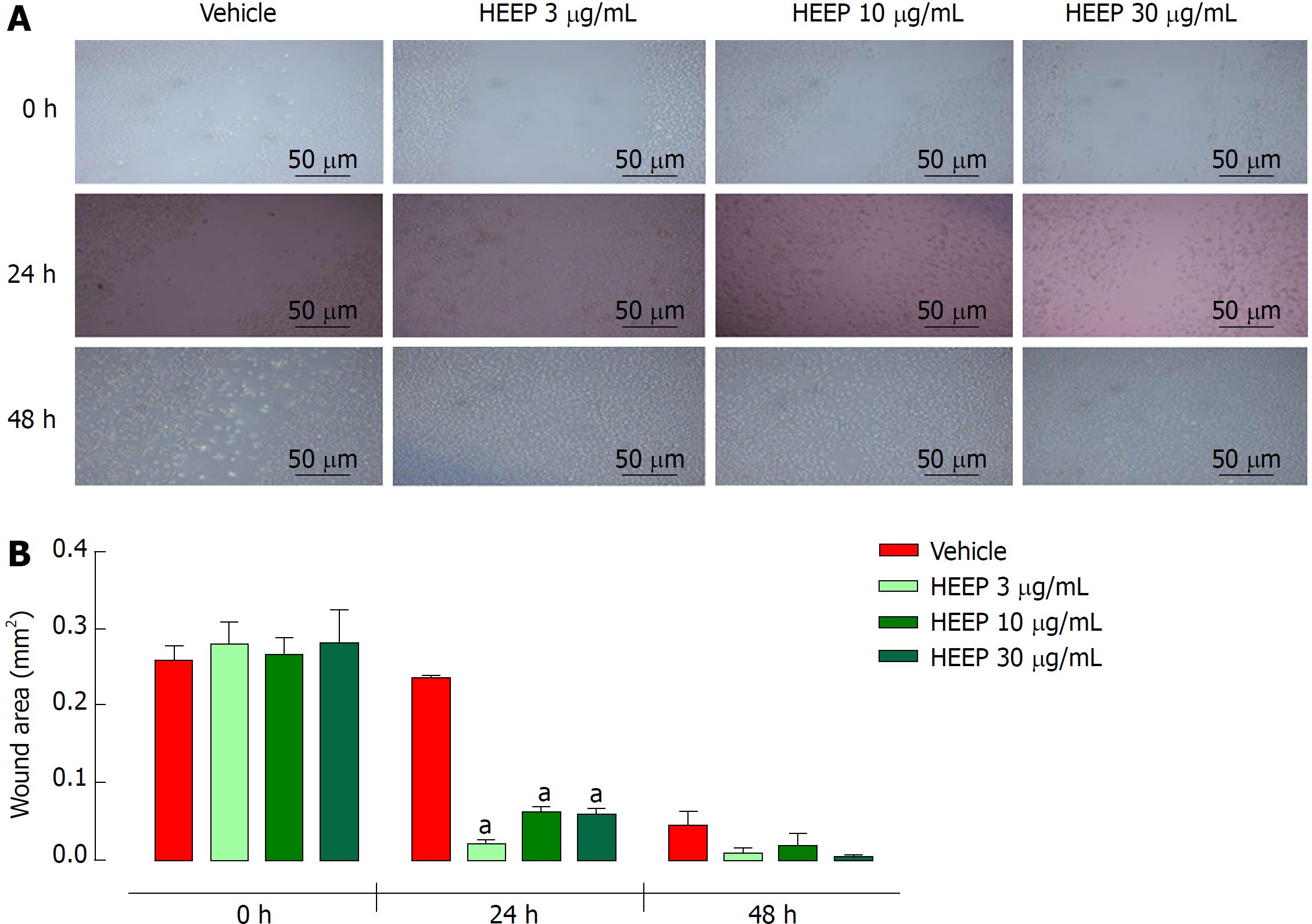
We performed an in vitro scratch-wound test to determine whether HEEP possesses cell proliferation activity. Indeed, HEEP promoted proliferation and migration of the cells to cover the scratch wounds made on the L929 cell monolayer (Figure 3A). The HEEP-induced enhancement of proliferation and coverage of the scratch wounds was found to decrease with increasing HEEP concentration (coverage achieved for HEEP concentration of 3 μg/mL, 10 μg/mL, and 30 μg/mL: 92.14%, 74.31%, and 75.25%, respectively; all significantly higher vs time-matched vehicle) (Figure 3B).
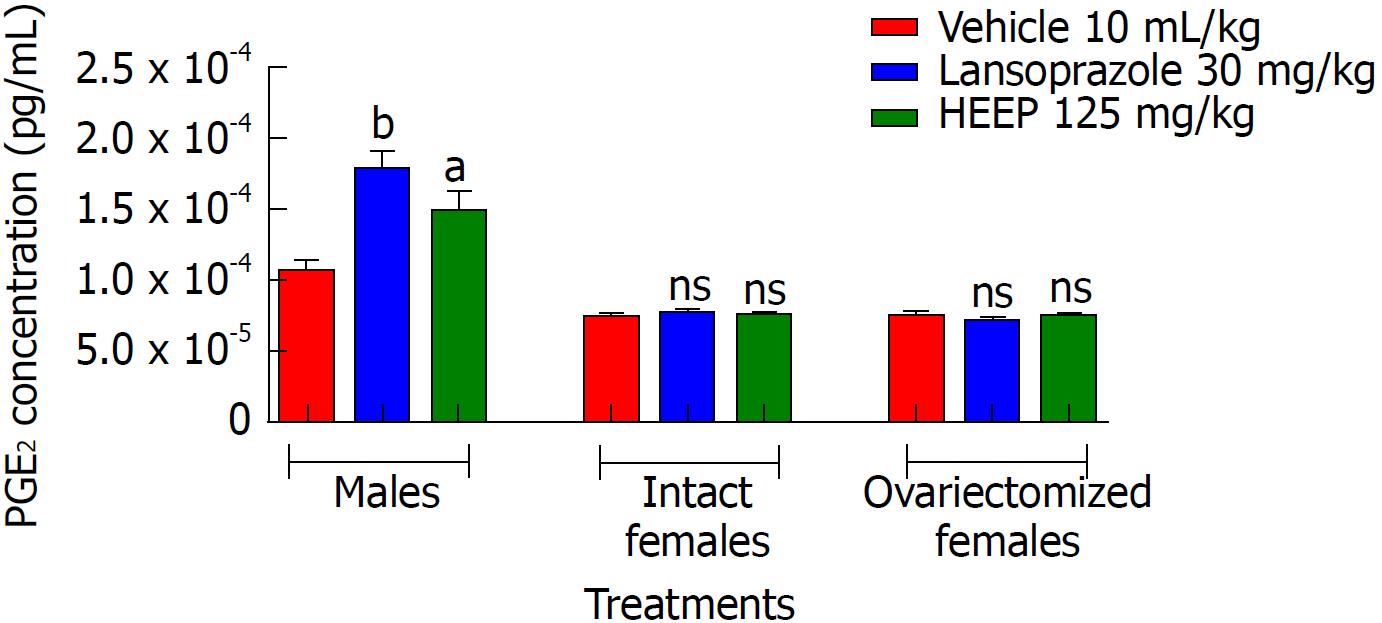
To gauge whether PGE2 plays a role in HEEP-mediated healing of gastric ulcers, we measured the levels of PGE2 at the end of treatment. In males, 14-d treatment with HEEP or LZ was associated with elevation of serum PGE2 levels (P-values vs vehicle: < 0.05 for HEEP, < 0.001 for LZ) (Figure 4). However, no increase in PGE2 levels was noted in intact or OVZ females treated with HEEP or LZ (P > 0.05 vs vehicle).
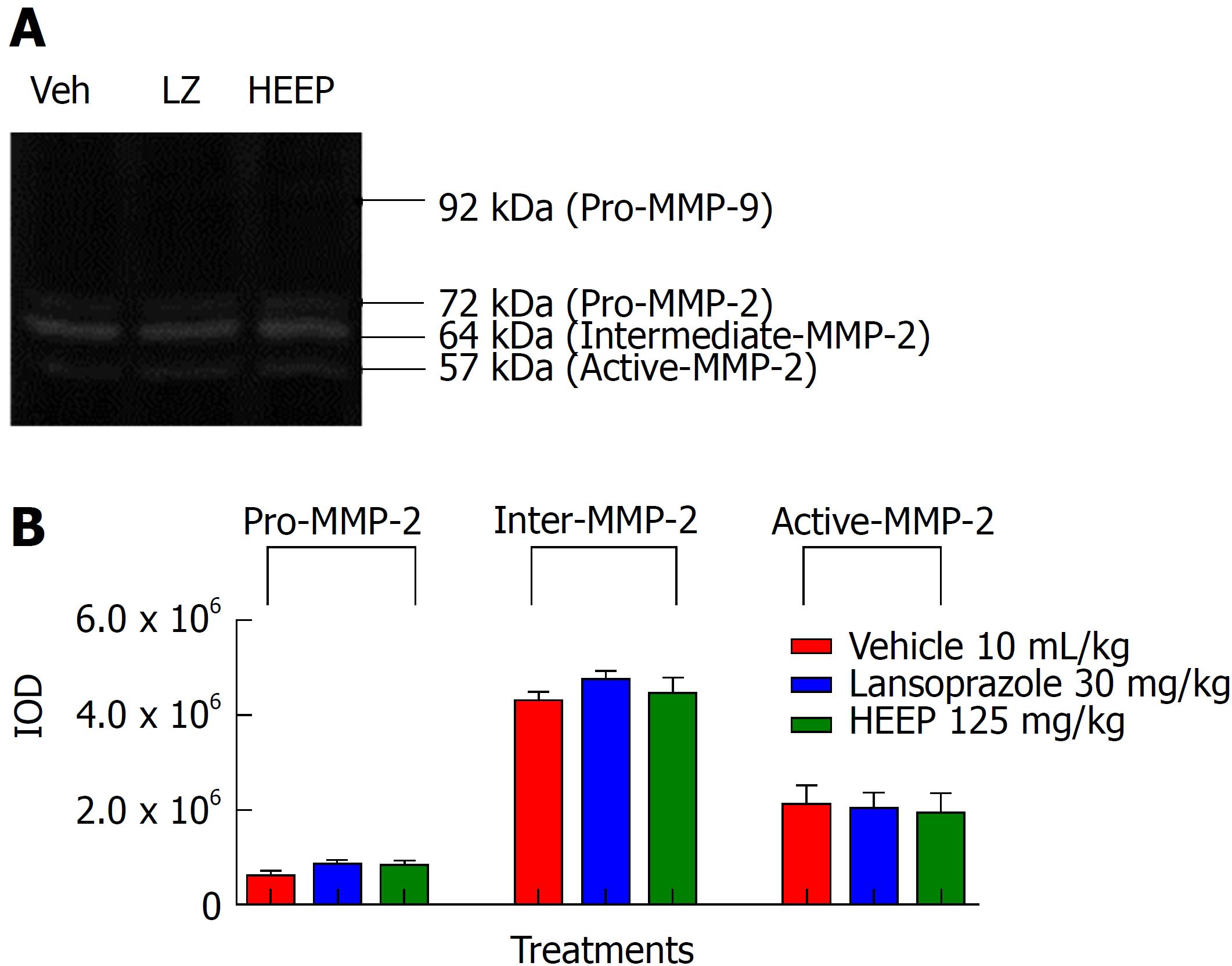
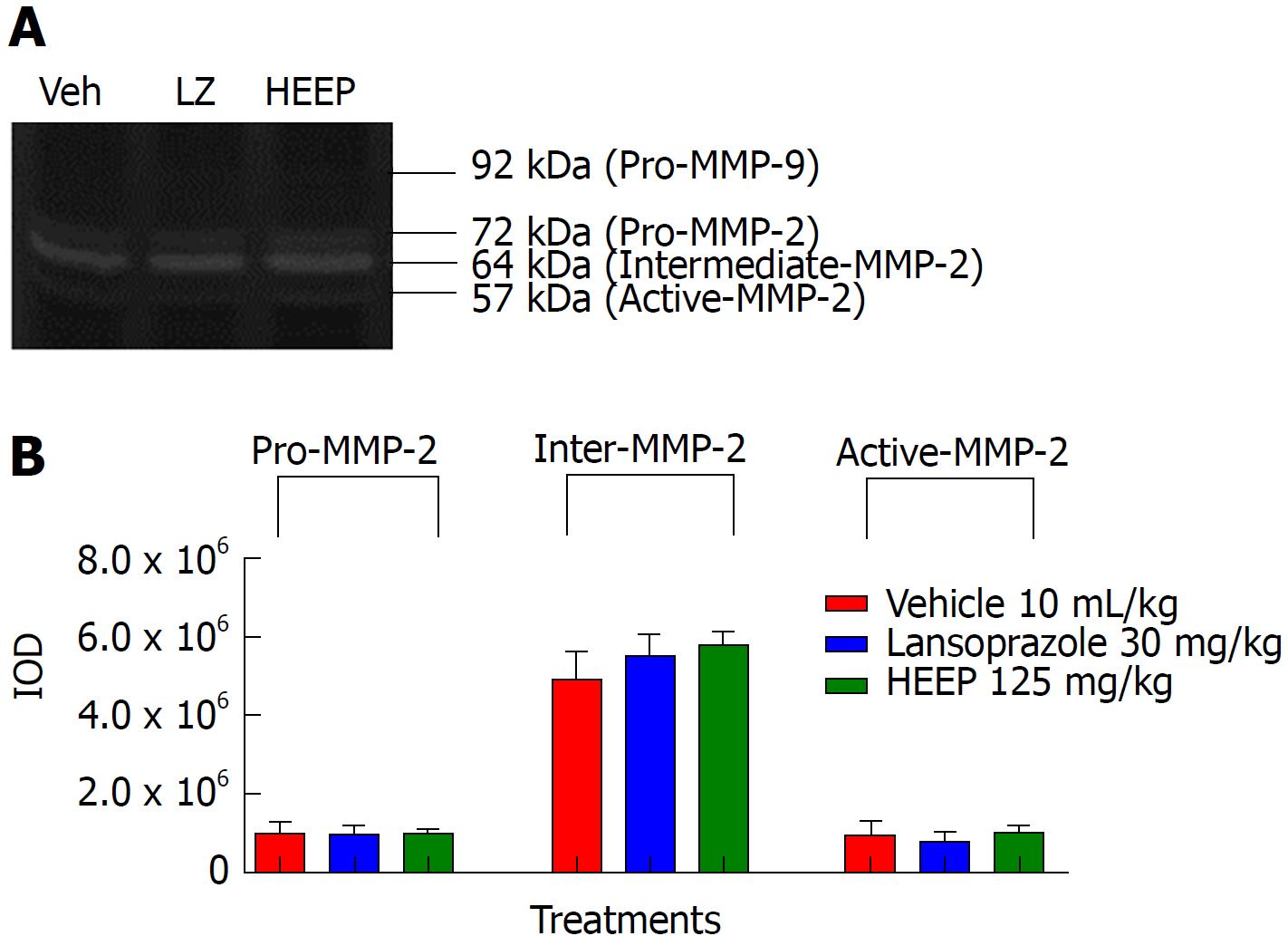
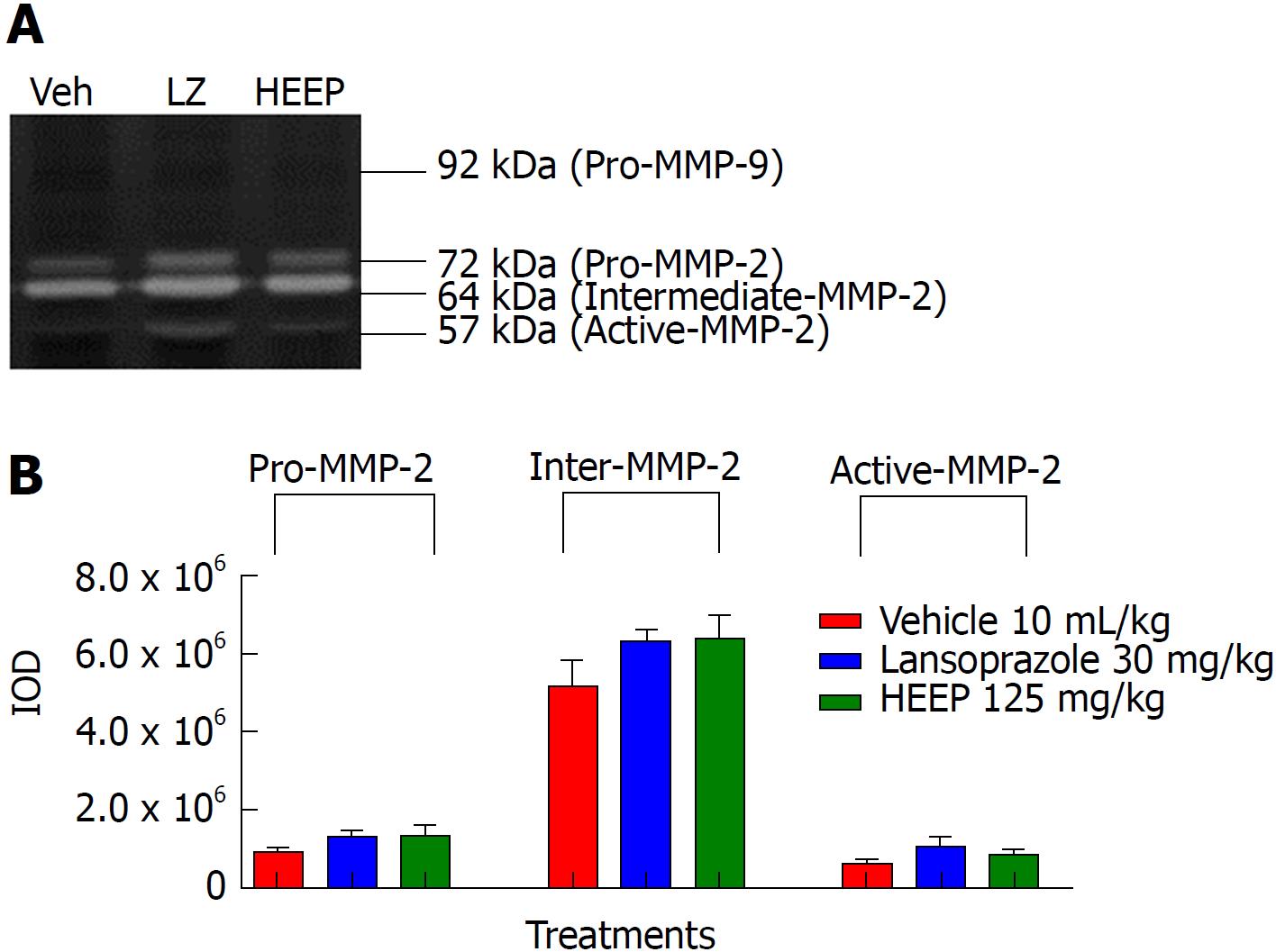
To clarify the nature of the HEEP-mediated healing process of gastric ulcers, we performed zymography analysis of the gastric ulcer tissue and evaluated the activities of MMP-2 and MMP-9 on the gastric mucosa after treatment with vehicle, LZ, and HEEP. No band corresponding to MMP-9 was observed, indicating that MMP-9 activity is absent in all rats. In contrast, MMP-2 activity was present in all rats. Specifically, males (Figure 5), intact females (Figure 6), and OVZ females (Figure 7) exhibited pro-MMP-2, intermediate MMP-2, and active MMP-2 activities. There is no difference regarding MMP-2 activity between rats treated with vehicle and those treated with HEEP or LZ (P > 0.05 vs vehicle).
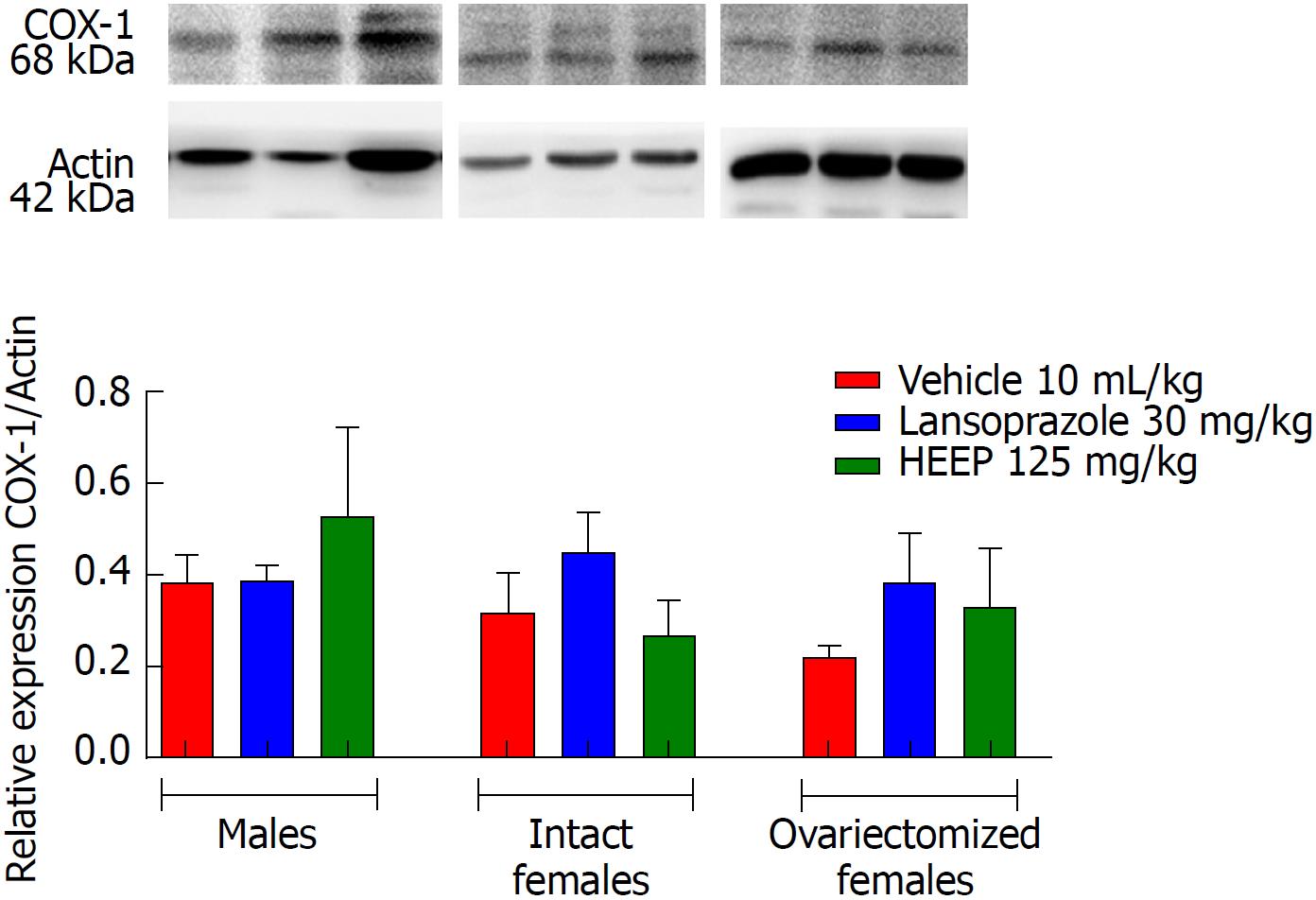
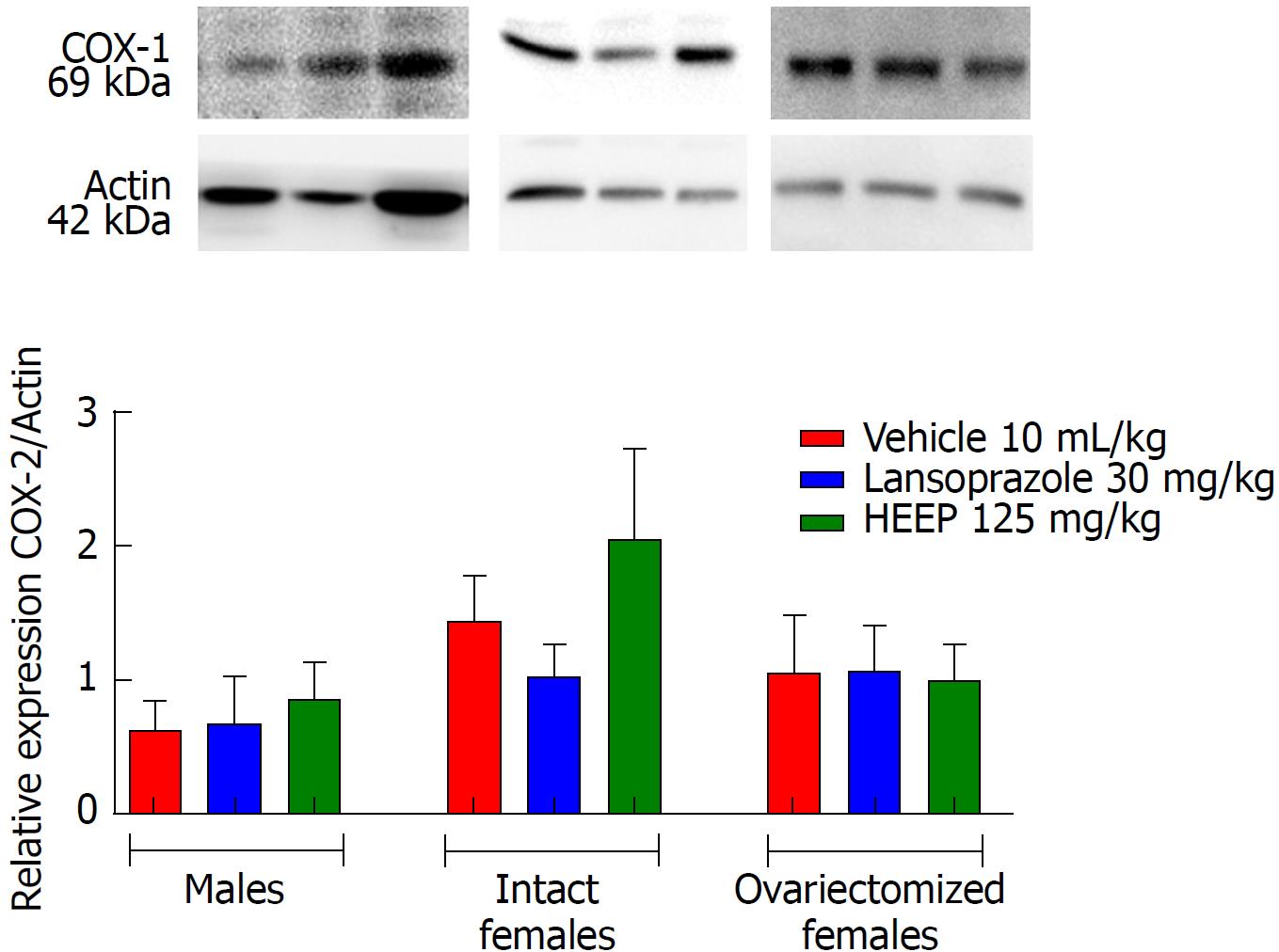
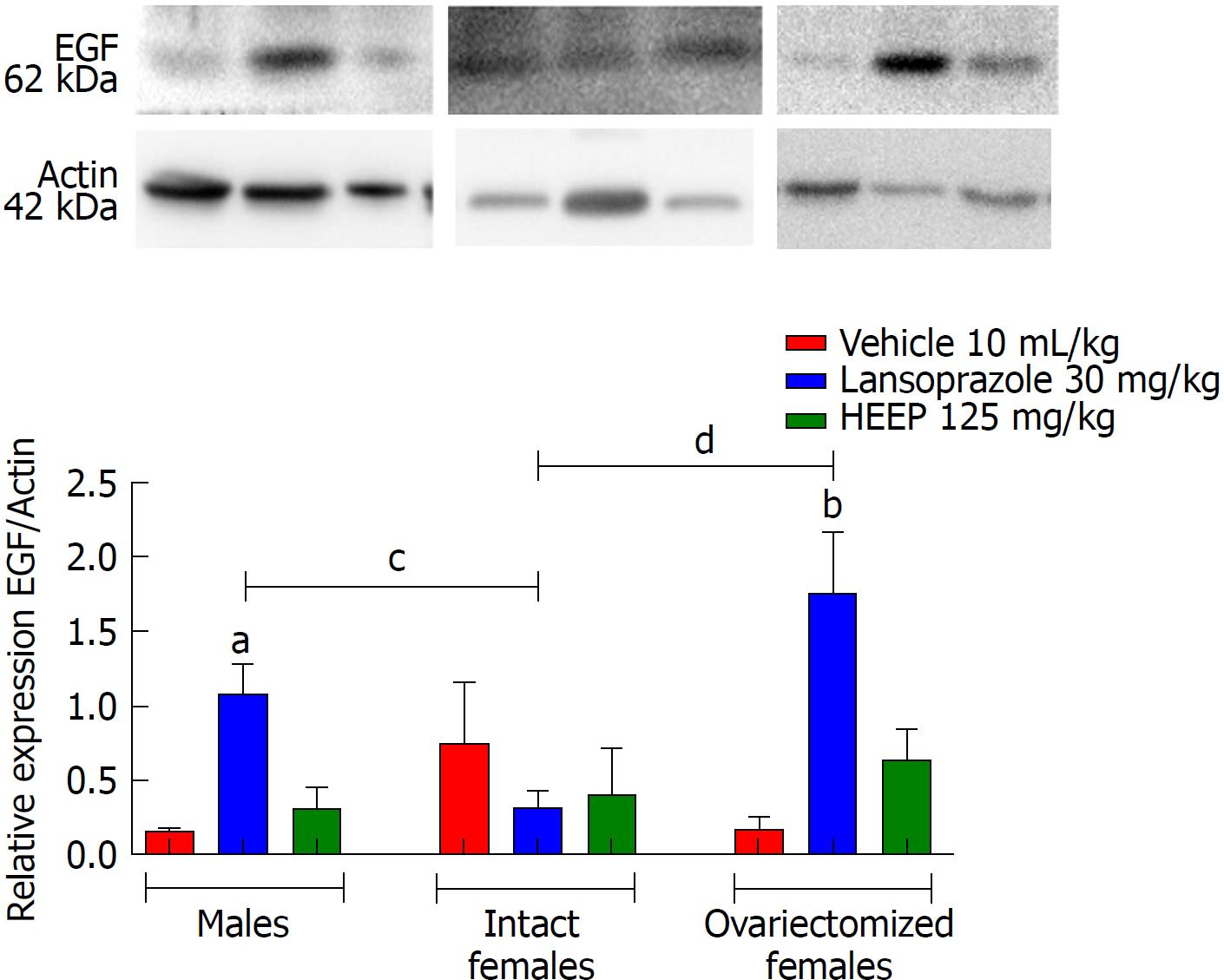
To determine the protein expression profiles associated with HEEP-mediated healing of gastric ulcers, we examined the western blotting gels for several important contributors to cell growth and cell death. Western blotting revealed that oral treatment with HEEP or LZ for 14 d after gastric exposure to absolute acetic acid does not affect the expression of COX-1 (Figure 8) or COX-2 (Figure 9) in males, intact females, or OVZ females (vs corresponding vehicle). LZ treatment was associated with increased EGF expression in males and in OVZ females (P < 0.01 vs corresponding to the respective vehicle; P < 0.05 vs intact females) (Figure 10). No effect on EGF expression was noted for HEEP treatment.
Western blot analysis revealed that male rats and OVZ rats had low VEGF expression at 14 d after induction of gastric ulcers, regardless of treatment (low-signal band; Figure 11). Neither HEEP nor LZ treatment induced significant changes in VEGF expression (P > 0.05 vs corresponding vehicle); however, higher VEGF expression was noted in intact females vs OVZ females treated with HEEP or LZ (P < 0.05).
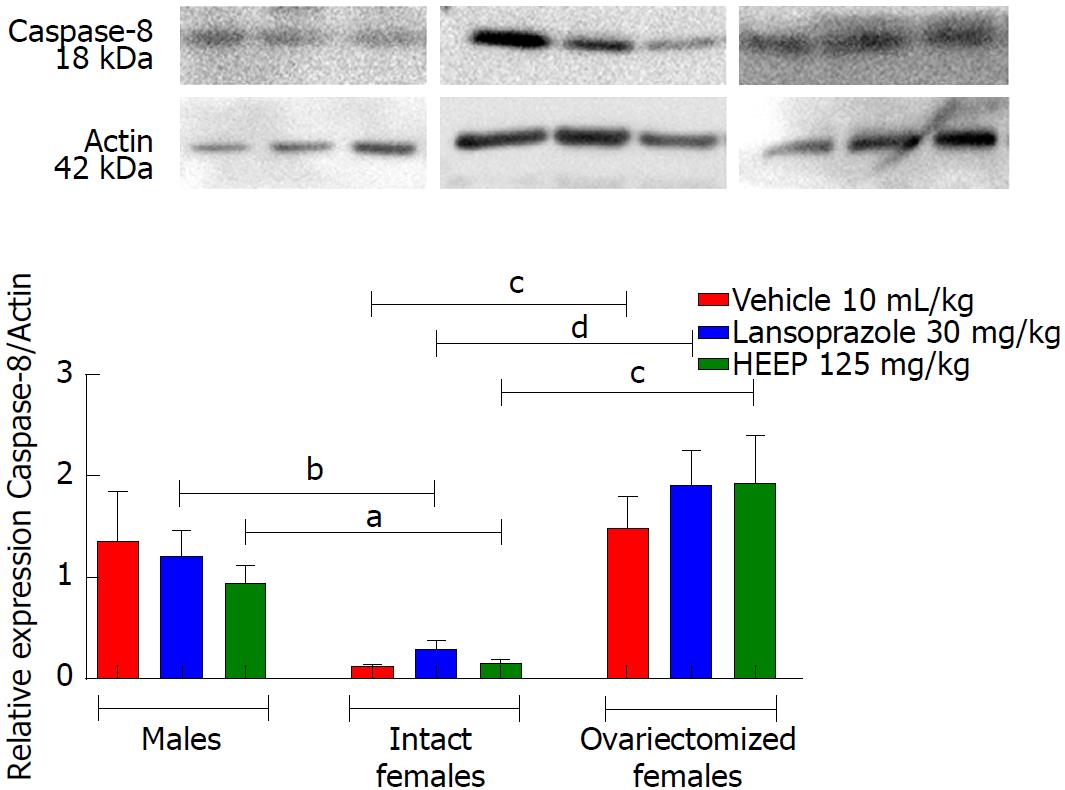
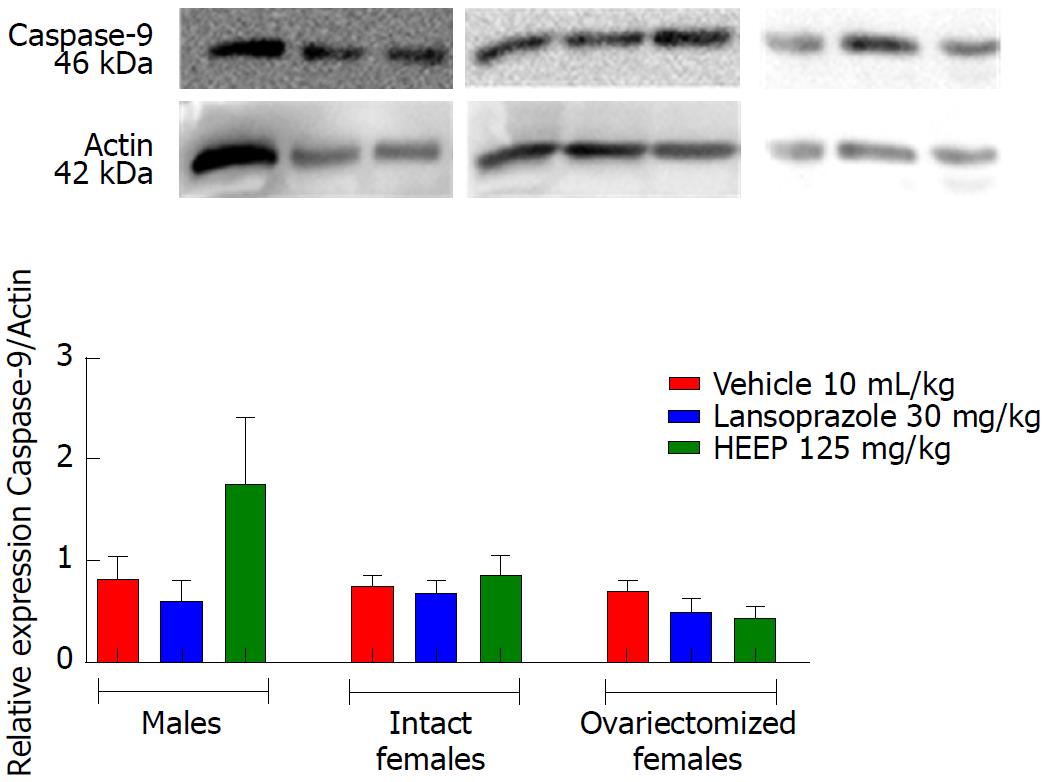
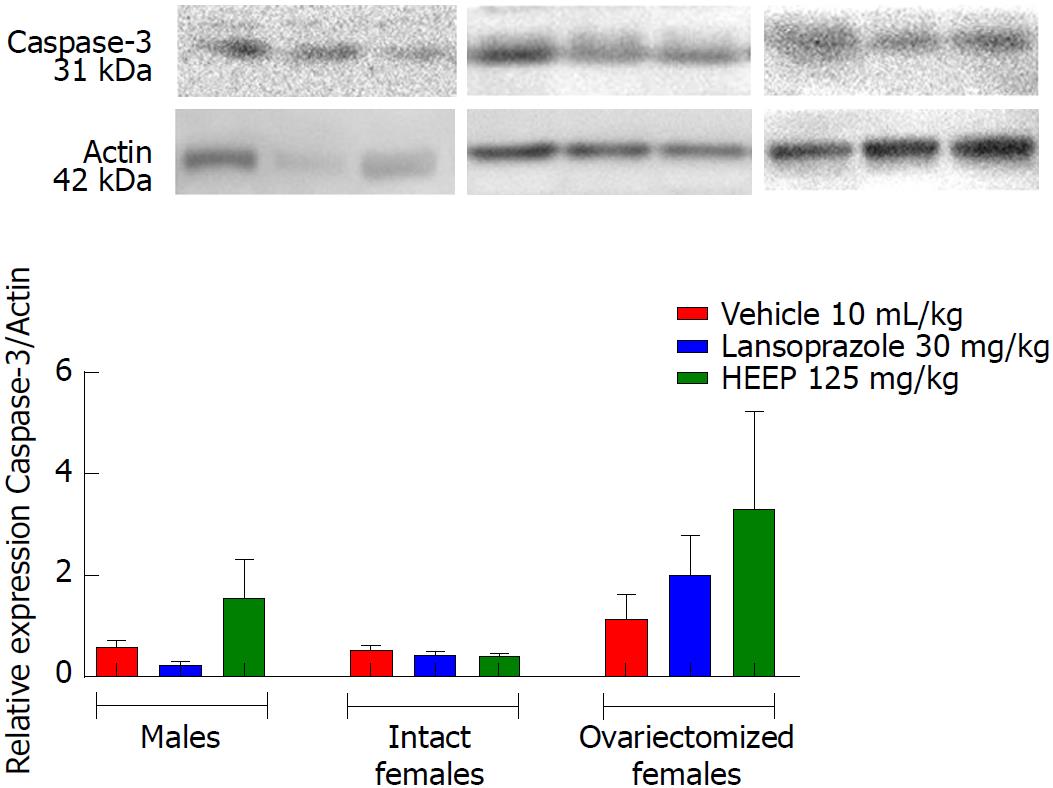
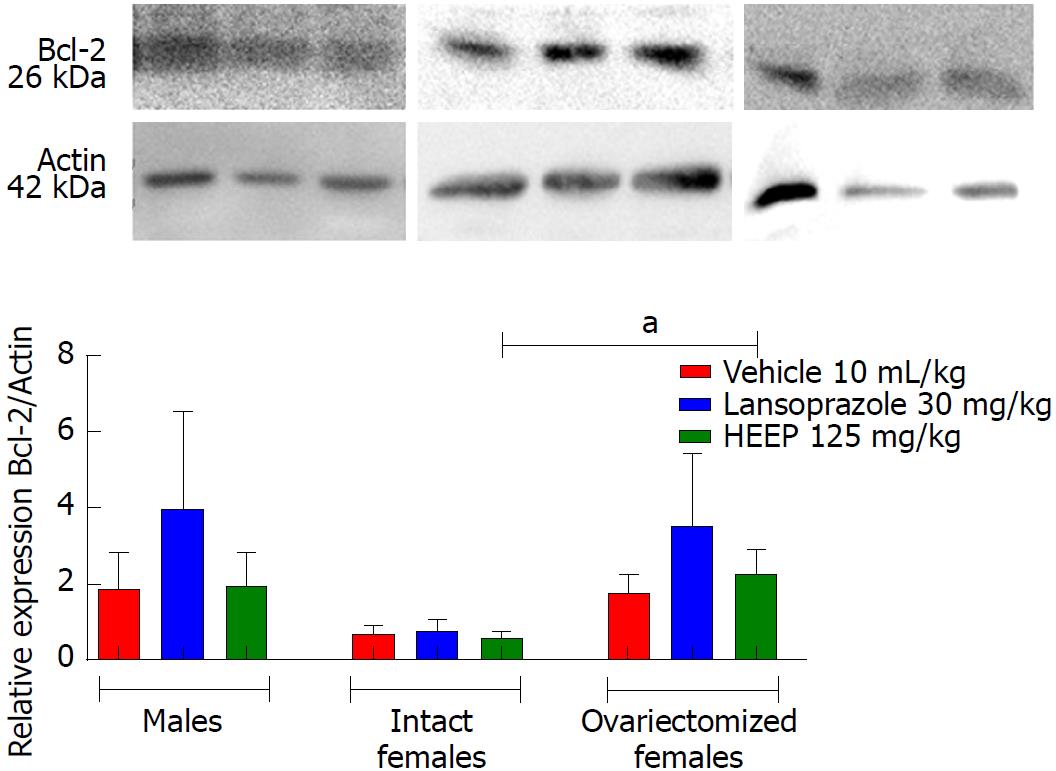
In addition to growth factors, we evaluated apoptotic factors including caspase-8 (Figure 12), caspase-9 (Figure 13), and caspase-3 (Figure 14), as well as the anti-apoptotic factor Bcl-2 (Figure 15). Neither HEEP nor LZ treatment altered the expression of the apoptotic factors (P > 0.05 vs corresponding vehicle). However, expression of caspase-8 (Figure 12) was significantly lower in intact females vs males treated with HEEP or LZ. Furthermore, compared to intact females, OVZ females had higher expression of caspase-8 for all treatments (P < 0.01). Similarly, neither HEEP nor LZ treatment altered the expression of the non-apoptotic factor Bcl-2 (P > 0.05 vs corresponding vehicle). However, HEEP treatment was associated with higher expression of Bcl-2 in OVZ females vs intact females (Figure 15).
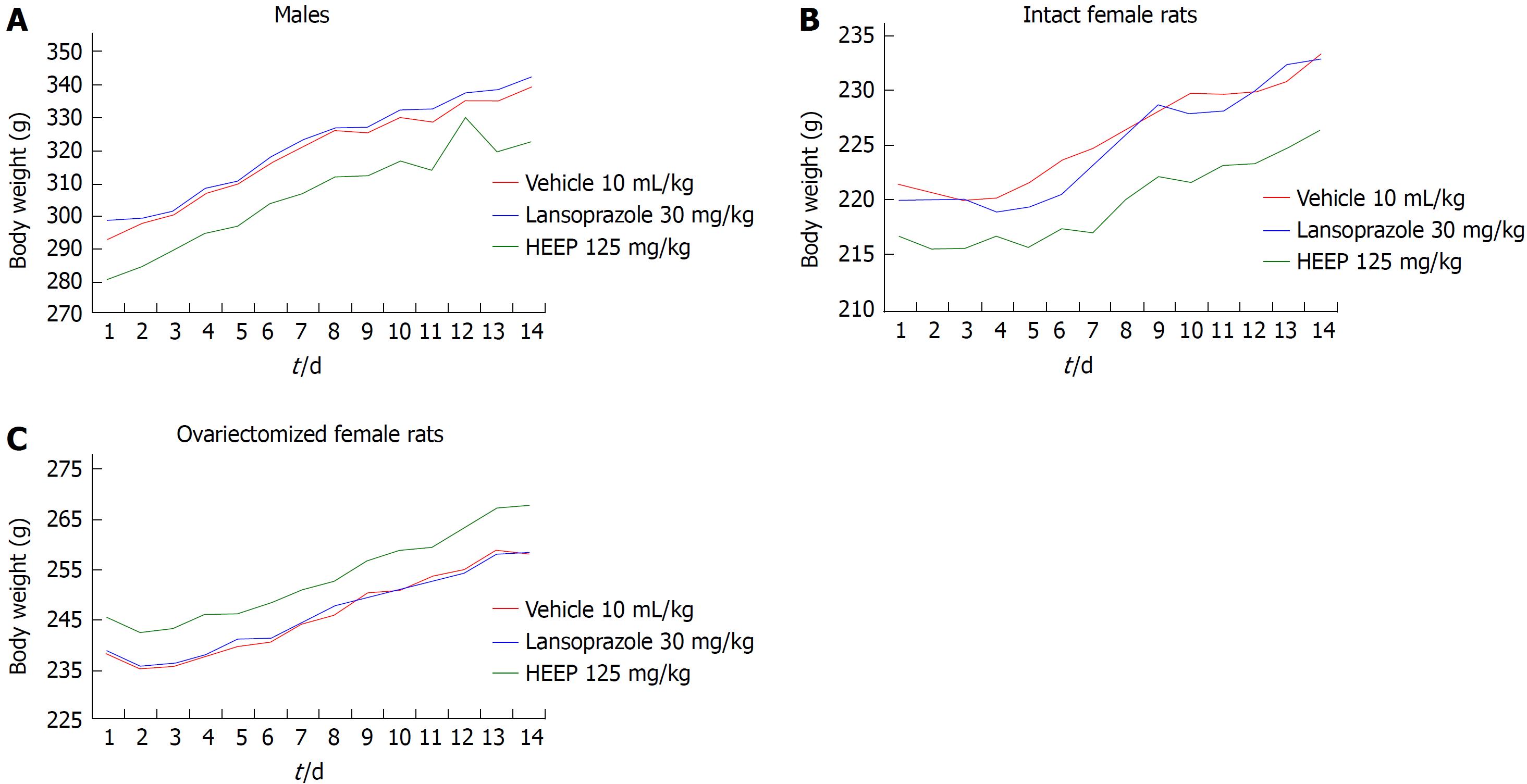
In male rats, oral administration of HEEP was not associated with any deaths or significant changes in body weight throughout the 14 d of treatment (Figure 16). Additionally, no significant changes in organ weight or biochemical parameters were noted for HEEP-treated males (P > 0.05 vs vehicle), whereas significantly higher glucose levels were noted in males treated with LZ (P < 0.05 vs vehicle) (Table 1).
| Treatment | |||
| Vehicle | Lansoprazole | HEEP | |
| Organ weights, g | |||
| Heart | 3.15 ± 0.03 | 3.13 ± 0.03 | 3.14 ± 0.06 |
| Kidneys | 4.81 ± 0.05 | 4.79 ± 0.04 | 4.84 ± 0.05 |
| Lung | 3.62 ± 0.08 | 3.73 ± 0.10 | 3.59 ± 0.06 |
| Liver | 11.56 ± 0.18 | 11.55 ± 0.10 | 11.26 ± 0.13 |
| Spleen | 2.94 ± 0.02 | 2.98 ± 0.05 | 2.99 ± 0.04 |
| Biochemical parameters | |||
| Glucose, mg/dL | 150.10 ± 6.04 | 170.90 ± 4.73a | 154.70 ± 3.80 |
| Creatinine, mg/dL | 0.34 ± 0.02 | 0.34 ± 0.02 | 0.33 ± 0.01 |
| Urea, mg/dL | 54.75 ± 3.15 | 52.13 ± 1.71 | 54.13 ± 1.94 |
| γ-GT, U/L | < 1.0 | < 1.0 | < 1.0 |
| AST, U/L | 137.80 ± 18.43 | 163.60 ± 22.33 | 144.50 ± 10.76 |
| ALT, U/L | 42.88 ± 3.55 | 48.88 ± 3.67 | 46.25 ± 3.09 |
Oral administration of HEEP (125 mg/kg) did not cause any sign of acute toxicity in intact females (Figure 16, Table 2) or OVZ females (Figure 16, Table 3). None of the female animals died during the treatment period, and no significant changes in body weight were noted. Finally, no differences in organ weights, hepatic enzyme levels (γ-GT, AST, and ALT), renal function (creatinine and urea levels), or glucose levels were observed in female rats at the end of the 14 d treatment period (P > 0.05 vs corresponding vehicle).
| Treatment | |||
| Vehicle | Lansoprazole | HEEP | |
| Organ weights, g | |||
| Heart | 3.43 ± 0.05 | 3.50 ± 0.03 | 3.44 ± 0.06 |
| Kidneys | 4.74 ± 0.03 | 4.81 ± 0.04 | 4.69 ± 0.06 |
| Lung | 4.23 ± 0.07 | 4.53 ± 0.06 | 4.01 ± 0.30 |
| Liver | 10.67 ± 0.14 | 10.90 ± 0.10 | 10.61 ± 0.09 |
| Spleen | 2.56 ± 0.06 | 2.56 ± 0.07 | 2.48 ± 0.06 |
| Biochemical parameters | |||
| Glucose, mg/dL | 119.10 ± 3.27 | 116.90 ± 3.21 | 122.30 ± 3.34 |
| Creatinine, mg/dL | 0.45 ± 0.02 | 0.41 ± 0.02 | 0.45 ± 0.01 |
| Urea, mg/dL | 49.50 ± 3.36 | 53.29 ± 1.11 | 53.70 ± 3.20 |
| γ-GT, U/L | < 1.0 | < 1.0 | < 1.0 |
| AST, U/L | 202.70 ± 12.73 | 198.40 ± 18.84 | 162.10 ± 7.24 |
| ALT, U/L | 57.00 ± 3.40 | 60.57 ± 3.59 | 65.86 ± 2.79 |
| Treatment | |||
| Vehicle | Lansoprazole | HEEP | |
| Organ weights, g | |||
| Heart | 3.35 ± 0.04 | 3.39 ± 0.05 | 3.49 ± 0.09 |
| Kidneys | 4.52 ± 0.07 | 4.56 ± 0.07 | 4.61 ± 0.05 |
| Lung | 4.28 ± 0.11 | 4.30 ± 0.10 | 4.47 ± 0.17 |
| Liver | 10.56 ± 0.16 | 10.61 ± 0.10 | 10.60 ± 0.11 |
| Spleen | 2.59 ± 0.06 | 2.68 ± 0.08 | 2.65 ± 0.05 |
| Biochemical parameters | |||
| Glucose, mg/dL | 117.30 ± 4.36 | 126.70 ± 4.48 | 119.00 ± 5.46 |
| Creatinine, mg/dL | 0.42 ± 0.00 | 0.43 ± 0.01 | 0.43 ± 0.01 |
| Urea, mg/dL | 55.08 ± 2.24 | 48.97 ± 1.67 | 55.37 ± 2.92 |
| γ-GT, U/L | < 1.0 | < 1.0 | < 1.0 |
| AST, U/L | 176.80 ± 15.37 | 192.70 ± 14.77 | 191.00 ± 21.04 |
| ALT, U/L | 62.17 ± 3.68 | 57.57 ± 2.36 | 56.43 ± 1.92 |
The acetic acid-induced gastrointestinal ulcer model is a classical model that has proven suitable for investigating the effect of treatment on the healing process of chronic gastrointestinal ulcers[28], provided that the wounds resemble human ulcers that do not heal naturally[29,30,31]. Such data can also help in the discovery of new anti-ulcer drugs or treatment targets. This ulcer-induction model provides an easy and reliable technique for producing round, deep ulcers in the stomach of rats, and such ulcers do not heal naturally. Furthermore, ulcers obtained using this model resemble human ulcers, and this model was successfully used to assess agents with potential therapeutic effects in chronic gastric ulcers.
One of the least understood aspects of gastric ulcers is the chronicity of the disease, which is characterized by repeated episodes of healing and re-exacerbation, posing a challenge to physicians and a burden for patients[31]. The healing process of ulcers can be divided into three phases: ulcer development phase (0-3 d), involving tissue necrosis, ulcer implantation, inflammatory infiltration, and ulcer margin formation; rapid healing phase (3-10 d), involving migration of epithelial cells and contraction of the ulcer base; slow healing phase (10-20 d), involving angiogenesis, remodeling of granulation tissues, and complete re-epithelialization of the ulcer[32].
To the best of our knowledge, the present study is the first to show that 14 d treatment with HEEP achieves healing of established gastric ulcers in all groups of rats studied (males, females, and OVZ females) (Figure 1). Specifically, HEEP treatment achieved a 52.44% lesion area reduction in males, compared to 85.22% and 65.47% in intact and OVZ females, respectively (all P < 0.05 vs corresponding vehicle) (Figure 1). A significant decrease in lesion area was also noted for LZ treatment in females (84.21%), OVZ females (49.40%), and males (40.81%) (Figure 1). Intact females treated with HEEP or LZ showed significantly greater healing effects than that noted in males or OVZ females who received the same treatment. This beneficial effect of HEEP in intact females is confirmed by the increase in VEGF expression associated with HEEP treatment in this group. Furthermore, there was no significant difference in lesion area between males and OVZ females who received the same treatment (Figure 1), suggesting that the absence of female sex hormones diminishes healing. Our results corroborate the findings of Günal et al[33], who demonstrated that the severity of gastric ulcers induced by the application of acetic acid is attenuated by the effect of estrogen. The protective role of female sex hormones likely accounts for the higher incidence of gastric ulcers in men vs women[34]. The anti-ulcerogenic activity of estrogen was previously explained by its stimulating effect on parietal cell activity and maintenance of mucus integrity[34]. The protective effect of estrogens on endothelial function includes antioxidant properties, vasodilator action, prevention of the formation of platelet thrombi, and angiogenesis promotion[35]. Results from several studies support the idea that the estrogen-induced vasoprotective effect may be due to the release of nitric oxide from the vascular endothelium[36].
Gastric ulcer healing is a complex and genetically-programmed dynamic process[37]. It is well-established that myofibroblasts are an important component of wound healing[38], responsible for extracellular matrix production, morphogenesis, and the inflammatory process involved in tissue repair[38-40]. Previous studies confirmed that fibroblasts play an important role in gastric and esophageal ulcer healing in mice and rats[37,41-42]. Similar to da Silva et al[43] (2015), the murine fibroblast L929 (NCTC clone 929) cells were used as a complementary in vitro model to confirm the cell proliferative effect of HEEP. Fibroblast cell culture has been proposed as a useful method for testing wound healing activity in vitro[44]. We found that incubation with HEEP led to adequate coverage of the scratch-wound areas on the L929 cell monolayer after only 24 h (Figure 3), with no sign of cytotoxicity in the MTT assay (Figure 2). Thus, the HEEP effect of enhancing cell proliferation and migration to cover the scratch wounds reinforces the healing potential of HEEP.
Another step taking place in this process is the mucosal reconstruction by formation of granulation tissue at the base of the ulcer, formation of new vessels, and restoration of the glandular architecture[45]. The integrity of the gastric mucosa is highly dependent on the continuous generation of prostaglandins by COX-1 and COX-2[46]. COX-1 is expressed constitutively in the gastric mucosa and mediates the synthesis of prostaglandins, which regulate blood flow in the mucosa and promote the secretion of mucus and bicarbonate. COX-2 also plays an important role in the healing of gastric ulcers by initiating cell proliferation, promoting angiogenesis, and restoring mucosal integrity[47,48]; COX-2 inhibition is associated with delayed healing. Upon evaluating PGE2 levels in the present model of acetic acid-induced gastric ulcers, we found increased PGE2 levels following HEEP or LZ treatment in male (P < 0.05 vs vehicle; Figure 4) but not female rats. This finding demonstrates the participation of PGE2 in the healing process of gastric ulcers in male rats treated with HEEP for 14 consecutive days.
The injection of acetic acid into the gastric mucosa induces the development of deep gastric ulceration and gastric mucosal damage directly associated with extracellular matrix degradation, in which zinc-dependent MMPs play a crucial role. Several animal studies of gastric ulcers have focused on the role of MMPs, mainly MMP-2 and MMP-9[49]. MMPs are divided into several groups based on their substrate specificity and cellular localization; such groups include collagenases, gelatinases, stromelysins, membrane-type MMPs, and others[50]. MMP-2 (72 kDa) and MMP-9 (92 kDa) are gelatinases that function in wound formation and subsequent healing[51,52]. Wound formation and subsequent healing represent dynamic processes of extracellular matrix remodeling, mainly influenced by MMP-2 and MMP-9.
Li et al[49] reported enhanced expression of MMP-9 in the margin of the ulcer and suggested that this finding may be indicative of inflammation and poor wound healing. Our results suggest that treatment with HEEP or LZ may inhibit MMP-9 activity, as no band corresponding to MMP-9 was detected in males (Figure 5), intact females (Figure 6) and OVZ females (Figure 7). This finding may also be explained by the fact that, at 14 d after induction with acetic acid, the ulcers were in the slow-healing phase, whereas MMP-9 is important in the early phase of gastric ulcer formation[49]. Neither HEEP nor LZ treatment affected MMP-2 activity in this model of acetic acid-induced gastric ulcers in rats. These results corroborate the findings of Baragi et al[53], namely that the expression of MMP-2 remained constant throughout the ulcer healing phase. Fini and Girard[54] reported that the expression of MMP-2 was the same in controls and in animals injected with acetic acid, concluding that MMP-2 may not have a direct role in the formation or healing phases of the ulcer, but rather may help in maintaining the integrity of the matrix structure by aiding in the proper assembly of new collagen fibrils.
Ulcer healing requires filling of the defect with cells and connective tissue, which is accomplished by cell migration, proliferation (mediated by EGF), apoptosis (mediated by caspases and Bcl-2), angiogenesis (mediated by VEGF) and remodeling (mediated by MMPs), ultimately leading to scar formation. All of these processes are controlled by growth factors (prostaglandins produced by COX-1 and COX-2), which stimulate cell proliferation and division. At the ulcer margin, epithelial cells proliferate and migrate to the granulation tissue to cover (re-epithelialize) the ulcer and initiate reconstruction of the glands within the ulcer scar[55,56]. Hence, we performed western blotting to evaluate the expression levels of COX-1, COX-2, EGF, VEGF, caspase-8, caspase-9, caspase-3, and Bcl-2 after 14 d of treatment with HEEP or LZ in animals with gastric ulcers induced by acetic acid (Figures 8-15). While HEEP did not alter the levels of these proteins (P > 0.05 vs vehicle), VEGF expression was higher in intact females than in OVZ females treated with HEEP. On the other hand, LZ treatment was associated with increased expression of EGF in males and OVZ females (P < 0.05 vs corresponding vehicle) (Figure 10). Growth factors such as EGF activate epithelial cell migration and proliferation, accelerating wound and ulcer healing in vivo and in vitro[57,37]. The increased expression of EGF in rats treated with LZ may reflect the ongoing healing of gastric ulcers in these animals, with intact females having progressed further than males and OVZ females. Since no significant increase in EGF levels was noted in rats treated with HEEP, we may infer that healing had progressed further and was potentially stabilized in such rats; on the other hand, rats treated with LZ may have experienced re-exacerbation, which represents the main challenge in the treatment of lesions in poorly vascularized and epithelialized gastric tissue.
The results of western blotting analysis revealed that HEEP treatment was not associated with altered expression of cell growth and death factors at 14 d after ulcer induction, suggesting rather that the expression levels of such factors had already normalized. Furthermore, we found that female sex hormones interfered with the expression of EGF, VEGF, caspase-8, and Bcl-2 (Figures 10-12 and 15). Compared to males and OVZ females receiving the same treatment, intact females showed a greater healing effect, decreased expression of EGF, apoptotic factors such as caspase-8, and anti-apoptotic factors such as Bcl-2, as well as increased VEGF expression.
Little is known about gender differences in the gastrointestinal tract because the studies that correlate anti- and pro-apoptotic protein expression with female sex hormones in normal gastric mucosal tissue are limited. Qin et al[58] shows that estrogen can induce apoptosis in gastric cancer cells, and that Bcl-2 might be involved in this effect.
Despite the lack of studies that provide an explanation about the gastric healing action related to female sex hormones, Kumtepe et al[59] showed the beneficial effect of acute and chronic administration of progesterone, estrogen, FSH and LH in rat gastric tissue, indicating the interference of the hormonal factor in this process.
In this model of acetic acid-induced gastric ulcers, we also evaluated the safety of HEEP treatment. No deaths or changes in body mass were noted throughout the treatment (Figures 16). The weights of the organs were not detrimentally affected by HEEP administration (Tables 1-3). In addition, evaluation of biochemical parameters indicated no detrimental effect of HEEP administration on hepatic integrity (AST, ALT, and γ-GT), renal integrity (urea and creatinine), or glucose levels (Tables 1-3). We found that HEEP treatment (125 mg/kg daily) did not alter any of the biochemical parameters analyzed (P > 0.05 vs vehicle). In addition, LZ treatment (30 mg/kg daily) was associated with significantly higher elevated serum glucose levels (P < 0.05 vs vehicle), but within the normal reference values[60]. According to the Clinical Laboratory Parameters for Crl:WI (Han) Rats, the reference values for glucose levels in male rats aged 8-16 wk range from 70-208 mg/dL. Thus, despite the variations between the groups (LZ vs vehicle), the values were within the normal range. Taken together, these findings indicate that 14 d oral treatment with HEEP (125 mg/kg daily) has no subacute toxicity in male or female (intact or OVZ) rats.
In conclusion, our findings suggest that HEEP treatment during 14 consecutive days can achieve the healing of gastric ulcer lesions in all groups defined in terms of sex (male vs female) and hormonal status (intact vs OVZ females). This healing effect is reinforced by the enhancement of cell proliferation and migration. The anti-ulcer effect of HEEP is mediated by PGE2 only in males. Compared to OVZ females and males treated with HEEP, intact females treated with HEEP are expected to have improved healing (higher reduction in lesion area), higher VEGF expression, and decreased expression of caspase-8 and Bcl-2. Finally, HEEP treatment is safe and unlikely to exhibit subacute toxicity or cytotoxicity.
Gastric ulcers refer to acid injury in the digestive tract, resulting in a mucosal break that reaches to the submucosal layer. Acid peptic disorders are very common in the United States, with four million individuals (new cases and recurrences) affected per year. This disease occurs more often in men than in women, but these sex differences are less pronounced after the age of 45 years, probably because there is an increased incidence of ulcers in menopausal women.
The conventional treatment of gastric ulcers is based on the inhibition of gastric acid secretion. However, this therapy is associated with several side effects and poor quality of ulcer healing. Therefore, alternative therapies are desirable. We investigated the effects of extracts from the leaves of E. punicifolia (HEEP), which is a medicinal plant popularly used to treat inflammation and wounds.
We evaluated the sex-specific effects of HEEP in the healing of gastric ulcers induced by acetic acid in rats.
We used a rat model of acetic acid-induced gastric ulcers to evaluate the healing effect of HEEP. We measured prostaglandin levels, analyzed the extracellular matrix by zymography, evaluated the quality of ulcer healing by western blot, and assessed the healing activity by scratch assay. Subacute toxicity (in vivo) and cytotoxicity (in vitro) were also investigated.
HEEP demonstrated a high healing capacity, with substantial reduction of lesion area in all groups studied (males, intact females, ovariectomized females). HEEP accelerated the healing of gastric lesions, and this effect was modulated by female sex hormones. The curative role of HEEP is due to an increase in PGE2 (only in males), as well as an inhibition (MMP-9) and maintenance (MMP-2) of the extracellular matrix in the gastric mucosa. The HEEP healing properties were also confirmed by the enhancement of proliferation and coverage of scratched wounds in a fibroblast monolayer (in vitro). No sign of toxicity was observed in this study.
This was the first study to prove the healing activity of HEEP in acetic acid-induced gastric ulcers for both sexes (males vs females) and irrespective of hormonal status (cycling vs ovariectomized females). This effect is modulated by female sex hormones. The curative effect of HEEP is also mediated by prostaglandin, the remodeling of the extracellular matrix, and both cell proliferation and migration in the gastric mucosa. Additionally, HEEP does not cause subacute toxicity or cytotoxicity.
The present findings support the popular use of E. punicifolia in the treatment of gastrointestinal ulcers, and contribute to the search for new therapies for diseases of the gastrointestinal tract.
We are grateful for the technical support kindly provided by the members of the Laboratory of Extracellular Matrix of the Department of Morphology, Institute of Biosciences, UNESP, Botucatu, São Paolo, Brazil.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Nakajima N, Soriano-Ursua MA S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Bian YN
| 1. | Gurusamy KS, Pallari E. Medical versus surgical treatment for refractory or recurrent peptic ulcer. Cochrane Database Syst Rev. 2016;3:CD011523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Venzon L, Mariano LNB, Somensi LB, Boeing T, de Souza P, Wagner TM, Andrade SF, Nesello LAN, da Silva LM. Essential oil of Cymbopogon citratus (lemongrass) and geraniol, but not citral, promote gastric healing activity in mice. Biomed Pharmacother. 2018;98:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Monici LT, Zeitune JMR, Lorena SLS, Mesquita MA, Magalhães AFN. de Úlcera péptica. Rev Bras Med. 2003;60:25-32. |
| 4. | Yu LY, Sun LN, Zhang XH, Li YQ, Yu L, Yuan ZQ, Meng L, Zhang HW, Wang YQ. A Review of the Novel Application and Potential Adverse Effects of Proton Pump Inhibitors. Adv Ther. 2017;34:1070-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Young Oh T, Ok Ahn B, Jung Jang E, Sang Park J, Jong Park S, Wook Baik H, Hahm KB. Accelerated Ulcer Healing and Resistance to Ulcer Recurrence with Gastroprotectants in Rat Model of Acetic Acid-induced Gastric Ulcer. J Clin Biochem Nutr. 2008;42:204-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Tarnawski A, Stachura J, Krause WJ, Douglass TG, Gergely H. Quality of gastric ulcer healing: a new, emerging concept. J Clin Gastroenterol. 1991;13 Suppl 1:S42-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Kangwan N, Park JM, Kim EH, Hahm KB. Quality of healing of gastric ulcers: Natural products beyond acid suppression. World J Gastrointest Pathophysiol. 2014;5:40-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Fornai M, Colucci R, Antonioli L, Awwad O, Ugolini C, Tuccori M, Fulceri F, Natale G, Basolo F, Blandizzi C. Effects of esomeprazole on healing of nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers in the presence of a continued NSAID treatment: Characterization of molecular mechanisms. Pharmacol Res. 2011;63:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Yang RQ, Mao H, Huang LY, Su PZ, Lu M. Effects of hydrotalcite combined with esomeprazole on gastric ulcer healing quality: A clinical observation study. World J Gastroenterol. 2017;23:1268-1277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Abdel-Aziz H, Kelber O, Lorkowski G, Storr M. Evaluating the Multitarget Effects of Combinations through Multistep Clustering of Pharmacological Data: the Example of the Commercial Preparation Iberogast. Planta Med. 2017;83:1130-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Estrella E. Plantas Medicinales Amazonicas: realidad y perspectivas. Lima, Peru. Secretaria Pro Tempore. 1995;1995:302. |
| 12. | de Oliveira RN, Dias IJM, Câmara CAG. Estudo comparativo do óleo essencial de Eugenia punicifolia (HBK) DC. de diferentes localidades de Pernambuco. 2005;15:39-43. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Maia JGS, Zoghbi MGB, Andrade EHA. Plantas aromáticas na Amazônia e seus óleos essenciais. Belém: Museu Paraense Emílio Goeldi. 2001;1-173. |
| 14. | Chaves EMF, Barros RFM. Diversidade e uso de recursos medicinais do carrasco na APA da Serra da Ibiapaba, Piauí, Nordeste do Brasil. Rev Bras Plantas Med. 2012;14:476-486. [DOI] [Full Text] |
| 15. | Silva S. Plantas do cerrado utilizadas pelas comunidades da região do Grande Sertão Veredas. Funatura. 1998;1-109. |
| 16. | Basting RT, Nishijima CM, Lopes JA, Santos RC, Lucena Périco L, Laufer S, Bauer S, Costa MF, Santos LC, Rocha LR. Antinociceptive, anti-inflammatory and gastroprotective effects of a hydroalcoholic extract from the leaves of Eugenia punicifolia (Kunth) DC. in rodents. J Ethnopharmacol. 2014;157:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Périco LL, Heredia-Vieira SC, Beserra FP, de Cássia Dos Santos R, Weiss MB, Resende FA, Dos Santos Ramos MA, Bonifácio BV, Bauab TM, Varanda EA. Does the gastroprotective action of a medicinal plant ensure healing effects? An integrative study of the biological effects of Serjania marginata Casar. (Sapindaceae) in rats. J Ethnopharmacol. 2015;172:312-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Long JA, Evans HM. The oestrous cycle in the rat and its associated phenomena. Memories of University of California. University of California Press. 1922;6:1-148. |
| 19. | Mandl AM. The Phases of the Oestrous Cycle in the Adult White Rat. J Exp Biol. 1951;28:576-584. |
| 20. | Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 1009] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 21. | Okabe S, Roth JL, Pfeiffer CJ. A method for experimental, penetrating gastric and duodenal ulcers in rats. Observations on normal healing. Am J Dig Dis. 1971;16:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 168] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Konturek SJ, Dembinski A, Warzecha Z, Brzozowski T, Gregory H. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology. 1988;94:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 198] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Chen W, Wang Z, Zhang Y. The effect of zinc on the apoptosis of cultured human retinal pigment epithelial cells. J Huazhong Univ Sci Technolog Med Sci. 2003;23:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Balekar N, Katkam NG, Nakpheng T, Jehtae K, Srichana T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J Ethnopharmacol. 2012;141:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Parvin R, Pande SV, Venkitasubramanian TA. On the colorimetric biuret method of protein determination. Anal Biochem. 1965;12:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Brooks SP, Lampi BJ, Sarwar G, Botting HG. A comparison of methods for determining total body protein. Anal Biochem. 1995;226:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Justulin LA Jr, Della-Coleta HH, Taboga SR, Felisbino SL. Matrix metalloproteinase (MMP)-2 and MMP-9 activity and localization during ventral prostate atrophy and regrowth. Int J Androl. 2010;33:696-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Takagi K, Okabe S, Saziki R. A new method for the production of chronic gastric ulcer in rats and the effect of several drugs on its healing. Jpn J Pharmacol. 1969;19:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 219] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Okabe S, Amagase K. An overview of acetic acid ulcer models--the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005;28:1321-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Okabe S, Roth JL, Pfeiffer CJ. Differential healing periods of the acetic acid ulcer model in rats and cats. Experientia. 1971;27:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Adinortey MB, Ansah C, Galyuon I, Nyarko A. In Vivo Models Used for Evaluation of Potential Antigastroduodenal Ulcer Agents. Ulcers. 2013;2013:1-12. [DOI] [Full Text] |
| 32. | Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:43S-51S; discussion 79S-80S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Günal O, Oktar BK, Ozçinar E, Sungur M, Arbak S, Yeğen B. Estradiol treatment ameliorates acetic acid-induced gastric and colonic injuries in rats. Inflammation. 2003;27:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Aguwa CN. Effects of exogenous administration of female sex hormones on gastric secretion and ulcer formation in the rat. Eur J Pharmacol. 1984;104:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Lindh A, Carlström K, Eklund J, Wilking N. Serum steroids and prolactin during and after major surgical trauma. Acta Anaesthesiol Scand. 1992;36:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Shan J, Resnick LM, Liu QY, Wu XC, Barbagallo M, Pang PK. Vascular effects of 17 beta-estradiol in male Sprague-Dawley rats. Am J Physiol. 1994;266:H967-H973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50 Suppl 1:S24-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 38. | Chai J, Norng M, Tarnawski AS, Chow J. A critical role of serum response factor in myofibroblast differentiation during experimental oesophageal ulcer healing in rats. Gut. 2007;56:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 480] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 40. | Mutoh H, Sashikawa M, Hayakawa H, Sugano K. Monocyte chemoattractant protein-1 is generated via TGF-beta by myofibroblasts in gastric intestinal metaplasia and carcinoma without H. pylori infection. Cancer Sci. 2010;101:1783-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Konturek SJ, Brzozowski T, Majka J, Szlachcic A, Bielanski W, Stachura J, Otto W. Fibroblast growth factor in gastroprotection and ulcer healing: interaction with sucralfate. Gut. 1993;34:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Nishida T, Tsuji S, Kimura A, Tsujii M, Ishii S, Yoshio T, Shinzaki S, Egawa S, Irie T, Yasumaru M. Endothelin-1, an ulcer inducer, promotes gastric ulcer healing via mobilizing gastric myofibroblasts and stimulates production of stroma-derived factors. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1041-G1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | da Silva LM, Boeing T, Somensi LB, Cury BJ, Steimbach VM, Silveria AC, Niero R, Cechinel Filho V, Santin JR, de Andrade SF. Evidence of gastric ulcer healing activity of Maytenus robusta Reissek: In vitro and in vivo studies. J Ethnopharmacol. 2015;175:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Abe Y, Inagaki K, Fujiwara A, Kuriyama K. Wound healing acceleration of a novel transforming growth factor-beta inducer, SEK-1005. Eur J Pharmacol. 2000;408:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Perini RF, Ma L, Wallace JL. Mucosal repair and COX-2 inhibition. Curr Pharm Des. 2003;9:2207-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 490] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 47. | Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Pajdo R, Drozdowicz D, Ptak A, Hahn EG. Classic NSAID and selective cyclooxygenase (COX)-1 and COX-2 inhibitors in healing of chronic gastric ulcers. Microsc Res Tech. 2001;53:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Tatsuguchi A, Sakamoto C, Wada K, Akamatsu T, Tsukui T, Miyake K, Futagami S, Kishida T, Fukuda Y, Yamanaka N. Localisation of cyclooxygenase 1 and cyclooxygenase 2 in Helicobacter pylori related gastritis and gastric ulcer tissues in humans. Gut. 2000;46:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Li SL, Zhao JR, Ren XY, Xie JP, Ma QZ, Rong QH. Increased expression of matrix metalloproteinase-9 associated with gastric ulcer recurrence. World J Gastroenterol. 2013;19:4590-4595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Parks WC, Mecham RP. Matrix metalloproteinase. Academic Press. 1998;. |
| 51. | Lempinen M, Inkinen K, Wolff H, Ahonen J. Matrix metalloproteinases 2 and 9 in indomethacin-induced rat gastric ulcer. Eur Surg Res. 2000;32:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Gyenge M, Amagase K, Kunimi S, Matsuoka R, Takeuchi K. Roles of pro-angiogenic and anti-angiogenic factors as well as matrix metalloproteinases in healing of NSAID-induced small intestinal ulcers in rats. Life Sci. 2013;93:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Baragi VM, Qiu L, Gunja-Smith Z, Woessner JF Jr, Lesch CA, Guglietta A. Role of metalloproteinases in the development and healing of acetic acid-induced gastric ulcer in rats. Scand J Gastroenterol. 1997;32:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Fini ME, Girard MT. The pattern of metalloproteinase expression by corneal fibroblasts is altered by passage in cell culture. J Cell Sci. 1990;97:373-383. [PubMed] |
| 55. | Tarnawski A, Tanoue K, Santos AM, Sarfeh IJ. Cellular and molecular mechanisms of gastric ulcer healing. Is the quality of mucosal scar affected by treatment? Scand J Gastroenterol Suppl. 1995;210:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Sánchez-Fidalgo S, Martín-Lacave I, Illanes M, Motilva V. Angiogenesis, cell proliferation and apoptosis in gastric ulcer healing. Effect of a selective cox-2 inhibitor. Eur J Pharmacol. 2004;505:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Tarnawski AS, Jones MK. The role of epidermal growth factor (EGF) and its receptor in mucosal protection, adaptation to injury, and ulcer healing: involvement of EGF-R signal transduction pathways. J Clin Gastroenterol. 1998;27 Suppl 1:S12-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Qin J, Liu M, Ding Q, Ji X, Hao Y, Wu X, Xiong J. The direct effect of estrogen on cell viability and apoptosis in human gastric cancer cells. Mol Cell Biochem. 2014;395:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Kumtepe Y, Borekci B, Karaca M, Salman S, Alp HH, Suleyman H. Effect of acute and chronic administration of progesterone, estrogen, FSH and LH on oxidant and antioxidant parameters in rat gastric tissue. Chem Biol Interact. 2009;182:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Giknis ML, Clifford CB. Clinical laboratory parameters For Crl: WI (Han) rats. Charles River Press. 2008;1-17. |