Published online Sep 21, 2018. doi: 10.3748/wjg.v24.i35.4054
Peer-review started: May 4, 2018
First decision: June 6, 2018
Revised: June 17, 2018
Accepted: June 25, 2018
Article in press: June 25, 2018
Published online: September 21, 2018
Processing time: 139 Days and 17.4 Hours
To investigate the incidence and risk factors of portosplenomesenteric vein thrombosis (PSMVT) in the early stage of severe acute pancreatitis (SAP).
Patients with SAP in a tertiary care setting from January 2014 to December 2016 were retrospectively reviewed. All contrast-enhanced computed tomography (CT) studies were reassessed and reviewed. Clinical outcome measures were compared between SAP patients with and without PSMVT in the early stage of the disease. Univariate and multivariate logistic regression analyses were sequentially performed to assess potential risk factors for the development of PSMVT in SAP patients. A receiver operating characteristic (ROC) curve was generated for the qualifying independent risk factors.
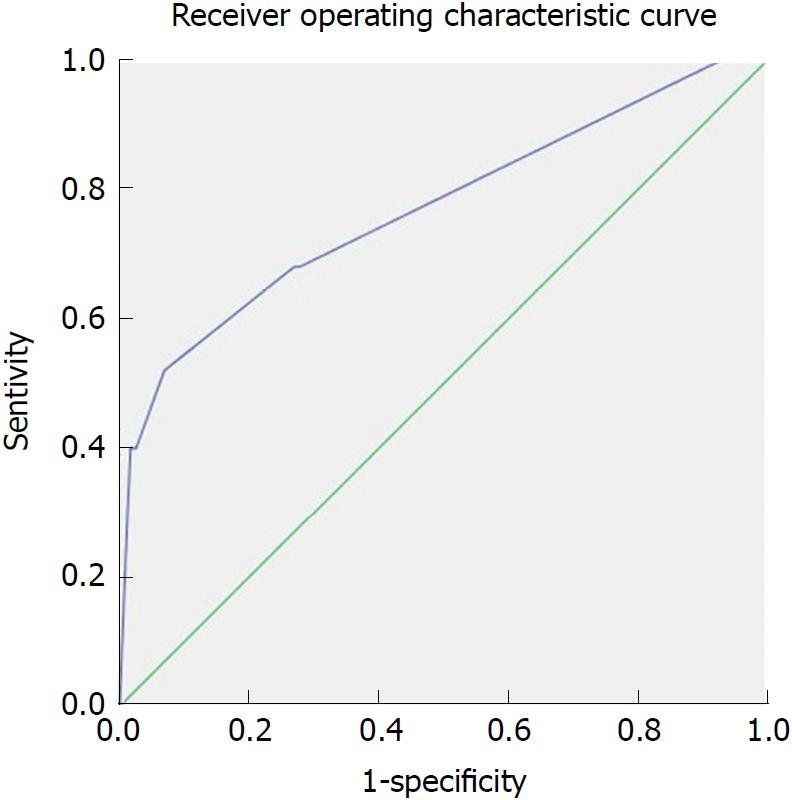
Twenty-five of the one hundred and forty (17.86%) SAP patients developed PSMVT 6.19 ± 2.43 d after acute pancreatitis (AP) onset. PSMVT was confirmed by contrast-enhanced CT. Multivariate stepwise logistic regression analyses showed that Balthazar’s CT severity index (CTSI) scores [odds ratio (OR): 2.742; 95% confidence interval (CI): 1.664-4.519; P = 0.000], hypoalbuminemia (serum albumin level < 25 g/L) (OR: 32.573; 95%CI: 2.711-391.353; P = 0.006) and gastrointestinal wall thickening (OR: 4.367, 95%CI: 1.218-15.658; P = 0.024) were independent risk factors for PSMVT developed in patients with SAP. The area under the ROC curve for Balthazar’s CTSI scores was 0.777 (P = 0.000), the sensitivity was 52%, and the specificity was 93% at a cut-off value of 5.5.
High Balthazar’s CTSI scores, hypoalbuminemia and gastrointestinal wall thickening are independent risk factors for PSMVT developed in the early stage of SAP.
Core tip: Studies on portosplenomesenteric vein thrombosis (PSMVT) occurring in the early stage of acute pancreatitis (AP) were rare. We found that 17.86% of severe AP patients developed PSMVT 6.19 d after AP onset. High Balthazar’s computed tomography severity index (CTSI) scores, hypoalbuminemia and gastrointestinal wall thickening are independent risk factors for the development of PSMVT, and Balthazar’s CTSI scores can predict the occurrence of PSMVT with a high degree of specificity which indicated that a low Balthazar’s CTSI score could be a good indicator that PSMVT would not occur.
- Citation: Ding L, Deng F, Yu C, He WH, Xia L, Zhou M, Huang X, Lei YP, Zhou XJ, Zhu Y, Lu NH. Portosplenomesenteric vein thrombosis in patients with early-stage severe acute pancreatitis. World J Gastroenterol 2018; 24(35): 4054-4060
- URL: https://www.wjgnet.com/1007-9327/full/v24/i35/4054.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i35.4054
Portosplenomesenteric vein thrombosis (PSMVT) involves the portal vein (PV), splenic vein and superior mesenteric vein (SMV), either separately or in combination. In most cases, PSMVT does not cause any additional symptoms, but may sometimes generate localized portal hypertension, leading to gastrointestinal and intra-abdominal hemorrhage, ascites, splenomegaly, and splenic infarction, among other problems[1,2]. The previous studies reported that PSMVT mostly occurs late during acute pancreatitis (AP)[3-5], and is usually detected incidentally on radiological imaging performed to evaluate the severity of AP[6].
Until now, the detailed natural history of PSMVT has remained unknown, and the pathogenesis underlying PSMVT in patients with AP has remained unclear and may involve many clinical factors. There is little data regarding the risk factors for this complication in the early stage of AP. The results about the risk factors of PSMVT in AP patients vary among studies[3,5,7-10]. Therefore, in this study, we analyzed the data to explore the incidence of PSMVT in the early stage of severe AP (SAP), the risk factors for the development of PSMVT, and the clinical outcomes of PSMVT.
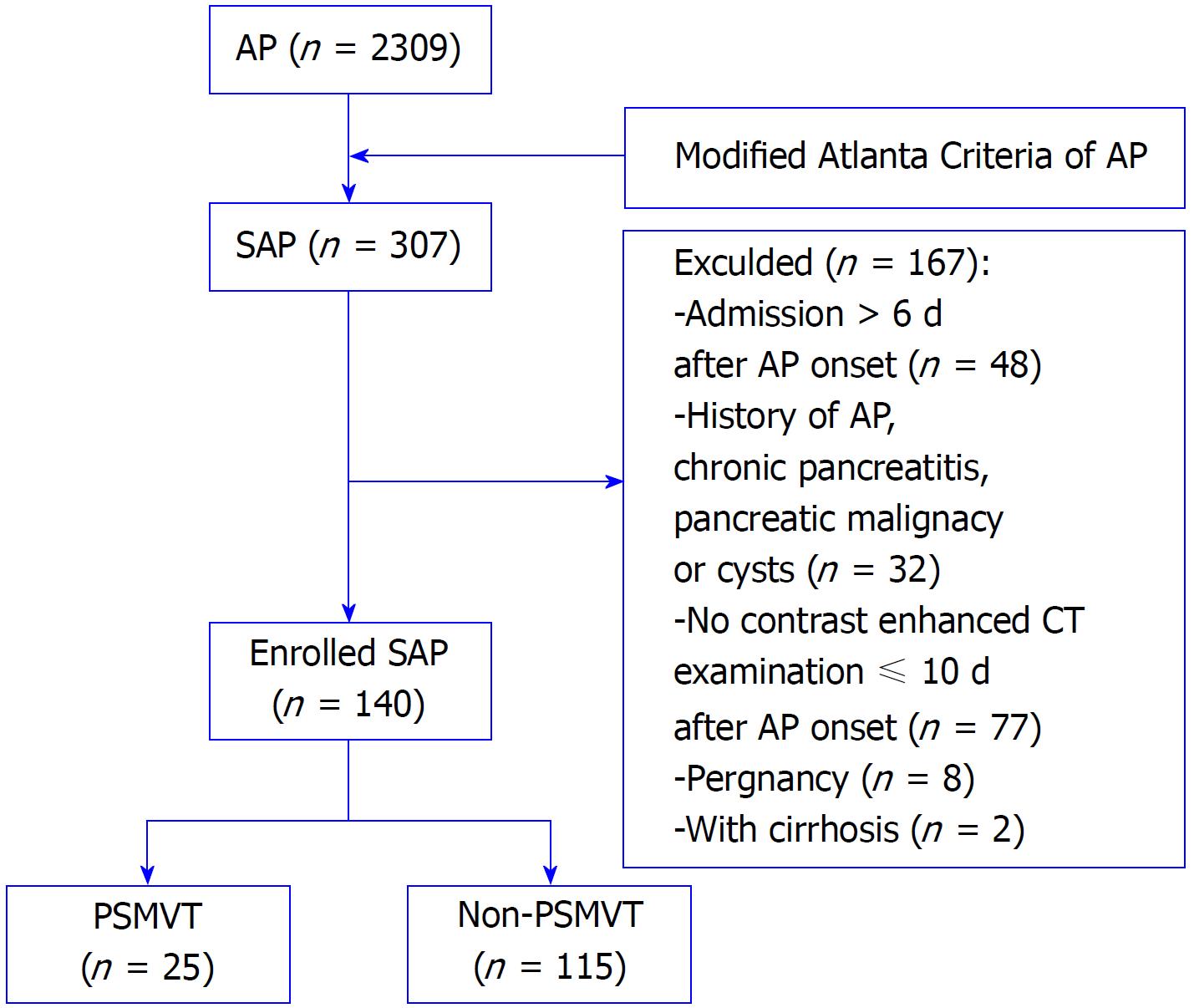
Consecutive adult patients (age ≥ 18 years old) with SAP who were admitted to the First Affiliated Hospital of Nanchang University between January 2014 and December 2016 were enrolled in this study. Exclusion criteria include the following: (1) Admission > 6 d after AP onset; (2) history of AP, chronic pancreatitis, pancreatic malignancy or cysts; (3) no contrast-enhanced CT examination ≤ 10 d after AP onset; (4) pregnancy; and (5) cirrhosis or coagulopathy disorder diseases or other systemic tumors.
The following data were collected: (1) General characteristics, including age, gender, etiology of AP, modified Marshall score, systemic inflammatory response syndrome score, acute physiology and chronic health evaluation II score, and Balthazar’s computer tomography severity index (CTSI)[11] at admission; (2) clinical outcomes, including microbiological outcomes, ascites, and intra-abdominal pressure measured with a catheter inserted into the bladder according to the guidelines[12], complications including gastrointestinal hemorrhage, intra-abdominal hemorrhage, multiple organ dysfunction syndrome, and infected pancreatic necrosis, and clinical treatments including minimally invasive and surgical interventions and hospital and intensive care unit lengths of stay and mortality; and (3) laboratory markers, including hypoalbuminemia (serum albumin level < 25 g/L), hematocrit, cholesterol, triglycerides, inflammation markers including leucocytes, C-reactive protein and procalcitonin, coagulation tests including platelets, prothrombin time, activated partial thromboplastin time, fibrinogen and D-dimer during the 24 h following admission. Anticoagulation therapy was not included in the manage protocol of PSMVT at early stage (within 10 d after AP onset).
All contrast-enhanced CT studies were reassessed and reviewed by a radiologist who specialized in abdominal imaging and was blinded to the clinical data. Contrast-enhanced CT was generally performed approximately 3-10 d after AP onset, with an average of 7 d. Thus, PSMVT, which occurred within 10 d after AP onset, was detected. All contrast-enhanced CT examinations were performed using a 64-channel multidetector CT scanner (Somatom Definition AS+, Siemens Medical Systems, Erlangen, Germany). After noncontrast scans were obtained, 1.3 mL/kg iopromide (Ultravist 370; Bayer Schering Pharma, Berlin, Germany) was intravenously injected at a rate of 3 mL/s with a high-pressure injector. Then, contrasted arterial and portal phase scans were obtained when the attenuation of the aorta at the thoracolumbar junction had reached 180 HU with a fixed 60 s delay. The scanned area extended from the diaphragmatic domes to the pubic symphysis.
The diagnosis of PSMVT was based on the results of the contrasted-enhanced CT. Thrombosis was defined as a filling defect within the lumen of the vessel seen on contrast-enhanced CT images. SAP was defined by persistent organ failure, that was, organ failure for longer than 48 h13]. Extrapancreatic necrosis alone was defined as extrapancreatic morphological changes exceeding fat stranding with complete enhancement of the pancreatic parenchyma without signs of focal or diffuse nonenhancement that could be determined on contrast-enhanced CT images[13]. Pancreatic parenchymal necrosis was defined as focal or diffuse nonenhancement of the pancreatic gland. When pancreatic necrosis was present, its location (head, neck, body, tail) and amount (< 30%, 30%-50%, > 50%) were noted. Acute peripancreatic fluid collections (APFCs) were defined as peripancreatic fluid collections with homogeneous liquefied components and without well-defined walls. The anatomical locations of APFCs included the anterior and posterior renal spaces, perirenal space, great and lesser omentum, paracolic sulci, mesenteric root, and transverse mesocolon. APFCs were recorded when the diameter was > 1 cm. Gastrointestinal wall thickening was defined as the thickening and edema of the gastrointestinal wall, with wall thickness greater than 4 mm, and as robust enhancement of the mucosa with reduced enhancement of the submucosa[13,14].
Continuous variables are described as the mean ± SD, and categorical variables are described as the absolute numbers and percentages. Continuous variables were compared using t-tests, and categorical data were analyzed with the chi-squared test. Variables found to be statistically significant in the univariate logistic regression analysis were introduced into a multivariate logistic analytic model (stepwise regression) to identify independent risk factors with odds ratios (ORs) and 95% confidence intervals (CIs). Furthermore, receiver operating characteristic (ROC) curves were generated for each of the qualified independent risk factors. A P-value < 0.05 was considered statistically significant. Data were analyzed using SPSS software (v17.0; SPSS Inc., Chicago, IL, United States).
As shown in the flow chart in Figure 1, 140 eligible patients were enrolled in this study. Twenty-five patients developed PSMVT (17.86%), and all the PSMVT cases were confirmed 6.19 ± 2.43 d after AP onset by contrast-enhanced CT. Supplementary Table 1 shows the demographic characteristics of all patients. The average age of all patients was 54.59 ± 15.90 years, and the study included 67 males (47.85%) and 73 females (52.14%). The PSMVT and non-PSMVT groups were matched by age, sex, AP etiology, Marshall score, systemic inflammatory response syndrome score, and acute physiology and chronic health evaluation II score. Balthazar’s CTSI score was higher in the PSMVT group than in the non-PSMVT group (7.20 ± 2.65 vs 4.63 ± 1.45, P = 0.000).
| Variables | OR | 95%CI | P value |
| Age | 0.974 | 0.948-1.002 | 0.065 |
| Sex | 0.828 | 0.347-1.976 | 0.670 |
| Etiology | 0.775 | 0.405-1.483 | 0.441 |
| APACHEII score | 1.03 | 0.924-1.148 | 0.590 |
| Modified Marshall score | 1.06 | 0.816-1.377 | 0.660 |
| SIRS score | 1.369 | 0.873-2.147 | 0.171 |
| Balthazar’s CTSI score | 1.78 | 1.419-2.232 | 0.000 |
| 1Hypoalbuminemia | 21.143 | 2.25-198.68 | 0.008 |
| Hematocrit | 1.027 | 0.974-1.083 | 0.331 |
| Leucocyte | 1.021 | 0.955-1.092 | 0.539 |
| C-reactive protein | 1.001 | 0.997-1.005 | 0.596 |
| Procalcitonin | 0.988 | 0.955-1.022 | 0.485 |
| Platelet | 1 | 0.995-1.006 | 0.928 |
| Prothrombin time | 0.973 | 0.875-1.081 | 0.606 |
| APTT | 1.01 | 0.981-1.040 | 0.504 |
| Fibrinogen | 0.984 | 0.865-1.120 | 0.806 |
| D-dimer | 1.008 | 0.954-1.064 | 0.782 |
| Cholesterol | 1.034 | 0.893-1.198 | 0.654 |
| Triglyceride | 1.006 | 0.952-1.063 | 0.831 |
| 2Average of IAP | 1.161 | 0.947-1.423 | 0.151 |
| 2Highest value of IAP | 1.174 | 0.985-1.400 | 0.074 |
| 3Culture positive of drainage fluid | 3.97 | 1.566-10.067 | 0.004 |
| Culture positive of blood | 1.889 | 0.766-4.655 | 0.167 |
| APFC on Mesenteric root | 2.765 | 1.126-6.791 | 0.026 |
| Extrapancreatic necrosis alone | 0.314 | 0.125-0.787 | 0.013 |
| Pancreatic parenchymal necrosis | 0 | - | 0.999 |
| Extrapancreatic and parenchymal necrosis | 6.021 | 2.357-15.379 | 0.000 |
| Location of necrosis | |||
| Head | 4.58 | 1.514-13.855 | 0.007 |
| Neck | 4.413 | 1.624-11.997 | 0.004 |
| Body | 6.431 | 2.536-16.306 | 0.000 |
| Tail | 8.5 | 3.209-22.514 | 0.000 |
| Amount of necrosis | |||
| < 30% | 0.722 | 0.226-2.304 | 0.582 |
| 30%-50% | 3 | 0.668-13.482 | 0.152 |
| > 50% | 24.889 | 6.148-100.750 | 0.000 |
| Gastrointestinal wall thickening | 4.25 | 1.725-10.474 | 0.002 |
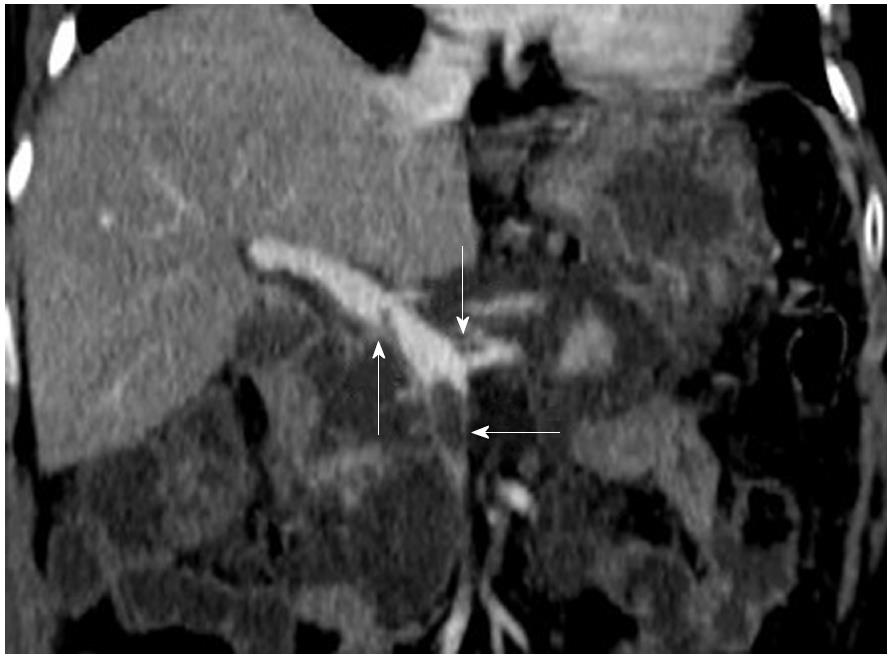
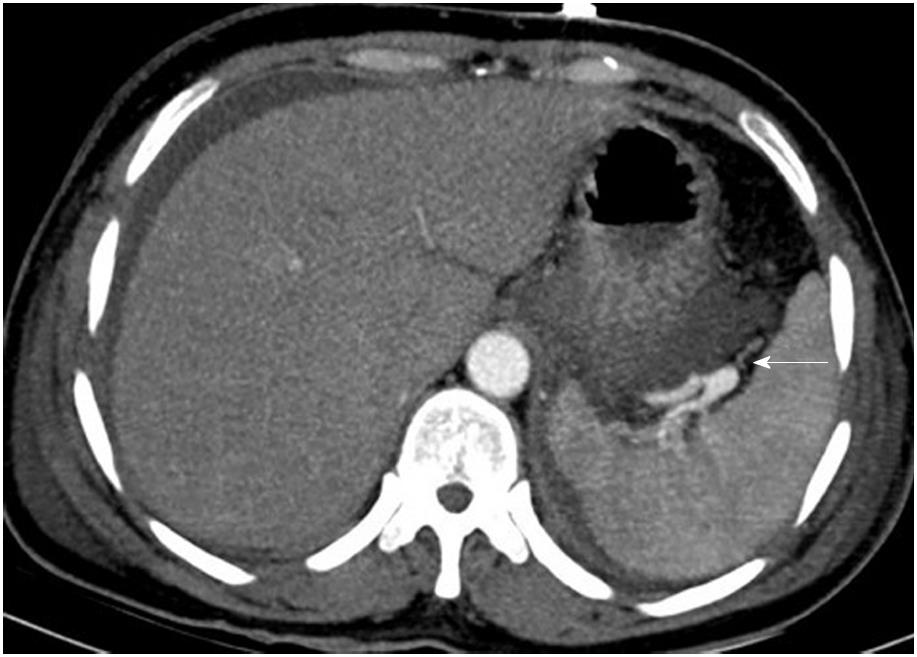
The most commonly involved vessel was the splenic vein, which was detected in ten patients, followed by the SMV in five patients, the PV in four patients, the splenic vein and PV in three patients, the splenic vein and SMV in one patient, and the splenic vein, SMV and PV in two patients (Figure 2). In addition, we further detected other vascular complications and local complications, which are shown in Supplementary Table 2. There were pseudoaneurysms (Figure 3) in two patients, and CT revealed a hematoma in one of them, who was subsequently discharged without any treatment.
| Variables | OR | 95%CI | P value |
| Balthazar’s CTSI score | 2.742 | 1.664-4.519 | 0.000 |
| hypoalbuminemia | 32.573 | 2.711-391.353 | 0.006 |
| Gastrointestinal wall thickening | 4.367 | 1.218-15.658 | 0.024 |
Supplementary Table 3 shows the relationship of a variety of clinical variables with the clinical outcomes. Twenty-one of the one hundred and forty patients (15%) died during hospitalization. Three patients experienced gastrointestinal hemorrhage. In one patient, digital subtraction angiography detected that the gastrointestinal hemorrhage was the result of the duodenal artery branch breaking; in the second patient, gastroscopy detected an acute gastric mucosal injury; and in the third patient, no reason was found. Intra-abdominal hemorrhage was detected in eight patients by abdominal CT, digital subtraction angiography, or during invasive interventions and surgical processes. Patients with PSMVT were more severely ill, as evidenced by their greater need for minimally invasive interventions, higher rates of multiple organ dysfunction syndrome, infected pancreatic necrosis, and longer durations of hospital and intensive care unit stays.
Table 1 shows the results of the univariate regression analysis of PSMVT. The following were potential risk factors: Higher Balthazar’s CTSI score (OR: 1.780, P = 0.000); hypoalbuminemia (OR: 0.047, P < 0.01); culture-positive drainage fluid (OR: 3.97, P < 0.01); the APFC at the mesenteric root (OR: 2.765, P < 0.05); extrapancreatic necrosis alone (OR: 0.314, P < 0.05); peripancreatic and pancreatic parenchymal necrosis (OR: 6.021, P = 0.000); necrosis located in the head (OR: 4.580, P < 0.01), neck (OR: 4.413, P < 0.01), body (OR: 6.431, P = 0.000), or tail (OR: 8.500, P = 0.000) of the pancreas; an extent of pancreatic necrosis > 50% (OR: 24.889, P = 0.000); and gastrointestinal wall thickening (OR: 4.053, P = 0.000).
Multivariate stepwise logistic regression analyses showed that higher Balthazar’s CTSI score (OR: 2.742; 95%CI: 1.664-4.519; P = 0.000), hypoalbuminemia (serum albumin level < 25 g/L) (OR:32.573; 95%CI: 2.711-391.353; P = 0.006) and gastrointestinal wall thickening (OR: 4.367, 95%CI: 1.218-15.658; P = 0.024) were independent risk factors for PSMVT in patients with SAP (Table 2). The area under the ROC curve for Balthazar’s CTSI score was 0.777 (P = 0.000), the sensitivity was 52%, and the specificity was 93% at a cut-off value of 5.5 (Figure 4).
There is a lack of data on the prevalence and natural course of PSMVT in AP. It has been reported that the incidence of vascular abnormalities varies from 1.8% to 57% because of the different vascular abnormalities described, and differences in the severity of AP, and time or method of detecting vascular abnormalities[3-5,10,15-18]. In the present study, PSMVT developed in 25 of 140 (17.86%) patients with early-stage SAP. It has been reported that most PSMVTs have been clinically confirmed in the late stage of AP onset[3-5,10]. This study is the first report to focus on PSMVT occurring as early as within 10 d (average 6 d) from AP onset and to indicate that high Balthazar’s CTSI, hypoalbuminemia and gastrointestinal wall thickening are independent prognostic factors of PSMVT in the early stage of the onset of SAP.
The development of PSMVT is associated with the presence, location, and extent of pancreatic necrosis[5,19]. In contrast, some papers showed a weaker association between Balthazar’s CTSI and superficial venous thrombosis (SVT) or PSMVT in AP[3,15]. In our study, Balthazar’s CTSI was an independent risk factor of PSMVT and the area under the ROC curve for Balthazar’s CTSI score was 0.777 at a cut-off value of 5.5. The high specificity indicated that a low Balthazar’s CTSI score could be a good indicator that PSMVT would not occur. The splenic vein runs behind the tail and the body of the pancreas. Behind the neck of the pancreas, the splenic vein joins the SMV to form the hepatic PV. Necrosis could directly impact the vascular system, resulting in the formation of PSMVT. In addition, pancreatic necrosis is thought to be associated with a severe local inflammatory response and may surround the vein directly, causing impaired vasomotor function, reduced capillary perfusion, and enhanced thrombosis. However, the appropriate time for drainage is controversial, and it is recommended that drainage should be delayed until the fluid is well encapsulated or there is necrosis[20]. In our study, we found that PSMVT could form in the early stage of AP and hence, for these patients, early drainage might alleviate local inflammation and lower the risk of PSMVT.
Low albumin status reflects poor nutritional status. Low albumin levels are thought to be associated with increased venous thromboembolism in head and neck surgery patients[21], hepatocellular carcinoma patients[22], colorectal surgery patients[23], and thoracolumbar surgery patients[24]. A prospective study determined that lower serum albumin levels were more accurate than clinical scores for diagnosing DVT in patients with several comorbidities[25]. In our study, hypoalbuminemia with albumin levels ≤ 25 g/L was a good predictor of PSMVT, with a very high OR (32.573). Thus, we speculate that correcting hypoalbuminemia during the early stage of AP may help prevent the occurrence of PSMVT. Low albumin levels may lead to peripheral edema and possibly to increased venous stasis. However, the exact mechanism needs further study.
This study revealed that gastrointestinal wall thickening was an independent risk factor for PSMVT. Gastrointestinal wall thickening is represented by the thickening and edema of the gastrointestinal wall, which means that the intestinal barrier is more vulnerable. There is speculation that intestinal bacteria and their products can infiltrate the blood circulation through the damaged intestinal barrier[26], causing severe local inflammation, which is related to venous thrombosis. The level of D-dimer is mostly used as an effective diagnostic tool to rule out deep vein thrombosis (DVT) and pulmonary embolism[27], and it has been reported to have great predictive power in the early phase of AP[28]. However, our study showed that coagulation tests including platelets, prothrombin time, activated partial thromboplastin time, fibrinogen and D-dimer were not independent risk factors in the logistic regression analysis, suggesting that coagulative disturbance may not be a direct cause of PSMVT, as reported before[3].
There were several limitations of our study. First, it was a retrospective study with a limited sample size. Second, we focused on the vascular complications during the early stage of AP; thus, whether more patients experienced PSMVT in later stages was unknown. Third, anticoagulation therapy was not included in the management protocol of PSMVT at the early stage (within 10 d after AP onset). However, when and whether anticoagulants and other treatments were given 10 d after AP onset depended on the decisions made by the attending doctors. Whether these interventions could have affected the long-term prognosis is unclear.
In conclusion, we demonstrated that the occurrence rate of PSMVT is high in SAP patients in the early stage, and high Balthazar’s CTSI scores, hypoalbuminemia and gastrointestinal wall thickening were independent prognostic factors.
The natural history of portosplenomesenteric vein thrombosis (PSMVT) was mostly reported to occur late during acute pancreatitis (AP). There is little data regarding risk factors for this complication in the early stage of severe acute pancreatitis (SAP).
We wanted to determine the potential risk factors for the development of PSMVT in SAP patients and further qualify independent risk factors.
To investigate the incidence and risk factors of PSMVT in the early stage of SAP.
Clinical outcomes and imaging measures were compared between 25 patients with PSMVT developed in the early stage of SAP and 115 patients without PSMVT. Independent risk factors were assessed by logistic regression analyses.
Twenty-five of the one hundred and forty (17.86%) SAP patients developed PSMVT 6.19 ± 2.43 d after AP onset. Balthazar’s computed tomography (CT) severity index scores, hypoalbuminemia (serum albumin level < 25 g/L) and gastrointestinal wall thickening were independent risk factors for PSMVT developed in patients with SAP. The area under the receiver operating characteristic (ROC) curve for Balthazar’s CT severity index scores was 0.777 with high specificity at a cut-off value of 5.5.
High Balthazar’s CT severity index scores, hypoalbuminemia and gastrointestinal wall thickening are independent risk factors for PSMVT developed in the early stage of SAP.
High Balthazar’s CT severity index scores, hypoalbuminemia and gastrointestinal wall thickening may be good forecasting indictors for PSMVT at the early stage of SAP. We hope that a future prospective, multicenter study will study the prevention and therapy of PSMVT at the early stage of AP.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kitamura K, Sharma V, Wang W, Wilcox CM S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Yin SY
| 1. | Heider TR, Azeem S, Galanko JA, Behrns KE. The natural history of pancreatitis-induced splenic vein thrombosis. Ann Surg. 2004;239:876-880; discussion 880-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Zhou J, Ke L, Yang D, Chen Y, Li G, Tong Z, Li W, Li J. Predicting the clinical manifestations in necrotizing acute pancreatitis patients with splanchnic vein thrombosis. Pancreatology. 2016;16:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Zhou J, Ke L, Tong Z, Li G, Li W, Li N, Li J. Risk factors and outcome of splanchnic venous thrombosis in patients with necrotizing acute pancreatitis. Thromb Res. 2015;135:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Harris S, Nadkarni NA, Naina HV, Vege SS. Splanchnic vein thrombosis in acute pancreatitis: a single-center experience. Pancreas. 2013;42:1251-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Easler J, Muddana V, Furlan A, Dasyam A, Vipperla K, Slivka A, Whitcomb DC, Papachristou GI, Yadav D. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Nadkarni NA, Khanna S, Vege SS. Splanchnic venous thrombosis and pancreatitis. Pancreas. 2013;42:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Fei Y, Gao K, Hu J, Tu J, Li WQ, Wang W, Zong GQ. Predicting the incidence of portosplenomesenteric vein thrombosis in patients with acute pancreatitis using classification and regression tree algorithm. J Crit Care. 2017;39:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Fei Y, Hu J, Gao K, Tu J, Wang W, Li WQ. Risk Prediction for Portal Vein Thrombosis in Acute Pancreatitis Using Radial Basis Function. Ann Vasc Surg. 2018;47:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Tripodi A, Legnani C, Palareti G. The risk of a first and a recurrent venous thrombosis associated with an elevated D-dimer level and an elevated thrombin potential: results of the THE-VTE study: comment. J Thromb Haemost. 2015;13:2283-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Toqué L, Hamy A, Hamel JF, Cesbron E, Hulo P, Robert S, Aube C, Lermite E, Venara A. Predictive factors of splanchnic vein thrombosis in acute pancreatitis: A 6-year single-center experience. J Dig Dis. 2015;16:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 960] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 12. | Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 364] [Article Influence: 28.0] [Reference Citation Analysis (1)] |
| 13. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4342] [Article Influence: 361.8] [Reference Citation Analysis (45)] |
| 14. | Vikram R, Balachandran A, Bhosale PR, Tamm EP, Marcal LP, Charnsangavej C. Pancreas: peritoneal reflections, ligamentous connections, and pathways of disease spread. Radiographics. 2009;29:e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Mortelé KJ, Mergo PJ, Taylor HM, Wiesner W, Cantisani V, Ernst MD, Kalantari BN, Ros PR. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol. 2004;52:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Dörffel T, Wruck T, Rückert RI, Romaniuk P, Dörffel Q, Wermke W. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas. 2000;21:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Butler JR, Eckert GJ, Zyromski NJ, Leonardi MJ, Lillemoe KD, Howard TJ. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB (Oxford). 2011;13:839-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 18. | Gonzelez HJ, Sahay SJ, Samadi B, Davidson BR, Rahman SH. Splanchnic vein thrombosis in severe acute pancreatitis: a 2-year, single-institution experience. HPB (Oxford). 2011;13:860-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Park DE, Chae KM. Chylous ascites caused by acute pancreatitis with portal vein thrombosis. J Korean Surg Soc. 2011;81 Suppl 1:S64-S68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-14115; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1385] [Article Influence: 115.4] [Reference Citation Analysis (3)] |
| 21. | Mowery A, Light T, Clayburgh D. Venous thromboembolism incidence in head and neck surgery patients: Analysis of the Veterans Affairs Surgical Quality Improvement Program (VASQIP) database. Oral Oncol. 2018;77:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers. 2017;32:e391-e396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Moghadamyeghaneh Z, Hanna MH, Carmichael JC, Nguyen NT, Stamos MJ. A nationwide analysis of postoperative deep vein thrombosis and pulmonary embolism in colon and rectal surgery. J Gastrointest Surg. 2014;18:2169-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Sebastian AS, Currier BL, Kakar S, Nguyen EC, Wagie AE, Habermann ES, Nassr A. Risk Factors for Venous Thromboembolism following Thoracolumbar Surgery: Analysis of 43,777 Patients from the American College of Surgeons National Surgical Quality Improvement Program 2005 to 2012. Global Spine J. 2016;6:738-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Malatino L, Cardella AM, Puccia G, Cilia C, Terranova V, Cataudella E, Buonacera A, Tripepi G, Di Marca S, Mastrosimone G. Testing Clinical Scores to Diagnose Incident Deep Vein Thrombosis in Patients Hospitalized in a Department of Medicine: Can Biomarkers Improve Accuracy? Angiology. 2016;67:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Cicalese L, Sahai A, Sileri P, Rastellini C, Subbotin V, Ford H, Lee K. Acute pancreatitis and bacterial translocation. Dig Dis Sci. 2001;46:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 902] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 28. | Salomone T, Tosi P, Palareti G, Tomassetti P, Migliori M, Guariento A, Saieva C, Raiti C, Romboli M, Gullo L. Coagulative disorders in human acute pancreatitis: role for the D-dimer. Pancreas. 2003;26:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |