Published online Sep 28, 2018. doi: 10.3748/wjg.v24.i36.4164
Peer-review started: July 16, 2018
First decision: July 24, 2018
Revised: August 15, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: September 28, 2018
Processing time: 74 Days and 17.3 Hours
To determine tissue expression (mRNA, protein) of two types of mucins [mucin 1 (MUC1) and mucin 2 (MUC2)] in patients with colorectal cancer (CRC).
Expression of membrane-bound mucin (MUC1) and secretory mucin (MUC2) in CRC (mRNA, protein) were analyzed in tissue material including fragments of tumors obtained from CRC patients (n = 34), and fragments of normal colorectal tissue from the same patients (control). The analysis was conducted using real-time quantitative polymerase chain reaction (RT-qPCR) (transcripts), immunohistochemistry (IHC) (apomucins), and the modern approach for morphometric analysis of IHC reaction (HSV filter software). Results on tissue expression of both mucins (mRNA, protein) were compared to histological alterations in colorectal cancer samples and correlated with selected clinical data in the patients. The statistical analysis was conducted using Statistica PL v. 12.0 software.
Significantly higher expression of the MUC1 mRNA in the CRC, compared with the control and the borderline correlation of mRNA expression with MUC1 protein levels in colorectal samples was observed. The expression of apomucins concerned cell membranes (MUC1) and cytoplasm (MUC2) and occurred both in control tissues and in most cancerous samples. There were no significant relationships between MUC1 (mRNA, protein) and the clinicopathological data of patients. MUC2 protein expression was significantly lower as compared to the control, while MUC2 mRNA expression was comparable in both groups. The MUC1/MUC2 ratio was significantly higher in CRC tissues than in the control. The higher expression of MUC2 was a feature of mucinous CRC subtypes, and characterized higher histological stage of tumors. Negative correlations have been obtained between MUC2 and the Ki-67 antigen, as well as between MUC2 and p53 protein expressions in CRC.
A combination of tissue overexpression of MUC1, reduced MUC2 expression, and high ratio of MUC1/MUC2 is a factor of poor prognosis in CRC patients. MUC2 tissue expression allows to differentiate mucinous and nonmucinous CRC subtypes.
Core tip: Colorectal cancers (CRC) represent the second most widely manifested malignant tumor worldwide in women and third in men. The evident expression of two mucins [mucin 1 (MUC1) and mucin 2 (MUC2)] occurs in a normal and cancerous large intestine. Using RT-qPCR analysis and immunohistochemistry we confirmed higher expression of the MUC1 mRNA, lower MUC2 protein, and higher MUC1/MUC2 expression ratio in CRC samples as compared to the control. MUC2 protein expression correlates with increased cellular proliferation. A combination of tissue overexpression of MUC1, reduced MUC2 expression, and high ratio of MUC1/MUC2 may be a useful factor of poor prognosis in CRC patients.
- Citation: Kasprzak A, Siodła E, Andrzejewska M, Szmeja J, Seraszek-Jaros A, Cofta S, Szaflarski W. Differential expression of mucin 1 and mucin 2 in colorectal cancer. World J Gastroenterol 2018; 24(36): 4164-4177
- URL: https://www.wjgnet.com/1007-9327/full/v24/i36/4164.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i36.4164
Colorectal cancer (CRC) is diagnosed in more than 1.3 million people worldwide, annually, with the number steadily increasing. Currently globally, this cancer is the third most common cancer in men and second most common in women[1]. In Poland CRC is the second most common cancer in men and woman, with the third leading causes of cancer deaths in Greater Poland Region[2]. While genetic factors play a major role in etiopathogenesis of CRC, the basis of most the cases of that cancer is unclear. Considering mutation source, CRC is classified as sporadic (70%), hereditary (25%) and congenital (3%-5%)[1,3]. Among the main pathologic alterations in CRC are quantitative and qualitative changes in glycoproteins called mucins[4-6]. Qualitative alterations of mucins include carbohydrate groups, as well as apomucin molecules[4,7-9]. The majority of CRC are nonmucinous adenocarcinomas (approximately 80%). A mucinous adenocarcinoma is a histological subtype of CRC with poorer prognosis than aforementioned. Quantitative changes identified in nonmucinous adenocarcinomas concern a reduction in total mucus output. In contrast, mucinous carcinomas are hypersecretory for mucus[4].
According to modern proteomics, the secreted mucin, mucin 2 (MUC2) is the main constituent of intestinal mucus, produced mainly by the goblet cells of the small and large intestine and playing a critical protective role[4,10-12]. Membrane-associated mucin 1 (MUC1) (episialin), in contrast, is widely expressed by normal glandular epithelial cells, with its high expression in malignant cells[4,6]. Structural changes of the MUC1, observed in the course of carcinogenesis, lead to the activation of signaling pathways such as: MAPK, PI3K/Akt, and Wnt[6,13]. In the blood serum of cancer patients, the MUC1-N subunits, CA 15.3 and CA 19.9 antigens can be detected, while the MUC1 itself was second among the top 75 Tumor-Associated antigens[6].
In the carcinogenesis initiation and CRC progression, overexpression of MUC1 and the decline in MUC2 expression is most commonly described[14-20]. These observations are also confirmed by meta-analysis[21-23]. However, knowledge of the role of tissue mucins expression, at various stages of the colon carcinogenesis is incomplete. Poorly known is the prognostic role of mucins in the mucinous subtypes of CRC, which generally have a worse clinical course and a worse response to chemotherapy[24,25]. Sporadic mucinous CRC had a worse survival rate than its nonmucinous counterpart[26], and mucinous differentiation results in a 2%-8% increased hazard of death, which persists after correction for stage[27]. Unlike the nonmucinous CRC, the mucinous subtype is correlated with higher MUC2 and lower MUC1 expression[4,21,24,28]. Research into the role of mucins in pathogenesis and CRC clinical studies (especially in mucinous subtypes) are also current topics from a methodological point of view. The lack of standardized methods of quantitative evaluation of mucins expression (especially at tissue level) and/or frequent lack of control groups, are a great difficulty in comparative analysis[15,29,30].
The goal of the present work was the verification of the hypothesis, that the examination of the tissue expression of selected mucins (mRNA, protein), using modern methods of quantitative assessment [real-time quantitative polymerase chain reaction (RT-qPCR), HSV filter software], could improve the diagnostic/prognostic usefulness of these markers of CRC. The specific aim of the study was to evaluate tissue expression (mRNA, protein) of two mucins (MUC1 and MUC2) in patients with colorectal carcinoma, and to assess the relationship between tissue expression of mucins and selected clinicopathological data.
The examined CRC group included 34 patients (27 men, 7 women) from Greater Poland Region, 32 to 89 years of age from the Chair and Department of General Surgery, Endocrinological and Gastroenterological Oncology, Poznan University of Medical Sciences, who were diagnosed and subjected to surgery between 2010-2015. We arbitrarily selected patients with CRC only from the Greater Poland Region, not treated before (radio- or chemotherapy), without significant additional systemic diseases, from whom consent was obtained, the perioperative tissue material met the requirements for scientific research and with available clinicopathological data.
Patients affected by diabetes, active chronic organ diseases (heart, kidney, liver), including autoimmune diseases and other cancers, have been excluded from the study. In three patients, hyperglycemia was observed in fasting, four patients were in hypertension treatment. There have been mild premalignant lesions (mainly adenomatous colon), that have been surgically removed as a preventive measure in the past, in 14/34 (41%) of patients.
The available clinical data for the study group, than was taken into account, included: descriptive histopathological diagnosis, histologic grade and stage on Dukes, Astler and Coller’s modified Dukes’ scales, and TNM system classification[31,32], age, patient sex and basic laboratory studies (complete blood count, number of leukocytes and platelets, as well as glucose levels). Seven patients (21%) of the entire study group died during the analysis period. Duration of patient’s survival reflected the time between the date of operation for colorectal cancer and the establishing diagnosis (October 1, 2010), and October 1, 2015.
Locations of the colorectal tumors were divided into proximal (right) colon (caecum, ascending, transverse colon) and distal (left) colon (descending, sigmoid colon and rectum). Macroscopic types were divided into protruded type (height of tumor ≥ 3 mm) and flat type (height of tumor < 3 mm).
Thirty-four paired specimens of colorectal tumor and non-tumor tissues were obtained during surgical treatment. For the CRC, colon mucosa and, depending on the depth of tumor invasion, submucosal layers approximately 15 cm from the tumor site, served as control tissues. In no case was tissue additional to that which would be removed normally during a particular surgical procedure.
The tissue samples were stored in RNA Stabilization Solution (RNAlater®, Applied Biosystems) at -80 °C until use. Additionally, formalin-fixed paraffin-embedded tumor specimens of 34 colorectal carcinomas and fragments of the confirmed control specimens were obtained from patients.
Informed consent was obtained from every subject, and the institutional review committee approved this study (No. 924/14).
CRC tumoral fragments and control tissues from 23 patients were qualified for the experiments that used the RT-qPCR technique as previously described[33].
One microliter of given cDNA or DNA was added to the reaction mixture, composed of 12.5 μL 2 × Maxima® SYBR Green/ROX qPCR Master Mix (Fermentas), 1 μL specific primer pair (f.c. 0.3 μmol/L) and 10.5 μL H2O. Primers for studies on expression of MUC1 and MUC2 mRNA expression are indicated in Table 1. β-actin, glycerylaldehyde-3-phosphate dehydrogenase (GADPH), and hypoxanthine-guanine phosphoribosyltransferase 1 (HPRT1) served as the housekeeping genes (geometric mean) for the gene expression analysis. All the primers were purchased from the Laboratory of DNA Sequencing and Oligonucleotide Synthesis, Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw (Table 1).
| Transcript | Sequence (5’-3’ direction) | ENST number http://www.ensembl.org | Product size |
| MUC1 | TCCAATATTAAGTTCAGGCCAGGA | 00000185499.16 | 768 bp |
| CACATCACTCACGCTGACGT | |||
| MUC2 | TGAAGACCTGCGGCTGTGT | 00000198788.8 | 3108 bp |
| CAGTCGAACTCGAAGTGCTCC | |||
| β-actin | TCTGGCACCACACCTTCTAC | 00000298556 | 169 bp |
| GATAGCACAGCCTGGATAGC | |||
| GADPH | GAAGGTGAAGGTCGGAGTCA | 00000229239 | 199 bp |
| GACAAGCTTCCCGTTCTCAG | |||
| HRPT1 | CTGAGGATTTGGAAAGGGTG | 00000298556 | 156 bp |
| AATCCAGCAGGTCAGCAAAC |
The reactions were driven in twin.tec real-time PCR plates with PCR Film (Eppendorf) using Mastercycler ep-realplex2 (Eppendorf). The PCR program was as followed: (1) Initial denaturation, 95 °C, 10 min; (2) Denaturation, 95 °C, 15 s; (3) Annealing, 60 °C, 30 s; (4) Extension, 72 °C, 30 s. The number of cycles was 40-50. Melting curves were made and 2% agarose gel electrophoresis was used to verify the amplification product specificity and size, respectively. All samples were amplified in duplicate or triplicate, and in case when results varied by more than 15%, the reactions were repeated.
Absolute quantitation method was used to quantify mRNA copy numbers of MUC1 and MUC2. Absolute quantification determines the exact copy concentration of a target gene by relating the Ct value to a standard curve. Prior to absolute quantification, the Ct values were normalized by comparison to the average of Ct’s obtained for three housekeeping genes (β-actin, GAPDH, and HPRT1).
Evaluation of alterations in expression of MUC1 and MUC2 mRNA, involved a comparison of mRNA copy numbers for those mucins per microgram of RNA, between the tumor and control samples from the same patient.
Tissue sections, 5 μm thick, were deposited onto SuperFrost/Plus microscope slides. In order to qualify the material for the study, routine staining of the sections with hematoxylin and eosin (HE) was performed. Anti-human mouse monoclonal antibodies (mAbs) specific for human Ki-67 antigen (clone MIB-1) (Dako Denmark A/S, Glostrup, Denmark, ready to use), anti-p53 (clone DO-7) (Dako), as well as the anti-MUC1 (clone Ma552) and anti-MUC2 (clone Ccp58) (both from NovocastraTM, both in 1:100 dilution) antibodies were used. The sections were incubated with these primary mAbs through the night, at 4 °C, and afterwards with dextran backbone, to which horseradish peroxidase (HRP) was attached, and with secondary biotinylated link anti-rabbit and anti-mouse IgG (Dako REAL™ EnVision™ Detection System peroxidase/DAB+, Rabbit/Mouse, Dako), with microwave-oven pre-treatment for antigen retrieval. Positive reaction manifested, in at least three sequential sections, as a dark brown or black precipitate in the cell nucleus (Ki-67, p53) and cell membrane/cytoplasm (MUC1 and MUC2). The preparations were counterstained using hematoxylin. Every test was accompanied by a negative control, in which specific antibodies were supplemented by a normal serum of a respective species in 0.05 mol/L Tris-HCl, pH approximately 7.6, supplemented with 0.1% bovine serum albumin (BSA) and 15 mmol/L sodium azide (internal negative control). All the steps of immunocytochemistry (IHC) technique were previously described[34]. Histological slides with IHC expression were examined under the optical Olympus BH-2 microscope, coupled to a digital camera. Color microscope images were recorded and archived using a 40 × objective (at least 10 fields in every microscope slide with an IHC positive reaction), with the use of LUCIA Image 5.0 computer software.
Expression of Ki-67 antigen and p53 (only clearly labelled cell nuclei were considered), was calculated, taking mean proportion of immunopositive cells in 10 light microscope fields into account. Expression was evaluated using the modified semi-quantitative scale[35], in which the score of 1 corresponded to up to 10% positive cells; the scores of 2, 3 and 4 corresponded to 11%-25%, 26%-50% and ≥ 51% positive cells, respectively.
The images with positive IHC reaction, 2560 × 1920 pixels in size, recorded in the LUCIA Image 5.0 software, were subjected to morphometric analysis, using the quantitative morphometric HSV Filter software, originally developed in the Department of Bioinformatics and Computational Biology, Poznan University of Medical Sciences, according to the following formula: (area of positive IHC reaction/area studied) × 100%.
