Published online Sep 21, 2018. doi: 10.3748/wjg.v24.i35.4086
Peer-review started: June 7, 2018
First decision: June 20, 2018
Revised: July 17, 2018
Accepted: August 1, 2018
Article in press: August 1, 2018
Published online: September 21, 2018
Processing time: 105 Days and 20.1 Hours
Steroid 5β-reductase [aldo-keto reductase family 1 member D1 (AKR1D1)] is essential for bile acid biosynthesis. Bile acid deficiency caused by genetic defects in AKR1D1 leads to life-threatening neonatal hepatitis and cholestasis. There is still limited experience regarding the treatment of this disease. We describe an infant who presented with hyperbilirubinemia and coagulopathy but normal bile acid and γ-glutamyltransferase. Gene analysis was performed using genomic DNA from peripheral lymphocytes from the patient, his parents, and his elder brother. The patient was compound heterozygous for c.919C>T in exon 8 and exhibited a loss of heterozygosity of the AKR1D1 gene, which led to an amino acid substitution of arginine by cysteine at amino acid position 307 (p.R307C). Based on these mutations, the patient was confirmed to have primary 5β-reductase deficiency. Ursodeoxycholic acid (UDCA) treatment did not have any effect on the patient. However, when we changed to chenodeoxycholic acid (CDCA) treatment, his symptoms and laboratory tests gradually improved. It is therefore crucial to supplement with an adequate dose of CDCA early to improve clinical symptoms and to normalize laboratory tests.
Core tip: We report a case of an infant with primary 3-oxo-Δ4-steroid 5β-reductase deficiency with a novel missense mutation in the aldo-keto reductase family 1 member D1 (AKR1D1) gene. The patient was successfully treated by early adequate supplementation with chenodeoxycholic acid (CDCA). This case suggests that a novel compound heterozygous R307C mutation and loss of heterozygosity in the AKR1D1 gene play a pathogenic role in congenital bile acid synthesis defect type 2. Accurate diagnosis of the disease and early adequate supplementation with CDCA are vital for the amelioration of symptoms in clinical practice.
- Citation: Wang HH, Wen FQ, Dai DL, Wang JS, Zhao J, Setchell KD, Shi LN, Zhou SM, Liu SX, Yang QH. Infant cholestasis patient with a novel missense mutation in the AKR1D1 gene successfully treated by early adequate supplementation with chenodeoxycholic acid: A case report and review of the literature. World J Gastroenterol 2018; 24(35): 4086-4092
- URL: https://www.wjgnet.com/1007-9327/full/v24/i35/4086.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i35.4086
Congenital bile acid synthesis defect type 2 (CBS2) is a rare and autosomal recessive inherited disease presenting with infant intrahepatic cholestasis, normal or slightly elevated total bile acids and γ-glutamyltransferase in serum[1,2]. This inborn error of bile acid synthesis is caused by a defect in the aldo-ketoreductase family 1 member D1 (AKR1D1) gene, which encodes Δ4-3-oxosteroid 5β-reductase, the key enzyme involved in bile acid biosynthesis[3]. This enzyme catalyzes the reduction of the Δ4-3-ketosteroid to form the AB cis ring structure; its deficiency results in a lack of primary bile acids and an increase in the synthesis of 3-oxo-Δ4 bile and allo-bile acids[4].
In 1988, Clayton et al[5] reported that severe liver disease in pediatric patients was detected with predominant unusual 3-oxo-Δ4 bile acids secondary to 5β-reductase deficiency. Primary 5β-reductase deficiency was first characterized by Setchell et al[6] the same year. It is difficult to distinguish primary 5β-reductase deficiency from another cholestasis secondary to a variety of severe liver diseases based on clinical symptoms and regular laboratory tests[1,7,8]. Thus, genetic analysis of the AKR1D1 gene is essential for the accurate diagnosis of primary 5β-reductase deficiency. Thus far, more than 20 cases of this inborn error have been reported, and over ten variant mutations of the AKR1D1 gene are attributed to a defect in 5β-reductase[1,7-13]. Most of these mutations are missense mutations, causing an amino acid alteration in the protein. Drury et al[3] further investigated five reported point mutations (L106F, P133R, P198L, G223E, and R261C) in the AKR1D1 gene to evaluate their effects on the enzymatic properties of 5β-reductase. They found that these mutations result in significantly decreased 5β-reductase activity and subsequently contribute to the progression of bile acid deficiency.
Primary bile acid supplementation can ameliorate the symptoms of CBSA2 and normalize liver function by offering feedback repression of the cholesterol 7α-hydroxylase gene and improving the absorption of fat and fat-soluble vitamins[14]. Treatment with primary bile acids includes monotherapy or the combination of cholic acid (CA), ursodeoxycholic acid (UDCA) and chenodeoxycholic acid (CDCA). Early treatment of these bile acids, especially CA and CDCA, is essential to reserve liver function and avoid liver transplantation. A delayed diagnosis would lead to a poor response to primary bile acid treatment and an unfavorable prognosis. There is still limited experience with the treatment of this disease. Here, we describe a case of CBS2 diagnosed by genetic analysis with a novel compound heterozygous mutation in the AKR1D1 gene, and review both the treatments and prognoses of genetically diagnosed CBS2 cases.
A male patient was delivered via Caesarean section at term after an uneventful pregnancy with a birth weight of 3400 g. He was the second child of his family and the third pregnancy of his mother. One of his mother’s pregnancies was terminated by abortion for social reasons. His parents were non-consanguineous and healthy, his elder brother was healthy, and none of them presented with any liver disease. The patient soon developed progressive jaundice after birth, with dark urine and pale stool. He was referred to our hospital at the age of two months. Laboratory tests indicated total bilirubin levels of 204.8 μmol/L, direct bilirubin levels of 112.4 μmol/L, alanine aminotransferase levels of 339 IU/L, aspartate aminotransferase levels of 619 IU/L, γ-glutamyltransferase levels of 50 IU/L, total bile acid levels of 1.8 μmol/L, an activated partial thromboplastin time of 62.6 s, a prothrombin time (PT) of 23.6 s, and an international normalized ratio of 2.1. Chronic hepatitis virus tests, including hepatitis B, hepatitis C and cytomegalovirus, were negative, and autoimmune hepatitis was ruled out by an appropriate laboratory test. Abdominal ultrasound showed a visible gallbladder and hepatomegaly; no other bile duct dysplasia was observed. Analysis of the amino acid and acylcarnitine spectrum of genetic metabolic diseases showed elevated tyrosine, which was speculated to be secondary to impaired liver function. Comprehensive analysis of urinary organic acids was normal.
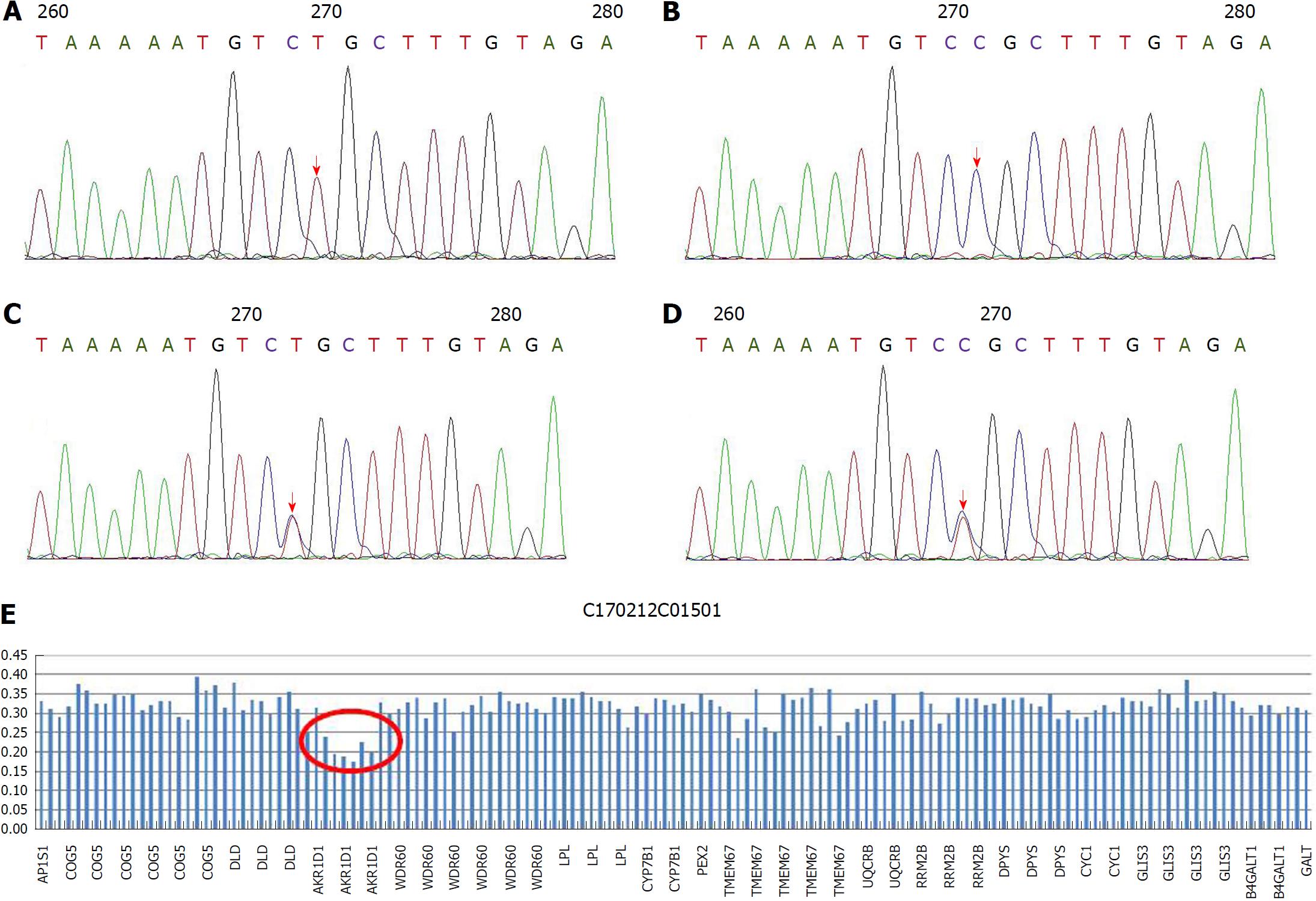
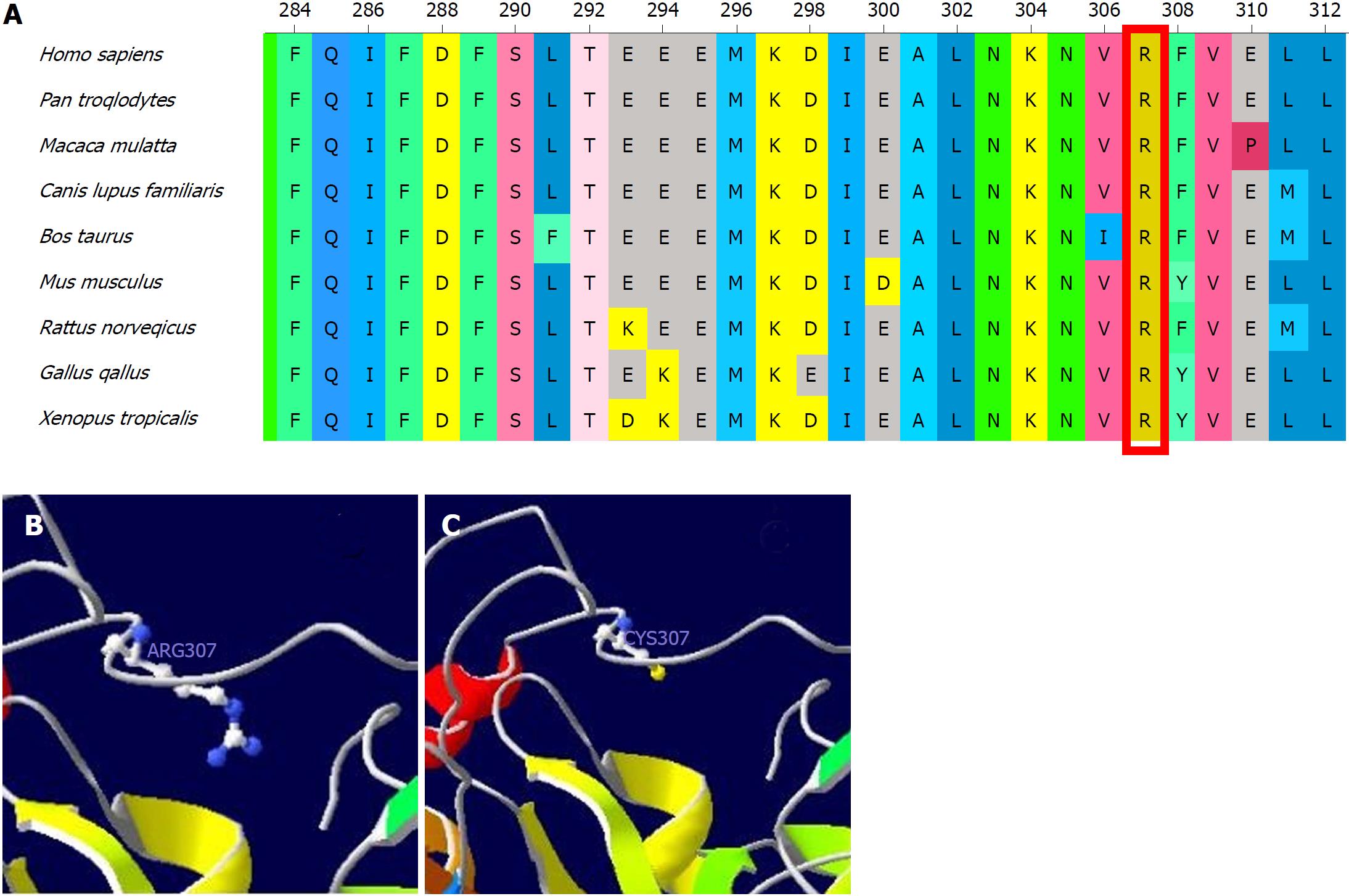
Since we were unavailable to perform bile acid analysis in our hospital, we performed genetic analysis with a cholestasis panel (Supplementary Table 1), which included prevalent pathogenic genes associated with infant cholestasis, to confirm the patient’s diagnosis. With informed consent, gene analysis was performed using genomic DNA from peripheral lymphocytes from the patient (Figure 1A), his parents (Figure 1B and C), and his elder brother (Figure 1D). The patient was compound heterozygous for c.919C>T in exon 8 (Figure 1A) and exhibited loss of heterozygosity of the AKR1D1 gene (Figure 1E), leading to an amino acid substitution of arginine by cysteine at amino acid position 307 (p. R307C) (Figure 2).
| Variant | Zygotic type | Age | Sex | Treatment | Outcome | INR | Ref. |
| c.662C > T (p. P198L) | Homozygote | 8 mo | F | CDCA 8 mg/kg/d CA 8 mg/kg/d | Alive and well | 1.00 | [7] |
| c.511delT (frameshift) | Homozygote | 8 wk | M | CDCA 8 mg/kg/d CA 8 mg/kg/d | Liver transplantation; alive and well | 1.40 | |
| c.385C > T (p. L106F) | Homozygote | 6 wk | F | UDCA 60 mg/d CDCA 30 mg/d | Liver transplantation and died | 2.00 | |
| c.467C > G (p. P133R) c.850C > T (p. R261C) | Heterozygote | 8 mo | F | CA 10 mg/kg/d | Alive and well | / | [10] |
| c.737G > A (p. G223Q) | Heterozygote | 3 mo | F | UDCA 5-10 mg/kg/d | Alive and well | / | [8] |
| c.217C > T (Arg50 stop) | Heterozygote | 2 mo | F | CDCA 12 mg/kg/d | Liver transplantation | / | |
| c.850C > T (p.R261C) | Homozygote | 6 mo | / | CA 8 mg/kg/d | Died | 2.50 | [1] |
| c.797G > A (p. R266Q) | Heterozygote | 11 mo | M | UDCA 40 mg/kg/d | Alive and well | / | [13] |
| c.396C > A (nonsense mutation) c.722A >T (p. D241V) | Heterozygote | 11wk | M | UDCA 40 mg/kg/d for 4 mo; CDCA 25 mg/kg/d | Alive and cerebral dysplasia | / | |
| c.866G > A (p. R266Q) | Heterozygote | 6 mo | M | UDCA 7.5 mg/kg/d; CDCA 5 mg/kg/d | Alive and well | / | [12] |
| c.737G >A (p. G223E) c.850C >T (p. R261C) | Heterozygote | 8 mo | F | UDCA 7.5 mg/kg/d; CDCA 10 mg/kg/d | Alive and well | / | |
| c.587delG (frameshift) | Homozygote | 9 wk | F | UCDA | Died | / | [11] |
| c.587delG (frameshift) | Homozygote | 6 mo | F | UCDA | Died | / | |
| c.587delG (frameshift) | Homozygote | 5 wk | F | CA 15 mg/kg/d | Alive and well | / | |
| c.579 + 2delT, c.853C > T (p. Q285X) | Heterozygote | 8 mo | M | CDCA | Alive and well | / | [9] |
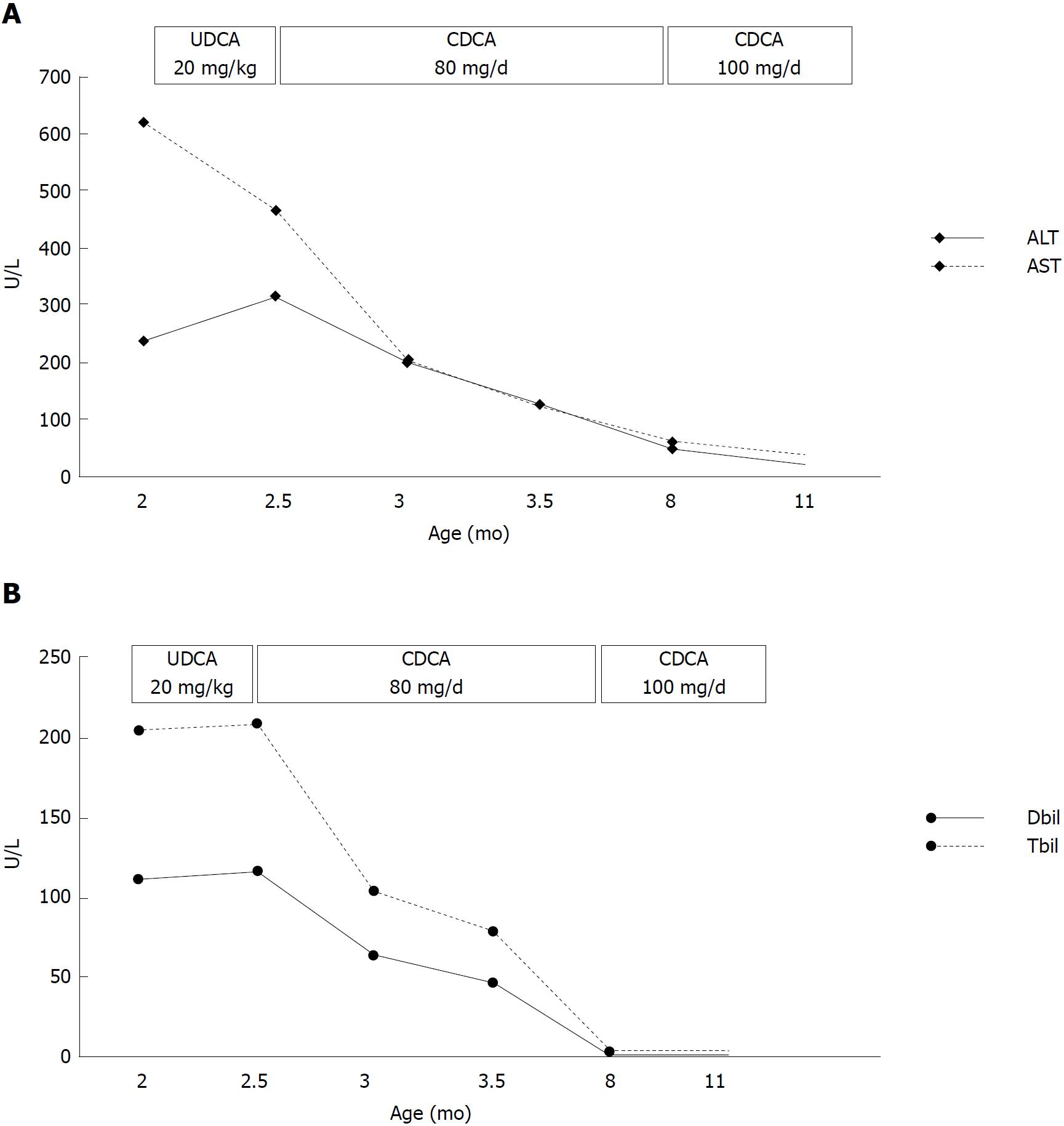
The patient was initially given UDCA treatment; however, there was no improvement in his clinical symptoms or liver function. UDCA was then changed to CDCA (80 mg/d) after one week of UCDA treatment. The jaundice began to alleviate after five days of CDCA treatment and his liver function gradually improved (Figure 3). To evaluate the response of bile acid metabolism subsequent to CDCA treatment, we sent the patient’s urine sample to Cincinnati Children’s Hospital Medical Center via the Children’s Hospital of Fudan University. Urine bile acid analysis was performed using fast atom bombardment ionization mass spectrometry after two months of CDCA treatment (80 mg/d). The profile revealed significant elevations in taurine and glycine conjugates of unsaturated oxo-dihydroxy and oxo-trihydroxy bile acids. Ions at m/z 444, 460, 494 and 510 reflected the presence of Δ4-3-oxo bile acids that are characteristic of the bile acid synthetic disorder involving a deficiency in the activity of the Δ4-3-oxosteroid 5β-reductase enzyme. Although these are not exceptionally high in concentration, it is difficult to know how responsive the patient was to CDCA therapy because we had no record of having analyzed a urine sample before treatment began. There is clear evidence of compliance to therapy from the presence of ions that reflect metabolites of CDCA. However, based on this mass spectrum, it appeared that the current dose of CDCA was not sufficient to complete the suppression of atypical bile acids. Thus, we increased the dose of CDCA to 100 mg/d and sent a second urine sample for bile acid analyses one month later. The profile showed a good response in terms of the down-regulation of hepatic bile acid synthesis. Thus, the increased dose of CDCA appeared adequate.
We summarized published CBS2 cases with a confirmed AKR1D1 mutation reported in the NCBI database through the end of December 2017 (Table 1). As demonstrated, missense mutations were present in 11 of 15 cases; the other four cases had a frameshift mutation. These cases consisted of seven homozygous and eight heterozygous mutations. All four cases in which the patient was deceased were homozygous and had a remarkably prolonged international normalized ratio (INR) (1.8 or above), which comprised three frameshift mutations and one missense mutation. In the other three homozygous cases, two showed a good response to primary bile acid treatment and had good prognoses; one patient was referred for liver transplantation and remains alive. All heterozygous cases remain alive and were effectively treated with primary bile acid treatment; only one patient required liver transplantation.
The patient we describe herein developed progressive jaundice in early infancy, with elevated direct bilirubin and alanine aminotransferase but normal total bile acids and γ-glutamyltransferase. After exclusion of bile duct dysplasia, metabolic disorder, viral hepatitis and autoimmune hepatitis, we highly suspected hereditary cholestasis. We were unable to perform bile acid profile analyses in our hospital at that time. To identify the cause of cholestasis, we screened gene disorders using a hereditary cholestasis panel. Genetic analyses revealed that the patient had one heterozygous mutation (R307C) in the AKR1D1 gene from his mother and loss of heterozygosity in the AKR1D1 gene from his father, making him compound heterozygous. Family genetic analyses indicated that the R307C mutation in the AKR1D1 gene was heterozygous both in the patient’s mother and brother but absent in his father. On the other hand, the loss of heterozygosity in the AKR1D1 gene was found in the patient and his father but was absent in his mother and brother. As predicted by SWISS-MODEL Homology Modeling, the R307C mutation could cause an alteration in the amino acid side chain, which may subsequently lead to 5β-reductase deficiency.
However, the patient’s brother did not develop cholestasis even though he also had the heterozygous R307C mutation, but without loss of heterozygosity in the AKR1D1 gene. Accordingly, we speculate that the combination of the R307C mutation and loss of heterozygosity cause the loss of 5β-reductase function.
The patient described herein showed an effective response to CDCA monotherapy (80 mg/d), consistent with a previous report[12]. After two months of oral CDCA treatment, the laboratory tests and clinical presence of the patient improved. However, urine bile acid analyses indicated that the CDCA dose of 80 mg/d was insufficient to complete the suppression of atypical bile acids. Thus, we increased the dose of CDCA to 100 mg/d, which proved adequate to down-regulate hepatic bile acid synthesis according to the second urine bile acid analyses. All laboratory tests had normalized when the patient was eight months old, and 100 mg/d CDCA was used to maintain treatment. Seki et al[12] reported that 5 mg/kg/d CDCA may not be able to induce negative feedback, and Gonzales et al[15] suggested a CDCA dose of 10 mg/kg/d may provide effective negative feedback against cholesterol 7α-hydroxylase. Our case required an even higher dose of CDCA to maintain effective feedback repression of 7α-hydroxylase. CA is considered more effective than CDCA in activating negative feedback of 7α-hydroxylase and is less hepatotoxic[15]. Clayton et al[16] reported that 5β-reductase deficiency was responsive to the combination of CDCA and CA treatment, but irresponsive to UCDA. As illustrated in Table 1, Lemonde et al[7] was also successful when combining CDCA (8 mg/kg/d) and CA (8 mg/kg/d) to treat a homozygous patient with normal PT. Nevertheless, the same treatment failed in two other homozygous patients with prolonged PT. The combination of CDCA and CA requires a smaller dose of CDCA, which may reduce the accumulation of potential hepatotoxic CDCA metabolites. According to our experience, an adequate dose of CDCA monotherapy was effective in alleviating clinical symptoms and normalizing laboratory tests of AKR1D1 deficiency, and the adjustment of bile acid dose should be based on urine bile acid analyses. Long-term follow-up, including liver function monitoring and urine bile acid analyses, are required to evaluate the hepatotoxicity of CDCA monotherapy and dose regulation. Although it is well-accepted that UCDA is not an optimal choice for the treatment of 5β-reductase deficiency[16,17], some reported cases, all of which were heterozygous, still benefited from UCDA treatment[8,13]. The natural immaturity of 5β-reductase during early infancy may promote the advancement of cholestasis caused by a defect in AKR1D1[18,19]. Thus, the presence of cholestasis and liver dysfunction in cases with a heterozygous mutation in the AKR1D1 gene may not require bile acid supplementation due to the natural physiological maturation of 5β-reductase.
Clayton et al[1] reported that patients with an INR of 1.4 or above at diagnosis were not responsive to bile acid treatment and had unfavorable outcomes. As more cumulative cases have been reported, it has been revealed that patients with significantly prolonged INR are predisposed to bad prognoses. Moreover, all reported cases in which the patients are deceased were homozygous and had an INR of 1.8 or above. However, although the case we encountered had an INR of 2.1, the patient had a good response to primary bile acid treatment. Due to the suspicion of an inborn error of bile acid synthesis, we soon substituted UDCA with CDCA after one week of invalid UDCA treatment. We believe that early supplementation with CDCA in our case may have prevented the deterioration of the patient’s liver function despite impaired coagulation function.
In conclusion, the case described herein was confirmed to involve a novel compound heterozygous R307C mutation and loss of heterozygosity in the AKR1D1 gene. Both early supplementation with and an adequate dose of CDCA monotherapy showed a favorable response, resulting in both improved clinical symptoms and the normalization of laboratory tests.
A 2 mo old male infant presented with hyperbilirubinemia and coagulopathy, but normal bile acid and γ-glutamyltransferase.
Infant cholestatic liver disease, diagnosed by elevated direct bilirubin and alanine aminotransferase.
Virus hepatitis, congenital bile duct dysplasia, genetic metabolic diseases, and autoimmune hepatitis.
Hyperbilirubinemia, coagulopathy, and impaired liver function.
The patient was initially given ursodeoxycholic acid (UDCA) treatment. We changed UDCA to chenodeoxycholic acid (CDCA) (80 mg/d) after one week of ineffective UCDA treatment. After two months of oral CDCA treatment, urine bile acid analyses indicated that the CDCA dose of 80 mg/d was insufficient to complete the suppression of atypical bile acids. We thus increased the dose of CDCA to 100 mg/d, which proved adequate to down-regulate hepatic bile acid synthesis based on the second urine bile acid analyses.
More than 20 cases of primary 5β-reductase deficiency have been reported, and over ten variant mutations in the aldo-ketoreductase family 1 member D1 (AKR1D1) gene are attributed to a defect in 5β-reductase.
Aldo-ketoreductase family 1 member D1 (AKR1D1) encodes Δ4-3-oxosteroid 5β-reductase; its deficiency results in a lack of primary bile acids and increased synthesis of 3-oxo-Δ4 bile and allo-bile acids.
Gene analysis is essential for the accurate diagnosis of primary 3-oxo-Δ4-steroid 5β-reductase deficiency. Early diagnosis and adequate supplementation with CDCA are vital for the amelioration of clinical symptoms.
We thank the patient’s family for providing background information and allowing us to publish this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013). The manuscript was prepared and revised according to the CARE Checklist (2013).
P- Reviewer: Deneau M, Schwarz SM S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Yin SY
| 1. | Clayton PT. Disorders of bile acid synthesis. J Inherit Metab Dis. 2011;34:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Heubi JE, Setchell KD, Bove KE. Inborn errors of bile acid metabolism. Semin Liver Dis. 2007;27:282-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Drury JE, Mindnich R, Penning TM. Characterization of disease-related 5beta-reductase (AKR1D1) mutations reveals their potential to cause bile acid deficiency. J Biol Chem. 2010;285:24529-24537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Kondo KH, Kai MH, Setoguchi Y, Eggertsen G, Sjöblom P, Setoguchi T, Okuda KI, Björkhem I. Cloning and expression of cDNA of human delta 4-3-oxosteroid 5 beta-reductase and substrate specificity of the expressed enzyme. Eur J Biochem. 1994;219:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Clayton PT, Lake BD, Hjelm M, Stephenson JB, Besley GT, Wanders RJ, Schram AW, Tager JM, Schutgens RB, Lawson AM. Bile acid analyses in “pseudo-Zellweger” syndrome; clues to the defect in peroxisomal beta-oxidation. J Inherit Metab Dis. 1988;11 Suppl 2:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Setchell KD, Suchy FJ, Welsh MB, Zimmer-Nechemias L, Heubi J, Balistreri WF. Delta 4-3-oxosteroid 5 beta-reductase deficiency described in identical twins with neonatal hepatitis. A new inborn error in bile acid synthesis. J Clin Invest. 1988;82:2148-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 173] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Lemonde HA, Custard EJ, Bouquet J, Duran M, Overmars H, Scambler PJ, Clayton PT. Mutations in SRD5B1 (AKR1D1), the gene encoding delta(4)-3-oxosteroid 5beta-reductase, in hepatitis and liver failure in infancy. Gut. 2003;52:1494-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Ueki I, Kimura A, Chen HL, Yorifuji T, Mori J, Itoh S, Maruyama K, Ishige T, Takei H, Nittono H. SRD5B1 gene analysis needed for the accurate diagnosis of primary 3-oxo-Delta4-steroid 5beta-reductase deficiency. J Gastroenterol Hepatol. 2009;24:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Cheng Y, Guo L, Deng M, Song YZ. [Clinical feature and genetic analysis of a family affected by congenital bile acid synthesis defect type 2: identification of 2 novel mutations in AKR1D1 gene]. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:734-740. [PubMed] |
| 10. | Gonzales E, Cresteil D, Baussan C, Dabadie A, Gerhardt MF, Jacquemin E. SRD5B1 (AKR1D1) gene analysis in delta(4)-3-oxosteroid 5beta-reductase deficiency: evidence for primary genetic defect. J Hepatol. 2004;40:716-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Morgan NV, Hartley JL, Setchell KD, Simpson MA, Brown R, Tee L, Kirkham S, Pasha S, Trembath RC, Maher ER. A combination of mutations in AKR1D1 and SKIV2L in a family with severe infantile liver disease. Orphanet J Rare Dis. 2013;8:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Seki Y, Mizuochi T, Kimura A, Takahashi T, Ohtake A, Hayashi S, Morimura T, Ohno Y, Hoshina T, Ihara K. Two neonatal cholestasis patients with mutations in the SRD5B1 (AKR1D1) gene: diagnosis and bile acid profiles during chenodeoxycholic acid treatment. J Inherit Metab Dis. 2013;36:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Zhao J, Fang LJ, Setchell KD, Chen R, Li LT, Wang JS. Primary Δ4-3-oxosteroid 5β-reductase deficiency: two cases in China. World J Gastroenterol. 2012;18:7113-7117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Russell DW, Setchell KD. Bile acid biosynthesis. Biochemistry. 1992;31:4737-4749. [PubMed] |
| 15. | Gonzales E, Gerhardt MF, Fabre M, Setchell KD, Davit-Spraul A, Vincent I, Heubi JE, Bernard O, Jacquemin E. Oral cholic acid for hereditary defects of primary bile acid synthesis: a safe and effective long-term therapy. Gastroenterology. 2009;137:1310-1320.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Clayton PT, Mills KA, Johnson AW, Barabino A, Marazzi MG. Delta 4-3-oxosteroid 5 beta-reductase deficiency: failure of ursodeoxycholic acid treatment and response to chenodeoxycholic acid plus cholic acid. Gut. 1996;38:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Setchell KD, Heubi JE. Defects in bile acid biosynthesis--diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2006;43 Suppl 1:S17-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Inoue T, Kimura A, Aoki K, Tohma M, Kato H. Developmental pattern of 3-oxo-delta 4 bile acids in neonatal bile acid metabolism. Arch Dis Child Fetal Neonatal Ed. 1997;77:F52-F56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kimura A, Mahara R, Inoue T, Nomura Y, Murai T, Kurosawa T, Tohma M, Noguchi K, Hoshiyama A, Fujisawa T. Profile of urinary bile acids in infants and children: developmental pattern of excretion of unsaturated ketonic bile acids and 7beta-hydroxylated bile acids. Pediatr Res. 1999;45:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |