Published online Sep 21, 2018. doi: 10.3748/wjg.v24.i35.4069
Peer-review started: May 30, 2018
First decision: July 4, 2018
Revised: August 6, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: September 21, 2018
Processing time: 113 Days and 17.7 Hours
To evaluate the use of chromoendoscopy for surveillance of ulcerative colitis in a real-life community hospital setting.
Patients with extensive ulcerative colitis, having disease duration of more than 8 years and who presented between the years of 1999 to 2013, were offered enrolment in this single cohort prospective study. All participants underwent standard bowel preparation with sodium phosphate and chromoendoscopy. Two expert endoscopists, novice to chromoendoscopy, evaluated each segment of the colon with standard-definition colonoscopes after spray application of 0.4% indigo carmine. All observed lesions were recorded and evaluated before being removed and/or biopsied. In addition, nontargeted biopsies were taken from each segment of the colon. The dysplasia detection rate and dysplasia detection yield were ascertained.
A total of 21 neoplastic lesions (2 carcinomas, 4 of high-grade dysplasia and 15 of low-grade dysplasia) and 27 nondysplastic lesions were detected in 16 of the total 67 patients (70% male; median disease duration: 17 years; median age at diagnosis: 25 years; 92% aminosalicylate-treated). The dysplasia detection rate was 10.5% (7/67 patients). The dysplasia detection yield was 20.8% (10/48) for targeted biopsies and 3.5% (11/318) for nontargeted biopsies. The sensitivity and specificity for the macroscopic evaluation of neoplasia using chromoendoscopy were 48% [95% confidence interval (CI): 26%-70%] and 96% (95%CI: 93%-98%), respectively. The positive predictive and negative predictive values were 42% (95%CI: 27%-59%) and 97% (95%CI: 95%-98%), respectively. A total of 19/21 dysplastic lesions were detected in mucosa with histologic inflammation.
Chromoendoscopy seems to be of value for dysplasia surveillance of ulcerative colitis in a community hospital setting. The yield of non-targeted biopsies is negligible.
Core tip: Patients with longstanding and extensive ulcerative colitis are at increased risk of developing colonic neoplasia and are advised to undergo regular colonoscopic surveillance. Current clinical guidelines favour chromoendoscopy with targeted biopsies, as it detects dysplasia more accurately and thus requires fewer biopsies than white-light endoscopy. However, these recommendations are based on studies performed in advanced endoscopic units and chromoendoscopy is not routinely applied in everyday clinical practice. This prospective cohort study suggests that, although novice to chromoendoscopy, endoscopists can accurately evaluate the absence of neoplasia. The yield of nontargeted biopsies was also found to be negligible.
- Citation: Klepp P, Tollisen A, Røseth A, Cvancarova Småstuen M, Andersen SN, Vatn M, Moum BA, Brackmann S. Real-life chromoendoscopy for dysplasia surveillance in ulcerative colitis. World J Gastroenterol 2018; 24(35): 4069-4076
- URL: https://www.wjgnet.com/1007-9327/full/v24/i35/4069.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i35.4069
Patients with extensive and long-standing ulcerative colitis (UC) carry an increased risk of developing colonic neoplastic lesions[1]. Carcinoma in UC is thought to develop through a stepwise progression from inflammation to low-grade dysplasia and finally to carcinoma[2]. Patients are therefore advised to undergo periodic colonoscopic surveillance, so as to detect neoplasia at an early stage[3]. Visualization of dysplastic lesions in UC represents a challenge as they may be flat or obscured by inflammatory changes and/or pseudopolyps[4]. Dysplasia surveillance using white-light endoscopy relies on random 4-quadrant biopsies taken every 10 cm, being a laborious and costly method[5]. Under-sampling is common with that technique and even if the recommended 30-40 biopsies are harvested, only a fraction of the entire mucosal surface of the colon is examined[6].
Chromoendoscopy (CE), on the other hand, uses a topical dye, which highlights mucosal abnormalities and allows for more precise biopsies[7]. Targeted biopsies are considered superior to random biopsies of apparently unaffected mucosa, as the latter are of little additional value since they have poor diagnostic yield[8]. Thus, recommendations for surveillance using CE are based on the assumption that CE requires fewer biopsies and is more cost effective than standard white-light endoscopy[7,9-11]. European clinical guidelines recommend CE with targeted biopsies as the favoured technique for dysplasia surveillance[3]. It is important to note that these guidelines are based on studies that were performed in endoscopic units with highly advanced expertise. However, in many countries, such as Norway, CE is not routinely applied for surveillance of UC patients. A retrospective multicentre study conducted over a 14-year period of CE implementation also did not show significant increase in the detection of dysplastic lesions[12].
The aim of this study was to assess the macroscopic and histologic evaluation of CE when implemented in real-life surveillance of patients with long-standing UC in a community hospital in Norway.
The study protocol was designed according to the combined knowledge and expertise of Assistant Professor Stephan Brackmann (Akershus University Hospital), Professor Bjørn A Moum (Oslo University Hospital) and Professor Morten Vatn (University of Oslo). The study protocol was approved by the Regional Committee for Medical and Health Research Ethics (Project NO. 2010/1093). Written informed consent was collected from all subjects prior to study inclusion.
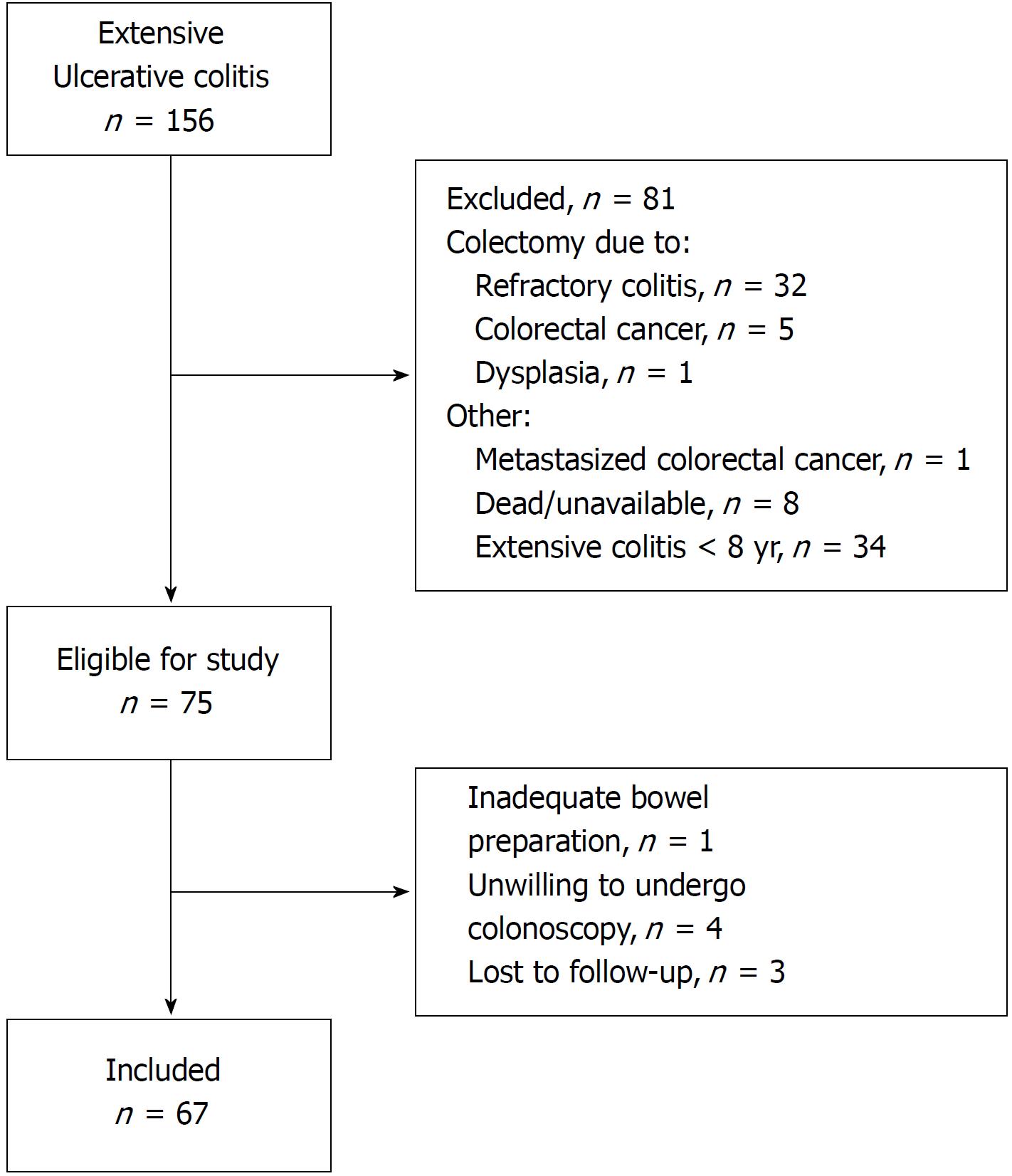
Patients registered in the database of Lovisenberg Hospital from 1999-2013 were invited to participate in the present study if they had (1) extensive UC, documented by endoscopy at any time during the course of disease, and (2) disease duration of 8 years or more. Exclusion criteria included colectomy at any time during follow-up and poor bowel preparation (Figure 1). Demographic and clinical data were extracted from digital medical journals and by interview of the patients (Table 1).
| Feature | Data |
| Study patients | 67 |
| Age (yr) | 40 (27-73) |
| Male sex | 46 (70) |
| Disease duration (yr) | 17 (8-51) |
| Age at diagnosis (yr) | 25 (12-59) |
| Colitis activity index score2 | 0 (0-8) |
| Primary sclerosing cholangitis | 3 (5), missing n = 2 |
| Colorectal cancer in first degree relative | 3 (5), missing n = 5 |
| Previous dysplasia in colon | 3 (5), missing n = 3 |
| Treatment | |
| Aminosalicylate1 | 58 (92), missing n = 3 |
| Steroids1 | 12 (18), missing n = 4 |
| Azathioprine1 | 4 (6) |
| Antitumour necrosis factor1 | 4 (6), missing n = 1 |
Patients underwent standard bowel preparation with sodium phosphate. CE was performed with standard definition endoscope (CF190 colonoscope; Olympus, Tokyo, Japan) and only carried out when the quality of bowel preparation was adequate (n = 67/68). The colonoscope was advanced to the cecum and during the extubation each segment (cecum, ascending colon, transverse colon, descending sigmoid and rectum) was scrutinised for lesions after the spray catheter application of 0.3% indigo-carmine. Extensive colitis was defined as endoscopic inflammation proximal to the splenic flexure. Endoscopic degree of inflammation was classified according to the Mayo endoscopic score for UC[13].
The location and size of all lesions identified after spray catheter application of 0.3% indigo-carmine dye were reported. Also the appearance of the lesions was classified according to terminology adapted from the Scenic Consensus[14] as either nonpolypoid flat or elevated or polypoid pedunculated or nonpedunculated before the lesions were biopsied or removed.
In addition, after spray catheter application of 0.3% indigo-carmine dye, a minimum of one nontargeted biopsy was taken from each of the six segments (cecum, ascending, transverse and descending colon, sigmoid and rectum) to determine the extent of disease and grade of inflammation.
Independent and blind analyses of the formalin-fixed paraffin-embedded biopsies were performed by two expert gastropathologists. The histologic degree of inflammation was graded based on the histological activity index[15]. Mucosal biopsies were classified as either negative for neoplasia, indefinite for dysplasia, or positive for low-grade dysplasia (LGD), high-grade dysplasia (HGD) or adenocarcinoma[16]. Neoplasia was considered proximal or distal according to its anatomic location to the splenic flexure. The dysplasia detection yield was defined as the proportion of bioptic sites/lesions containing dysplasia or invasive colorectal cancer (CRC) in relation to the total number of bioptic sites/lesions. The dysplasia detection rate was defined as the proportion of patients who had at least one dysplastic lesion or invasive CRC in relation to the total number of screened patients.
The statistical methods of this study were reviewed by Milada Cvancarova Småstuen (Institute of Clinical Medicine, University of Oslo, PO Box 1122 Blinderen, 0317 Oslo, Norway). All statistical analyses were carried out with the SPSS software, version 24 (IBM Corp, Armonk, NY, United States). Patient demographic characteristics were summarized as median (interquartile range) for continuous variables and as percentage of subgroup totals for categorical variables. Sensitivity and specificity were estimated with corresponding 95% confidence intervals (CIs) that were calculated using the exact binomial distribution.
CE was performed in 67 patients with extensive UC. A total of 21 neoplastic lesions were detected in 7 of the 67 patients, including 10 (comprised of 2 CRC, 4 HGD and 4 LGD) identified by targeted biopsies and 11 (all LGD) by nontargeted biopsies.
Neoplasia was detected in 7 of the total 67 patients, giving a dysplasia detection rate of 10.5%. In 4, neoplasia was detected from both targeted and nontargeted biopsies. In 2, dysplasia was detected by nontargeted biopsies alone. In 1, adenocarcinoma was diagnosed after partial colonic resection.
A total of 48 lesions were visualised in 16 of the total 67 patients. The median number of lesions per patient was 1 (range: 0-6). The distribution and findings from endoscopic and histologic evaluations of the lesions are described in detail in Figure 2 and Tables 2 and 3. Ten of the visualised lesions harboured neoplasia, resulting in a dysplasia detection yield of 20.8% for targeted biopsies. Among the 318 nontargeted biopsies, 11 harboured LGD, resulting in a dysplasia detection yield of 3.5% for nontargeted biopsies.
Correct classification by the endoscopists was achieved for 307 of the 345 nondysplastic sites/lesions and 10 of the 21 dysplastic sites/lesions. On the other hand, 11 of the 21 dysplastic lesions were assessed as nondysplasia, whereas 38 of the 345 nondysplastic sites /lesions were assessed as dysplasia. As a result, the sensitivity and specificity for the macroscopic evaluation of neoplasia using CE were 48% (95%CI: 26%-70%) and 96% (95%CI: 93%-98%), respectively. The positive predictive value was 42% (95%CI: 27%-59%) and the negative predictive value (NPV) was 97% (95%CI: 95%-98%).
In 2 of the 7 patients with LGD, the dysplasia was detected solely by nontargeted biopsies taken during a CE described as macroscopically normal. Follow-up with colonoscopy neither confirmed nor revealed any further dysplasia for either patient. In another 2 of the 7 patients, the LGD was detected solely by targeted biopsies. In yet another 2 of the 7 patients, nontargeted biopsies confirmed a field effect by detecting LGD when dysplastic lesions were identified elsewhere in the colorectum by targeted biopsies; during intensified follow-up colonoscopy, no further dysplasia was detected after 2.5 years follow-up (range: 2-5 years) in these patients.
In 1 of the 7 patients with neoplasia, nontargeted biopsies showed multifocal fields of LGD synchronous with targeted biopsies that showed multifocal lesions with HGD and adenocarcinoma. The patient had primary sclerosing cholangitis and proctocolectomy was performed. Finally, adenocarcinoma was detected after partial resection of the colon in 1 of the 7 patients for who endoscopy had raised suspicion of malignancy, whereas targeted biopsies of this area were normal on two consecutive colonoscopies. After surgery, no further neoplasia was detected during 3 years of intensified follow-up colonoscopies.
No signs of histologic inflammation were recorded in 93 of the 366 bioptic sites. A total of 237 had Mayo grade 0-1 and 39 had Mayo grade 2-3. The presence of neoplasia in relation to histologic inflammatory changes is described in detail in Table 4.
The median time from the prior “prestudy” surveillance colonoscopy (n = 61/67) until the next scheduled colonoscopy was 24 mo (range: 0-96 mo). The median time from the prior “prestudy” surveillance colonoscopy (n = 66/67) until the study CE was 26 mo (range: 1-105 mo).
This prospective cohort study performed in a community hospital suggests that, although novice to CE, endoscopists were able to accurately evaluate the absence of neoplasia during real-life surveillance of patients with UC. Neoplasia was detected by targeted biopsies in 5 of the 67 total patients, of whom 2 had a field effect confirmed by nontargeted biopsies. Two additional patients were diagnosed with LGD in the colon by nontargeted biopsies alone. The neoplasia detection rate for the 7 of the 67 total patients in the present study is similar to that found in studies performed in tertiary referral centres (11.2%). It is important to note, however, that the present study was conducted in a community hospital in which patients may present with a less aggressive UC than seen in advanced units.
The neoplasia detection yield of the present study was 20.8%, which is lower than the average rate of 14% found by Mooiweer et al[12] in several prior randomized trials. The endoscopists in our study were able to accurately rule-out neoplasia (NPV = 97%); thus, when the endoscopists evaluated the lesion as benign, the probability of dysplasia was minimal (3%). These results are in line with a recent prospective multicentre cohort study in which both CE novice endoscopists and CE expert endoscopists evaluated lesions, and had a high NPV[17]. That same study found a sensitivity of 70% for CE, which is lower than the pooled sensitivity of 91% reported from a recent meta-analysis[17,18]. In the present study, the sensitivity for the detection of LGD by CE was modest, which could be related to the endoscopists’ lack of prior CE experience.
Alternatively, these results support the presence of “invisible” dysplasia. In our cohort, “invisible” dysplasia was rare in the absence of dysplastic lesions elsewhere in the colon, similar to findings reported by Matsumoto et al[9,19] and underlining the concept of field cancerization in UC[9,19]. The results must, however, be evaluated with caution due to the size of the study sample and the low observed rate of cases.
The clinical importance of dysplasia detected through random biopsies is debatable. In the present study, the dysplasia detection yield was 3.5% for nontargeted biopsies compared to a 20.8% for targeted biopsies. The nontargeted biopsies were primarily taken not to detect neoplasia but to evaluate the grade of mucosal inflammation. In line with previous studies, the low dysplasia yield of nontargeted biopsies leads to questions about their clinical value[7,8,17,20,21]. Also, the follow-up of the patients in which LGD was detected by nontargeted biopsy alone did not reveal any further dysplasia. However, a recent study has suggested that despite a low bioptic neoplasia yield, nontargeted biopsies are advisable in patients with inflammatory bowel disease and related high risk of CRC[21].
Clinical guidelines recommend the first surveillance colonoscopy to be performed between 8-10 years after the diagnosis of UC, with ensuing colonoscopies based on individual risk. In our cohort, the median time until the next scheduled colonoscopy was 24 mo, which is in accordance with guidelines[3].
The visualisation of small lesions harbouring dysplasia may have been hampered by inflammation surrounding multifocal lesions. However, the minimal level of inflammatory changes in those patients in who dysplasia was diagnosed by nontargeted biopsies only did not likely impede the detection.
In the present study, current or previous inflammatory changes were found to be present around all dysplastic lesions, including for nontargeted biopsies harbouring lesions with LGD. These results support the findings of Watanabe et al[22], who suggested that random biopsies may be omitted in the absence of previous or current inflammation.
We recognize that the present study has several limitations. The adenoma detection rate of the endoscopists is not currently implemented in Norway and therefore not available for the endoscopists in the present study. Both endoscopists had however substantial experience in non-CE endoscopy. The present study reflects the everyday clinical world in this location. Each CE was performed with standard and not high-definition endoscopes, which may have affected the detection of lesions. The prospective cohort design of the study minimizes patient selection bias; however, the sample size is moderate, although considerable effort was spent on the inclusion of patients. Patients may have been reluctant to undergo colonoscopy. Low compliance rates for surveillance endoscopy have previously been described, although the reasons remain unclear[23]. Additionally, although all the patients in the sample carry an increased risk of colonic neoplasia, the low observed rate of neoplasia resulted in wide CIs around sensitivity estimates for the detection of dysplastic lesions.
Despite the limitations outlined above, the results of the present study are thought to reflect the real-life scenario of dysplasia surveillance in UC in a community-based hospital in Norway, which could represent the typical setting in similar hospitals in Europe where CE is not routinely applied.
In conclusion, the present study suggests that, although lacking in previous CE expertise, the endoscopists were able to accurately evaluate the absence of neoplasia. The yield of nontargeted biopsies with LGD was negligible, and LGD appears to be present in mucosa with signs of histologic inflammation. Finally, dysplasia in endoscopically unsuspicious appearing mucosa seems to occur mostly when visible neoplasia is diagnosed elsewhere in the colon. Although further larger studies are needed, CE seems to be of value for surveillance of neoplasia in UC in a community hospital setting.
Patients with longstanding and extensive ulcerative colitis carry an increased risk of developing colonic neoplasia and are advised to undergo regular colonoscopic surveillance.
The current clinical guidelines favour chromoendoscopy with targeted biopsies for dysplasia surveillance of ulcerative colitis. These recommendations, however, are based on studies performed in advanced endoscopic units and chromoendoscopy is not routinely applied in everyday clinical practice.
Our aim was to evaluate chromoendoscopy for real-life dysplasia surveillance for cases of long-standing ulcerative colitis in a community hospital.
Patients with extensive ulcerative colitis, with disease duration of more than 8 years, were prospectively included in this single cohort study. The chromoendoscopies were performed by two expert endoscopists novice to the method. Lesions were evaluated macroscopically and removed and/or biopsied. Nontargeted biopsies were also taken from each segment of the colon.
A total of 21 neoplastic lesions (consisting of 2 carcinomas, 4 high-grade dysplasias and 15 low-grade dysplasias) and 27 nondysplastic lesions were detected in 16 of the 67 total patients included in the study. The dysplasia detection rate was 10.5% (for 7 of the 67 patients). The dysplasia detection yield was 20.8% (10/48) for targeted biopsies and 3.5% (11/318) for nontargeted biopsies. The endoscopists accurately evaluated the absence of neoplasia (specificity of 96%, with 95% confidence interval of 93-98; and a negative predictive value of 97%, with 95% confidence interval of 95%-98%).
Although novice to chromoendoscopy, the endoscopists in this Norwegian community hospital accurately evaluated the absence of neoplasia. In addition, the yield of nontargeted biopsies was negligible.
Chromoendoscopy appears to be of value for dysplasia surveillance for cases of long-standing ulcerative colitis who are treated in a community hospital setting in which endoscopists are novice to the technique.
The authors thank the staff at the Unger-Vetlesen Research Institute, Lovisenberg Diaconal Hospital and Lars Gustav Lyckander at the Department of Pathology, Akershus University Hospital.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Norway
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hiraoka S, Luo H, Tandon RK, Tang ZP S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Rubin DT, Huo D, Kinnucan JA, Sedrak MS, McCullom NE, Bunnag AP, Raun-Royer EP, Cohen RD, Hanauer SB, Hart J. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol. 2013;11:1601-1608.e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 855] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 3. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1293] [Article Influence: 161.6] [Reference Citation Analysis (0)] |
| 4. | Velayos FS, Loftus EV Jr, Jess T, Harmsen WS, Bida J, Zinsmeister AR, Tremaine WJ, Sandborn WJ. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Annese V, Beaugerie L, Egan L, Biancone L, Bolling C, Brandts C, Dierickx D, Dummer R, Fiorino G, Gornet JM. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2015;9:945-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 323] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 6. | Eaden JA, Ward BA, Mayberry JF. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointest Endosc. 2000;51:123-128. [PubMed] |
| 7. | Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | van den Broek FJ, Stokkers PC, Reitsma JB, Boltjes RP, Ponsioen CY, Fockens P, Dekker E. Random biopsies taken during colonoscopic surveillance of patients with longstanding ulcerative colitis: low yield and absence of clinical consequences. Am J Gastroenterol. 2014;109:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Matsumoto T, Nakamura S, Jo Y, Yao T, Iida M. Chromoscopy might improve diagnostic accuracy in cancer surveillance for ulcerative colitis. Am J Gastroenterol. 2003;98:1827-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Marion JF, Waye JD, Israel Y, Present DH, Suprun M, Bodian C, Harpaz N, Chapman M, Itzkowitz S, Abreu MT. Chromoendoscopy Is More Effective Than Standard Colonoscopy in Detecting Dysplasia During Long-term Surveillance of Patients With Colitis. Clin Gastroenterol Hepatol. 2016;14:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Konijeti GG, Shrime MG, Ananthakrishnan AN, Chan AT. Cost-effectiveness analysis of chromoendoscopy for colorectal cancer surveillance in patients with ulcerative colitis. Gastrointest Endosc. 2014;79:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Mooiweer E, van der Meulen-de Jong AE, Ponsioen CY, Fidder HH, Siersema PD, Dekker E, Oldenburg B. Chromoendoscopy for Surveillance in Inflammatory Bowel Disease Does Not Increase Neoplasia Detection Compared With Conventional Colonoscopy With Random Biopsies: Results From a Large Retrospective Study. Am J Gastroenterol. 2015;110:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2250] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 14. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489-501.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 15. | Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099-1105; quiz 1340-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 570] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 16. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1348] [Cited by in RCA: 1213] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 17. | Carballal S, Maisterra S, López-Serrano A, Gimeno-García AZ, Vera MI, Marín-Garbriel JC, Díaz-Tasende J, Márquez L, Álvarez MA, Hernández L. Real-life chromoendoscopy for neoplasia detection and characterisation in long-standing IBD. Gut. 2018;67:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Wu L, Li P, Wu J, Cao Y, Gao F. The diagnostic accuracy of chromoendoscopy for dysplasia in ulcerative colitis: meta-analysis of six randomized controlled trials. Colorectal Dis. 2012;14:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Leedham SJ, Graham TA, Oukrif D, McDonald SA, Rodriguez-Justo M, Harrison RF, Shepherd NA, Novelli MR, Jankowski JA, Wright NA. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;136:542-550.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Marion JF, Waye JD, Present DH, Israel Y, Bodian C, Harpaz N, Chapman M, Itzkowitz S, Steinlauf AF, Abreu MT. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Moussata D, Allez M, Cazals-Hatem D, Treton X, Laharie D, Reimund JM, Bertheau P, Bourreille A, Lavergne-Slove A, Brixi H. Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy? Gut. 2018;67:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Watanabe T, Ajioka Y, Mitsuyama K, Watanabe K, Hanai H, Nakase H, Kunisaki R, Matsuda K, Iwakiri R, Hida N. Comparison of Targeted vs Random Biopsies for Surveillance of Ulcerative Colitis-Associated Colorectal Cancer. Gastroenterology. 2016;151:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | Vienne A, Simon T, Cosnes J, Baudry C, Bouhnik Y, Soulé JC, Chaussade S, Marteau P, Jian R, Delchier JC. Low prevalence of colonoscopic surveillance of inflammatory bowel disease patients with longstanding extensive colitis: a clinical practice survey nested in the CESAME cohort. Aliment Pharmacol Ther. 2011;34:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |