Published online Sep 28, 2018. doi: 10.3748/wjg.v24.i36.4186
Peer-review started: May 2, 2018
First decision: June 6, 2018
Revised: July 30, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: September 28, 2018
Processing time: 146 Days and 15.8 Hours
To specify the type and prevalence of anemia along with a treatment approach for inflammatory bowel disease (IBD).
We conducted a retrospective study on 465 patients who were diagnosed with IBD and followed up at our hospital from June 2015 to June 2016 [male: 254, female: 211; average age: 47 ± 14.4; Crohn’s disease (CD): 257, Ulcerative Colitis (UC): 208]. Epidemiological and clinical data, such as sex, age, age of diagnosis, type of IBD, disease extension, disease behavior and duration, treatments for IBD and anemia, and surgical history were obtained for each patient. Per World Health Organization guidelines, anemia was diagnosed for males if hemoglobin values were less than 13 g/dL and for females if hemoglobin values were less than 12 g/dL.
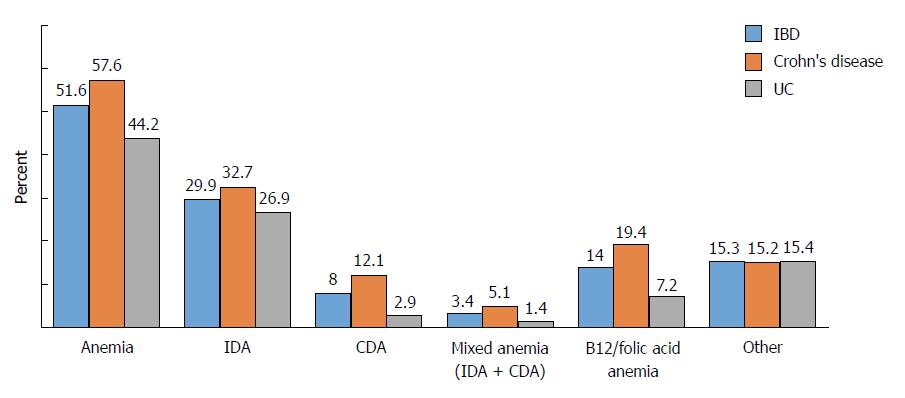
We determined that 51.6% of the patients had anemia, which was more frequent in women then men (64% vs 41.3%, P < 0.001). Anemia frequency was higher in CD cases (57.6%) than in UC cases (44.2%) (P = 0.004). CD involvements were as follows: 48.2% in ileal involvement, 19% in colonic involvement, and 32.8% in ileocolonic involvement. Furthermore, 27.5% of UC patients had proctitis (E1) involvement, 41% of them had involvement in left colitis (E2), and 31.5% had pancolitis involvement. There was no significant relationship between anemia frequency and duration of disease (P = 0.55). Iron deficiency anemia (IDA) was the most common type of anemia in this cohort. Moreover, because anemia parameters have not been evaluated during follow-up of 15.3% of patients, the etiology of anemia has not been clarified. Fifty percent of patients with anemia received treatment. Twenty-three percent of IDA patients had oral iron intake and forty-one percent of IDA patients had parenteral iron treatment. Fifty-three percent of patients who were suffering from megaloblastic anemia received B12/folic acid treatment.
We found out that almost half of all IBD patients (51.6%) had anemia, the most frequent of which was IDA. Almost half of these patients received treatment. We should increase the treatment rate in our IBD patients that have anemia.
Core tip: We conducted a retrospective study on 465 patients, who were diagnosed with inflammatory bowel disease (IBD). We determined that 51.6% of patients had anemia, which was more frequent in women then men. Anemia frequency was higher in Crohn’s disease cases than in ulcerative colitis cases. No relation has been found between the presence of anemia and disease duration. Iron deficiency anemia was the most common type of anemia. The factors that are related to anemia among IBD patients are being female, drug therapy (corticosteroids, AZA/MTX, Anti-TNF), and high C-reactive protein levels. Fifty percent of patients with anemia received treatment.
- Citation: Bengi G, Keyvan H, Durmaz SB, Akpınar H. Frequency, types, and treatment of anemia in Turkish patients with inflammatory bowel disease. World J Gastroenterol 2018; 24(36): 4186-4196
- URL: https://www.wjgnet.com/1007-9327/full/v24/i36/4186.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i36.4186
Inflammatory bowel disease (IBD) is a chronic idiopathic disease with a relapsing and remitting course. There are two major types of IBD, including Crohn’s disease (CD) and ulcerative colitis (UC). The most common extraintestinal finding seen in IBD patients is anemia, which decreases both the quality of life and the ability to work. Fatigue and weakness are the most common complaints reported in IBD-related anemia. Moreover, IBD-related anemia has been associated with frequent hospitalizations, late hospital discharge, increased health expenditures, co-morbidity for other diseases [e.g., transfusion-related Hepatitis C virus (HCV), etc.], and most importantly, a significant increase in the risk of mortality[1].
The prevalence of anemia is higher in IBD patients than in the general population, and ranges between 6% and 74%[2,3]. According to a review of 22 articles[2], the mean prevalence of anemia among IBD patients is 17% (95%CI: 16-18). In a meta-analysis[4], the prevalence of anemia in 2192 IBD patients was reported as 24% (27% in CD patients and 21% in UC patients). A recent study by Koutroubakis et al[5] including 1821 patients (1077 CD, 744 UC) reported the prevalence of anemia as 50.1% (CD: 53.3%, UC: 44.7%). The first study to report on the incidence of anemia in Turkish patients with IBD reported that 58.2% of 941 patients (62.1% of 375 CD patients and 55.7% of 566 UC patients) had anemia at least once during an 18-year follow-up period[6].
The most common causes of anemia in IBD are iron deficiency anemia (IDA) and chronic disease anemia (CDA)[6-10]. Other causes of anemia in IBD include macrocytic anemias (such as vitamin B12 deficiency and/or folate deficiency), hemolytic anemia, and drug-related bone marrow suppression.
Although IBD-related anemia has a relatively high prevalence, its diagnosis and treatment is generally overlooked. Iron therapy is recommended for all patients with IDA-related IBD, and the treatment should aim to return the patient to normal hemoglobin levels and provide adequate iron storage[11]. Recently, intravenous iron therapy has been recommended for the treatment of IDA-related IBD[12]. This is because intravenous iron treatment exerts its effects quickly, particularly among those who have active disease, have been intolerant to previous oral iron therapy, have severe anemia (Hb < 10 g/dL), and require erythropoiesis-stimulating agents[13].
The current study aimed to determine the frequency and types of anemia in IBD patients, to determine the relationship between anemia and disease characteristics, and to determine the most effective treatment approach.
Patients: This study retrospectively evaluated 465 patients who were diagnosed with IBD and followed-up between June 2015 and June 2016 in the Gastroenterology/IBD outpatient clinic or ward of Dokuz Eylül University, Medical Faculty Hospital.
The IBD diagnoses were made in accordance with the new European Crohn’s and Colitis Organization (ECCO) guidelines, and were confirmed according to standard clinical, endoscopic, histologic, and radiological criteria[14,15]. In order to obtain epidemiological and clinical data, the following data were recorded for each patient: sex, age, age at diagnosis, type of IBD, disease extension, disease behavior and duration, treatments for IBD and anemia, and surgical history. Patients were excluded from this study if they had indeterminate colitis, were pregnant, were monitored for less than one year, or had diseases such as chronic renal insufficiency, gastrectomy, hematological diseases, etc. Demographic and clinical data as well as endoscopic activities were obtained from hospital records.
Definition of anemia: We used the World Health Organization guidelines to diagnose anemia in our IBD patients. Males were diagnosed with anemia if they had hemoglobin values less than 13 g/dL, and females were diagnosed if they had hemoglobin values less than 12 g/dL[16]. Severe anemia was defined as having Hb values below 10 g/dL for both sexes. We evaluated the lowest hemoglobin levels of each patient during follow-up, as well as iron levels and other anemia parameters. The following were obtained from each patient’s laboratory records: hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), serum ferritin, serum iron level, transferrin saturation (TS), serum iron binding capacity (SIBC), folic acid, vitamin B12, CRP, and albumin.
Three main classifications of anemia were selected in accordance with the European consensus on anemia in IBD, including IDA, CDA, and mixed anemia[17] (Table 1). Aside from these, other causes of anemia were determined by examining the peripheral smear, and in cases with suspected macrocytic anemia, vitamin B12 and folic acid levels were evaluated. Additionally, medications that may cause macrocytic anemia (i.e., thiopurines and sulfasalazine) were also taken into account. In cases with suspected hemolytic anemia, reticulocyte ratio and haptoglobulin levels were evaluated.
| Ferritin (ng/mL) | Transferrin saturation (%) | CRP (5 mg/L) | |
| IDA | < 30 | < 20 | - |
| CDA | > 100 | < 20 | > 5 |
| Mixed anemia (IDA + CDA) | > 30 and < 100 | < 20 |
In our current study, variables indicated by census are presented as percentage distributions, and variables indicated by measurements are presented as means and standard deviations. In univariate analyses, Chi Square and Fisher’s exact test were used to compare the variables indicated by census. The variables indicated by measurement were compared by Student’s t-test. The logistic regression model was used in multivariate analysis. Values of P < 0.05 were considered significant. All analyses were made using the Statistical package for the Social Sciences (SPSS) (version 22.0; SPSS Inc., Chicago, IL, United States).
This study was approved by Dokuz Eylül University School of Medicine’s non-invasive clinical research ethics committee (15.06.2017 3387-GOA). Patient information was kept confidential, and the study was conducted according to the Helsinki declaration.
This study included the data from 465 IBD patients [254 male (54.6%) and 211 female (45.4%)] who were newly diagnosed or were being followed-up with in our hospital. Of these patients, 55.3% were diagnosed with CD and 44.7% with UC. The mean age at IBD diagnosis was 40.2 ± 13.9 years (39.8 ± 13.7 years in CD patients, 40.7 ± 14.6 years in UC patients, P = 0.46). The patient characteristics are presented in Table 2. There was no significant difference between mean disease duration of CD (6.45 years) and UC (7.36 years) (P = 0.07).
| IBD overall | Crohn’s disease | Ulcerative colitis | P value | |
| n | 465 | 257 (55.3) | 208 (44.7) | |
| Age (yr) mean (SD; range) | 47.1 (14.3; 18-83) | 46.2 (13.4; 17-78) | 48 (15.3; 12-83) | 0.183 |
| Sex (male/female) | 254 (54.6)/211 (45.4) | 153 (59.5)/104 (40.5) | 101 (48.6)/107 (51.4) | 0.019 |
| Disease characteristics | ||||
| Age at diagnosis (yr) | 40.2 | 39.7 | 40.7 | 0.46 |
| Location of the disease | ||||
| L1 | 122 (48.2) | |||
| L2 | 48 (19) | |||
| L3 | 83 (32.8) | |||
| L4 | - | |||
| Disease behavior | ||||
| B1 | 148 (60.9) | |||
| B2 | 40 (16.5) | |||
| B3 | 55 (22.6) | |||
| Disease extension | ||||
| E1 | 49 (27.5) | |||
| E2 | 73 (41) | |||
| E3 | 56 (31.5) | |||
| Drugs | ||||
| SZP/5-ASA | 360 (77.4) | 171 (66.5) | 189 (90.6) | < 0.001 |
| Corticosteroids | 55 (11.8) | 30 (11.7) | 25 (12) | 0.90 |
| Budenoside | 17 (3.7) | 15 (5.8) | 2 (1) | 0.005 |
| AZA / MTX | 200 (43) | 155 (60.3) | 45 (21.6) | < 0.001 |
| IFX/ADA | 81 (17.4) | 72 (28) | 9 (4.3) | < 0.001 |
| Antibiotic | 92 (19.8) | 68 (26.5) | 24 (11.5) | < 0.001 |
| Surgery | 70 (15.1) | 60 (23.3) | 10 (4.8) | < 0.001 |
Among patients with CD, 48.2% had ileal involvement (L1), 19% had colonic involvement (L2), and 32.8% had ileocolonic involvement (L3). Isolated upper GI involvement (L4) was not observed in any of the patients. In terms of CD behavior, 60.9% of patients had inflammatory type CD (B1), 16.5% had structuring type CD (B2), and 22.6% had penetrating type CD (B3).
In patients with UC, 27.5% had proctitis involvement (E1), 41% had left colitis involvement (E2), and 31.5% had pancolitis involvement (Table 2).
In our current study, 51.6% (n = 240) of the patients had anemia. Anemia frequency was higher in CD (57.6%) than in UC (44.2%) (P = 0.004). The mean hemoglobin concentration was 12.3 g/dL in IBD patients, and was significantly lower in those with CD (12.1 g/dL) than in those with UC (12.5 g/dL) (P = 0.03). The frequency of anemia and hematological profiles at the time of IBD diagnosis is shown in Table 3.
| IBD overall | Crohn’s disease | Ulcerative colitis | P value | |
| Anemia | 240 (51.6) | 148 (57.6) | 92 (44.2) | 0.005 |
| Severe anemia | 64 (16.4) | 47 (21.6) | 17 (9.8) | 0.002 |
| Hemoglobin (g/dL) mean (SD; range) | 12.3 (2.1; 5.3-17.9) | 12.1 (2.2; 5.3-16.8) | 12.5 (1.9; 7.1-17.9) | 0.03 |
| Hematocrit (%) mean (SD; range) | 37.41 (6.1; 10-53) | 36.8 (6.6; 10-52) | 38.16 (5.3; 22-53) | 0.02 |
| MCV (fL) mean (SD; range) | 84.54 (7.7; 57-112) | 84.75 (8.3; 58-112) | 84.28 (7; 57-100) | 0.46 |
| Iron (μg/dL) mean (SD; range) | 43.76 (30.3; 4-160) | 43.26 (30.4; 4-126) | 44.5 (30; 4-160) | 0.73 |
| Ferritin (μg/dL) mean (SD; range) | 40.3 (74; 2-754) | 48.96 (91; 2-754) | 27.26 (37; 2-167) | 0.01 |
| TS (%) mean (SD; range) | 15.35 (13; 2-100) | 15.14 (12.7; 2-100) | 15.67 (12; 2-92) | 0.9 |
| CRP (mg/L) mean (SD; range) | 13 (29; 1-289) | 17.1 (32.8; 0-289) | 7.9 (7.93; 1-242) | 0.001 |
| Albumin mean (SD; range) | 4.2 (0.4; 1.9-5.0) | 4.1 (0.4; 1.9-4.9) | 4.1 (0.4; 2.0-5.0) | 0.17 |
Anemia was more common among women than men (64% vs 41.3%, P < 0.001) (Table 4). Severe anemia (Hb < 10 g/dL) was observed in 21.6% of patients with CD and 9.8% of patients with UC (P = 0.002). No relationship was found between the presence of anemia and disease duration (P = 0.55).
| Anemia | IDA | CDA | Mixed anemia | B12/Folic acid anemia | Other anemia | |
| Sex | ||||||
| M | 105 (41.3) | 48 (18.9) | 18 (7.1) | 8 (3.8) | 34 (13.4) | 35 (13.8) |
| F | 135 (64) | 91 (43.1) | 19 (9) | 8 (3.1) | 31 (14.6) | 36 (17.1) |
| P value | < 0.001 | < 0.001 | 0.44 | 0.7 | 0.6 | 0.32 |
| Disease duration (yr) | ||||||
| A+ | 6.6 | 6.7 | 6.1 | 5.9 | 5.9 | 6.8 |
| A - | 7.1 | 6.9 | 6.9 | 6.9 | 7 | 6.9 |
| P value | 0.55 | 0.45 | 0.31 | 0.14 | 0.36 | |
| Disease type | ||||||
| CD | 148 (57.6) | 84 (32.7) | 31 (12.1) | 13 (5.1) | 50 (19.4) | 39 (15.2) |
| UC | 92 (44.2) | 55 (26.4) | 6 (2.9) | 3 (1.4) | 15 (7.2) | 32 (15.4) |
| P value | 0.004 | 0.14 | < 0.001 | 0.03 | 0.001 | 0.95 |
| Disease localization | ||||||
| L1 | 66 (54.1) | 34 (27.9) | 13 (10.7) | 5 (4.1) | 25 (20.5) | 19 (15.6) |
| L2 | 29 (60.4) | 17 (35.4) | 8 (16.7) | 1 (2.1) | 3 (6.3) | 9 (18.8) |
| L3 | 50 (60.2) | 31 (37.3) | 9 (13.8) | 6 (7.2) | 22 (26.5) | 10 (12) |
| P value | 0.6 | 0.32 | 0.51 | 0.02 | 0.56 | |
| Disease behavior | ||||||
| B1 | 90 (60.8) | 53 (35.8) | 18 (12.2) | 8 (5.4) | 23 (15.6) | 25 (16.9) |
| B2 | 27 (67.5) | 12 (30) | 5 (12.5) | 1 (2.5) | 14 (35) | 6 (15) |
| B3 | 26 (47.3) | 16 (29.1) | 8 (14.5) | 4 (7.3) | 12 (21.8) | 6 (10.9) |
| P value | 0.1 | 0.59 | 0.9 | 0.02 | 0.57 | |
| Disease extension | ||||||
| E1 | 16 (32.7) | 6 (12.2) | 1 (2) | 0 | 2 (4.1) | 9 (18.4) |
| E2 | 32 (48.8) | 19 (26) | 2 (2.7) | 2 (2.7) | 6 (8.2) | 12 (16.4) |
| E3 | 35 (62.5) | 26 (46.4) | 3 (5.4) | 1 (1.8) | 6 (10.7) | 6 (10.7) |
| P value | 0.008 | < 0.001 | 0.51 | |||
| Corticosteroid use | ||||||
| + | 44 (80) | 28 (50.9) | 10 (18.2) | 4 (7.3) | 15 (27.3) | 10 (18.2) |
| None | 196 (47.8) | 111 (27.1) | 27 (6.6) | 12 (2.9) | 50 (12.2) | 61 (14.9) |
| P value | < 0.001 | < 0.001 | 0.003 | 0.097 | 0.003 | 0.52 |
| AZA/MTX use | ||||||
| + | 129 (64.5) | 73 (36.5) | 25 (12.5) | 11 (5.5) | 45 (22.5) | 36 (18) |
| None | 111 (41.9) | 66 (24.5) | 12 (4.5) | 5 (1.9) | 20 (7.5) | 35 (13.2) |
| P value | < 0.001 | 0.007 | 0.002 | 0.03 | < 0.001 | 0.15 |
| Anti-TNF use | ||||||
| + | 56 (69.1) | 38 (46.9) | 13 (16) | 7 (8.6) | 21 (26) | 11 (13.6) |
| None | 184 (47.9) | 101 (26.3) | 24 (6.3) | 9 (2.3) | 44 (11.5) | 60 (15.6) |
| P value | 0.001 | < 0.001 | 0.003 | 0.01 | 0.002 | 0.64 |
| Surgery | ||||||
| + | 44 (62.9) | 23 (32.9) | 11 (15.7) | 5 (7.19) | 15 (21.4) | 14 (20) |
| None | 196 (49.6) | 116 (29.4) | 26 (6.6) | 11 (2.8) | 50 (12.6) | 57 (14.4) |
| P value | 0.041 | 0.55 | 0.009 | 0.07 | 0.55 | 0.23 |
| CRP (mg/L) | ||||||
| < 5 | 109 (41.3) | 70 (26.5) | 7 (2.7) | 3 (1.1) | 31 (11.8) | 29 (11) |
| > 5 | 120 (65.6) | 63 (34.4) | 29 (15.8) | 12 (6.6) | 30 (16.4) | 39 (21.3) |
| P value | 0.001 | 0.07 | 0.001 | 0.002 | 0.37 | 0.003 |
IDA was the most common type of anemia (29.9%). The frequencies of CDA and mixed anemia (IDA + CDA) were 8% and 3.4%, respectively. In addition, vitamin B12/folic acid deficiency anemia was observed in 14% of the patients (Figure 1). Moreover, since anemia parameters were not evaluated during the follow-up of 15.3% of our IBD patients, the etiology of anemia was not clarified in these patients.
Results of the current study indicate that anemia in IBD patients is more common among women than men, regardless of disease type or age. While elevated CRP levels (> 5 mg/L) are indicative of active disease, CD and UC patients with CRP levels > 5 mg/L had significantly higher rates of anemia compared to those with lower levels of CRP (70.4% vs 45.8% for CD patients, P < 0.001, and 54.7% vs 36.8% for UC patients, P = 0.006). The factors related to anemia in IBD are summarized in Table 5. Data from this study indicate that disease type and duration do not have a significant effect on the frequency of anemia in CD.
| Odds ratio | 95%CI | P value | |
| All IBD patients | |||
| Sex (female) | 3.19 | 2.07-4.91 | < 0.001 |
| Corticosteroids | 3.21 | 1.52-6.80 | 0.002 |
| AZA/MTX | 2.28 | 1.48-3.49 | < 0.001 |
| Anti-TNF (INF/ADA) | 2.32 | 1.30-4.11 | 0.004 |
| Elevated CRP | 2.75 | 1.78-4.24 | < 0.001 |
| Crohn’s disease | |||
| Sex (female) | 4.10 | 2.19-7.68 | < 0.001 |
| Corticosteroids | 4.06 | 1.27-12.96 | 0.018 |
| AZA/MTX | 2.30 | 1.26-4.20 | 0.006 |
| Anti-TNF (INF/ADA) | 2.34 | 1.20-4.53 | 0.012 |
| Elevated CRP | 3.05 | 1.67-5.58 | < 0.001 |
| Ulcerative colitis | |||
| Sex (female) | 3.12 | 1.58-6.18 | 0.001 |
| Corticosteroids | 3.62 | 1.22-10.67 | 0.02 |
| Elevated CRP | 2.03 | 0.96-4.26 | 0.062 |
| Disease extension | 3.79 | 1.51-9.55 | 0.005 |
Corticosteroid users had a higher frequency of anemia than non-users (80% vs 47.8%, P < 0.001). Further, those using immunomodulator therapy (such as AZA and MTX) also had higher rates of anemia than non-users (64.5% vs 41.9%, P < 0.001). Vitamin B12/folic acid deficiency anemia was the most common type of anemia in this immunomodulator users group. On the other hand, anemia rates were significantly higher among patients who did not use anti-TNF, compared to those who did use anti-TNF (69.1% vs 30.9%, P = 0.001). Further, those who had undergone previous surgeries had higher rates of anemia than those who did not (62.9 % vs 37.1%, P = 0.04).
In UC patients, left colon involvement and pancolitis involvement were associated with a higher incidence of anemia than proctitis involvement. Also in the UC patients, as disease duration increased, the incidence of anemia significantly increased (P = 0.008) (Table 4). IDA is significantly more prevalent in UC with pancolitis involvement compared to other types of disease involvement (P < 0.001).
Approximately 50.4% of all of the IBD patients who were diagnosed with anemia received treatment. Of the patients with IDA, 23% received oral iron therapy and 40.3% received parenteral iron preparations. Of those with B12/folic acid anemia, 53.3% received B12/folic acid treatment. None of the patients in the current study received blood transfusions or were given erythropoiesis stimulant agents.
Anemia is the most common extraintestinal finding in IBD. While anemia significantly impairs quality of life, the majority of IBD patients with anemia are not aware that some of their symptoms and/or complaints may be related to their anemia. In our current study, 52% of the IBD patients had anemia, and the rate of anemia was higher in CD patients compared to UC patients. These results are similar to those of previous studies[4,5,18,19]. In their population-based study including 756 patients (235 CD and 519 UC), Hoivik et al[18] found that 48.8% of CD patients and 20.2% of UC patients were diagnosed with anemia. Another population-based study[6] reported that among 749 IBD patients, 30% had anemia, and the rate was higher in CD patients (42%) compared to UC patients (24%). Another study conducted in Spain[19] revealed a similar incidence of anemia in IBD (41.2%). Half of the CD patients had anemia, and only one third of the UC patients were anemic. In a study conducted in Turkey[20], the anemia rate was found to be 22% in UC and 24% in CD among 398 patients with IBD. The higher rate of anemia observed repeatedly in CD may be explained not only by increased bleeding, but also by additional mechanisms such as systemic inflammation (which can be more severe in CD) and decreased iron absorption (due to involvement of the proximal gastrointestinal tract). However, it is important to note that some studies have reported no difference in anemia rates between IBD sub-types[21].
In our patient population, the severe anemia (Hb < 10 g/dL) rate was significantly higher in CD patients compared to UC patients. However, Lucendo et al[19] reported a lower rate of severe anemia, and that the rate of severe anemia between CD and UC was not significantly different. Another study conducted in Korea reported that the anemia rate in UC was 36.3% (similar to that of Western countries), while the rate in CD patients was 41.6%; however, the severe anemia rates between CD and UC were not significantly different[22].
While the underlying cause of anemia in IBD patients is most likely multi-factorial, the most common causes are IDA and CDA. Multivariate analyses from published studies have repeatedly shown that being female[5,23,24] and disease activity[18,25] are the most important determining factors that increase the frequency of anemia in both UC and CD. Our results are consistent with these previous studies. The fact that anemia is more common among young women could be related to blood loss during menstrual cycles, gestation, and lactation.
In our current study, anemia was detected at a higher rate in those with active disease (CRP levels higher than 5 mg/L) than in those without. Moreover, CRP level is suggested to be the predictive factor for unresponsiveness to oral iron therapy. Therefore, intravenous iron therapy is proposed to be the first line therapy in active disease[26]. Similar to our current study, previous reports have shown that the frequency of anemia increases with increased clinical activity in IBD. These data are supported by the results of the current study indicating a high incidence of anemia in patients taking corticosteroids or immunomodulator treatment due to active disease. Immune system activation and disease-related lesions in the gastrointestinal tract have been shown to contribute to the association between disease activity and anemia. It should be kept in mind that anemia is sometimes seen in IBD patients who are in remission (rate of 18%), and therefore, these patients should be evaluated for anemia as well[24].
Results of the current study revealed no difference in the frequency of anemia in CD patients with regards to disease behavior. However, in UC the rate of anemia was significantly increased in parallel with disease duration. The increased rate of anemia in the more extensive disease may be a result of both increased blood loss and increased burden of inflammation.
Smoking has been shown to have a low risk for causing anemia in IBD patients. This is due to these patients often developing compensatory polycythemia due to increased carbon monoxide consumption[18,24]. Previous studies have reported that while smoking is a risk factor for anemia in CD, it is a protective factor in UC[19]. Our current study did not elucidate the role of smoking in the development of anemia in IBD patients.
Although it is known that the incidence of anemia is higher in hospitalized patients than in outpatients, we could not perform a robust statistical comparison in our current study due to the low the number of hospitalized patients. In addition, the current study did not establish a significant relationship between surgery and frequency of anemia. It has been reported that the incidence of anemia decreases as IBD disease duration progresses. However, similar to the study of Koutroubakis et al[5], results of our current study indicate no relationship between disease duration and anemia.
In our current study, the most common cause of anemia was IDA. Similar to a previously published review[7], results of our current study reveal that IDA was more frequent in CD (32%) than in UC (26%). IDA can develop due to intestinal bleeding, dietary restrictions, or malabsorption[27]. Pro-inflammatory cytokines, such as IL-6 and bone morphogenetic protein, are increased in the circulation in active IBD. This causes increased secretion of hepcidin, which is produced in the liver and responsible for the absorption of iron. Increased hepcidin levels may cause the degradation of ferroportin channels, which allow iron to be transferred through the basolateral membrane of enterocytes, thus causing malabsorption of iron. In addition, it is known that iron accumulates in macrophages and monocytes. Basseri et al[28] revealed that hepcidin expression increases in parallel with increased levels of IL-6 in CD. Concordant with this finding, Semrin et al[29] showed that intestinal iron absorption is decreased in active CD compared to patients in remission.
While the diagnosis of IDA is routinely made via low serum ferritin levels (< 30 ng/mL) as well as a decrease in the TS and MCV indices, these criteria may not be valid in IBD patients, since ferritin is an acute phase reactant. When evaluating IBD patients, TS and disease activity status should always be considered along with ferritin levels[25]. In our current study, no difference was observed between CD and UC in terms of serum iron levels and mean TS. However, serum ferritin and CRP were significantly higher in CD (P = 0.001) (Table 3).
CDA is the second most common type of anemia in IBD patients. In our current study, CDA was seen a rate of 8%, while IDA was seen at a rate of 3.4%. In IBD patients, anemia often develops due to increased levels of cytokines [e.g., TNF-α, IL-1, interferon (IFN)-γ] and hepcidin, and decreased levels of erythropoietin[30,31]. IFN-γ is known to inhibit the development of erythroid progenitor cells and enhance erythrocyte destruction in the spleen[32], while TNF-α causes increased apoptosis in the erythroid progenitor leading to anemia[33].
In CD, vitamin B12 and folic acid deficiency due to malabsorption is reported to be 29%-33%[34]. However, in our current study, this rate was estimated to be 19%. Anemia in CD patients can be caused by dietary restrictions, malabsorption due to ileal inflammation, bacterial overgrowth, fistula development, and/or surgical resection. In UC, vitamin B12 deficiency has been reported at a rate of 16%, which is lower than that reported for CD[34]. According to a study conducted in Turkey, there is a higher rate of vitamin B12 deficiency in CD than UC[35]. In our current study, the rate of anemia due to vitamin B12 or folic acid deficiency in UC patients was 7%. The responsible mechanisms in this situation may include ileal dysfunction following proctocolectomy and ileal pouch-anal anastomosis, bacterial overgrowth, and reduced intestinal transit time[36].
Anemia may sometimes occur as a side effect of drugs used for the treatment of IBD. Anemia has been reported to occur at a rate of 3% per year due to the toxic effects of thiopurines on bone marrow[37]. Other reports indicate that sulfazalazine is rarely associated with reduced folate absorption, and does not often cause anemia due to aplasia or hemolysis[38]. Results of the current study indicate a 64% anemia rate among AZA users; this is often due to vitamin B12 and folic acid deficiency. The combination of IDA and megaloblastic anemia caused by thiopurines may present as normocytic normochromic anemia. Testa et al[23] observed only two UC patients had autoimmune hemolytic anemia due to antibody development caused by cross-reaction against erythrocytes as a result of AZA and INF administration. In the current study, we found no evidence of drug-related hemolytic anemia in our patients.
It is recommended that IBD patients with active inflammation (high inflammatory bio-markers or endoscopic evidence due to disease activity) be evaluated for signs of anemia at least once every three months, while those in remission should be checked every six months. If the patient has anemia, further tests should be performed to determine etiology. Regular follow-up is recommended, as there is a risk of B12 or folic acid deficiency in patients with small bowel disease or history of resection[12]. In our current study, further examinations were not performed in 15% of patients during follow-up, and therefore the etiology of anemia in these patients remains unknown.
Unfortunately, many clinicians still believe in the concept of asymptomatic anemia, in which anemia in IBD slowly progresses and may resolve once patients adapt to low Hb levels. In these cases, anemia is often not treated until it is severe. Only half of the patients in our follow-up were treated for anemia, which is better than the rates of previous studies.
In their 2013 study including gastroenterologists from nine European countries, Stein et al[8] reported that patients with IBD were not adequately monitored and treated for anemia. The diagnosis of anemia was made based on Hb levels in 88% of patients, serum ferritin levels in 75%, and on TS in 25%. Iron deficiency (ferritin < 30 ng/mL) was detected in 76% of the patients in that study, and only 28% of them were prescribed IV iron therapy.
Danese et al[1] reported that only 33% of IBD patients with anemia were being treated despite having been diagnosed. A recent study including 55 German gastroenterology centers reported that only 43.5% of IBD patients with anemia were treated, and 56% of those had received oral iron therapy[39]. In our current study, IV iron therapy was prescribed more often than oral iron therapy.
A sufficient response in the treatment of IDA after four weeks of treatment is indicated by an increase of 2 g/dL Hb or a > 30% increase in TS[40]. The target ferritin level in IV iron therapy is 400 µg/L. Oral iron replacement therapy alone is typically only successful in cases with mild disease activity and mild anemia[41]. Due to its low cost and safety, oral iron replacement therapy is usually used by clinicians as a first line treatment. However, oral iron therapy has been associated with some side effects and mucosal injury events, and therefore the efficacy and tolerability of this therapy must be monitored during treatment[42]. In animal studies, oral and rectal iron administration has been shown to increase disease activity because they increase pro-inflammatory cytokines, such as IL-1, IL-6, TNF-α, and IFN-α[43]. Therefore, IV iron therapy is the preferred treatment for IBD patients, especially those with severe anemia who have had an inadequate response to oral iron therapy or cannot tolerate oral iron therapy[44]. The benefits of IV iron therapy include quicker improvement of iron deficiency, quicker alleviation of patients’ complaints, and higher satisfaction rate from patients. Moreover, it has been shown that IV-administered iron has no effect on disease activity[45]. Since IDA has a tendency to recur in IBD, maintenance treatment should be continued for at least three months[46]. In cases of unresponsiveness to all types of anemia therapies, the patient should be referred to a hematologist[40].
In IBD patients with anemia, it is of utmost importance to treat the underlying cause(s) and to control inflammation. Over time, attacks of inflammation in IBD lead to decreased iron absorption. In addition, TNF-α is known to increase bone marrow suppression. In our current study, there was a lower rate of anemia among patients undergoing anti-TNF therapy compared to other treatment groups. Anti-TNF therapy is becoming an increasingly preferred treatment. These patients had a lower rate of anemia, which may be related to the fact that anti-TNF agents suppress adverse effects on bone marrow, decrease inflammation, and provide effective mucosal improvement. Similarly, another study reported that anti-TNF therapy improved anemia by controlling inflammation and disease activity in IBD patients[47]. It has been suggested that anti-TNF therapy regulates erythropoiesis at various levels. However, some studies have shown that the prevalence of anemia was higher in patients treated with anti-TNF agents than those treated with other drugs (e.g., immunomodulators, corticosteroids, aminosalicylates)[48]. The higher prevalence reported in those studies is associated with the fact that these drugs (anti-TNF agents) are often used in patients with increased disease severity.
One of the limitations of our current study is that it was retrospective. Therefore, we did not have any information about disease activity or smoking status. However, since we could not obtain or confirm the accuracy of the patients’ clinical activity indices, we utilized CRP levels to interpret disease activity. Moreover, we could not evaluate the etiology of anemia in 15.3% of our patients because their anemia parameters were not evaluated during follow-up. In addition, we did not separately evaluate the anemia rates of pediatric-onset IBD and adult-onset IBD, and therefore we could not compare the frequency of anemia between these groups. Further, we did not evaluate anemia-associated symptom rates or quality of life in this study. Lastly, the current study may not represent the general Turkish patient population as it was conducted with IBD patients with more severe and problematic conditions who were being followed at a tertiary referral university hospital.
In conclusion, because almost half of IBD patients have anemia and anemia causes a multitude of negative effects on patients, its presence should be further examined, and if necessary treated with regards to disease activity. It should be kept in mind that the most common cause of anemia is iron deficiency. Being female and disease activity are important risk factors in the development of anemia, and disease involvement is an additional factor in UC. Treatment rates should be increased in IBD patients with anemia. To conclude, anemia should be recognized, investigated, and treated in IBD patients.
Inflammatory bowel disease (IBD) is a chronic idiopathic disease with a relapsing and remitting course. The most common extraintestinal finding seen in IBD patients is anemia, which decreases both the quality of life and the ability to work. The first study to report the incidence of anemia in Turkish patients with IBD reported that 58.2% had anemia at least once during an 18-year follow-up period.
The prevalence of anemia is higher in IBD patients than in the general population. The most common causes of anemia in IBD are iron deficiency anemia (IDA) and chronic disease anemia (CDA). Although IBD-related anemia has a relatively high prevalence, its diagnosis and treatment is generally overlooked.
The current study aimed to determine the frequency and types of anemia in IBD patients, to determine the relationship between anemia and disease characteristics, and to determine the most effective treatment approach.
This study retrospectively evaluated 465 patients who were diagnosed with IBD and followed-up between June 2015 and June 2016 in the Gastroenterology/IBD outpatient clinic or ward of Dokuz Eylül University, Medical Faculty Hospital. The IBD diagnoses were made in accordance with the new European Crohn’s and Colitis Organization (ECCO) guidelines, and were confirmed according to standard clinical, endoscopic, histologic, and radiological criteria. Demographic and clinical data as well as endoscopic activities were obtained from hospital records. We used the World Health Organization guidelines to diagnose anemia in our IBD patients. Males were diagnosed with anemia if they had hemoglobin values less than 13 g/dL, and females were diagnosed if they had hemoglobin values less than 12 g/dL. Severe anemia was defined as having Hb values below 10 g/dL for both sexes. We evaluated the lowest hemoglobin levels of each patient during follow-up, as well as iron levels and other anemia parameters. Three main classifications of anemia were selected in accordance with the European consensus on anemia in IBD, including IDA, CDA, and mixed anemia.
This study included the data from 465 IBD patients (54.6% male and 45.4% female) who were newly diagnosed or were being followed-up with in our hospital. Of these patients, 55.3% were diagnosed with CD and 44.7% with UC. Approximately fifty-two percent of the IBD patients had anemia. Anemia frequency was higher in CD than in UC. Anemia was more common among women than men. Severe anemia was observed in 21.6% of patients with CD and 9.8% of patients with UC. IDA was the most common type of anemia (29.9%).
Approximately 50.4% of all of the IBD patients who were diagnosed with anemia in this study received treatment. Of the patients with IDA, 23% received oral iron therapy and 40.3% received parenteral iron preparations. Of those with B12/folic acid anemia, 53.3% received B12/folic acid treatment. None of the patients in the current study received blood transfusions or were given erythropoiesis stimulant agents.
Since almost half of IBD patients have anemia, and because anemia causes a multitude of negative effects on patients, its presence should be further examined, and if necessary, treated with regards to disease activity. It should be kept in mind that the most common cause of anemia is iron deficiency. Treatment rates should be increased in IBD patients with anemia.
Anemia in IBD patients must be monitored throughout active and remissive disease and treated accordingly.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiba T, Lin J, Serban ED, Sergi CM, Yücel O S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 1. | Danese S, Hoffman C, Vel S, Greco M, Szabo H, Wilson B, Avedano L. Anaemia from a patient perspective in inflammatory bowel disease: results from the European Federation of Crohn’s and Ulcerative Colitis Association’s online survey. Eur J Gastroenterol Hepatol. 2014;26:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Gisbert JP, Gomollón F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1507-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Filmann N, Rey J, Schneeweiss S, Ardizzone S, Bager P, Bergamaschi G, Koutroubakis I, Lindgren S, Morena Fde L, Moum B. Prevalence of anemia in inflammatory bowel diseases in european countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Koutroubakis IE, Ramos-Rivers C, Regueiro M, Koutroumpakis E, Click B, Schwartz M, Swoger J, Baidoo L, Hashash JG, Barrie A. Five-Year Period Prevalence and Characteristics of Anemia in a Large US Inflammatory Bowel Disease Cohort. J Clin Gastroenterol. 2016;50:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Atuğ Ö, Kani HT, Banzragch M, İmeryüz N, Akın H. Incidence rate of anemia in inflammatory bowel diseases. Turk J Gastroenterol. 2016;27:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Gomollón F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol. 2009;15:4659-4665. [PubMed] |
| 8. | Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 10. | Reinisch W, Staun M, Bhandari S, Muñoz M. State of the iron: how to diagnose and efficiently treat iron deficiency anemia in inflammatory bowel disease. J Crohns Colitis. 2013;7:429-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | de Silva AD, Mylonaki M, Rampton DS. Oral iron therapy in inflammatory bowel disease: usage, tolerance, and efficacy. Inflamm Bowel Dis. 2003;9:316-320. [PubMed] |
| 12. | Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (3)] |
| 13. | Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, Gomollon F, Iqbal T, Katsanos K, Koutroubakis I. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 14. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, Kupcinskas L, Mantzaris G, Travis S, Stange E; European Crohn’s and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 792] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 15. | Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 640] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 16. | WHO , UNICEF , UNU . Iron deficiency anemia: assessment, prevention and control. Report of a joint WHO/UNICEF/UNU consultation. Geneva: World Health Organization; 1998; . |
| 17. | Weiss G. Anemia of Chronic Disorders: New Diagnostic Tools and New Treatment Strategies. Semin Hematol. 2015;52:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Høivik ML, Reinisch W, Cvancarova M, Moum B; IBSEN study group. Anaemia in inflammatory bowel disease: a population-based 10-year follow-up. Aliment Pharmacol Ther. 2014;39:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Lucendo AJ, Arias Á, Roncero Ó, Hervías D, Verdejo C, Naveas-Polo C, Bouhmidi A, Lorente R, Alcázar LM, Salueña I, García-Quiñones JA, Carrillo-Ramos MJ. Anemia at the time of diagnosis of inflammatory bowel disease: Prevalence and associated factors in adolescent and adult patients. Dig Liver Dis. 2017;49:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Toruner M, Kalkan C, Keskin O, Cetinkaya H, Soykan I. P599. Prevalence of iron deficiency anemia and iron deficiency in a single center Turkish IBD cohort. J Crohns Colitis. 2014;S318-S319. |
| 21. | Portela F, Lago P, Cotter J, Gonçalves R, Vasconcelos H, Ministro P, Lopes S, Eusébio M, Morna H, Cravo M. Anaemia in Patients with Inflammatory Bowel Disease - A Nationwide Cross-Sectional Study. Digestion. 2016;93:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Lee DS, Bang KB, Kim JY, Jung YS, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. The prevalence and clinical characteristics of anemia in Korean patients with inflammatory bowel disease. Intest Res. 2016;14:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Testa A, Rispo A, Romano M, Riegler G, Selvaggi F, Bottiglieri E, Martorano M, Rea M, Gravina A, Nardone OM. The burden of anaemia in patients with inflammatory bowel diseases. Dig Liver Dis. 2016;48:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Antunes CV, Hallack Neto AE, Nascimento CR, Chebli LA, Moutinho IL, Pinheiro Bdo V, Reboredo MM, Malaguti C, Castro AC, Chebli JM. Anemia in inflammatory bowel disease outpatients: prevalence, risk factors, and etiology. Biomed Res Int. 2015;2015:728925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 348] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 26. | Herrera-deGuise C, Casellas F, Robles V, Navarro E, Borruel N. Iron Deficiency in the Absence of Anemia Impairs the Perception of Health-Related Quality of Life of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:1450-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Murawska N, Fabisiak A, Fichna J. Anemia of Chronic Disease and Iron Deficiency Anemia in Inflammatory Bowel Diseases: Pathophysiology, Diagnosis, and Treatment. Inflamm Bowel Dis. 2016;22:1198-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Basseri RJ, Nemeth E, Vassilaki ME, Basseri B, Enayati P, Shaye O, Bourikas LA, Ganz T, Papadakis KA. Hepcidin is a key mediator of anemia of inflammation in Crohn’s disease. J Crohns Colitis. 2013;7:e286-e291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Semrin G, Fishman DS, Bousvaros A, Zholudev A, Saunders AC, Correia CE, Nemeth E, Grand RJ, Weinstein DA. Impaired intestinal iron absorption in Crohn’s disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis. 2006;12:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 287] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Dallalio G, Law E, Means RT Jr. Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood. 2006;107:2702-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Libregts SF, Gutiérrez L, de Bruin AM, Wensveen FM, Papadopoulos P, van Ijcken W, Ozgür Z, Philipsen S, Nolte MA. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118:2578-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 33. | Felli N, Pedini F, Zeuner A, Petrucci E, Testa U, Conticello C, Biffoni M, Di Cataldo A, Winkles JA, Peschle C. Multiple members of the TNF superfamily contribute to IFN-gamma-mediated inhibition of erythropoiesis. J Immunol. 2005;175:1464-1472. [PubMed] |
| 34. | Ward MG, Kariyawasam VC, Mogan SB, Patel KV, Pantelidou M, Sobczyńska-Malefora A, Porté F, Griffin N, Anderson SH, Sanderson JD. Prevalence and Risk Factors for Functional Vitamin B12 Deficiency in Patients with Crohn’s Disease. Inflamm Bowel Dis. 2015;21:2839-2847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Yakut M, Ustün Y, Kabaçam G, Soykan I. Serum vitamin B12 and folate status in patients with inflammatory bowel diseases. Eur J Intern Med. 2010;21:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | M’Koma AE. Follow-up results of hematology data before and after restorative proctocolectomy. Clinical outcome. Dis Colon Rectum. 1994;37:932-937. [PubMed] |
| 37. | Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002;51:536-539. [PubMed] |
| 39. | Blumenstein I, Dignass A, Vollmer S, Klemm W, Weber-Mangal S, Stein J. Current practice in the diagnosis and management of IBD-associated anaemia and iron deficiency in Germany: the German AnaemIBD Study. J Crohns Colitis. 2014;8:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Akpınar H, Çetiner M, Keshav S, Örmeci N, Törüner M. Diagnosis and treatment of iron deficiency anemia in patients with inflammatory bowel disease and gastrointestinal bleeding: iron deficiency anemia working group consensus report. Turk J Gastroenterol. 2017;28:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D’Haens G, Gasche C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 42. | Lugg S, Beal F, Nightingale P, Bhala N, Iqbal T. Iron treatment and inflammatory bowel disease: what happens in real practice? J Crohns Colitis. 2014;8:876-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Oldenburg B, van Berge Henegouwen GP, Rennick D, Van Asbeck BS, Koningsberger JC. Iron supplementation affects the production of pro-inflammatory cytokines in IL-10 deficient mice. Eur J Clin Invest. 2000;30:505-510. [PubMed] |
| 44. | Lee TW, Kolber MR, Fedorak RN, van Zanten SV. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta-analysis. J Crohns Colitis. 2012;6:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Schröder O, Mickisch O, Seidler U, de Weerth A, Dignass AU, Herfarth H, Reinshagen M, Schreiber S, Junge U, Schrott M. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease--a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100:2503-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Schmidt C, Ahmad T, Tulassay Z, Baumgart DC, Bokemeyer B, Howaldt S, Stallmach A, Büning C; AEGIS Study Group. Ferric maltol therapy for iron deficiency anaemia in patients with inflammatory bowel disease: long-term extension data from a Phase 3 study. Aliment Pharmacol Ther. 2016;44:259-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Bergamaschi G, Di Sabatino A, Albertini R, Ardizzone S, Biancheri P, Bonetti E, Cassinotti A, Cazzola P, Markopoulos K, Massari A. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica. 2010;95:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 48. | Magro F, Ramos J, Correia L, Lago P, Peixe P, Gonçalves AR, Rodrigues Ã, Vieira C, Ferreira D, Pereira Silva J, Túlio MA, Salgueiro P, Fernandes S. [Portuguese Consensus on the Diagnosis, Prevention and Treatment of Anaemia in Inflammatory Bowel Disease]. Acta Med Port. 2016;29:144-156. [PubMed] |