Published online Oct 7, 2018. doi: 10.3748/wjg.v24.i37.4243
Peer-review started: June 12, 2018
First decision: August 1, 2018
Revised: August 6, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: October 7, 2018
Processing time: 110 Days and 23 Hours
Although colonoscopy has been proven effective in reducing the incidence of colorectal cancer through the detection and removal of precancerous lesions, it remains an imperfect examination, as it can fail in detecting up to almost one fourth of existing adenomas. Among reasons accounting for such failures, is the inability to meticulously visualize the colonic mucosa located either proximal to haustral folds or anatomic curves, including the hepatic and splenic flexures. In order to overcome these limitations, various colonoscope attachments aiming to improve mucosal visualization have been developed. All of them - transparent cap, Endocuff, Endocuff Vision and Endorings - are simply mounted onto the distal tip of the scope. In this review article, we introduce the rationale of their development, present their mode of action and discuss in detail the effect of their implementation in the detection of lesions during colonoscopy.
Core tip: Colonoscopy is the modality of choice for the detection and removal of precancerous lesions. However, almost one fourth of adenomas can be lost during conventional colonoscopy. Their location proximal to the colonic folds or in proximity to anatomic flexures is one of the reasons for this particular detection failure. To overcome this caveat, various single-use devices mounted onto the tip of the scope have been developed. They facilitate lesions’ detection by manipulating and flattening the haustral folds. In this Minireview we present the development of these devices (Cap, Endocuff, Endocuff Vision and Endorings) and their effectiveness in improving detection rates of lesions during colonoscopy.
- Citation: Gkolfakis P, Tziatzios G, Spartalis E, Papanikolaou IS, Triantafyllou K. Colonoscopy attachments for the detection of precancerous lesions during colonoscopy: A review of the literature. World J Gastroenterol 2018; 24(37): 4243-4253
- URL: https://www.wjgnet.com/1007-9327/full/v24/i37/4243.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i37.4243
Colorectal cancer (CRC) is the second most lethal cancer among common cancers and more than 140200 new CRC cases are expected to be diagnosed in the United States by the end of 2018[1]. Colonoscopy has been proven efficient for both diagnosis and screening of colorectal cancer. It allows the detection and consequent removal of adenomas, the most well-known precancerous lesions, preventing CRC-associated death[2]. Adenoma detection rate (ADR)-the percentage of colonoscopies with at least one adenoma- has been associated with both decreased risk of interval CRC (i.e., CRC that is diagnosed in the meantime between a screening colonoscopy and the next recommended surveillance examination) incidence and death[3-5]. Thus, ADR has been established as the core quality indicator for colonoscopy[6]. However, colonoscopy stands far from being the perfect examination. Back-to-back studies have shown that endoscopists fail to detect almost 25% of existing polyps and adenomas[7,8]. These miss rates are higher in the right colon, where a variant of precancerous lesions (the sessile serrated adenomas) that does not follow the classic adenoma-carcinoma pathway of carcinogenesis occurs more frequently[9,10]. To a great extent, missed lesions like these have been held responsible for the aforementioned interval cancers[11]. Inadequate bowel preparation, lack of physician’s expertise, inability to accurately visualize the colonic mucosa located proximal to the haustral folds or in proximity to anatomic flexures have been listed among the main reasons such lesions can be missed during a colonoscopy[12]. Lately, several devices-ranging from complex endoscopic systems to simple plastic attachments- have been developed, in an attempt to address this problem[13]. They promise to flatten the mucosa during scope withdrawal and facilitate maneuverability around anatomic flexures offering meticulous mucosal visualization and detection of “hidden” lesions. In this review we aim to present the rationale that led to the development of these detachable devices, their evolution through time, their main mode of action and technical characteristics, as well as their impact on various patient-related colonoscopy outcomes. A comprehensive review of English literature published in MEDLINE until May 2018 was conducted. We aimed to identify high quality studies (randomized controlled trials and meta-analyses) using the following key words: “Cap”, “Cap-assisted colonoscopy”, “Endocuff”, “Endocuff-Vision”, “Endocuff-assisted colonoscopy” and “Endorings”. Apart from ADR the following measures were assessed: polyp detection rate (PDR), i.e., the percentage of colonoscopies with at least one polyp, mean number of adenomas detected per colonoscopy (MAC), adenoma miss rate-the percentage of adenomas missed by the index examination and detected by the tandem colonoscopy- and advanced ADR (the percentage of colonoscopies with at least one advanced adenoma).
The transparent cap is a simple, single-use device made of thermoplastic elastomer (Figure 1). It was initially designed to facilitate endoscopic mucosectomy, since it enables an optimal field of view by maintaining an appropriate distance between the endoscope tip and the intervention site[14]. Originally launched by Olympus (Olympus America Inc., Center Valley, PA, United States), it is available in various sizes, in order to accommodate all types of endoscopes. Its rounded edge prevents tissue damage during contact, while its side hole allows fluid draining. Endoscope functions such as suction and air insufflation remain undisturbed. The distal end of the cap protrudes from the scope’s tip (protruding length ranges from 2 mm to 7 mm). Its basic characteristics are outlined in Table 1. It is the protruding edge that allows manipulation and flattening of the colonic folds in the field of view. During the last 10 years numerous randomized control trials[15-27] have been conducted to evaluate the usefulness of cap-assisted colonoscopy (CAC) in improving colonoscopy outcomes, including potential augmentation of the detection of precancerous lesions. Beyond ADR and PDR, additional outcomes such as cecal intubation rate and cecal intubation time were assessed as well.
| Cap | Endocuff | Endocuff Vision | Endorings | |
| Manufacturer | Olympus, Centre Valley, Pennsylvania | Arc Medical Leeds, United Kingdom | Norgine Pharmaceuticals Ltd, Uxbridge, United Kingdom | EndoAid, Caesarea, Israel |
| Launched in market | 1993 | 2011 | 2016 | 2015 |
| Short description | Transparent, single-use distal attachment with side hole for draining of fluid | Single-use, soft, radiopaque, 2 cm long cylindrical sleeve with flexible projections arranged in 2 rows of 8, emerging from gaps on the shaft of the device | Single-use, device with single row of 8 flexible 15 mm spikes | Single-use device composed of 2 layers of flexible, soft circular rings, placed on a cylindrical cuff |
| Material | Thermoplastic elastomer | Core: Non-latex, biocompatible polymer; Projections: thermoplastic elastomer | Latex free, polypropylene | Silicone |
| Dimensions | Outer diameter ranging from 13.9-16.1 mm according to each type of cap | Finger projections: proximal 8.15 mm, distal 5mm; core length: 23.8 mm; diameter: 16.1, 16.7, 17.2, and 18.5 mm (hairs folded back) and 32.6, 33.1, 33.6, and 34.8 mm (hairs opened out) | Diameter: 16.1, 16.7, 17.2, and 18.5mm (spikes folded back) and 39.07, 39.07, 39.07, and 39.66 mm (spikes opened out) | 22-50 mm diameter |
| Mode of action | Protruding cap manipulates and flattens haustral folds to inspect the mucosa on the proximal side of the fold maintaining optimal field of view | Hinged projections flatten and spread mucosa and folds | Hinged projections flatten and improve visibility behind the colon folds | Sequential rings stretches out the folds of the colon during withdrawal for a clear view |
| Interfere with view of field | Edge of the hood comes into the vision field of the colonoscope, but lesions can be seen through the transparent wall | No interference of vision | No interference with vision | No interference with vision |
| Compatible scopes | Adult, pediatric: Ten different sizes, to fit all scopes | Adult, pediatric: 4 color-coded sizes (purple, orange, green and blue) to fit all scopes | Adult, pediatric: 4 color-coded sizes (purple, orange, green and blue) to fit all scopes | Scope Distal End Diameter [mm]; Adult colonoscope 12.8-14.5 mm; Slim Adult colonoscope 11.5-13.0 mm |
| Advantages | Resection of wider areas; Suction and insufflation of air unaffected | Folds movement provides a dynamic picture - even the smallest polyps can be identified; Centers the scope in the middle of the lumen preventing sudden slip back and “red-out”; Projections allow traction to avoid sudden slippage around turns and flexures, improving scope’s stability; Helps perform EMR | Delivers more tip control without compromising intubation - improving loop management; Early and controlled view of the upstream surface of large folds - no need for repeated intubation; Prevents sudden slip back and red out; Optimizes tip position during therapy and polyp retrieval | Maintains position during loop reduction, decreases slippage, anchoring during endoscopic therapy; Maintains identical depth and breadth of scope's viewing field; Minimal resistance on insertion; Easy ileum intubation |
| Disadvantages | Interfere with the field of view | Petechial marks on colon; Potential dislodgement; Larger model more effective than smaller; Ileum intubation may be difficult | Potential dislodgement | Ileum intubation may be difficult |
Kondo et al[15] evaluated colonoscopy with two types of caps (2 mm-short or 4 mm-transparent) vs conventional colonoscopy without a cap. More than 200 patients undergoing colonoscopy for various indications were randomized in each of the three groups. The use of the transparent 4mm cap was associated with decreased cecal intubation time compared to the 2 mm-short cap and the control (11.5 min vs 13.5 min vs 15 min; P = 0.008). At the same time, PDR was significantly increased in the transparent cap group (49.3%) compared to the controls (39.1%; P = 0.04)[15]. In another Japanese randomized controlled trial (RCT) with 592 patients, CAC with a 2 mm short cap was also related to a shorter cecal intubation rate, but without any difference in the rate of polyp detection[17]. Horiuchi et al[16] randomized 60 patients diagnosed with colonic adenomas to repeat colonoscopy in three months, with or without a 7 mm cap; all lesions were removed during the second examination. Cap-assisted colonoscopy detected 20% more adenomas compared to a 4% increase in adenoma detection without the cap[16]. Moreover, a small back-to-back RCT of 67 screening/surveillance patients demonstrated a reduced adenoma miss rate associated to cap-assisted colonoscopy compared to conventional colonoscopy (21% vs 33%; P = 0.04)[19].
In terms of ADR and MAC, evidence remains controversial. A study from Japan[21], evaluating the efficacy of autofluorescence imaging with a transparent cap in a cancer referral center, found that CAC leads to an increased ADR (62% vs 56%; P = 0.023) compared to conventional white-light imaging. A few years later, in the first USA. study, Rastogi et al[23] randomized 420 screening/surveillance individuals (210 in each group) to undergo either CAC using a 4 mm transparent cap or a conventional examination. Investigators concluded that CAC not only shortened the cecal intubation time (3.3 min vs 4 min; P < 0.001), but also increased ADR (69% vs 56%; P = 0.009) and MAC (2.3 vs 1.4; P < 0.001) compared to colonoscopy without the cap[23]. In a study that randomized 1113 patients with various indications to undergo either CAC (4 mm cap) or conventional colonoscopy[24], cecal intubation was faster in the CAC arm (4.9 min vs 5.8 min; P < 0.001), but both arms had similar ADR (42% vs 40%; P = 0.452) and MAC (0.89 vs 0.82; P = 0.432)[24]. It is of great interest that among the 10 participating endoscopists the effect of CAC in terms of ADR ranged from a 15% decrease to a 20% increase[24]. Looking at individual endoscopists’ performance, the authors concluded that CAC may be beneficial especially for endoscopists who spend more time during scope withdrawal since the cap may further enhance their already meticulous examination[24]. Recently, Othman et al[26] showed that CAC compared to conventional colonoscopy is related to an increased advanced ADR - the detection rate of advanced adenomas- (9.9% vs 4.6%; P = 0.049) and detection of more polyps larger than 9mm (9.5% vs 3.7%; P = 0.026)[26]. However, in this RCT of 440 screening/surveillance participants no difference between the two groups in terms of ADR and PDR was found[26].
In two RCTs[20,22], 400 individuals of mixed indications[20] and 1380 screening participants[22] were allocated either to CAC or conventional examination. A 4-mm cap was used in both studies. The first study[20] did not show any benefit of CAC in terms of PDR (32.8% vs 31.3%; P = 0.75) and cecal intubation time (9.9 min vs 10.3 min; P = 0.21), while in the second study[22] CAC was associated with a shorter intubation time (7.7 min vs 8.9 min; P < 0.001), but ADR (29% vs 29%; P = 0.96) and MAC (0.52 vs 0.50; P = 0.83) did not differ between the two groups.
Two studies involving endoscopy trainees provided similar results; trainees had a higher cecal intubation rate (CIR) and reached the cecum faster using the cap[27,28]. However, CAC did not improve trainees’ detection rates (ADR and advanced ADR)[27,28].
Paradoxically, in a large RCT (1000 patients recruited) from Hong Kong, ADR was lower in the cap-assisted arm compared to the standard one (30.5% vs 37.5%; P = 0.018), but there was no difference regarding advanced lesions[18]. Shorter withdrawal times and inadequate bowel preparation in the CAC arm were postulated by the authors as potential explanations for this finding[18]. In accordance with previous results, cecal intubation time was shorter in the cap arm (6 min vs 7.2 min; P < 0.001) with no difference in the CIR[18].
Seven meta-analyses[29-35] that attempt to summarize the role of CAC in improving colonoscopy outcomes have been published so far (Table 2). Despite their different designs and inclusion criteria, one can figure out a couple of mutual conclusions. Four[30,31,34,35] out of five meta-analyses reporting on cecal intubation time, conclude that CAC significantly shortens it (mean difference ranging from -0.93 min to -0.64 min), while three of them[30,34,35] did not show any increase of CIR associated to CAC. Moreover, six[29-32,34,35] and four[30,32,34,35] meta-analyses examined PDR and ADR, respectively. The majority of these[29-31,34] link CAC to a higher PDR. On the contrary, none of the relevant meta-analyses showed a benefit in terms of ADR with CAC[29,30,32,34,35]. However, in the most recent meta-analysis that included 23 RCTs and almost 13000 participants[34], sensitivity analysis showed that the exclusion of one large study[18]-in which the quality of bowel preparation was significantly worse in the CAC arm- not only eliminated the existing heterogeneity, but also altered the synthetic outcome direction, showing a significant benefit of CAC vs conventional colonoscopy regarding ADR [OR (95%CI): 1.17 (1.04-1.33)][34]. Finally, one meta-analysis[33] has evaluated the effect of CAC on the ADR of the right colon. Pooled data from 4 studies (2546 and 2547 patients in the CAC and the conventional arms, respectively) associated CAC with an increased right colon ADR [OR (95%CI): 1.49 (1.08-2.05)] compared to conventional colonoscopy[33].
| Author (yr) | Device vs comparator | Included Studies (n) | Included studies’ design | Patients (n) | ADR | PDR | MAC | CIR | CIT |
| Westwood 2012 | CAC vs CC | 12 (9 FP, 3 AB) | RCTs | 6185 | NR | aOR (95%CI): 1.13 (1.02-1.26) | NR | aOR (95%CI): 1.36 (1.06-1.74) | MD (95%CI): 0.04 (-0.03 to 0.12) min |
| Ng 2012 | CAC vs CC | 16 (13 FP, 3 AB) | RCTs | 8991 | RR (95%CI): 1.04 (0.90-1.19) | aRR (95%CI): 1.08 (1.00-1.17) | NR | RR (95%CI): 1.00 (0.90-1.02) | aMD (95%CI): -0.64 (-1.19 to -0.10) min |
| He 2012 | CAC vs CC | 19 (14 FP, 5 AB) | RCTs | 9235 | NR | aOR (95%CI): 1.12 (1.02-1.22) | NR | aOR (95%CI): 1.36 (1.13-1.64) | aMD (95%CI): -0.65 (-0.85 to −0.44) min |
| Omata 2014 | CAC vs CC | 10 (10 FP) | RCTs | 5219 | RR (95%CI): 1.07 (0.94-1.23) | RR (95%CI): 1.00 (0.86-1.16) | NR | NR | NR |
| Desai 2017 | CAC vs CC | 4 (4 FP) | 2 RCTs; 2 retrospective | 5093 | a1OR (95%CI): 1.49 (1.08-2.05) | NR | NR | NR | NR |
| Mir 2017 | CAC vs CC | 23 (18 FP, 5 AB) | RCTs | 12947 | OR (95%CI): 1.11 (0.95-1.30) | aOR (95%CI): 1.17 (1.06-1.29) | NR | OR (95%CI): 1.32 (0.94-1.87) | aMD (95%CI): -0.82 (-1.20 to -0.44) min |
| Chin 2016 | 2EAC vs CC | 9 (4FP, 5 AB) | 4 RCTs; 1 prospective observational; 4 retrospective | 5624 | aOR (95%CI): 1.49 (1.23-1.80) | NR | NR | OR (95%CI): 1.26 (0.70-2.27) | NR |
| Williet 2018 | 2EAC vs CC | 12 (7 FP, 5 AB) | RCTs | 8376 | aRR (95%CI): 1.20 (1.06-1.36) | aRR (95%CI): 1.20 (1.06-1.36) | MD (95%CI): 0.11 (-0.17-0.38) | RR (95%CI): 0.99 (0.97- 1.00) | MD (95%CI): -0.57 (-1.43 to 0.28) min |
| 3Facciorusso 2017 | CAC vs CC | 14 (14 FP) | RCTs | 8306 | RR (95%CI): 1.07 (0.96-1.19) | RR (95%CI): 1.08 (0.99-1.18) | NR | RR (95%CI): 1.00 (1.00- 1.01) | aMD (95%CI): -0.68 (-1.11 to -0.24) min |
| 2EAC vs CC | 9 (4FP, 5 AB) | RCTs | 7072 | aRR (95%CI): 1.21 (1.03-1.41) | aRR (95%CI): 1.22 (1.07-1.40) | NR | RR (95%CI): 1.00 (0.98- 1.01) | aMD (95%CI): -0.93 (-1.55 to -0.30) min | |
| Endorings vs CC | 1 (1 FP) | RCTs | 116 | RR (95%CI): 1.70 (0.86-3.36) | RR (95%CI): 1.68 (0.94-2.99) | NR | NR | MD (95%CI): 0.90 (-1.47 to 3.27) min |
Endocuff (Arc Medical Design, Leeds, United Kingdom) is a single-use soft, radiopaque device that consists of a cylindrical polypropylene core and 2 rows of flexible thermoplastic elastomer-made projections. Each row counts 8 projections that emerge from gaps on the shaft of the device (Figure 2A and B). It is available in 4 different color-coded sizes to fit all scopes and its technical characteristics are presented in Table 1. Its designers were inspired through the practical difficulties that occur during a conventional colonoscopy, including the scope slipping back, difficulties in tip stabilization and inability to inspect the mucosa located behind folds, to mention a few. Endocuff was launched in 2012 and its use was reported for the first time in a small retrospective feasibility study where it facilitated endoscopic access for complex polypectomy and scar assessment in the sigmoid colon[36]. The projections move independently from another in a passive way when in contact with the mucosa and during withdrawal, they extend radially manipulating colonic folds away from the field of view, allowing a more meticulous mucosa inspection (Figure 2C). Moreover, the device stabilizes the scope in the middle of the lumen and allows traction against sudden slippage around flexures. Moreover, the examiner’s visibility is not affected, since the device does not extend beyond the tip of the scope and thus does not interfere with suction, flushing or the working channel.
There are enough data regarding the effect of Endocuff on colonoscopy outcomes, since seven RCTs of parallel[37-43] and one of tandem[44] design have been published. The first German studies[37,38]-each recruiting almost 500 patients who underwent colonoscopy for various indications (screening included)-showed a significant benefit of Endocuff-assisted colonoscopy (EAC) compared to the conventional one regarding ADR (35.4% vs 20.7%, P < 0.0001 and 36% vs 28%, P = 0.043, respectively)[37,38]. Similar results regarding PDR were also achieved (55.4% vs 38.4%, P < 0.0001 and 56% vs 42%, P = 0.001, respectively), while only the second study[38] detected a difference in the mean number of adenomas detected per colonoscopy [2 (IQR: 1-3) vs 1 (IQR: 1-2), P = 0.002]. Regarding polyp location, both studies identified a superiority of EAC for the detection of polyps located in the sigmoid and the cecum. Moreover, no major adverse events related to EAC were reported and there were no differences between overall procedure and withdrawal times[37,38].
Similar positive results associated to Endocuff use were reported from Japan[39] (477 patients, mixed indications for colonoscopy) and Mexico[40] (337 screening individuals), where two single-centre RCTs demonstrated increase of ADR (55.2% vs 39.2%, P = 0.0002 and 22.4% vs 13.5%, P = 0.02, respectively), PDR (61.9% vs 49.2%, P = 0.003 and 29.9% vs 16%, P = 0.002, respectively) and MAC (1.11 vs 0.66, P < 0.01 and 0.29 vs 0.22, P = 0.04) in the device arms. An Italian single-centre study by De Palma et al[41] enrolled 288 patients with mixed indications and reported that EAC increased ADR by 3.3% (29.6% vs 26.3%) compared to the conventional colonoscopy. However, use of Endocuff was associated with mucosal erosions in 7 (2.5%) cases, with one of them needing to be treated with adrenaline solution injection at the site of bleeding[41].
Additionally, in a recently published 4-arm multicenter parallel-group study comparing Endocuff, Endorings, FUSE and conventional colonoscopy, 299 and 295 patients underwent Endocuff-assisted and conventional high definition colonoscopy, respectively[42]. EAC performed significantly better compared to conventional colonoscopy in terms of ADR (64% vs 56%, P = 0.003), PDR (83% vs 77%, P = 0.001) and MAC (1.82 ± 2.58 vs 1.53 ± 2.33, P = 0.014)[42]. However, Endocuff did not enhance the detection rate of sessile serrated polyps (11% vs 12%, P = 0.047)[42]. There were no differences between the mean insertion time (354 s ± 216 s vs 422 s ± 319 s) for Endocuff-assisted and conventional colonoscopy respectively and no adverse events were reported[42]. On the contrary, a benefit regarding sessile serrated adenoma/polyp detection was shown in a retrospective veterans’ study[45] which included almost 500 participants: Endocuff detected 50 sessile serrated adenomas/polyps compared to 8 detected by conventional colonoscopy (detection rate 15% vs 3%, P < 0.0001).
So far, the largest parallel RCT[43] failed to confirm the positive results reported in the abovementioned studies. In this multicentre study from the Netherlands[43] more than 1000 patients of various indications were randomized to undergo either Endocuff-assisted or conventional colonoscopy; MAC and ADR consisted the primary outcomes. ADR was the same in both groups (52%, P = 0.92), whereas the higher number of adenomas per patient in the Endocuff group (1.36 ± 2.10 vs 1.17 ± 1.65) did not reach statistical significance (P = 0.08). Interestingly, detection rates did not differ either according to indication or between academic and non-academic centres[43]. Cecal intubation time was significantly shorter in the Endocuff arm [median (IQR) 7 min (5-10) vs 8.3 min (6-12), P < 0.001] and there were no Endocuff-associated adverse events[43].
Finally, a multicentre back-to-back study[44] assessed Endocuff in terms of adenoma miss rates. Two hundred patients (86.5% were screening and surveillance cases) were randomized (1:1) to undergo either initial EAC followed by a conventional one or vice versa[44]. EAC was associated with lower adenoma miss rates, both overall and in the proximal colon compared to conventional colonoscopy (14.7% vs 38.4% and 10.4% vs 38.9%, respectively)[44]. It is worthy to note that all examinations were performed by endoscopists with an historical ADR > 35%, suggesting that the device could enhance detection ability even of experienced and skilled endoscopists. Despite the fact that there were no serious adverse events, in three index Endocuff examinations cecal intubation failed to be achieved, compared to none with the conventional scope (P = 0.08)[44].
Despite its revolutionary design, Endocuff was associated with a couple of drawbacks (mucosal erosions and difficulties in terminal ileum intubation) that paved to way for its descendant, namely Endocuff Vision (Norgine Pharmaceuticals Ltd, Uxbridge, United Kingdom). This single-use device is made of a polypropylene cylinder and a single row of 8-longer than in the first generation Endocuff-thermoplastic elastomer-made “spikes” (Figure 3). There are 4 different sizes with respective colors to fit in all scopes ranging from pediatric to adult ones (Table 1). Endocuff Vision is also mounted onto the tip of the scope before insertion and its “spikes” fold around the scope while it advances in the colon due to a hinge at the base of each spike that thins progressively. On the other hand, the “spikes” evert during withdrawal (Figure 3). This leads to an early and controlled view of the upstream surface of the large colonic folds in the right colon and prevents sudden scope slip-back. Moreover, when in the sigmoid colon, the device facilitates the opening of contracted folds, permitting a clearer view of the in-between mucosa. Similar to the first generation Endocuff it optimizes the tip’s position during endoscopically applied therapy (e.g., polypectomy).
Endocuff Vision has been evaluated only in two parallel multicenter RCTs from the United Kingdom[46,47]. The “ADENOMA” study[46] recruited 1772 adult patients (45% screening). Of them, 884 underwent conventional colonoscopy and 886 Endocuff Vision-assisted colonoscopy. ADR was significantly higher with EAC compared to conventional colonoscopy (40.9% vs 36.2%, P = 0.02). The benefit of Endocuff Vision was even higher in patients participating in the screening program, where ADR was 61.5% for EAC compared to 50.9% (P < 0.001) for the conventional colonoscopy arm. Similar results in favor of EAC were also reported regarding PDR (54.1% vs 48%; P = 0.005 and 73.9% vs 63.3%; P < 0.001 for the whole and the screening cohorts, respectively). Of note, EAC showed a statistically significant increase in the detection rate in the left colon (26.1% vs 2.2%; P = 0.03), of small (10.6% vs 7.7%; P = 0.02) and of diminutive adenomas (34.6% vs 30.8%; P = 0.04). It should be underlined that in this study[46] EAC detected significantly more cancers both in the whole cohort (4.1% vs 2.3%; P = 0.02) as well as in the screening participants (6.6% vs 3.7%; P = 0.03). Moreover, median insertion time was shorter with Endocuff Vision compared to conventional colonoscopy (8 min vs 9 min; P = 0.001). The investigators did not report any adverse event related to use of Endocuff Vision; however the device had to be removed in 4.1% of the cases mostly due to acute angulation in a fixed sigmoid colon.
On the contrary, the “E-cap” study failed to show any benefit in terms of ADR (60.9% vs 63%, P = 0.85), PDR (70.3% vs 69.8%, P = 0.93) and MAC (1.3 ± 1.8 vs 1.4 ± 1.5, P = 0.54)[47]. This single center study had PDR as the primary endpoint. Only patients attending the national screening program with a positive FOBT test were enrolled and all four participating endoscopists had an extremely high pre-study ADR (58.9%)[47]. All these reasons may attribute to the lack of any significant benefit deriving from application of Endocuff Vision and should be considered in the design of future “real-life” studies, which should possibly include both endoscopists with an average or even a low ADR and patients with various indications for colonoscopy.
Finally, a pilot evaluation study[48] demonstrated that Endocuff Vision was associated with an improvement in endoscopists’ performance measured as increased ADR, increased MAC and decreased insertion time. In this non-randomized study[48], the investigators performed 410 screening colonoscopies in three periods (137 pre-Endocuff, 136 using Endocuff Vision and 137 post-Endocuff). Overall, an increase in ADR (16%, P < 0.03) and MAC (83%, P = 0.007) was noted between the pre-Endocuff and the Endocuff period; this benefit was maintained in the post-Endocuff period, where the device was not available. A potential explanation could be that during the Endocuff period the endoscopists had the chance to comprehend their flaws during the withdrawal phase, look for adenomas in more detail and improve their skills[49]. Interestingly, insertion time was statistically lower during the Endocuff period compared to pre- and post-Endocuff one (7 min vs 8 min, P = 0.002 and 7 min vs 9 min, P = 0.002, respectively)[48]; no adverse events were reported[48].
To date, three meta-analyses attempting to summarize the impact of Endocuff devices on colonoscopy outcomes have already been published[35,50,51] (Table 2).
The earliest one[50] meta-analyzed data from three published papers and six studies presented as abstracts, four of which with a prospective and five with a retrospective design. Eight studies (n = 4387) of mixed populations reported on ADR, which was measured to be higher for the Endocuff group [OR (95%CI): 1.49 (1.23-1.80), I2 = 50%][50]. In this pooled analysis, 27 patients (2.3%) in the Endocuff group experienced superficial mucosal lacerations[50].
A recently published meta-analysis updated these data by including only RCTs (7 published and 5 presented as abstracts)[51]. Regarding ADR, data from more than 8370 patients demonstrated a benefit of EAC compared to conventional colonoscopy [RR (95%CI): 1.20 (1.06-1.36), P = 0.003, I2 = 79%]. Of interest, this benefit was lower in the subgroup of studies with a mean conventional arm ADR > 45% [RR (95%CI): 1.01 (0.93-1.09), P = 0.087, I2 = 0], while it was maximized in the subgroup of studies with a respective ADR lower than 35% [RR (95%CI): 1.51 (1.35-1.69), P < 0.001, I2 = 43%]. These data imply a potential ancillary role of the device especially for lower detectors[51]. Furthermore, a numerical higher MAC was detected in the Endocuff-assisted colonoscopy group, but this difference did not reach statistical significance [mean difference (95%CI): 0.11(-0.17 to 0.38][51]. Mean insertion times did not differ between the two groups and 4% of the Endocuff patients experienced adverse events (exclusively minor lacerations)[51]. This meta-analysis reported on additional outcomes such as advanced ADR and right colon ADR, with no difference detected between the two groups [RR (95%CI): 0.93 (0.76-1.13), P = 0.47 and RR (95%CI): 1.36 (0.80-2.34), P = 0.26, respectively]. However, the small number of studies included in the analysis regarding these outcomes warrants caution when attempting to generalize the respective results.
Finally, similar results were shown in a network meta-analysis investigating the comparative efficacy of distal attachments in increasing detection rates during colonoscopy[35]. The mixed effect estimate (including both pairwise and indirect treatment effects) supported that ADR increased significantly with EAC compared to the conventional examination [RR (95%CI): 1.21 (1.03-1.41)][35]. Interestingly and contrary to the meta-analysis from Williet et al[51] this network meta-analysis[35] calculated a very modest benefit of Endocuff regarding low (baseline ADR 10%) detectors [anticipated ADR (95%CI): 11 (10-12)%] compared to a more considerable effect [anticipated ADR (95%CI): 48 (14-56)%] on ADR of high detectors (baseline ADR 40%).
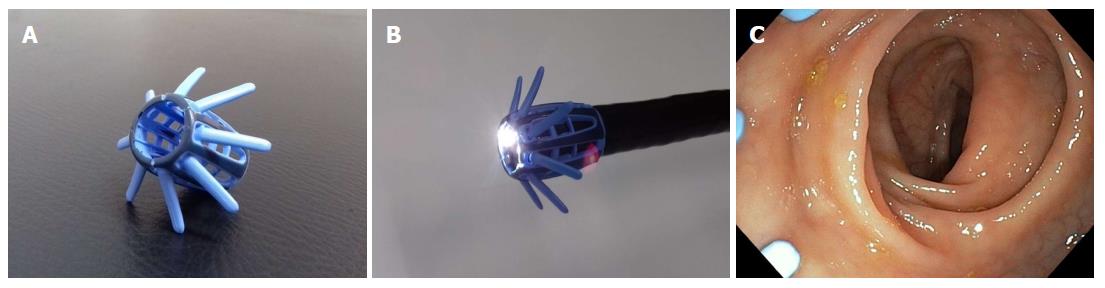
EndoRings (EndoAid Ltd., Caesarea, Israel) is a single-use scope attachment consisting of 2 layers of flexible, soft circular silicon rings placed on a cylindrical cuff (Figure 4A and B). Endorings fit on scopes of an outer diameter ranging from 12.8 mm to 14.5 mm and two sizes for adult and slim adult scopes are available (Table 1). The flexible rings deflect to the opposite direction during scope manipulation. In that way scope insertion is not affected -minimal resistance may be noted- as the rings fold at the side of the scope’s shaft without projecting beyond the distal end of the scope. During withdrawal, the two rings deploy with the proximal-most circular ring creating a wider lumen fenestration by stretching the mucosa and colonic folds (Figure 4C) assisting the detection of otherwise “hidden” lesions. Moreover, the device maintains identical depth and width of scope viewing by stabilizing the scope, maintaining position during loop reduction, deceasing slippage and finally by anchoring during application of endoscopic therapy. Terminal ileum intubation is reported not to be limited by use of the device. Endorings has been evaluated in two RCTs[42,52]. In the first one, a multicentre back-to-back study[52], 116 patients of mixed indications were randomized to undergo initial examination using the Endorings followed by conventional colonoscopy or vice versa. Applying Endorings on the tip of the scope was associated with a statistically significant lower adenoma miss rate compared to conventional colonoscopy (10.4% vs 48.3%, P < 0.001). A similar benefit was also noted for polyp miss rates (9.1% vs 52.8%, P < 0.001). Endorings significantly decreased adenoma miss rates both in the proximal (10.6% vs 58.1%, P < 0.001) and the distal colon (10% vs 37%, P < 0.001). Cecal intubation time was shorter with conventional colonoscopy compared to EndoRings-assisted colonoscopy (8.4 min vs 9.3 min), but this difference did not achieve statistical significance.
In the aforementioned 4-arm parallel group multicenter study[42], 295 and 295 patients were allocated in the in Endorings and the conventional colonoscopy arms respectively. ADR did not differ between the two groups (57% vs 56%). Moreover, in one of the participating centers, insertion time was significantly increased when Endorings was used (251 s vs 170 s, P = 0.003), but this finding was not uniform for all participating centers (overall time to cecum: 263 s vs 319 s, no statistical difference). Of note, the device was not able to pass the sigmoid colon in 6 patients, thus authors commented that the larger the diameter of Endorings, the greater the difficulty of scope insertion.
Detecting and removing precancerous lesions remains the mainstay of screening colonoscopy. In this review, data on how 4 different colonoscope attachments influence detection rates were presented. The transparent cap was the first device launched in the market. CAC achieves a better orientation during insertion by pushing away the folded mucosa and reduces the time needed to reach the cecum. During scope withdrawal, the cap opens and manipulates colonic folds, revealing otherwise obscured lesions[23]. CAC has been validated extensively and pooled data from numerous studies have linked it with a significant increase in polyp detection[34]. Taking into account its simplicity in use, it can be an interesting and accessible solution for colonoscopy outcome improvement.
Endocuff and Endocuff Vision - two similar detachable attachments - have been commercialized during the last years. They are fitted at the tip of the scope and enhance mucosa visualization through their opened-out projections that flatten the colonic folds. Data from studies conclude that their application increases ADR[35,50,51]. Furthermore, they can prove to be beneficial for both low adenoma detectors[51] (increase in ADR) as well as in high ones[44] (decrease in miss rates). Moreover, Endocuff and Endocuff Vision are safe, user-friendly and affordable devices. Taking this into account, Endocuff-assisted colonoscopy should be considered as a pivotal part of the physicians’ arsenal in their effort to diminish missed precancerous lesions during colonoscopy.
Endorings is the last device that was presented. It functions in a -more or less- similar way to EAC. Its double-layered rings stretch the mucosa and optimize visualization. While significantly decreased adenoma miss rates have been reported[52], this device has been validated in only two studies[42,52] and more data are definitely needed to reach safe and generalizable conclusions regarding its effectiveness.
An issue that remains to be highlighted is the efficacy of these devices in terms of screening colonoscopy. To the best of our knowledge, no study has been conducted in an exclusively screening population yet. Thus, conclusions are extrapolated only from studies enrolling mixed populations (screening, symptomatic, surveillance). Although promising, more robust data are definitely needed in order to systematically assess the performance of these mechanical novelties overall and particularly in specific populations. Moreover, it is still unknown whether all levels of endoscopists are to benefit from their implementation. Finally, optimizing several useful and simultaneously low-cost existing alternatives (e.g., water-aided colonoscopy, second observer, dynamic position change) may be as effective as these add-on devices in improving colonoscopy and patient outcomes. All the aforementioned reasons probably prevent these devices from being integrated into current everyday clinical practice worldwide.
In conclusion, technological advancements offer physicians various choices to improve their performance during colonoscopy. Undoubtedly, they can be considered an adjuvant approach to improve colonoscopy outcomes as they are feasible, easy-to-use, affordable and effective. At the same time, these devices should not be considered a panacea and physicians should always pay attention to other well-established quality measures such as high level education, adequate bowel preparation, and adequate withdrawal time to ensure that they offer their patients optimal clinical services.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arya V, Qayed E, Rerknimitr R S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | SEER Cancer Stat Facts: Colorectal Cancer. 2018;. |
| 2. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2273] [Article Influence: 174.8] [Reference Citation Analysis (1)] |
| 3. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1548] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 4. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1461] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 5. | Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, Kobiela J, Franczyk R, Rupinska M, Kocot B. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology. 2017;153:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 369] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 6. | Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, Hoff G, Jover R, Suchanek S, Ferlitsch M. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 7. | Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 369] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Leufkens AM, van Oijen MG, Vleggaar FP, Siersema PD. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy. 2012;44:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 9. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1048] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 10. | Lee J, Park SW, Kim YS, Lee KJ, Sung H, Song PH, Yoon WJ, Moon JS. Risk factors of missed colorectal lesions after colonoscopy. Medicine (Baltimore). 2017;96:e7468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, Cross AJ, Zauber AG, Church TR, Lance P. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 12. | Xiang L, Zhan Q, Zhao XH, Wang YD, An SL, Xu YZ, Li AM, Gong W, Bai Y, Zhi FC. Risk factors associated with missed colorectal flat adenoma: a multicenter retrospective tandem colonoscopy study. World J Gastroenterol. 2014;20:10927-10937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Gkolfakis P, Tziatzios G, Dimitriadis GD, Triantafyllou K. New endoscopes and add-on devices to improve colonoscopy performance. World J Gastroenterol. 2017;23:3784-3796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Tada M, Inoue H, Yabata E, Okabe S, Endo M. Colonic mucosal resection using a transparent cap-fitted endoscope. Gastrointest Endosc. 1996;44:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Kondo S, Yamaji Y, Watabe H, Yamada A, Sugimoto T, Ohta M, Ogura K, Okamoto M, Yoshida H, Kawabe T. A randomized controlled trial evaluating the usefulness of a transparent hood attached to the tip of the colonoscope. Am J Gastroenterol. 2007;102:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Horiuchi A, Nakayama Y. Improved colorectal adenoma detection with a transparent retractable extension device. Am J Gastroenterol. 2008;103:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Harada Y, Hirasawa D, Fujita N, Noda Y, Kobayashi G, Ishida K, Yonechi M, Ito K, Suzuki T, Sugawara T. Impact of a transparent hood on the performance of total colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2009;69:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Lee YT, Lai LH, Hui AJ, Wong VW, Ching JY, Wong GL, Wu JC, Chan HL, Leung WK, Lau JY. Efficacy of cap-assisted colonoscopy in comparison with regular colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2009;104:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Hewett DG, Rex DK. Cap-fitted colonoscopy: a randomized, tandem colonoscopy study of adenoma miss rates. Gastrointest Endosc. 2010;72:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Tee HP, Corte C, Al-Ghamdi H, Prakoso E, Darke J, Chettiar R, Rahman W, Davison S, Griffin SP, Selby WS. Prospective randomized controlled trial evaluating cap-assisted colonoscopy vs standard colonoscopy. World J Gastroenterol. 2010;16:3905-3910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 21. | Takeuchi Y, Inoue T, Hanaoka N, Higashino K, Iishi H, Chatani R, Hanafusa M, Kizu T, Ishihara R, Tatsuta M. Autofluorescence imaging with a transparent hood for detection of colorectal neoplasms: a prospective, randomized trial. Gastrointest Endosc. 2010;72:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | de Wijkerslooth TR, Stoop EM, Bossuyt PM, Mathus-Vliegen EM, Dees J, Tytgat KM, van Leerdam ME, Fockens P, Kuipers EJ, Dekker E. Adenoma detection with cap-assisted colonoscopy versus regular colonoscopy: a randomised controlled trial. Gut. 2012;61:1426-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Rastogi A, Bansal A, Rao DS, Gupta N, Wani SB, Shipe T, Gaddam S, Singh V, Sharma P. Higher adenoma detection rates with cap-assisted colonoscopy: a randomised controlled trial. Gut. 2012;61:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Pohl H, Bensen SP, Toor A, Gordon SR, Levy LC, Berk B, Anderson PB, Anderson JC, Rothstein RI, MacKenzie TA. Cap-assisted colonoscopy and detection of Adenomatous Polyps (CAP) study: a randomized trial. Endoscopy. 2015;47:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Huang RX, Xiao ZL, Li F, Ji DN, Zhou J, Xiang P, Bao ZJ. Black hood assisted colonoscopy for detection of colorectal polyps: a prospective randomized controlled study. Eur Rev Med Pharmacol Sci. 2016;20:3266-3272. [PubMed] |
| 26. | Othman MO, Zhang D, Elhanafi S, Eloliby M, Davis B, Guererro R, Alvarado L, Sanchez L, Dwivedi A, Zuckerman MJ. Cap-Assisted Colonoscopy Increases Detection of Advanced Adenomas and Polyps. Am J Med Sci. 2017;353:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Tang Z, Zhang DS, Thrift AP, Patel KK. Impact of cap-assisted colonoscopy on the learning curve and quality in colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2018;87:723-732.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Park SM, Lee SH, Shin KY, Heo J, Sung SH, Park SH, Choi SY, Lee DW, Park HG, Lee HS. The cap-assisted technique enhances colonoscopy training: prospective randomized study of six trainees. Surg Endosc. 2012;26:2939-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Westwood DA, Alexakis N, Connor SJ. Transparent cap-assisted colonoscopy versus standard adult colonoscopy: a systematic review and meta-analysis. Dis Colon Rectum. 2012;55:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Ng SC, Tsoi KK, Hirai HW, Lee YT, Wu JC, Sung JJ, Chan FK, Lau JY. The efficacy of cap-assisted colonoscopy in polyp detection and cecal intubation: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2012;107:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | He Q, Li JD, An SL, Liu SD, Xiao B, Zhang YL, Jiang B, Bai Y, Zhi FC. Cap-assisted colonoscopy versus conventional colonoscopy: systematic review and meta-analysis. Int J Colorectal Dis. 2013;28:279-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Omata F, Ohde S, Deshpande GA, Kobayashi D, Masuda K, Fukui T. Image-enhanced, chromo, and cap-assisted colonoscopy for improving adenoma/neoplasia detection rate: a systematic review and meta-analysis. Scand J Gastroenterol. 2014;49:222-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Desai M, Sanchez-Yague A, Choudhary A, Pervez A, Gupta N, Vennalaganti P, Vennelaganti S, Fugazza A, Repici A, Hassan C. Impact of cap-assisted colonoscopy on detection of proximal colon adenomas: systematic review and meta-analysis. Gastrointest Endosc. 2017;86:274-281.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Mir FA, Boumitri C, Ashraf I, Matteson-Kome ML, Nguyen DL, Puli SR, Bechtold ML. Cap-assisted colonoscopy versus standard colonoscopy: is the cap beneficial? A meta-analysis of randomized controlled trials. Ann Gastroenterol. 2017;30:640-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Facciorusso A, Del Prete V, Buccino RV, Della Valle N, Nacchiero MC, Monica F, Cannizzaro R, Muscatiello N. Comparative Efficacy of Colonoscope Distal Attachment Devices in Increasing Rates of Adenoma Detection: A Network Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1209-1219.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Tsiamoulos ZP, Saunders BP. A new accessory, endoscopic cuff, improves colonoscopic access for complex polyp resection and scar assessment in the sigmoid colon (with video). Gastrointest Endosc. 2012;76:1242-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Floer M, Biecker E, Fitzlaff R, Röming H, Ameis D, Heinecke A, Kunsch S, Ellenrieder V, Ströbel P, Schepke M. Higher adenoma detection rates with endocuff-assisted colonoscopy-a randomized controlled multicenter trial. PLoS One. 2014;9:e114267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Biecker E, Floer M, Heinecke A, Ströbel P, Böhme R, Schepke M, Meister T. Novel endocuff-assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol. 2015;49:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Wada Y, Fukuda M, Ohtsuka K, Watanabe M, Fukuma Y, Wada Y, Wada M. Efficacy of Endocuff-assisted colonoscopy in the detection of colorectal polyps. Endosc Int Open. 2018;6:E425-E431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | González-Fernández C, García-Rangel D, Aguilar-Olivos NE, Barreto-Zúñiga R, Romano-Munive AF, Grajales-Figueroa G, Zamora-Nava LE, Téllez-Avila FI. Higher adenoma detection rate with the endocuff: a randomized trial. Endoscopy. 2017;49:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | De Palma GD, Giglio MC, Bruzzese D, Gennarelli N, Maione F, Siciliano S, Manzo B, Cassese G, Luglio G. Cap cuff-assisted colonoscopy versus standard colonoscopy for adenoma detection: a randomized back-to-back study. Gastrointest Endosc. 2018;87:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Rex DK, Repici A, Gross SA, Hassan C, Ponugoti PL, Garcia JR, Broadley HM, Thygesen JC, Sullivan AW, Tippins WW. High-definition colonoscopy versus Endocuff versus EndoRings versus full-spectrum endoscopy for adenoma detection at colonoscopy: a multicenter randomized trial. Gastrointest Endosc. 2018;88:335-344.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 43. | van Doorn SC, van der Vlugt M, Depla A, Wientjes CA, Mallant-Hent RC, Siersema PD, Tytgat K, Tuynman H, Kuiken SD, Houben G. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: a multicentre randomised controlled trial. Gut. 2017;66:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 44. | Triantafyllou K, Polymeros D, Apostolopoulos P, Lopes Brandao C, Gkolfakis P, Repici A, Papanikolaou IS, Dinis-Ribeiro M, Alexandrakis G, Hassan C. Endocuff-assisted colonoscopy is associated with a lower adenoma miss rate: a multicenter randomized tandem study. Endoscopy. 2017;49:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Baek MD, Jackson CS, Lunn J, Nguyen C, Shah NK, Serrao S, Juma D, Strong RM. Endocuff assisted colonoscopy significantly increases sessile serrated adenoma detection in veterans. J Gastrointest Oncol. 2017;8:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Ngu WS, Bevan R, Tsiamoulos ZP, Bassett P, Hoare Z, Rutter MD, Clifford G, Totton N, Lee TJ, Ramadas A. Improved adenoma detection with Endocuff Vision: the ADENOMA randomised controlled trial. Gut. 2018; Epub ahead of print. [PubMed] |
| 47. | Bhattacharyya R, Chedgy F, Kandiah K, Fogg C, Higgins B, Haysom-Newport B, Gadeke L, Thursby-Pelham F, Ellis R, Goggin P. Endocuff-assisted vs. standard colonoscopy in the fecal occult blood test-based UK Bowel Cancer Screening Programme (E-cap study): a randomized trial. Endoscopy. 2017;49:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Tsiamoulos ZP, Misra R, Rameshshanker R, Elliott TR, Beintaris I, Thomas-Gibson S, Haycock A, Suzuki N, Rees C, Saunders BP. Impact of a new distal attachment on colonoscopy performance in an academic screening center. Gastrointest Endosc. 2018;87:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Bugajski M, Kaminski MF. Devices for adenoma detection rate: Holy Grail or training tool? Gastrointest Endosc. 2018;87:241-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Chin M, Karnes W, Jamal MM, Lee JG, Lee R, Samarasena J, Bechtold ML, Nguyen DL. Use of the Endocuff during routine colonoscopy examination improves adenoma detection: A meta-analysis. World J Gastroenterol. 2016;22:9642-9649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 51. | Williet N, Tournier Q, Vernet C, Dumas O, Rinaldi L, Roblin X, Phelip JM, Pioche M. Effect of Endocuff-assisted colonoscopy on adenoma detection rate: meta-analysis of randomized controlled trials. Endoscopy. 2018;50:846-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 52. | Dik VK, Gralnek IM, Segol O, Suissa A, Belderbos TD, Moons LM, Segev M, Domanov S, Rex DK, Siersema PD. Multicenter, randomized, tandem evaluation of EndoRings colonoscopy--results of the CLEVER study. Endoscopy. 2015;47:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |