Published online Oct 7, 2018. doi: 10.3748/wjg.v24.i37.4217
Peer-review started: July 5, 2018
First decision: July 25, 2018
Revised: July 30, 2018
Accepted: August 1, 2018
Article in press: August 1, 2018
Published online: October 7, 2018
Processing time: 87 Days and 8.3 Hours
The brain-gut axis serves as the bidirectional connection between the gut microbiome, the intestinal barrier and the immune system that might be relevant for the pathophysiology of inflammatory demyelinating diseases. People with multiple sclerosis have been shown to have an altered microbiome, increased intestinal permeability and changes in bile acid metabolism. Experimental evidence suggests that these changes can lead to profound alterations of peripheral and central nervous system immune regulation. Besides being of pathophysiological interest, the brain-gut axis could also open new avenues of therapeutic targets. Modification of the microbiome, the use of probiotics, fecal microbiota transplantation, supplementation with bile acids and intestinal barrier enhancers are all promising candidates. Hopefully, pre-clinical studies and clinical trials will soon yield significant results.
Core tip: Many studies suggest that the brain-gut connection can contribute to our knowledge of the pathophysiology of neurological conditions. Recent evidence suggests that people with multiple sclerosis have changes in their gut microbiome, their intestinal barrier and even in the metabolism of bile acids. All of these represent relevant therapeutic targets that could feasibly be addressed by pre-clinical and clinical studies. This knowledge acquired in the bench might soon be translated to the bedside.
- Citation: Camara-Lemarroy CR, Metz LM, Yong VW. Focus on the gut-brain axis: Multiple sclerosis, the intestinal barrier and the microbiome. World J Gastroenterol 2018; 24(37): 4217-4223
- URL: https://www.wjgnet.com/1007-9327/full/v24/i37/4217.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i37.4217
Clinical and preclinical studies have shown bidirectional interactions within the brain-gut axis and the gut microbiome, the intestinal barrier and the immune system, both in health and disease. These complex interactions might be relevant for the pathophysiology of inflammatory demyelinating diseases, and in particular, multiple sclerosis, where much interest has been placed in the recent literature.
Much interest has been placed recently on the possible role of the gut microbiome in multiple sclerosis (MS) pathophysiology. Many review articles on this subject have recently been published[1-3], perhaps more than original research articles that actually characterize the gut microbiome in patients with MS. This research is in keeping with the essential role that the gut microbiome has in regulating the development of the immune system[4]. This area of research has also been the subject of recent symposia in international MS conferences[5,6].
Much of the experimental evidence is derived from studies using the experimental autoimmune encephalomyelitis (EAE) mouse model of MS. Modifying the gut microbiota with either antibiotic cocktails or probiotics leads to EAE attenuation as well as a multitude of regulatory immune responses[7-9]. Animals bred in germ-free conditions are resistant to EAE induction and show an attenuated immunological response[10,11], an effect lost when mice are repopulated with gut commensals[11]. In recent intriguing experiments, transgenic mice prone to spontaneous brain autoimmunity developed severe disease when transplanted with fecal microbiota from MS patients, as opposed to mice that received fecal microbiota from healthy matching twins[12]. Germ-free mice receiving similar transplants also developed severe EAE, while showing altered peripheral immune responses[13].
From studies attempting to characterize the composition of the microbiome, it is clear there are some differences in people with MS compared to controls. People with relapsing-remitting MS (RRMS) have an abundance of Anaerostipes, Faecalibacterium, Pseudomonas, Mycoplasma, Haemophilus, Blautia, and Dorea and a relative decrease of Bacteroides, Prevotella, Parabacteroides and Adlercreutzia[14-16]. In pediatric MS, patients have higher levels of members of Desulfovibrionaceae and depletion in Lachnospiraceae and Ruminococcaceae[17,18]. Issues are further complicated by complex analyses at the taxa, phylum and species levels, and a myriad of microbes have been implicated. For example, studies have found a significant depletion in clostridial species[15,19], Butyricimonas[20], Roseburia[21] and an increase in Streptococcus[22], Methanobrevibacter, Akkermansia and Coprococcus[14,20].
However, there are some limitations to these studies. The methods used to analyze the microbiome have been heterogeneous, with most (but not all) studies using a variation of 16S sequencing. There are differences in sample processing, DNA extraction, choice of primers, databases and hyper-variable regions analyzed across studies. Furthermore, close to two thirds of patients with MS have gastrointestinal symptoms such as constipation, dyspepsia and other functional gastrointestinal disorders[23], and many of these have been also associated with an altered gut microbiota[24]. Studies so far have not properly accounted for these symptoms or other relevant variables such as diet. An ongoing International MS Microbiome study aims to define a “core microbiome”[3]. It might shine some light into this complicated field.
Nonetheless, there is mounting experimental evidence that the gut microbiome may play a role in MS pathophysiology and human studies suggest that patients have a different microbiome compared to controls. Of course, the true significance of the results obtained so far is unclear, considering that there has often been a failure to replicate microbiome animal studies in humans. But the next question that comes to mind is whether this can also constitute a relevant therapeutic target. Although this appears to be the case in experimental models, translation to clinical practice may prove challenging.
Modifying the microbiome through medications, possibly antibiotics, could be the simplest method, but several issues arise that question the feasibility of this approach. Targeting specific commensals might prove difficult and requires appropriate identification of these targets. The case of minocycline is an interesting example. Recently shown to delay the occurrence of a second demyelinating event in patients with a clinically isolated syndrome[25], minocycline is an antibiotic known to alter the gut microbiome[26]. Whether this is an additional mechanism of action remains unknown; it is noteworthy that the initial rationale for testing minocycline in early MS is based on its various immune-modifying properties[27]. On the other hand, there is also evidence that MS disease modifying therapies (DMTs) may alter the microbiome directly[26], and indeed, it also appears that a multitude of other medications such as antidepressants, antipsychotics and immune modulators may also do so[28]. Issues such as the generation of resistant strains are also worthy of consideration.
Probiotics are a popular option but there are various issues with their practical implementation. Probiotics do not modify the host microbiome in a robust and persistent manner, although they are purported to be able to influence gut immunity and homeostasis. Despite success in showing a benefit for probiotics in animal models[29,30], there are only a handful of clinical trials in MS. Results have been preliminary, with some modest beneficial trends in clinical variables and some biochemical markers of changes in peripheral immune function[31-33]. However, they have included very small numbers of patients and the duration of these trials have been too short to shed any light onto clinically meaningful outcomes. There are many barriers to be overcome, such as selecting the appropriate formulation, dose and study design. There is also a lesson to be learned from the multiple clinical trials in inflammatory bowel disease (IBD), where despite a wealth of available studies (although heterogeneous in design and quality), the evidence supporting their clinical use is limited to carefully selected subpopulations[34,35].
Fecal microbiota transplantation (FMT) would constitute the optimal strategy to modify the gut microbiome. It has proven to be remarkably effective in managing C. difficile colitis, and isolated case reports describe beneficial effects over MS disease course, through mechanisms that remain unclear[36,37]. A clinical trial of FMT is underway[38], but even before its completion, many questions arise. It is unclear which population should be studied and what characterizes an ideal donor, not to mention the dose, route of administration and dose scheduling (single dose vs multiple doses). Patient with C. diff colitis who undergo FMT have been previously treated with antibiotics such as vancomycin and metronidazole, and presumably, have had some of their microbiota depleted. Would patients with MS require “ablation” of their microbiome before FMT? DMTs have immune modulating properties and they may also directly alter the microbiome[26], so their possible effects on the “engraftment” cannot be underestimated.
The intestinal barrier is the physical and functional zone of interaction between the gut microbiome and the organism. It is a complex multi-layered structure that includes major portions of the gut immunological system and is essential for homeostasis[26]. However, it has been comparatively ignored regarding its possible role in MS pathophysiology.
In experimental models, mice undergoing EAE show an altered intestinal barrier, with increased permeability and various gross morphological changes, as well as alterations in the expression of tight junction proteins in the intestinal mucosa[39]. The peak of intestinal barrier dysfunction mirrors the peak of EAE clinical severity and preventing intestinal barrier breakdown leads to attenuation of EAE[40]. These alterations have also been associated with several abnormal immunological responses.
Patients with MS also have an altered intestinal barrier. Almost 2 decades ago, investigators found that patients with MS had increased intestinal permeability when compared to controls, using an in vivo mannitol/lactulose ratio test[41]. Increased intestinal permeability was also found to be associated with the number of peripheral CD45RO+ B cells[41]. A more recent study confirmed this finding; up to 70% of MS patients had increased intestinal permeability[42]. It has been hypothesized that an altered intestinal barrier might lead to bacterial translocation thus allowing the passage of noxious molecules such as microbial associated molecular patterns. This could then alter peripheral immune responses or allow these molecules to enter the CNS and alter neuroimmunity[26,43].
Although the evidence linking the intestinal barrier with MS is much more limited than evidence linking MS with alterations of the gut microbiome, the question of whether it constitutes a viable therapeutic target is the same. Of course, the issue is complicated by the fact that the microbiome is essential in the regulation of intestinal barrier function, so it could be arbitrary to think of them as separate entities. An altered intestinal barrier is also a crucial aspect of the pathophysiology of IBD and celiac disease, so research from these fields has shed light on possible strategies to maintain intestinal barrier integrity.
One of the first components of the intestinal barrier is a thick mucus layer forming a protective film, enriched by secretory IgA and antimicrobial peptides and proteins. Oral supplementation with lecithin and phosphatidylcholine can adhere to the intestinal mucosa, strengthening the mucus layer and improving barrier function[44-46]. Regulators of tight junctions, such as larazotide, are under development. Larazotide is a peptide able to re-arrange tight junctions and prevent intestinal barrier dysfunction. It has been studied in patients with celiac disease with promising results[47-49]. Designing pre-clinical studies using these methods to enhance barrier function in the setting of autoimmune neurological disease should be straightforward.
Although probiotics may not be the ideal method to modify the microbiome, they have been suggested to play a significant role in modulating barrier function. E. coli strain nissle has been marketed in Europe for many years as a probiotic with beneficial effects on the intestinal barrier[50]. It has moderate evidence from randomized trials showing it may lead to remission in ulcerative colitis[51] and in the EAE mouse model of MS it reduced disease severity by maintaining intestinal barrier function[40]. VSL#3 is another probiotic mixture with putative barrier-protecting properties[52]. There is evidence of clinical effectiveness in the management of chronic pouchitis in patients with ulcerative colitis[35,53]. VSL#3 administered to a small number of MS patients leads to an anti-inflammatory peripheral immune response[33]. These two probiotic agents would be good candidates for a large, well-designed clinical trial.
Finally, we go full circle and return to FMT. It is believed that after successful modification of the microbiome, this strategy might lead to improved intestinal barrier function[54]. The gut microbiome is essential for the regulation of intestinal barrier homeostasis[55], partly through the production of short chain fatty acids (SCFA) such as butyrate, propionate and acetate. SCFAs can modulate tight junctions in the gut and modulate inflammatory responses in the intestinal mucosa[44,55]. Other interesting alternatives have also recently been described including the use of stool substitute preparations made from purified intestinal bacterial cultures derived from a single healthy donor[56]. Of course, many questions would need to be settled before clinical trials as discussed above.
Bile acids are the main regulators of fat and fat-soluble vitamins digestion. They also significantly affect gut physiology and homeostasis. Bile acids can modulate the intestinal barrier function through complex mechanisms[57,58], and can shape the gut microbiota community. In turn, the microbiome can change bile acid metabolism[59]. Remarkably, bile acids may also modulate inflammatory signaling in the central nervous system. Ursodeoxycholic acid can inhibit the inflammatory activity of microglia in vitro[60], and tauroursodeoxycholic acid can shift microglia phenotypes towards an anti-inflammatory state through activation of the G protein-coupled bile acid receptor 1/Takeda G protein-coupled receptor 5[61]. Bile acids are also agonists of the nuclear hormone receptor farnesoid X receptor. Bile acid farnesoid X agonism led to attenuation of EAE and modulation of neuroinflammatory responses[62]. Mice fed a high-fat diet show dysregulated bile acid synthesis, gut dysbiosis and increased microglial activation[63]. Furthermore, metabolomics studies have found alterations in bile acids in patients with MS compared to healthy controls[64]. Conjugated bile acids such as ursodeoxycholic acid have been used in the management of some gastrointestinal diseases for decades. A clinical trial of bile acid supplementation in MS is underway[65].
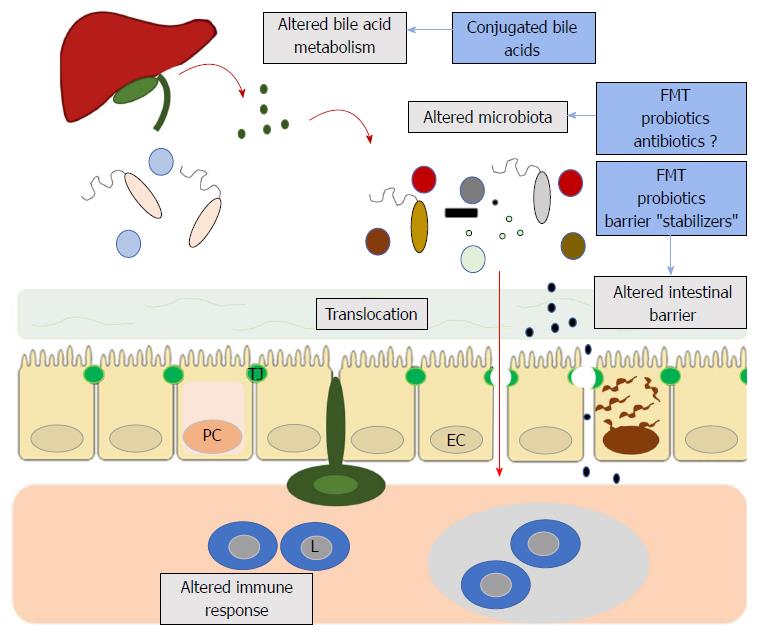
Exciting research suggests that the brain-gut axis, once an almost esoteric concept, might yield novel therapeutic targets in neuroimmunological diseases such as MS (Figure 1). The often-symbiotic roles of the gut microbiome, intestinal barrier and even bile acids in the regulation of neuroimmune responses is beginning to be elucidated. If future pre-clinical and clinical studies confirm the relevance of intestinal barrier dysfunction, bile acid metabolism and the gut microbiome in the pathophysiology of MS, the next step will be to translate these findings into therapeutics. Only well designed clinical trials will answer whether interventions such as FMT, probiotics or barrier protectors yield clinically meaningful results. The time is right to assess whether the gut-brain axis can be transferred from the bench to the bedside.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abalo R, Quigley EM S- Editor: Gong ZM L- Editor: A E- Editor: Bian YN
| 1. | Freedman SN, Shahi SK, Mangalam AK. The “Gut Feeling”: Breaking Down the Role of Gut Microbiome in Multiple Sclerosis. Neurotherapeutics. 2018;15:109-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 2. | Calvo-Barreiro L, Eixarch H, Montalban X, Espejo C. Combined therapies to treat complex diseases: The role of the gut microbiota in multiple sclerosis. Autoimmun Rev. 2018;17:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Pröbstel AK, Baranzini SE. The Role of the Gut Microbiome in Multiple Sclerosis Risk and Progression: Towards Characterization of the “MS Microbiome”. Neurotherapeutics. 2018;15:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1350] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 5. | Hot Topic 6: Gut microbiota in multiple sclerosis. Symposium held at the 7th Joint ECTRIMS-ACTRIMS meeting, 25-28 October, Paris, France. Available from: https://www.ectrims-congress.eu/2017/scientific-programme/scientific-programme.html. |
| 6. | Brain-Gut Axis: a focus on finding a cure for Multiple Sclerosis. University of Alberta MS Centre Research Symposium, held May 4, 2018, Edmonton, Alberta, Canada. Available from: https://cloudfront.ualberta.ca/-/media/medicine/ms-centre/2018-ualberta-ms-centre-program-25apr18-pdfa.pdf. |
| 7. | Ochoa-Repáraz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes. 2010;1:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Colpitts SL, Kasper EJ, Keever A, Liljenberg C, Kirby T, Magori K, Kasper LH, Ochoa-Repáraz J. A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes. 2017;8:561-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Begum-Haque S, Telesford KM, Ochoa-Repáraz J, Christy M, Kasper EJ, Kasper DL, Robson SC, Kasper LH. A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes. 2014;5:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 935] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 11. | Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4615-4622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 1023] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 12. | Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA. 2017;114:10719-10724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 641] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 13. | Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114:10713-10718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 704] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 14. | Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, Venkatesan A, Fraser CM, Mowry EM. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med. 2015;63:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 15. | Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim SW. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 2015;10:e0137429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 567] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 16. | Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, Luckey DH, Marietta EV, Jeraldo PR, Chen X. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 613] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 17. | Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, Graves J, Lynch S, Waubant E; US Network of Pediatric MS Centers. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol. 2016;23:1308-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 265] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 18. | Tremlett H, Fadrosh DW, Faruqi AA, Hart J, Roalstad S, Graves J, Lynch S, Waubant E; US Network of Pediatric MS Centers. Gut microbiota composition and relapse risk in pediatric MS: A pilot study. J Neurol Sci. 2016;363:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 19. | Rumah KR, Linden J, Fischetti VA, Vartanian T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS One. 2013;8:e76359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 934] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 21. | Swidsinski A, Dörffel Y, Loening-Baucke V, Gille C, Göktas Ö, Reißhauer A, Neuhaus J, Weylandt KH, Guschin A, Bock M. Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet. Front Microbiol. 2017;8:1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, Radice E, Mariani A, Testoni PA, Canducci F. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. 2017;3:e1700492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 23. | Levinthal DJ, Rahman A, Nusrat S, O’Leary M, Heyman R, Bielefeldt K. Adding to the burden: gastrointestinal symptoms and syndromes in multiple sclerosis. Mult Scler Int. 2013;2013:319201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | De Palma G, Collins SM, Bercik P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes. 2014;5:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Metz LM, Li DKB, Traboulsee AL, Duquette P, Eliasziw M, Cerchiaro G, Greenfield J, Riddehough A, Yeung M, Kremenchutzky M. Trial of Minocycline in a Clinically Isolated Syndrome of Multiple Sclerosis. N Engl J Med. 2017;376:2122-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 26. | Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain. 2018;141:1900-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 27. | Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 393] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 28. | Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1273] [Article Influence: 181.9] [Reference Citation Analysis (0)] |
| 29. | Kwon HK, Kim GC, Kim Y, Hwang W, Jash A, Sahoo A, Kim JE, Nam JH, Im SH. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin Immunol. 2013;146:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Weström B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010;5:e9009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 31. | Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, Tajabadi-Ebrahimi M, Jafari P, Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 32. | Tamtaji OR, Kouchaki E, Salami M, Aghadavod E, Akbari E, Tajabadi-Ebrahimi M, Asemi Z. The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin, and Lipids in Patients With Multiple Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Trial. J Am Coll Nutr. 2017;36:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Tankou SK, Regev K, Healy BC, Cox LM, Tjon E, Kivisakk P, Vanande IP, Cook S, Gandhi R, Glanz B. Investigation of probiotics in multiple sclerosis. Mult Scler. 2018;24:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Abraham BP, Quigley EMM. Probiotics in Inflammatory Bowel Disease. Gastroenterol Clin North Am. 2017;46:769-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 35. | Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (1)] |
| 36. | Borody TJ, Leis SM, Campbell J, Torres M, Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS). Am J Gastroenterol. 2011;106:S352. |
| 37. | Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm. 2018;5:e459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 38. | Kremenchutzky M. Fecal Microbial Transplantation in Relapsing Multiple Sclerosis Patients. cited 2018-05-22; In ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03183869. |
| 39. | Nouri M, Bredberg A, Weström B, Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One. 2014;9:e106335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | Secher T, Kassem S, Benamar M, Bernard I, Boury M, Barreau F, Oswald E, Saoudi A. Oral Administration of the Probiotic Strain Escherichia coli Nissle 1917 Reduces Susceptibility to Neuroinflammation and Repairs Experimental Autoimmune Encephalomyelitis-Induced Intestinal Barrier Dysfunction. Front Immunol. 2017;8:1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 41. | Yacyshyn B, Meddings J, Sadowski D, Bowen-Yacyshyn MB. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig Dis Sci. 1996;41:2493-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Buscarinu MC, Cerasoli B, Annibali V, Policano C, Lionetto L, Capi M, Mechelli R, Romano S, Fornasiero A, Mattei G. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult Scler. 2017;23:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 43. | Buscarinu MC, Romano S, Mechelli R, Pizzolato Umeton R, Ferraldeschi M, Fornasiero A, Reniè R, Cerasoli B, Morena E, Romano C. Intestinal Permeability in Relapsing-Remitting Multiple Sclerosis. Neurotherapeutics. 2018;15:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 44. | Sun J, Shen X, Li Y, Guo Z, Zhu W, Zuo L, Zhao J, Gu L, Gong J, Li J. Therapeutic Potential to Modify the Mucus Barrier in Inflammatory Bowel Disease. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis. 2014;32:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 46. | Stremmel W, Gauss A. Lecithin as a therapeutic agent in ulcerative colitis. Dig Dis. 2013;31:388-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Pearce SC, Al-Jawadi A, Kishida K, Yu S, Hu M, Fritzky LF, Edelblum KL, Gao N, Ferraris RP. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biol. 2018;16:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 48. | Gopalakrishnan S, Tripathi A, Tamiz AP, Alkan SS, Pandey NB. Larazotide acetate promotes tight junction assembly in epithelial cells. Peptides. 2012;35:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Khaleghi S, Ju JM, Lamba A, Murray JA. The potential utility of tight junction regulation in celiac disease: focus on larazotide acetate. Therap Adv Gastroenterol. 2016;9:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Sonnenborn U. Escherichia coli strain Nissle 1917-from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol Lett. 2016;363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 51. | Scaldaferri F, Gerardi V, Mangiola F, Lopetuso LR, Pizzoferrato M, Petito V, Papa A, Stojanovic J, Poscia A, Cammarota G. Role and mechanisms of action of Escherichia coli Nissle 1917 in the maintenance of remission in ulcerative colitis patients: An update. World J Gastroenterol. 2016;22:5505-5511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 117] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (2)] |
| 52. | Dai C, Zhao DH, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med. 2012;29:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 53. | Singh S, Stroud AM, Holubar SD, Sandborn WJ, Pardi DS. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2015;CD001176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Reinisch W. Fecal Microbiota Transplantation in Inflammatory Bowel Disease. Dig Dis. 2017;35:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A, de Vos WM. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171-G193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 417] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 56. | Petrof EO, Gloor GB, Vanner SJ, Weese SJ, Carter D, Daigneault MC, Brown EM, Schroeter K, Allen-Vercoe E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013;1:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 523] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 57. | Pavlidis P, Powell N, Vincent RP, Ehrlich D, Bjarnason I, Hayee B. Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Aliment Pharmacol Ther. 2015;42:802-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 58. | Keating N, Keely SJ. Bile acids in regulation of intestinal physiology. Curr Gastroenterol Rep. 2009;11:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Li Y, Tang R, Leung PSC, Gershwin ME, Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev. 2017;16:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 60. | Joo SS, Won TJ, Lee DI. Potential role of ursodeoxycholic acid in suppression of nuclear factor kappa B in microglial cell line (BV-2). Arch Pharm Res. 2004;27:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Yanguas-Casás N, Barreda-Manso MA, Nieto-Sampedro M, Romero-Ramírez L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J Cell Physiol. 2017;232:2231-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 62. | Ho PP, Steinman L. Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2016;113:1600-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 63. | Jena PK, Sheng L, Di Lucente J, Jin LW, Maezawa I, Wan YY. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity. FASEB J. 2018;32:2866-2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 64. | Bhargava P, Mowry E, Calabresi P. Global metabolomics identifies perturbation of multiple metabolic pathways in Multiple Sclerosis (Abstract). Neurology. 2015;84:P5.242. |
| 65. | Bhargave P. A Trial of Bile Acid Supplementation in Patients With Multiple Sclerosis. Cited 2018-05-22; In ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03423121. |