In the Results section, values of average IHC expression of both mucins were presented, expressed in percentages, manifested by the IHC reactions per field of colorectal cancer/control sample area.
At the first stage of statistical analysis, consistency of all of the results with normal distribution of Gauss was verified using the Shapiro-Wilk test. Parameters of descriptive statistics (mean value, median value, SD, and minimum and maximum value) were calculated.
Data related to quantitative mucin expression (mRNA, protein), in CRC group, were compared with the data obtained for the control samples of the same patients (linked variables) with the Wilcoxon test. In cases of unlinked variables in two groups, the non-parametric Mann-Whitney’s test was applied. The t-Student test was applied in case of consistency of the results with normal Gaussian distribution.
The percentage shares of IHC positivity of both mucins were evaluated, using the difference test between two proportions.
Correlations between data rows were determined employing Spearman’s rank correlation index. The Kaplan-Meier survival curves and Log-rank test were used to compare overall survival rates. The results were accepted to be significant at the level of P value less than 0.05. The statistical analysis was conducted using Statistica PL v. 12.0 software (StatSoft Inc., Tulsa, OK, United States). The statistical analysis of the study was performed by biomedical statistician (AS-J). The statistical method of the study was reviewed by a statistician (Kaczmarek E) from the Department of Bioinformatics and Computational Biology, Chair of Pathology, Poznan University of Medical Science.
Patients over 50 years of age were predominant in the Study Group (94%). The cancer was primarily located in the distal part of the colon (left colon) (62%). In 3 patients, the tumor was localized in the rectum. In 21 patients (62%), protruded type of tumor was observed, while the flat type was seen in 13 patients (38%).
The majority of patients were diagnosed with tubular adenocarcinoma located in the colon or rectum, and nonmucinous subtype of CRC (71%) prevailed. Among these patients one had a mixed-type tumor with the neuroendocrine component, the other was diagnosed as adenocarcinoma in situ. Mucinous subtype of CRC was diagnosed in 10/34 patients.
The histopathological study showed a majority of moderately differentiated adenocarcinoma of the colon or rectum [grade 2 (G2)] (68%) compared to other grades. On a Dukes scale and in its modified form (Astler and Coller scale), most tumors were assessed at Stage C/C1-C3. In five patients from the whole Study Group (15%), there were distant metastases present (all to the liver). The vast majority of patients (85%) was classified stage III and IV on the TNM classification system.
The clinicopathological characteristics of CRC patients were collected in Table 2.
| Variable | CRC (n = 34) | |
| Age (yr) | < 50 | 2 (6) |
| ≥ 50 | 32 (94) | |
| Sex | male | 27 (79) |
| female | 7 (21) | |
| Tumor location | Right colon | 10 (29) |
| Left colon | 21 (62) | |
| Rectum | 3 (9) | |
| Mucin content | Nonmucinous | 24 (71) |
| Mucinous | 10 (29) | |
| Histologic grade (G) | Carcinoma in situ | 1 (3) |
| Well differentiated (G1) | 1 (3) | |
| Moderately differentiated (G2) | 23 (68) | |
| Poorly differentiated (G3) | 9 (26) | |
| Gross morphology | Protruded | 21 (62) |
| Flat | 13 (38) | |
| Dukes/Astler and Coller stage | Carcinoma in situ | 1 (3) |
| A/B1 | 4 (12) | |
| B/B2, B3 | 10 (29) | |
| C/C1, C2, C3 | 14 (41) | |
| D | 5 (15) | |
| TNM classification system | Carcinoma in situ | 1 (3) |
| I and II | 4 (12) | |
| III and IV | 29 (85) | |
| Status | Survival | 27 (79) |
| Death | 7 (21) | |
The expression of the MUC1 and MUC2 transcripts was present in all control and cancerous tissue samples. Our study showed that the expression of the MUC1 mRNA in the CRC tissues (75095 ± 72149 copies/μg RNA) was significantly higher when compared with the control tissue (32413 ± 44486 copies/μg RNA) (P = 0.004), and the expression of MUC2 mRNA was comparable in the study and control group (350227 ± 529270 vs 219744 ± 324252 copies/μg RNA) (P = 0.274).
The MUC1/MUC2 transcripts ratio in the test group, although higher (1.56 ± 4.50), did not differ significantly from the one obtained in the control tissue (0.28 ± 0.40) (P = 0.128) (Table 3).
| Group | Number | Mean | Median | Min | Max | SD | aP value | ||
| MUC1 | mRNA | CRC | 23 | 75095 | 49309 | 5648 | 267473 | 72149 | 0.004 |
| Control | 23 | 32413 | 20075 | 2 | 199681 | 44486 | |||
| Protein | CRC | 34 | 2.57 | 1.64 | 0.33 | 9.80 | 2.24 | 0.627 | |
| Control | 32 | 2.16 | 1.72 | 0.00 | 6.54 | 1.64 | |||
| MUC2 | mRNA | CRC | 23 | 350227 | 191457 | 2806 | 2399156 | 529270 | 0.274 |
| Control | 23 | 219744 | 130691 | 1 | 1509936 | 324252 | |||
| Protein | CRC | 34 | 4.94 | 2.15 | 0.20 | 32.30 | 7.24 | 0.035 | |
| Control | 32 | 7.40 | 5.15 | 0.73 | 31.60 | 6.77 | |||
| MUC1/MUC2 ratio | mRNA | CRC | 23 | 1.56 | 0.25 | 0.06 | 21.30 | 4.50 | 0.128 |
| Control | 23 | 0.28 | 0.18 | 0.04 | 2.00 | 0.40 | |||
| Protein | CRC | 34 | 1.84 | 0.80 | 0.04 | 10.35 | 2.54 | 0.003 | |
| Control | 32 | 0.52 | 0.37 | 0.00 | 1.95 | 0.51 |
No significant differences could be disclosed in the amount of MUC1 and MUC2 transcripts on one hand and CRC subtype (mucinous vs nonmucinous), colon tumor size, anatomical location of the CRC (proximal vs distal section of the colon), histologic grade or stage in the Dukes, or Astler and Coller scale, on the other (data not shown).
The comparison of the mRNA expression of both mucins, depending on the parameters in the TNM classification system was possible only for patients with N0 and N1, with no significant differences observed in this case as well (data not shown).
Using immunohistochemistry, a positive MUC1 immunoexpression was detected in all CRC samples (100%) and in 29/32 control colorectal samples (91%), thus the detectability of the positive expression of both mucins was similar. The immunoexpression of MUC2 was present in all CRC samples and in all samples of the colorectal control.
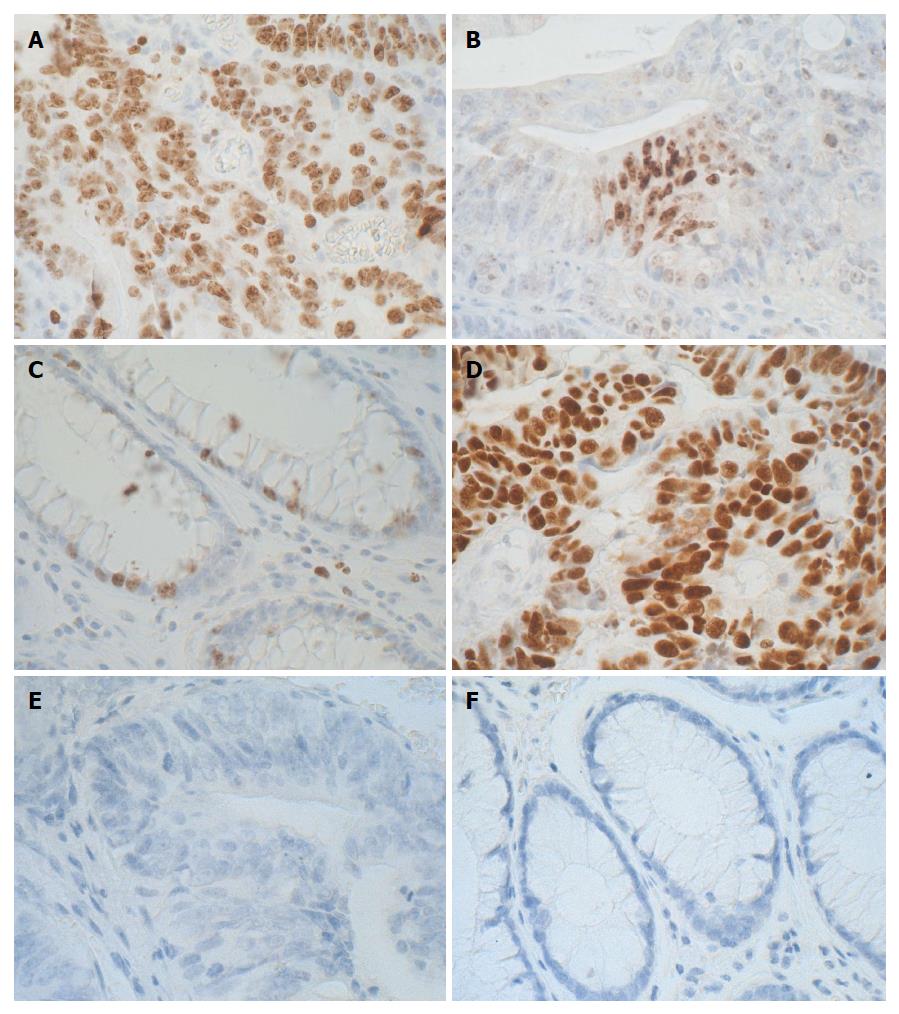
MUC1 tissue expression in CRC was pronounced and related mostly to cell membranes on the apical surface of the neoplastic cells lining the glandular structures and in the lumen of altered intestinal crypts (extracellular mucins fields) (Figure 1A and B). In the control tissue of large intestine, membranous expression of MUC1 prevailed and was observed mainly on the surface of normal intestinal crypts (Figure 1C).
In contrast, MUC2 expression was mainly related to the cytoplasm of neoplastic cells with differentiated expression of this mucin, from single immunopositive cells (Figure 1D) to intense reaction in the cytoplasm of numerous cancer cells and/or localized extracellularly (Figure 1E). In the normal intestinal mucosa (control), cytoplasmic expression of MUC2 prevailed and was observed in normal intestinal crypts (Figure 1F).
No preferred detection sites were observed for both mucins (MUC1 and MUC2) within the evaluated area of the colorectal tumor (center, periphery).
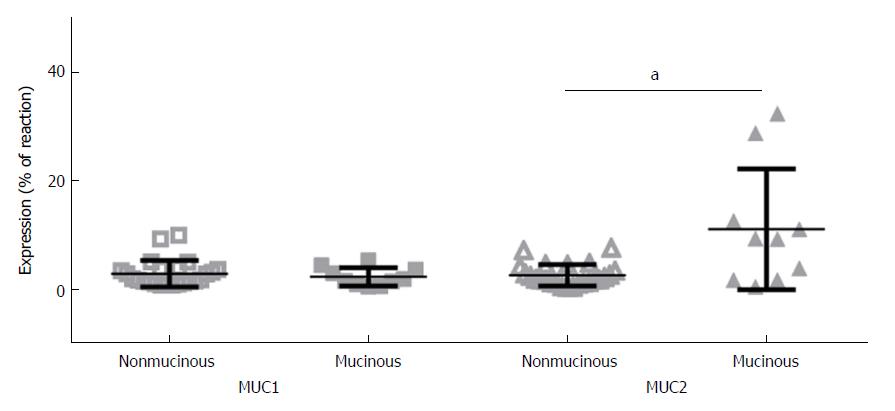
The mean expression of MUC1 in colorectal tumors (2.57% ± 2.24 % of IHC reaction) and in the normal large intestine (2.16% ± 1.64%) was comparable (P = 0.627). In the case of MUC2, significantly lower expression of this glycoprotein in CRC (4.94% ± 7.24%) than in the control (7.40% ± 6.77%) has been shown (P = 0.035) (Table 3).
The MUC1/MUC2 expression ratio was significantly higher in the CRC tissues (1.84 ± 2.54), than in the control (0.52 ± 0.51) (P = 0.003) (Table 3).
A significantly higher expression of MUC2 in mucinous CRC (10.97% ± 11.17% of IHC reaction), compared to the rest of the CRC (2.40% ± 2.00%), was shown (P = 0.018). No such differences were observed with MUC1 expression (Figure 2). No significant differences could be disclosed in the expression of both mucins from one hand and tumor size, the anatomical location of the CRC, histologic grade (G2 vs G3) and in patients with N0 and N1 in the TNM classification system (data not shown).
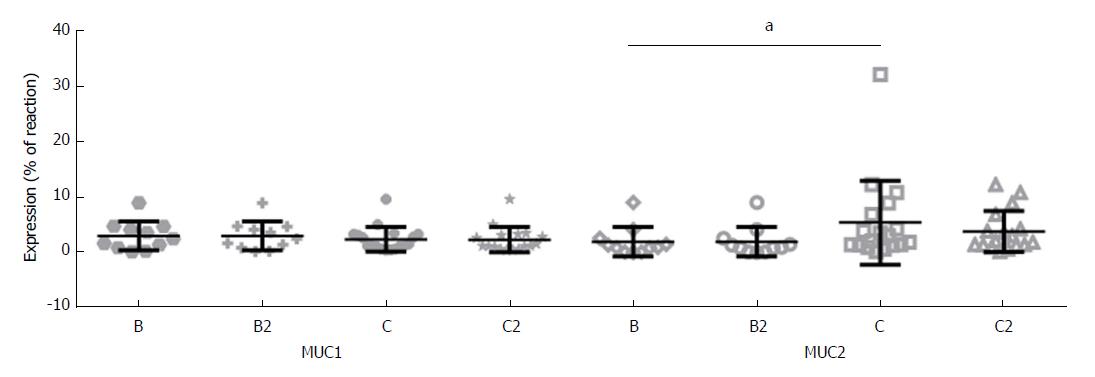
In the case of MUC2 expression, significantly higher expression of this mucins in colorectal tumors in Stage C was shown (5.54% ± 7.57%), compared with Stage B (2.12% ± 2.64%) (P = 0.044) (Figure 3). The analysis of mucins expression in tumors of Stage B2 and C2 on Astler and Coller scale, confirmed these results, although only a borderline statistical significance (P = 0.066) was obtained for MUC2 expression (data not shown).
High positive Spearman’s correlation was observed in patients affected by CRC, between mutual expression of both analyzed mucin transcripts (r = 0.602; P < 0.05), but not between protein expression itself (r = 0.046) (Table 4). Additionally, in CRC tumor tissues, borderline positive Spearman’s correlation, between mRNA and protein expression of MUC1 (r = 0.405; P = 0.055). MUC2 didn’t show a statistically significant correlation between mRNA and protein expression (Table 4).
Negative correlation between MUC1 mRNA expression and the age of patients was observed (r = -0.481; P < 0.05). Furthermore, positive correlations considered the expression of both mucins (MUC1 and MUC2) and the blood leukocyte count (r = 0.465 and r = 0.474 respectively; P < 0.05 in both cases). Additionally, the expression of MUC1 protein was positively correlated with thrombocyte numbers in patients affected by CRC (r = 0.474; P < 0.05) (Table 5).
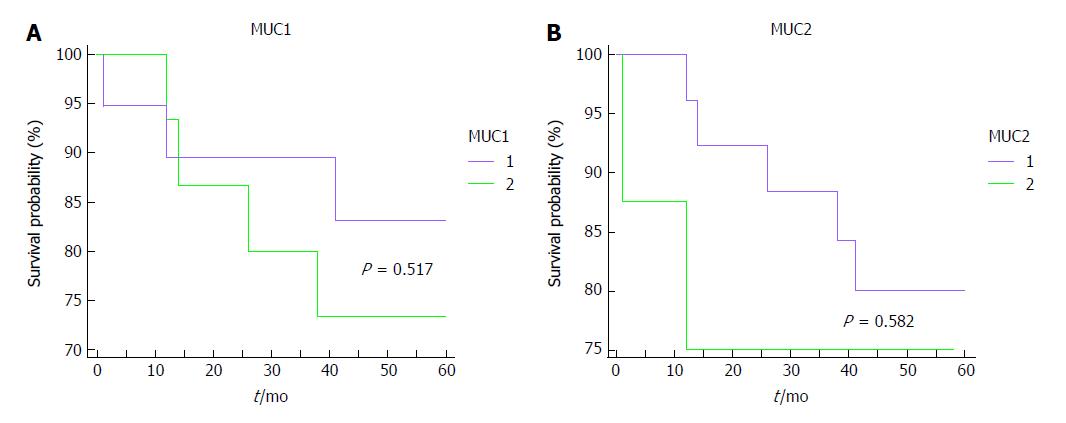
Mean survival time of patients affected by CRC was 52 ± 3 mo. The Kaplan-Meier analysis shows that neither MUC1, nor MUC2 apomucins expression were significantly associated with survival probability in patients with CRC (Figure 4A and B). Survival curves of 34 patients with CRC showed that also expression of mRNA for both mucins in tissue samples was not associated with the prognosis of CRC (data not shown).
The positive expression of Ki-67 proliferating antigen was detected in 28/34 (82%) of CRC tissue samples. Additionally, a significantly higher expression of Ki-67 in CRC as compared with control was demonstrated (P < 0.001) (data not shown).
Only the nuclear location of Ki-67 within different percentages of immunopositive tumor cells was observed (Figure 5A and B). The Ki-67 antigen expression in the control samples was evident mainly in the individual basally located nuclei of the goblet cells lining the unaltered intestinal crypts (Figure 5C).
Positive expression of p53 was demonstrated in 19/34 (56%) patients. Similar to Ki-67 antigen, only the nuclear location was observed and, as a rule, a very intense IHC reaction concerning the majority of polymorphic cell nuclei in the evaluated samples (Figure 5D). 44% of CRC patients did not show the presence of the protein in tumor samples (Figure 5E). In the healthy colorectal samples (control), p53 expression was not detected in any specimen (Figure 5F).
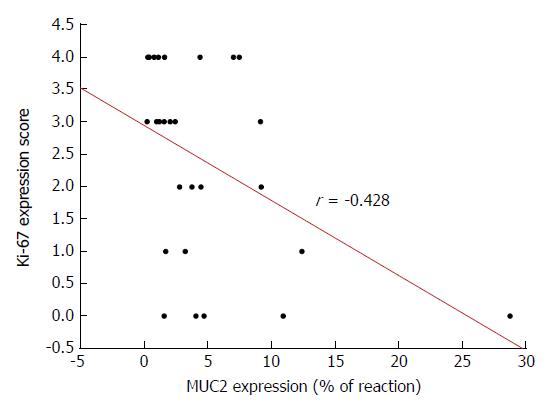
A significant, relatively high, negative Spearman’s correlation, between the expression of MUC2 apomucin and Ki-67 (r = -0.428; P < 0.05) was observed (Figure 6). In contrast, relatively weak, negative correlation between MUC2 protein and p53 expression (r = -0.389; P < 0.05), was shown in CRC tissues (data not shown). Additionally, a high positive correlation was observed for Ki-67 antigen and p53 expression in study group (r = 0.603; P < 0.05) (data not shown).
Some discrepancies are present between the results of MUC1 expression detection, in healthy colon and rectum, with the use of immunohistochemistry. Some scientific publications, notably the recent ones, document lack of MUC1 expression in control large intestine in adults[18-20], or emphasize low detectability (10%) of this apomucin[36].
In this study, positive MUC1 expression (mRNA, protein) was observed in almost all of the control samples of large intestinal tissue. These results are coherent with the findings of other authors, conducted with the use of light and electron microscopy[37,38]. Descriptions of clear, membranous expression of MUC1, on the luminal surface of glandular cells of normal colon epithelium, are available also in interactive databases[39]. Therefore, our own research using IHC technique confirms both, detectability and evident membranous expression of this mucin, in tissues of healthy large intestine.
In neoplastic CRC tissues collected in this study, detectability of MUC1 expression via IHC was 100%, being much higher than those achieved by other authors, which note it from below 20%[40], through 32%-40%[14,18,41], approximately 55%[20], to 70%-80%[29]. According to some publications, MUC1 expression was more commonly detected in CRC patients with lymph node metastases discovered during surgical procedures, than those without such metastases (84.2% vs 34.6%)[20], which is not confirmed by the current study. However, our results are similar to those obtained in CRC tissue microarrays, in which the authors also did not observe correlation between MUC1 expression and histologic grade, stage, vascular invasion, or cancer type[14,30]. The results of research by Matsuda et al[41], concerning more common expression of MUC1 in CRC of more severe histologic stages, were not confirmed in our studies. However, similarly to the authors[41], we have also not found any correlation between MUC1 and p53 expression. As in our studies, Kesari et al[19] did not observe differences in MUC1 expression depending on the histologic stage, but a higher incidence of expression of this mucin was described in G2, than in G1 of this cancer (55% vs 11%)[19]. The positive relationship between MUC1 expression and histologic grade and stage can also be found in other publications, with the appreciation of this expression as a high risk factor of death in Caucasian population (HR: 2.03; P = 0.038)[29,37]. The co-expression of MUC1 and p53 was a bad prognostic factor for the overall survival (OS) of these patients[29]. Our own research cannot confirm the above observations, as well as the results of other authors, where MUC1 expression was shown to be an independent marker of prognosis (HR: 1.339, 95%CI: 1.002-1.790; P = 0.048)[14]. The lack of dependence between MUC1 expression and histologic grade or stage, in the current study probably results from the very homogenous group of patients with CRC in the range of histologically assessed parameters [68% with grade 2; 56% with stage C (C1-C3)/D in Dukes/Astler and Coller scale; 85% with stage III and IV in TNM classification system]. Furthermore, it should be stressed, that the results of multiple authors are mainly based on the analysis of the detection (incidence) of MUC1 expression, rather than a reliable quantitative assessment[19,29]. In several works, MUC1 expression was admittedly evaluated, using semi-quantitative methods[20,30,36], but some of them did not have control groups[14,19,30]. There are publications that intensify the IHC reaction, and introduce the division into the so-called high, low and negative MUC1 expression, occurring in a different percentage of patients (12%, 52%, 36%, respectively)[30]. Hence, the overexpression of the MUC1 protein was observed in varying percentages in different patients, from 12%[30] to approximately 40%[19].
Most of the cited researchers, in their studies, used monoclonal, primary Ma695 antibody (Novocastra), in 1:100 dilution[18,19,30,40]. In the current study, antibodies from the same company have been used, also in 1:100 dilution. However, we have chosen another clone (Ma552). Furthermore, the use of a reliable, repeatable method of quantitative evaluation of IHC expression (HSV filter program), may explain the discrepancies obtained in the study results, at least partially, including the increased MUC1 expression in both control, and cancerous large intestine, presented in our study.
Current studies, employing the RT-qPCR method, have been shown a significantly higher mean expression of MUC1 mRNA in CRC, compared to the healthy tissue. This expression showed borderline correlation (P = 0.055) with MUC1 protein expression. It is difficult to relate this result to the literature data.
The results of correlation analysis, between the MUC1 expression (mRNA, protein) and the available patient clinical data, are not as spectacular as those obtained by other authors. In the case of MUC1, a negative correlation is shown between the mRNA (but not the protein itself) expression and the age of the CRC affected patients. The data on that specific relation was not found in literature. In addition, we have observed positive correlations between the MUC1 mRNA and the number of leukocytes, while the expression of the MUC1 apomucin itself, correlated with the number of platelets in the CRC. There are also no exact references to these results in literature. However, in mouse model, it was shown that carcinoma mucins (fragments from human colonic adenocarcinoma LS180 cells) initiate thrombosis through adhesion-dependent, reciprocal activation of neutrophils and platelets. These studies provide insights into mucin-dependent, thrombin-independent thrombosis in patients with Trousseau syndrome[42].
Similar to MUC1 expression, the detectability of MUC2 (mRNA, protein), obtained in the current, study was higher, than that presented in the literature data, which cite approximately 30%[14], approximately 50%-64%[20,30,43] and 92% of positive CRC cases[44]. Some sources document a higher incidence of MUC2 expression detection (72%-100%), only in the case of mucinous CRC subtypes[29,40]. The production of MUC2 mRNA in the healthy colorectal tissues is documented by numerous researchers[18,45,46], although some of the authors describe it in just 20% of the control tissues[44].
The cytoplasmic pattern of MUC2 expression, demonstrated in the present work, confirms previous observations in the healthy and cancer-altered large intestine[39,46,47]. We have described similar amounts of MUC2 mRNA in CRC and control, but a lower expression of the MUC2 protein in patients with CRC, compared to control. Confronting this with literature, the results of the IHC study are consistent with those obtained by many researchers[14-20,29,41,43,48], while in the case of MUC2 transcripts detection, few works record reduced expression of MUC2 mRNA in the CRC, compared to the normal tissue[45]. In the current work, higher expression of MUC2 in the mucinous CRC, as compared with nonmucinous subtypes of cancer, has been shown, which also confirms the results of other authors’ research[24,47,49].
In CRC patients gathered in this work, a higher expression of MUC2 was also observed in the more advanced histologic stages of the tumor. In addition, there have been significant negative correlations between MUC2 with Ki-67 and p53 expressions. This could indicate a significant relation between the decrease in the expression of this glycoprotein in the course of colon carcinogenesis, and the pro-proliferative activity (Ki-67), or deregulation of the tumor suppressive P53 signaling. This result is confirmed by the research of other authors[50,51]. However, similarly to MUC1, there was no significant correlation between MUC2 expression and histologic grade, size, or location of the tumor, which is also consistent with the literature data[43,52].
In the current study, it was also not possible to find statistically significant relationships between mucin expression (mRNA, protein) and survival of patients with CRC. In the 5-year period evaluated, seven people died of cancer (21%), the average survival was 52 mo from the time of surgery. The small number of patients analyzed, including the deceased, did not allow to draw binding conclusions on the predictive role of MUC1 and MUC2 tissue expression, in the CRC patients of the Greater Poland Region.
Research by other authors points to a link between MUC1 overexpression and poorer survival, especially in mucinous tumors. These authors prove, that higher frequency of MUC1 immunoreactivity in the mucinous subtype of CRC was independently related to greater rate of cancer death in colorectal patients[26]. In the case of MUC2, however, other studies have also shown important correlations between MUC2 expression reduction/loss, shorter survival time (OS), shorter progression-free/disease-free (PFS) in patients with stage II and III colorectal carcinomas[30,53] and longer disease-free (DFS) and disease-specific survival (DSS) in patients with positive MUC2 expression. The loss of expression of this mucin was correlated with the recurrence of cancer[52]. In some studies, the relationship between MUC2 expression and survival was not spectacular, but only borderline, and more often concerned well-to-moderately differentiated adenocarcinomas [P = 0.064 for recurrence/metastasis-free survival (RFS) and P = 0.172 for OS] but not for poorly differentiated adenocarcinomas[43].
Although the current study is based on a relatively small group of patients (n = 34), with the predominance of nonmucinous subtype of CRC, it can be assumed, that the expression of both mucins (MUC1 and MUC2), at the level of mRNA and protein, occurs in a normal and tumor-altered colon. Lower tissue expression of MUC2 in CRC, as compared with control, correlates with increased cellular proliferation and could become a marker of cancer progression. The intensity of MUC2 expression allows to differentiate mucinous and nonmucinous CRC subtypes.
The clinical limitations of the current study can be summarized as follows: (1) Most likely due to the homogeneous study group in the range of histologically assessed parameters (68% patients with G2 and 85% with stage III and IV in TNM classification), not all differences in MUC1 and MUC2 expression or correlations with clini–cal data have reached statistical significance. And (2) The small number of deceased patients (n = 7) analyzed in the current study, did not allow to draw binding conclusions on the predictive role of MUC1 and MUC2 tissue expression for the survival time of patients with CRC of the Greater Poland Region.
Future study is required and a larger number of patients should be evaluated to confirm our findings. Better characterization of the role of mucins in molecular mechanisms in colorectal carcinogenesis requires further testing, also on an in vitro model.
In conclusion, a combination of tissue overexpression of MUC1, reduced MUC2 expression, and high ratio of MUC1/MUC2 is a factor of poor prognosis in CRC patients. MUC2 tissue expression allows to differentiate mucinous and nonmucinous CRC subtypes.
In Poland, colorectal carcinoma (CRC) is the second most common cancer in men and woman, with the third leading causes of cancer deaths in Greater Poland Region. Altered mucin expression is correlated with the prognosis of this cancer. In vivo as well as in vitro studies on the expression of mucins may have also therapeutic implications.
The role of mucin expression at various stages of the colon carcinogenesis is incomplete. The prognostic role of mucins in the mucinous subtypes of CRC is poorly known. Research into the role of mucins in pathogenesis and CRC clinical studies (especially in mucinous subtypes) are also current topics from a methodological point of view. The lack of standardized methods of quantitative evaluation of mucins expression (especially at tissue level) and/or frequent lack of control groups, are a great difficulty in comparative analysis.
Current research determines tissue expression (mRNA, protein) of membrane-bound mucin [mucin 1 (MUC1)] and secretory mucin [mucin 2 (MUC2)] in healthy and colorectal cancer tissue samples and evaluates the relationship between tissue expression of both mucins and selected clinicopathological data of the patients with CRC.
The research on tissue expression of two types of mucins (MUC1 and MUC2) in cancerous and normal colorectal tissue samples was performed using real-time quantitative polymerase chain reaction (RT-qPCR) to evaluate expression of transcripts, immunohistochemistry (IHC) for demonstrating apomucins localization, and the morphometric analysis of intensity of IHC reaction using modern HSV filter software.
Significantly higher expression of the MUC1 mRNA in the CRC, while MUC2 transcript expression was comparable with the control colorectal samples. Using immunohistochemistry, we observed lower MUC2 protein as compared to control tissue. MUC2 protein expression correlated negatively with cellular proliferation (Ki-67 antigen expression) and expression of mutated form of p53. In neoplastic tissue of CRC it was observed also higher MUC1/MUC2 ratio as compared with healthy colorectal tissue. Higher expression of MUC2 was a feature of mucinous CRC subtypes, and characterized higher histological stage of tumors. Future study is required to explain molecular mechanisms of CRC carcinogenesis including mucins and TP53 pathway.
Our study confirmed that the colorectal carcinogenesis is closely related to overexpression of MUC1 and the decline in MUC2 expression. The use of increasingly repetitive and reliable method for the quantitative evaluation of mucins expression may prove useful to evaluate different patterns of IHC reaction (membranous, cytoplasmic, etc.) and can be useful in various subtype of colorectal cancer, as our research shows (mucinous vs nonmucinous CRC). Both the microscopic demonstration of evident MUC1 expression, especially in healthy colorectal tissue (control), and morphometric quantitative evaluation (Filter HSV program) of membranous (MUC1) and secreted (MUC2) expression is the novelty of the present work. The quantitative method used in the current study, can be used for further comparative research and to evaluate tissue expression of other types of mucins in CRC. The use of quantitative methods in immunocytochemistry can improve the detection of tissue markers in CRC and assess their true value in daily medical practice.
This study proposed, that the examination of the tissue expression of MUC1 and MUC2 (mRNA, protein), should use modern methods of quantitative assessment of transcripts (e.g. RT-qPCR), and more reliable morphometric methods (e.g. HSV filter software). Only these methods could improve the diagnostic/prognostic usefulness of mucins as tissue biomarkers in CRC patients. A better explanation of molecular mechanisms in the colorectal carcinogenesis with mucin involvement requires further testing, also on an in vitro model. These results could be the basis for further studies to understand the carcinogenesis of colorectal cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boscá L, Lim SC, Wang SK S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
| 1. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2294] [Article Influence: 208.5] [Reference Citation Analysis (1)] |
| 2. | Dyzmann-Sroka A, Malicki J. Cancer incidence and mortality in the Greater Poland Region-Analysis of the year 2010 and future trends. Rep Pract Oncol Radiother. 2014;19:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 907] [Article Influence: 113.4] [Reference Citation Analysis (2)] |
| 4. | Corfield AP, Carroll D, Myerscough N, Probert CS. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001;6:D1321-D1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Sheng YH, Hasnain SZ, Florin TH, McGuckin MA. Mucins in inflammatory bowel diseases and colorectal cancer. J Gastroenterol Hepatol. 2012;27:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 7. | Mastrodonato M, Mentino D, Liquori GE, Ferri D. Histochemical characterization of the sialic acid residues in mouse colon mucins. Microsc Res Tech. 2013;76:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Jain P, Mondal SK, Sinha SK, Mukhopadhyay M, Chakraborty I. Diagnostic and prognostic significance of different mucin expression, preoperative CEA, and CA-125 in colorectal carcinoma: A clinicopathological study. J Nat Sci Biol Med. 2014;5:404-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Mastrodonato M, Mentino D, Portincasa P, Calamita G, Liquori GE, Ferri D. High-fat diet alters the oligosaccharide chains of colon mucins in mice. Histochem Cell Biol. 2014;142:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1222] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 11. | Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 975] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 12. | Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EE. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 922] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 13. | Niv Y. MUC1 and colorectal cancer pathophysiology considerations. World J Gastroenterol. 2008;14:2139-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Duncan TJ, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J Surg Oncol. 2007;5:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Perçinel S, Savaş B, Ensari A, Kuzu I, Kuzu MA, Bektaş M, Cetinkaya H, Kurşun N. Mucins in the colorectal neoplastic spectrum with reference to conventional and serrated adenomas. Turk J Gastroenterol. 2007;18:230-238. [PubMed] |
| 16. | Perez RO, Bresciani BH, Bresciani C, Proscurshim I, Kiss D, Gama-Rodrigues J, Pereira DD, Rawet V, Cecconnello I, Habr-Gama A. Mucinous colorectal adenocarcinoma: influence of mucin expression (Muc1, 2 and 5) on clinico-pathological features and prognosis. Int J Colorectal Dis. 2008;23:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Saeland E, Belo AI, Mongera S, van Die I, Meijer GA, van Kooyk Y. Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients. Int J Cancer. 2012;131:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. Expression of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases. Int J Clin Exp Pathol. 2013;6:613-621. [PubMed] |
| 19. | Kesari MV, Gaopande VL, Joshi AR, Babanagare SV, Gogate BP, Khadilkar AV. Immunohistochemical study of MUC1, MUC2 and MUC5AC in colorectal carcinoma and review of literature. Indian J Gastroenterol. 2015;34:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Wang HS, Wang LH. The expression and significance of Gal-3 and MUC1 in colorectal cancer and colon cancer. Onco Targets Ther. 2015;8:1893-1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Debunne H, Ceelen W. Mucinous differentiation in colorectal cancer: molecular, histological and clinical aspects. Acta Chir Belg. 2013;113:385-390. [PubMed] |
| 22. | Xu F, Liu F, Zhao H, An G, Feng G. Prognostic Significance of Mucin Antigen MUC1 in Various Human Epithelial Cancers: A Meta-Analysis. Medicine (Baltimore). 2015;94:e2286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Zeng Y, Zhang Q, Zhang Y, Lu M, Liu Y, Zheng T, Feng S, Hao M, Shi H. MUC1 Predicts Colorectal Cancer Metastasis: A Systematic Review and Meta-Analysis of Case Controlled Studies. PLoS One. 2015;10:e0138049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Chiang JM, Yeh CY, Changchien CR, Chen JS, Tang R, Chen JR. Mucinous adenocarcinoma showing different clinicopathological and molecular characteristics in relation to different colorectal cancer subgroups. Int J Colorectal Dis. 2010;25:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Winder T, Lenz HJ. Mucinous adenocarcinomas with intra-abdominal dissemination: a review of current therapy. Oncologist. 2010;15:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | You JF, Hsieh LL, Changchien CR, Chen JS, Chen JR, Chiang JM, Yeh CY, Hsieh PS, Fan CW, Liu CT. Inverse effects of mucin on survival of matched hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer patients. Clin Cancer Res. 2006;12:4244-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012;65:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Benedix F, Kuester D, Meyer F, Lippert H. [Influence of mucinous and signet-ring cell differentiation on epidemiological, histological, molecular biological features, and outcome in patients with colorectal carcinoma]. Zentralbl Chir. 2013;138:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Manne U, Weiss HL, Grizzle WE. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res. 2000;6:4017-4025. [PubMed] |
| 30. | Betge J, Schneider NI, Harbaum L, Pollheimer MJ, Lindtner RA, Kornprat P, Ebert MP, Langner C. MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: expression profiles and clinical significance. Virchows Arch. 2016;469:255-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 31. | ASTLER VB, COLLER FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954;139:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 744] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Puppa G, Sonzogni A, Colombari R, Pelosi G. TNM staging system of colorectal carcinoma: a critical appraisal of challenging issues. Arch Pathol Lab Med. 2010;134:837-852. [PubMed] |
| 33. | Kasprzak A, Szaflarski W, Szmeja J, Andrzejewska M, Przybyszewska W, Kaczmarek E, Koczorowska M, Kościński T, Zabel M, Drews M. Differential expression of IGF-1 mRNA isoforms in colorectal carcinoma and normal colon tissue. Int J Oncol. 2013;42:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Gurgul E, Kasprzak A, Blaszczyk A, Biczysko M, Surdyk-Zasada J, Seraszek-Jaros A, Ruchala M. Ghrelin and obestatin in thyroid gland - immunohistochemical expression in nodular goiter, papillary and medullary cancer. Folia Histochem Cytobiol. 2015;53:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Gatter KC, Dunnill MS, Gerdes J, Stein H, Mason DY. New approach to assessing lung tumours in man. J Clin Pathol. 1986;39:590-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Lugli A, Zlobec I, Baker K, Minoo P, Tornillo L, Terracciano L, Jass JR. Prognostic significance of mucins in colorectal cancer with different DNA mismatch-repair status. J Clin Pathol. 2007;60:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Biemer-Hüttmann AE, Walsh MD, McGuckin MA, Ajioka Y, Watanabe H, Leggett BA, Jass JR. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Winterford CM, Walsh MD, Leggett BA, Jass JR. Ultrastructural localization of epithelial mucin core proteins in colorectal tissues. J Histochem Cytochem. 1999;47:1063-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | MUC1. Accessed March 20, 2018. Available from: http://www.humanproteinatlas.org. |
| 40. | Ishizu H, Kumagai J, Eishi Y, Takizawa T, Koike M. Mucin core protein expression by colorectal mucinous carcinomas with or without mucus hyperplasia. J Gastroenterol. 2004;39:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Matsuda K, Masaki T, Watanabe T, Kitayama J, Nagawa H, Muto T, Ajioka Y. Clinical significance of MUC1 and MUC2 mucin and p53 protein expression in colorectal carcinoma. Jpn J Clin Oncol. 2000;30:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, Xia L, Varki A, McEver RP. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood. 2011;118:4015-4023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | Imai Y, Yamagishi H, Fukuda K, Ono Y, Inoue T, Ueda Y. Differential mucin phenotypes and their significance in a variation of colorectal carcinoma. World J Gastroenterol. 2013;19:3957-3968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | El-Sayed IH, Lotfy M, Moawad M. Immunodiagnostic potential of mucin (MUC2) and Thomsen-Friedenreich (TF) antigens in Egyptian patients with colorectal cancer. Eur Rev Med Pharmacol Sci. 2011;15:91-97. [PubMed] |
| 45. | Ogata S, Uehara H, Chen A, Itzkowitz SH. Mucin gene expression in colonic tissues and cell lines. Cancer Res. 1992;52:5971-5978. [PubMed] |
| 46. | Sylvester PA, Myerscough N, Warren BF, Carlstedt I, Corfield AP, Durdey P, Thomas MG. Differential expression of the chromosome 11 mucin genes in colorectal cancer. J Pathol. 2001;195:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Hanski C, Hofmeier M, Schmitt-Gräff A, Riede E, Hanski ML, Borchard F, Sieber E, Niedobitek F, Foss HD, Stein H. Overexpression or ectopic expression of MUC2 is the common property of mucinous carcinomas of the colon, pancreas, breast, and ovary. J Pathol. 1997;182:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Al-Khayal K, Abdulla M, Al-Obaid O, Zubaidi A, Vaali-Mohammed MA, Alsheikh A, Ahmad R. Differential expression of mucins in Middle Eastern patients with colorectal cancer. Oncol Lett. 2016;12:393-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Blank M, Klussmann E, Krüger-Krasagakes S, Schmitt-Gräff A, Stolte M, Bornhoeft G, Stein H, Xing PX, McKenzie IF, Verstijnen CP. Expression of MUC2-mucin in colorectal adenomas and carcinomas of different histological types. Int J Cancer. 1994;59:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Li A, Goto M, Horinouchi M, Tanaka S, Imai K, Kim YS, Sato E, Yonezawa S. Expression of MUC1 and MUC2 mucins and relationship with cell proliferative activity in human colorectal neoplasia. Pathol Int. 2001;51:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Walsh MD, Clendenning M, Williamson E, Pearson SA, Walters RJ, Nagler B, Packenas D, Win AK, Hopper JL, Jenkins MA. Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod Pathol. 2013;26:1642-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 52. | Elzagheid A, Emaetig F, Buhmeida A, Laato M, El-Faitori O, Syrjänen K, Collan Y, Pyrhönen S. Loss of MUC2 expression predicts disease recurrence and poor outcome in colorectal carcinoma. Tumour Biol. 2013;34:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Kang H, Min BS, Lee KY, Kim NK, Kim SN, Choi J, Kim H. Loss of E-cadherin and MUC2 expressions correlated with poor survival in patients with stages II and III colorectal carcinoma. Ann Surg Oncol. 2011;18:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |