Published online Sep 28, 2018. doi: 10.3748/wjg.v24.i36.4197
Peer-review started: July 2, 2018
First decision: July 17, 2018
Revised: July 28, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: September 28, 2018
Processing time: 91 Days and 19.4 Hours
To evaluate the T stage of esophageal squamous cell carcinoma (ESCC) using preoperative low-dose esophageal insufflation computed tomography (EICT).
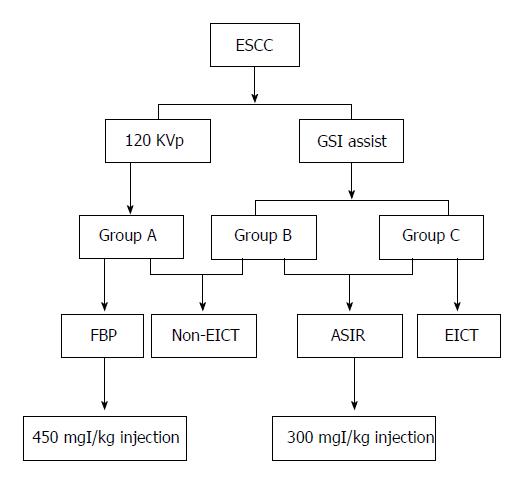
One hundred and twenty ESCC patients confirmed by surgery or esophagoscopy were divided into three groups. Groups B and C were injected with 300 mgI/kg contrast medium for automatic spectral imaging assist (GSI assist), while group A underwent a conventional 120 kVp computed tomography (CT) scan with a 450 mgI/kg contrast medium injection. EICT was performed in group C. Group A was reconstructed with filtered back projection, and groups B and C were reconstructed with 50% adaptive statistical iterative reconstruction. The contrast-to-noise ratio of lesion-to-mediastinal adipose tissue and the radiation dose were measured. Specific imaging features were observed, and T stage ESCCs were evaluated.
The sensitivity and accuracy of the T1/2 stage were higher in group C than in groups A and B (sensitivity: 43.75% vs 31.82% and 33.33%; accuracy: 54.29% vs 46.67% and 52.50%, respectively). With regard to the T3 stage, the sensitivity and specificity in group C were higher than those in groups A and B (sensitivity: 56.25% vs 41.17% and 44.44%; specificity: 73.68% vs 67.86% and 63.64%, respectively). The diagnostic sensitivity, specificity and accuracy of the T4 stage were similar among all groups. There were no significant differences in volume CT dose index [(5.91 ± 2.57) mGy vs (3.24 ± 1.20) vs (3.65 ± 1.77) mGy], dose-length product [(167.10 ± 99.08) mGy•cm vs (113.24 ± 54.46) mGy•cm vs (117.98 ± 32.32) mGy•cm] and effective dose [(2.52 ± 1.39) vs (1.63 ± 0.76) vs (1.73 ± 0.44) mSv] among the groups (P > 0.05). However, groups B and C received similar effective doses but lower iodine loads than group A [(300 vs 450) mgI/kg].
EICT combined with GSI assist allows differential diagnosis between the T1/2 and T3 stages. The ability to differentially diagnose the T3 and T4 stages of medullary ESCC can be improved by quantitatively and qualitatively analyzing the adipose tissue in front of the vertebral body.
Core tip: Esophageal insufflation computed tomography (EICT) is a method of insufflating air into the stomach before computed tomography examination, which fully expands the esophageal lumen. The optimal monochromatic energy level clearly displays esophageal lesions and surrounding adipose infiltration by means of effectively improving the image quality and resolution. Our study demonstrates that EICT combined with GSI assist technology contributes to better performance in the differential diagnosis between the T1/2 vs T3 stages and the T3 vs T4 stages in medullary esophageal cancer.
- Citation: Zhou Y, Liu D, Hou P, Zha KJ, Wang F, Zhou K, He W, Gao JB. Low-dose spectral insufflation computed tomography protocol preoperatively optimized for T stage esophageal cancer - preliminary research experience. World J Gastroenterol 2018; 24(36): 4197-4207
- URL: https://www.wjgnet.com/1007-9327/full/v24/i36/4197.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i36.4197
Esophageal carcinoma (EC) is a common malignant tumor of the digestive system. Mortality due to EC is approximately 300000 people per year worldwide[1]. The most common pathology subtype in Asia is esophageal squamous cell carcinoma (ESCC). As it is rare for patients to exhibit early symptoms of ESCC, patients typically receive treatment in the mid- and late stages (T3/4, N+ or M1)[2]. The overall five-year survival rate of progressive ESCC (T3/4 or N+) is only 42% after surgical resection or preoperative neoadjuvant chemotherapy[3]. Identification of the correct T stage of ESCC by preoperative imaging plays a critical role in the development, treatment and prognosis of patients.
Endoscopic ultrasonography (EUS) can be used clinically to determine the infiltration of ESCC and the possibility of surgical resection. However, the detection range is limited to centimeters from the center of the ultrasonic probe without interference or severe stenosis. Griffin et al[4] reported that inflammation or fibrous tissue surrounding ESCC tissue leads to over-staging of the local T stage. Currently, computed tomography (CT) and positron emission tomography/computed tomography (PET/CT) are common methods used to evaluate the T stage before ESCC treatment[5-7]. Variations in the sensitivity and specificity of these common methods is 27%-67% and 33%-93%, respectively[8,9]. Some studies continue to use traditional CT enhancement with low spatial and density resolutions. Konieczny et al[10] asserted that the accuracy of traditional 64-slice CT enhancement was 34% for EC. The sensitivity and specificity of CT or PET/CT are approximately 31% and 59% for diagnosis of the T1/2 stage, 60% and 64% for diagnosis of the T3 stage, and 100% and 4% for diagnosis of the T4 stage, respectively, which are not satisfactory.
Conventional CT has limitations for ESCC staging or restaging after treatment. Esophageal insufflation CT (EICT) is a method of insufflating air into the stomach before CT examination, which fully expands the esophageal lumen[11]. Diagnosis of the T1 or T2 stage has low accuracy because of the difficulty in visualizing the esophageal mucosa[12,13]. The optimal monochromatic energy level clearly displays esophageal lesions and the surrounding adipose infiltration by effectively improving the image quality and resolution. The optimal monochromatic energy level can be used for diagnosis, treatment selection, and therapeutic monitoring. Hence, we aimed to evaluate the T stage of ESCC using low-dose spectral insufflation CT, and we discuss the accuracy of this technique for diagnosing the T stage preoperatively.
This study was approved by the Institutional Review Board. All patients enrolled in this study provided informed consent.
In this single-institution study, 120 patients with a biopsy-proven esophageal malignancy who were being considered for radical treatment and who had already undergone EUS with a median age of 58 years (range, 48-83 years) were recruited from November 2015 to August 2017. The patients included 66 males and 54 females, with a median age of 55.4 years (range, 48-83 years). The typical clinical symptoms included vomiting, progressive dysphagia, intermittent sternal sensation, hematemesis and a sense of frustration. All patients considered for radical treatment were staged according to spectral CT and EUS within 6 wk.
The exclusion criteria included the following: (1) patients with esophageal cancer undergoing spectral CT to detect recurrence; (2) patients with a poor physical condition or a combination of severe heart, liver or kidney dysfunction; (3) patients with a history of iodine allergy, making them unsuitable for enhanced examination; and (4) patients with a history of other cancers.
Patients were divided into three groups that included 45 patients (group A), 40 patients (group B) and 35 patients (group C). Patients were required to fast for 6 h prior to the investigation and were administered an intramuscular injection of amidoamine (20 mg) 10-15 min before experimental procedures. EUS was performed using a PHILIP IU22 Color Doppler Diagnostic Apparatus (Philip, Eindhoven, The Netherlands) with a 5-10 Hz radical or linear endoscope. Then, a dual-phase contrast enhancement spectral spiral CT was performed with a spectral CT scanner (Discovery CT, GE Healthcare, Waukesha, WI, United States) from the thoracic inlet to the bottom of the lungs. The imaging parameters for group B were as follows: tube voltage: 80 kV and 140 kV with a fast kV-switching technique; tube current: auto mA with a slice thickness of 5 mm. Iobitrido (Guerbet, Paris, French), containing 350 mg/mL of iodine, was injected at a dose of 300 mgI/kg. The injection rate was calculated as the weight in kilograms divided by 30 s. A triggering scan was performed when the CT attenuation of the aortic arch reached the level of 100 HU. The starting time was 90 s after triggering. The saline tracer injection rate was similar to that of the contrast medium. For group A, conventional 120 kVp chest-enhanced CT scanning was performed with an injection dose of 450 mgI/kg. The remaining parameters were similar to those of group B.
For group C, a gastric tube was inserted into the stomach via the nasal cavity 10-15 min before the CT examination. The depth of insertion was referenced to the location of the esophageal lesion, and the end of the tube was fixed near the nostrils. Patients were asked to press a balloon to fill the stomach with air. Pressure was maintained between 4-4.67 kPa. Patients were required to keep their lips tightly closed and to fill the esophagus as much as possible during the process of filling. The rest of the parameters were similar to those of group B.
CT images (40-140 keV, monochromatic) were reconstructed using spectral imaging analysis software (GE Healthcare, Waukesha, WI, United States). The 50% adaptive statistical iterative reconstruction (ASIR) algorithm and standard filtered back projection reconstruction were applied to the decomposition images of group A and groups B and C, respectively. A flowchart of the study procedures is shown in Figure 1.
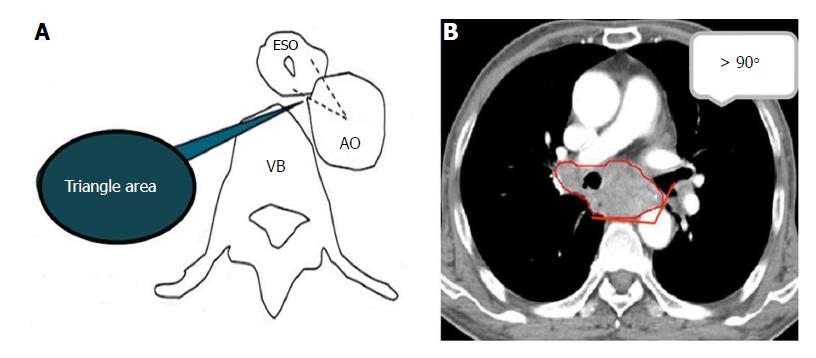
Two radiologists (Zha KJ and Zhou Y) with ten years of experience in CT diagnosis independently observed and recorded the special features of the CT images in a blinded and randomized manner using a dedicated workstation (Advantage Workstation 4.6, GE Healthcare, Waukesha, WI, United States). In the case of a discrepancy between interpretations, a consensus was reached by discussion. The main observations included enhanced features for ESCC (layered/unlayered enhancement) and morphological changes near ESCC tissue (a triangle area in front of the vertebral body, trachea, bronchus and aorta). A layered enhanced feature for ESCC was used to clearly identify the layers of the esophageal wall to effectively determine infiltration.
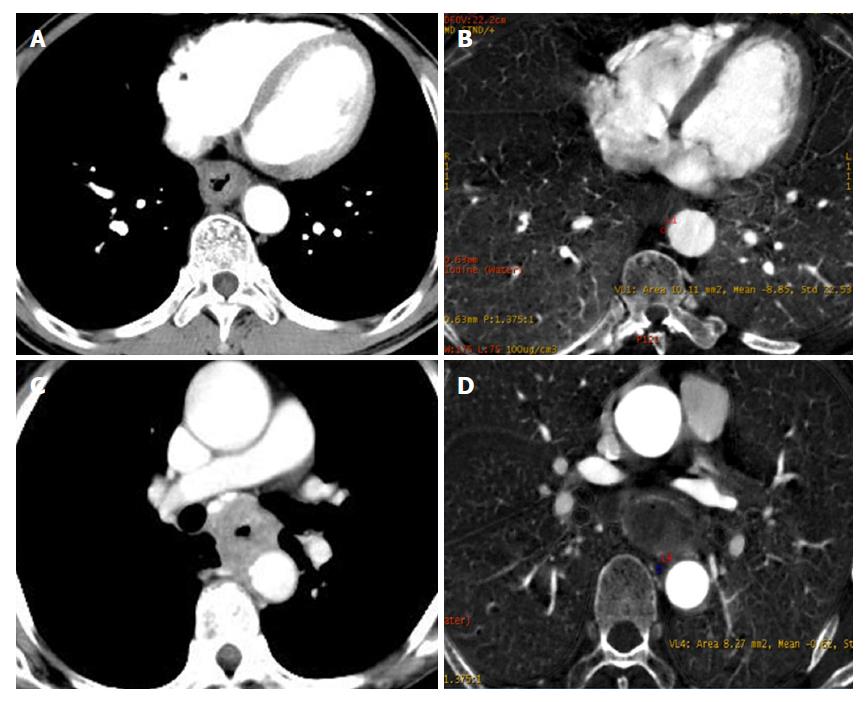
As described in Figure 2A, the triangular area in front of the vertebral body is surrounded by the outer esophageal membrane, the thoracic aorta and the vertebral body. Under normal circumstances, this area is filled with adipose tissue; however, when invaded by ESCC, this area is blurred or disappears. The narrow space between the anterior wall of the esophagus and the trachea is connected by loose connective tissue. When the tracheal bronchus is invaded, the space is depicted as having an ill-defined boundary or tracheal bronchus deformation and displacement. In general, the contact arc between the tumor and the thoracic aorta is less than 45°, while an arc of 45°-90° indicates invasion, and an arc larger than 90° indicates thoracic aorta invasion (Figure 2B).
From the monochromatic images, an analysis was performed to obtain the optimal energy level to provide the best contrast-to-noise ratio (CNR) between ESCC tissue and surrounding adipose tissue. Selected circular or oval-shaped areas from 70-80 mm2 were used for the regions of interest (ROI) measurement, which contained ESCC tissue and surrounding adipose tissue. The GSI Viewer software package automatically calculated the best CNR values from 101 sets of monochromatic images. The standard deviation (SD) of adipose tissue inside the mediastinal space at the same level represents image noise. The ROI was placed in the region as homogeneously as possible (an average of three ROIs). CNR was calculated using the following formula: CNR=(CTESCC-CTadipose)/SDadipose. The normalized iodine concentration was obtained by dividing the iodine concentration (IC) for ESCC tissue (ICESCC) by that for the aorta (ICaorta). The normalized iodine concentration (NIC) was calculated as NIC = ICESCC/ICaorta.
The volume CT dose index (CTDIvol, mGy) and dose-length product (DLP, mGy•cm) in the dose report were also recorded. The estimated effective dose (ED, mSv) was calculated by multiplying the DLP by 0.014 (as recommended by the International Commission on Radiological Protection (ICRP) for chest CT examinations).
The spectral CT results were compared with the results of other combined staging investigations, such as EUS and PET. For unresectable disease, sections were obtained for histological assessment, additional imaging [PET/CT, magnetic resonance imaging (MRI)] or clinical course determination, such as rapidly progressive disease or response to treatment. For potentially resectable disease, lesion sections were taken and frozen at the time of resection when appropriate, and information was obtained upon subsequent relapse and survival.
The T stage was reported according to the maximum wall thickness of ESCC tissue using the criteria of the classification system by Konieczny et al[10] and Jones et al[14], and consistent with the 7th TNM edition[4,15]. The T status for CT diagnosis was defined as follows: the T1 and T2 stages were combined because it was impossible to differentiate between the esophageal wall layers on MDCT images. The T1/2 stage was defined as a tumor wall thickness of at least 5-10 mm without evidence of mediastinal involvement. The T3 stage was defined as a tumor wall thickness greater than 10 mm with mediastinal involvement but no invasion of adjacent structures. The T4a (invasion of pleura, pericardium and diaphragm) and T4b (invasion of other structures, e.g., aorta, vertebral body and trachea) stages were defined as a tumor wall thickness greater than 10 mm and invaded adjacent structures.
The pathological subtype was classified based on the advanced esophagus cancer pathology classification criteria of the NCCN guidelines (2017. V3). The pathological subtypes included medullary type (wall thickness with symmetry or partial lumen stenosis), mushroom type (wall thickness similar to a flat mushroom mass), ulcer type (a larger and deeper ulcer on the surface of the wall) and narrowing type (narrow and obstructed lumen with a dilated upper segment).
The Statistical Package for the Social Sciences version 19.0 software program (SPSS, Inc., Chicago, IL, United States) was used for statistical analyses. Quantitative variables are expressed as the mean ± SD. Paired t-tests were used to compare age, BMI and radiation dose among the image reconstruction protocols. One-way analysis of variance was used to compare objective image noise. The least significant difference correction was used for multiple comparisons. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy for determining the T stage of the three groups were calculated, and the positive predictive value and negative predictive value were calculated. Inter-observer agreements between the two radiologists were based on the percentage agreement and simple Cohen’s kappa statistic. The significance level for all tests was 5% (two-sided).
The basic characteristics of the patients are summarized in Table 1. No significant differences were found in gender, tumor location, differentiation, and clinical symptoms among groups (P < 0.05 for all). The medullary type comprised the largest proportion in each group, followed by the mushroom and ulcer types; the narrowing type corresponded to the smallest proportion.
| Variable | Group A (n = 45) | Group B (n = 40) | Group C (n = 35) |
| Gender | |||
| Male | 25 (56) | 19 (48) | 22 (64) |
| Female | 50 (44) | 21 (52) | 13 (36) |
| Age (yr) | |||
| Medium | 67 | 61 | 63 |
| Range | 55-82 | 52-79 | 50-81 |
| Location | |||
| Upper esophagus | 11 (24) | 8 (20) | 6 (16) |
| Middle esophagus | 14 (32) | 11 (28) | 11 (32) |
| Lower esophagus | 20 (44) | 22 (56) | 18 (52) |
| Differentiation degree | |||
| High | 17 (38) | 9 (23) | 10 (28) |
| Medium | 23 (50) | 25 (62) | 14 (40) |
| Low | 5 (12) | 6 (15) | 11 (32) |
| Symptom | |||
| Progressive dysphagia | 18 (40) | 19 (48) | 21 (60) |
| Vomiting | 13 (28) | 8 (20) | 4 (12) |
| Intermittent sternal sensation | 9 (20) | 8 (20) | 6 (16) |
| Hematemesis and sense of frustration | 5 (12) | 5 (12) | 4 (12) |
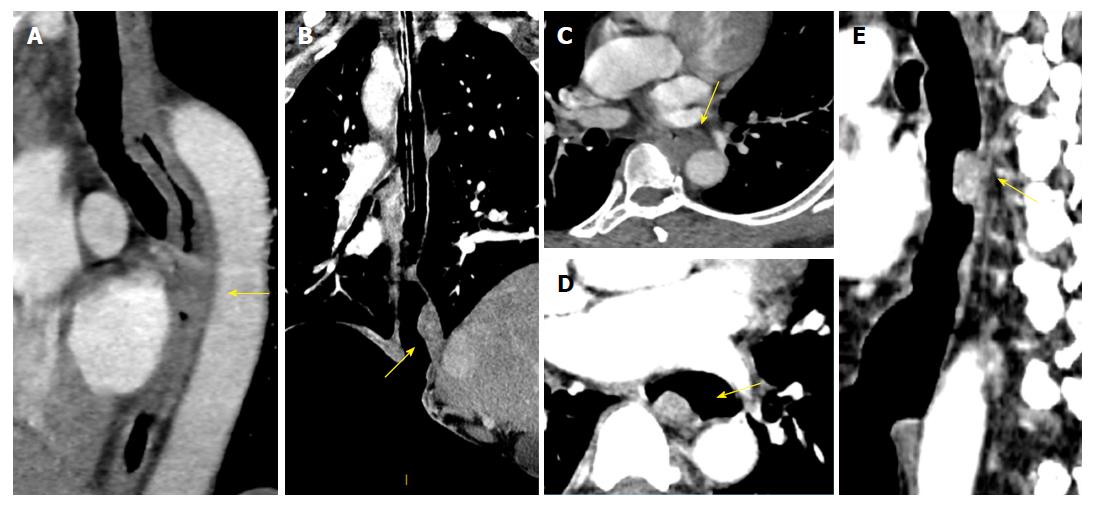
The proportions of layered enhancement in medullary ESCC tissue in groups A, B and C were 33%, 56% and 75% (for the T1/2 stage) and 20%, 20% and 11% (for the T3 stage), respectively; those for ulcer type were 33%, 0% and 33% (for the T1/2 stage) and 0%, 0% and 20% (for the T3 stage); and those for mushroom type were 20%, 0% and 60% (for the T1/2 stage) and 0% for all groups (for the T3 stage). The presentation of layered enhancement significantly differed between T1/2 and T3 stage medullary ESCC in group C (P < 0.05) but not between those stages in groups A and B (P > 0.05), and there was no significant difference between the T1/2 and T3 stages in the ulcer and mushroom types (P > 0.05 for all) (Table 2, Figures 3 and 4).
| Group | Enhancement feature | Medullary type | Ulcerative type | Mushroom type | Total | |||
| T1/2 | T3 | T1/2 | T3 | T1/2 | T3 | |||
| A | Layered | 3 | 2 | 2 | 0 | 1 | 0 | 8 |
| Unlayered | 6 | 8 | 6 | 4 | 4 | 3 | 31 | |
| Total | 9 | 10 | 8 | 4 | 5 | 3 | 39 | |
| B | Layered | 5 | 2 | 0 | 0 | 0 | 0 | 7 |
| Unlayered | 4 | 8 | 2 | 5 | 4 | 3 | 26 | |
| Total | 9 | 10 | 2 | 5 | 4 | 3 | 33 | |
| C | Layered | 6 | 1 | 1 | 1 | 3 | 0 | 12 |
| Unlayered | 2 | 8 | 2 | 4 | 2 | 2 | 20 | |
| Total | 8 | 9 | 3 | 5 | 5 | 2 | 22 | |
The optimal monochromatic image with the best CNR in groups B and C was mainly located at (50.18 ± 2.64) KeV. The CNRlesion-to-adipose at 50 keV in groups B and C was higher than that of group A (P < 0.05); however, there was no significant difference in the CNRlesion-to-adipose between groups B and C (P < 0.05). In terms of the morphological change of the triangular area in front of the vertebral body, the proportion of adipose blur or disappearance in groups A, B and C was 40%, 30% and 22% (for the T3 stage) and 50%, 57% and 54% (for the T4 stage) for medullary ESCC, respectively; 25%, 0% and 0% (for the T3 stage) and 0% in all groups (for the T4 stage) for the ulcer type; and 0% in all groups (for the T3 and T4 stage) for the mushroom type. There were no significant differences in morphological changes between the T3 and T4 stages for medullary type, ulcerative type and mushroom type ESCC (Table 3).
| Group | Morphological change of triangle area in front of vertebral body | Medullary type | Ulcerative type | Mushroom type | Total | |||
| T3 | T4 | T3 | T4 | T3 | T4 | |||
| A | Clear | 6 | 3 | 3 | 0 | 3 | 0 | 15 |
| Blurred or disappeared | 4 | 3 | 1 | 0 | 0 | 0 | 8 | |
| Total | 10 | 6 | 4 | 0 | 3 | 0 | 23 | |
| B | Clear | 7 | 3 | 5 | 0 | 3 | 0 | 18 |
| Blurred or disappeared | 3 | 4 | 0 | 0 | 0 | 0 | 7 | |
| Total | 10 | 7 | 5 | 0 | 3 | 0 | 25 | |
| C | Clear | 7 | 6 | 5 | 0 | 2 | 0 | 20 |
| Blurred or disappeared | 2 | 7 | 0 | 0 | 0 | 0 | 9 | |
| Total | 9 | 13 | 5 | 0 | 2 | 0 | 29 | |
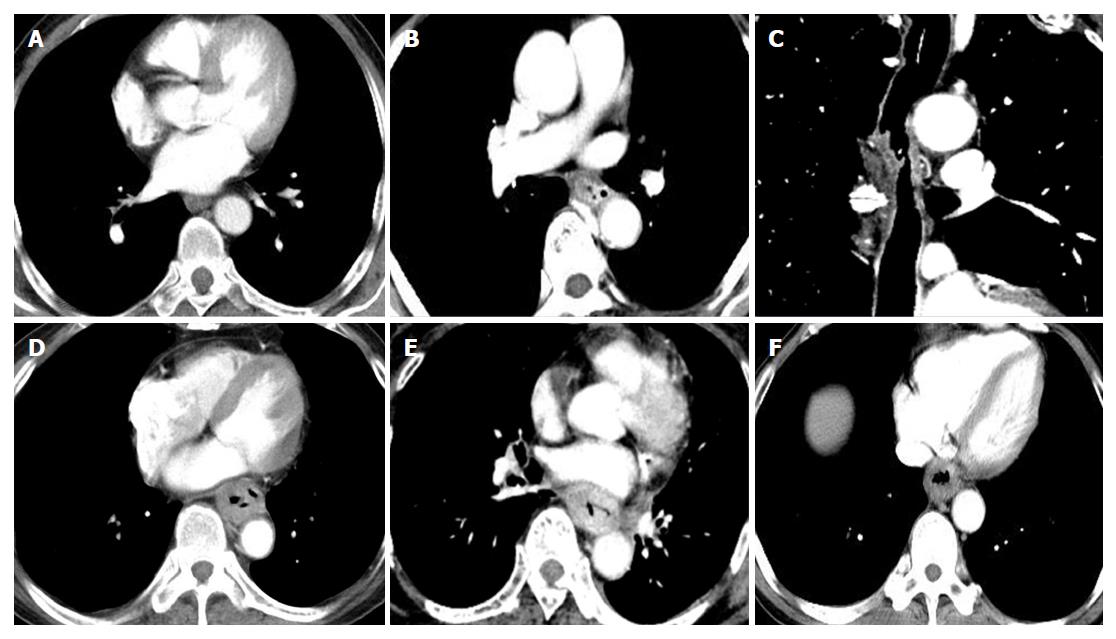
The quantitative parameters IC and NIC of adipose tissue in the triangular area in front of the vertebral body during arterial phase (AP) and venous phase showed significant differences in their ability to discriminate the T3 and T4 stages (P < 0.05). The receiver operating characteristic curve demonstrated that the area under the curve for NIC was higher than that for IC. When the threshold of NIC during AP was -0.03, the sensitivity and specificity for identifying the T3 stage were 83.30% and 83.33%, respectively (Figure 5).
Combined analyses of the morphological features and NIC during AP in the triangular area in front of the vertebral body highlighted a significant difference in discriminating T3 and T4 stage medullary ESCC in groups B and C (P < 0.05), and there were no significant differences between T3 and T4 stage ulcer and mushroom type ESCC (P > 0.05 for all) (Table 4, Figure 6).
| Group | T stage | Medullary type | Ulcerative type | Mushroom type | Total | |||
| T3 | T4 | T3 | T4 | T3 | T4 | |||
| A | T3 | 6 | 1 | 3 | 0 | 3 | 0 | 13 |
| T4 | 4 | 5 | 1 | 0 | 0 | 0 | 10 | |
| Total | 10 | 6 | 4 | 0 | 3 | 0 | 23 | |
| B | T3 | 8 | 1 | 5 | 0 | 3 | 0 | 17 |
| T4 | 2 | 6 | 0 | 0 | 0 | 0 | 8 | |
| Total | 10 | 7 | 5 | 0 | 3 | 0 | 25 | |
| C | T3 | 6 | 2 | 5 | 0 | 2 | 0 | 15 |
| T4 | 3 | 11 | 0 | 0 | 0 | 0 | 14 | |
| Total | 9 | 13 | 5 | 0 | 2 | 0 | 29 | |
The sensitivity and accuracy in group C in terms of diagnosing the T1/2 stage were higher than those in the other groups. With regard to diagnosing the T3 stage, the sensitivity and specificity were higher in group C than in the other groups. The accuracy of diagnosing the T4 stage between groups was similar (Table 5).
| Stage | Group | n | TP | TN | FP | FN | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) |
| A | 45 | 7 | 9 | 15 | 14 | 31.82 | 60.87 | 46.67 | 43.75 | 48.28 | |
| T1/2 | B | 40 | 5 | 9 | 10 | 16 | 33.33 | 64.00 | 52.50 | 35.71 | 61.54 |
| C | 35 | 7 | 7 | 9 | 12 | 43.75 | 63.16 | 54.29 | 50.00 | 57.14 | |
| A | 45 | 7 | 9 | 10 | 19 | 41.17 | 67.86 | 57.78 | 43.75 | 65.52 | |
| T3 | B | 40 | 8 | 8 | 10 | 14 | 44.44 | 63.64 | 55.00 | 50.00 | 58.33 |
| C | 35 | 9 | 5 | 7 | 14 | 56.25 | 73.68 | 65.71 | 60.00 | 66.67 | |
| A | 45 | 5 | 12 | 1 | 27 | 83.33 | 69.23 | 71.11 | 29.41 | 96.43 | |
| T4 | B | 40 | 6 | 10 | 1 | 23 | 85.71 | 69.70 | 72.50 | 37.50 | 95.83 |
| C | 35 | 11 | 7 | 2 | 15 | 84.62 | 68.18 | 74.29 | 61.11 | 94.12 |
Compared with the pathological results, mucosa enhancement was identified in 31.82% (7/22), 33.33% (5/15) and 43.75% (7/16) of cases for T1/2 stage ESCC in groups A, B and C, respectively; 68.18% (15/22), 66.67% (10/15) and 56.25% (9/16) of these cases were upstaged to T3, respectively.
There were 41.17% (7/17), 44.44% (8/18) and 56.25% (9/16) cases with T3 stage ESCC in groups A, B and C, respectively; 35.29% (6/17), 27.78% (5/18) and 25% (4/16) of these cases were upstaged to the T4 stage. Four cases were characterized by blurring of adipose tissue between tumor and adjacent structures, seven cases showed significant enhancement of the esophageal layer, three cases had an unclear boundary mass or ulcer, and 23.53% (4/17), 27.78% (5/18) and 18.75% (3/16) cases were down-staged to T1/2.
There were no significant differences in CTDIvol [(5.91 ± 2.57) mGy vs (3.24 ± 1.20) mGy vs (3.65 ± 1.77) mGy], DLP [(167.10 ± 99.08) mGy•cm vs (113.24 ± 54.46) mGy•cm vs (117.98 ± 32.32) mGy•cm] and ED [(2.52 ± 1.39) mSv vs (1.63 ± 0.76) mSv vs (1.73 ± 0.44) mSv] among the groups (P > 0.05). However, groups B and C received similar effective doses but lower iodine loads than group A [(300 vs 450) mgI/kg].
Reducing the radiation dose while maintaining image quality has become a key issue in CT research[16]. Reasonable adjustments of the ASIR-weighted value with the appropriate reduction in the scanning conditions are the main factors for low-dose scanning[17]. As a type of automatic dynamic real-time radiation dose control technology, GSI appropriately changes the tube to compensate for the loss of image contrast by adjusting the field of view, rotation speed and detector width[18,19]. Our study shows that the CNRs of groups B and C were superior to the CNR of group A. Thus, GSI assist combined with ASIR achieved equal or higher image quality than conventional scanning. Low-dose scanning brings benefits to patients with esophageal cancer[20]. Radiation from multiple follow-ups can be potentially harmful to patients who receive multiple radiation or chemotherapy treatments.
The esophageal wall is composed of a mucosal layer, a submucosal layer, a muscle layer and an outer membrane layer. The infiltration depth of ESCC determines the T stage. In fact, esophageal wall thickening observed on CT enhancement is largely dependent on the pathological classification. We found that T staging not only depends on the advantages of imaging techniques, but is also closely connected to the degree of esophageal lumen filling. In addition, esophageal wall shrinkage leads to incomplete lumen filling and makes it difficult to identify layers. In our study, the proportion of layered enhancement for the medullary T1/2 and T3 stages increased when we performed EICT in group C. However, no significant differences between the T1/2 and T3 stages in the ulcer and mushroom types were found. We argue that insufflation CT promotes the ability to identify lesions located on one side or around the lumen, but limitations for flat masses or local ulcers remain.
We reported that there were no significant differences between the T3 and T4 stages in all types of ESCC when only observing the morphological changes of the triangular area in front of the vertebral body. We inferred that the changes in the triangular area in medullary ESCC were affected by the size of the adipose tissue and connections with adjacent structures. Less adipose tissue and a close correlation with the triangular area are sometimes observed as blurring. Combined analysis of subjective observations and quantitative measurements revealed significant differences that could be used to discriminate T3 and T4 stage medullary ESCC in groups B and C. Hence, the better performance of the combined analysis in the triangular area was mainly attributed to the ability of NIC to discern invasion during the AP.
Based on the above analysis, the ability to diagnose T1-T3 stages is consistent with previous research[9,13]. The accuracy and sensitivity in distinguishing the T4 stage were similar to those reported by Cerfolio et al[13] and Konieczny et al[10], while the specificity was significantly higher than that reported by Konieczny et al[10]. Our study included more T4 stage patients than that of Konieczny et al[10], and, thus, our results may be more reliable than previous reports. Therefore, our findings demonstrate the relatively stable ability of our proposed technique to discriminate peripheral invasion, such as invasion of the trachea, aorta, muscle and pericardium.
There are several limitations of our study. First, the small sample size of T4 stage patients likely impacted the comparison of the diagnostic value in this preliminary study. Second, incomplete lumen filling associated with uncertain lesions restricts tumor localization. Third, nose or mouth leaks were unavoidable when patients held their breath for a long period of time. Fourth, there is still a certain false-positive rate when analyzing the triangular area in front of the vertebral body. Furthermore, the EICT process is influenced by the patient’s age and tolerance coordination. Lastly, other modalities, especially functional MRI, perform better in displaying the esophagus layer and distant metastases. Comparisons of EICT with EUS, MRI and PET-CT will be performed in the future. As mentioned above, fewer adipose tissue and close adjacent structural connections are easily misdiagnosed. The future direction of our research will focus on local expansion by lumen filling.
In conclusion, GSI optimizes the image contrast, maintains the radiation dose and reduces the contrast medium injection dose. EICT combined with GSI assist promotes differential diagnosis between T1/2 and T3 stage ESCC. The ability to differentially diagnose the T3 and T4 stages in medullary ESCC can be improved by quantitatively and qualitatively analyzing adipose tissue in front of the vertebral body.
Conventional computed tomography (CT) has limitations for esophageal cancer staging or restaging after treatment. Diagnoses of the T1 and T2 stages exhibit low accuracy due to difficulty visualizing the esophageal mucosa. The optimal monochromatic energy level clearly displays esophageal lesions and the surrounding adipose infiltration by effectively improving the image quality and resolution.
Radiation from multiple follow-ups can be potentially harmful to patients who receive multiple radiation or chemotherapy treatments. Low-dose scanning brings benefits to patients with esophageal cancer. GSI combined with ASIR achieved image quality equal to or greater than that of conventional scanning.
We aimed to evaluate the T stage of esophageal cancer using low-dose spectral insufflation CT, and we discuss the accuracy of this technique for preoperatively diagnosing the T stage.
One hundred and twenty patients with esophageal cancer were divided into three groups that included 45 patients (group A underwent conventional 120 kVp CT with 450 mgI/kg contrast medium injection), 40 patients (group B underwent GSI assist and 300 mgI/kg contrast medium injection) and 35 patients (group C underwent insufflation CT combined GSI assist and 300 mgI/kg contrast medium injection). Specific imaging features were observed, and the contrast-to-noise ratio of lesion-to-mediastinal adipose tissue was calculated for qualitative and quantitative T stage evaluation. The radiation dose was measured in each group.
When performed with insufflation CT combined with GSI assist technology, the ability to present layered enhancement was significantly different for the identification of T1/2 and T3 stage medullary esophageal cancer. Combined analyses of the morphological features and normalized iodine concentration during the arterial phase in the triangular area in front of the vertebral body highlighted a significant difference in discriminating T3 and T4 stage medullary esophageal cancer.
EUS can be clinically used to determine the infiltration of esophageal cancer and the possibility of surgical resection. However, the detection range is limited to centimeters from the center of the ultrasonic probe without interference or severe stenosis. Currently, CT and PET/CT are common methods used to evaluate the T stage before esophageal cancer treatment. Hence, we aimed to evaluate the T stage of esophageal squamous cell carcinoma using low-dose spectral insufflation CT, and we discuss the accuracy of this technique for preoperatively diagnosing the T stage. We propose the new idea that the T stage for esophageal cancer can be assessed quantitatively and qualitatively methods using low-dose spectral CT scanning. We found that insufflation CT combined GSI assist technology allows a differential diagnosis between the T1/2 and T3 stages. The ability to differentially diagnose the T3 and T4 stages in medullary esophageal cancer can be improved by analyzing the adipose tissue in front of the vertebral body.
Nose or mouth leaks are unavoidable when patients hold their breath for a long period of time. Furthermore, the process of insufflation CT is influenced by the patient’s age and ability to tolerate the procedures. The future direction of our research will focus on local expansion by lumen filling.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Eleftheriadis NP, Kim SM, Surucu E S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 1. | Roth MJ, Liu SF, Dawsey SM, Zhou B, Copeland C, Wang GQ, Solomon D, Baker SG, Giffen CA, Taylor PR. Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer. 1997;80:2047-2059. [PubMed] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8971] [Article Influence: 690.1] [Reference Citation Analysis (0)] |
| 3. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1266] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 4. | Griffin JM, Reed CE, Denlinger CE. Utility of restaging endoscopic ultrasound after neoadjuvant therapy for esophageal cancer. Ann Thorac Surg. 2012;93:1855-1859; discussion 1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Wolf MC, Stahl M, Krause BJ, Bonavina L, Bruns C, Belka C, Zehentmayr F. Curative treatment of oesophageal carcinoma: current options and future developments. Radiat Oncol. 2011;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Lorenzen S, von Gall C, Stange A, Haag GM, Weitz J, Haberkorn U, Lordick F, Weichert W, Abel U, Debus J. Sequential FDG-PET and induction chemotherapy in locally advanced adenocarcinoma of the Oesophago-gastric junction (AEG): the Heidelberg Imaging program in Cancer of the oesophago-gastric junction during Neoadjuvant treatment: HICON trial. BMC Cancer. 2011;11:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Yen TJ, Chung CS, Wu YW, Yen RF, Cheng MF, Lee JM, Hsu CH, Chang YL, Wang HP. Comparative study between endoscopic ultrasonography and positron emission tomography-computed tomography in staging patients with esophageal squamous cell carcinoma. Dis Esophagus. 2012;25:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Westerterp M, van Westreenen HL, Reitsma JB, Hoekstra OS, Stoker J, Fockens P, Jager PL, Van Eck-Smit BL, Plukker JT, van Lanschot JJ. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy--systematic review. Radiology. 2005;236:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Sloof GW. Response monitoring of neoadjuvant therapy using CT, EUS, and FDG-PET. Best Pract Res Clin Gastroenterol. 2006;20:941-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Konieczny A, Meyer P, Schnider A, Komminoth P, Schmid M, Lombriser N, Weishaupt D. Accuracy of multidetector-row CT for restaging after neoadjuvant treatment in patients with oesophageal cancer. Eur Radiol. 2013;23:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Liu BR, Liu BL, Wang XH, Zhan L, Liu LL, Song JT, Du B, Cui L, Liu SQ. Esophageal insufflation computed tomography for the diagnosis and management of esophageal submucosal tumors. Surg Endosc. 2017;31:2350-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Shi Q, Zhong YS, Yao LQ, Zhou PH, Xu MD, Wang P. Endoscopic submucosal dissection for treatment of esophageal submucosal tumors originating from the muscularis propria layer. Gastrointest Endosc. 2011;74:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA, Eloubeidi MA. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;129:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Jones DR, Parker LA Jr, Detterbeck FC, Egan TM. Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer. 1999;85:1026-1032. [PubMed] |
| 15. | Misra S, Choi M, Livingstone AS, Franceschi D. The role of endoscopic ultrasound in assessing tumor response and staging after neoadjuvant chemotherapy for esophageal cancer. Surg Endosc. 2012;26:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Yasaka K, Katsura M, Hanaoka S, Sato J, Ohtomo K. High-resolution CT with new model-based iterative reconstruction with resolution preference algorithm in evaluations of lung nodules: Comparison with conventional model-based iterative reconstruction and adaptive statistical iterative reconstruction. Eur J Radiol. 2016;85:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Uhrig M, Simons D, Kachelrieß M, Pisana F, Kuchenbecker S, Schlemmer HP. Advanced abdominal imaging with dual energy CT is feasible without increasing radiation dose. Cancer Imaging. 2016;16:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Zhu Z, Zhao XM, Zhao YF, Wang XY, Zhou CW. Feasibility Study of Using Gemstone Spectral Imaging (GSI) and Adaptive Statistical Iterative Reconstruction (ASIR) for Reducing Radiation and Iodine Contrast Dose in Abdominal CT Patients with High BMI Values. PLoS One. 2015;10:e0129201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Kulkarni NM, Uppot RN, Eisner BH, Sahani DV. Radiation dose reduction at multidetector CT with adaptive statistical iterative reconstruction for evaluation of urolithiasis: how low can we go? Radiology. 2012;265:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Zhao E, Ling T, Xu J, Zhao G, Cao H, Giacopuzzi S, Bencivenga M, de Manzoni G. Turning left or right? A comparative analysis in adenocarcinomas of the esophagogastric junction according to the seventh AJCC TNM classification for cancers of the esophagus and stomach: experience in a Chinese single institution. Int J Clin Exp Med. 2015;8:10668-10677. [PubMed] |