Published online Sep 7, 2018. doi: 10.3748/wjg.v24.i33.3776
Peer-review started: June 11, 2018
First decision: July 6, 2018
Revised: July 31, 2018
Accepted: August 1, 2018
Article in press: August 1, 2018
Published online: September 7, 2018
Processing time: 86 Days and 16.6 Hours
To investigate the feasibility and safety of secondary endoscopic submucosal dissection (ESD) for residual or locally recurrent gastric tumors.
Between 2010 and 2017, 1623 consecutive patients underwent ESD for gastric neoplasms at a single tertiary referral center. Among these, 28 patients underwent secondary ESD for a residual or locally recurrent tumor. Our analysis compared clinicopathologic factors between primary ESD and secondary ESD groups.
The en bloc resection and curative rate of resection of secondary ESD were 92.9% and 89.3%, respectively. The average procedure time of secondary ESD was significantly longer than primary ESD (78.2 min vs 55.1 min, P = 0.004), and the adverse events rate was not significantly different but trended slightly higher in the secondary ESD group compared to the primary ESD group (10.7% vs 3.8%, P = 0.095). Patients who received secondary ESD had favorable outcomes without severe adverse events. During a mean follow-up period, no local recurrence occurred in patients who received secondary ESD.
Secondary ESD of residual or locally recurrent gastric tumors appears to be a feasible and curative treatment though it requires greater technical efficiency and longer procedure time.
Core tip: Although secondary endoscopic submucosal dissection (ESD) is technically demanding, it can be applied to residual or recurrent tumors. We categorized secondary ESD into three groups according to the surgical strategy and analyzed them. There is no consensus on the timing of salvage ESD. This is the first study to report the feasibility and safety of secondary ESD according to the timing of ESD. Although secondary ESD requires greater technical efficiency and a longer procedure time, secondary ESD of residual or locally recurrent gastric tumors appears to be a feasible and curative treatment.
- Citation: Jung DH, Youn YH, Kim JH, Park JJ, Park H. Secondary endoscopic submucosal dissection for locally recurrent or incompletely resected gastric neoplasms. World J Gastroenterol 2018; 24(33): 3776-3785
- URL: https://www.wjgnet.com/1007-9327/full/v24/i33/3776.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i33.3776
Endoscopic submucosal dissection (ESD) technique has evolved, and various lesions can now be treated with ESD, regardless of their size and site, including ulcer scars with severe fibrosis[1,2]. To achieve curative resection, it is very important to accurately predict the margin of neoplasm before ESD. Therefore, chromoendoscopy, magnifying endoscopy, and magnifying image-enhanced endoscopy are widely used to determine the appropriate lesion margin[3,4]. However, despite these efforts, the positive lateral margins of a neoplasm can still occur after ESD. According to the Japanese gastric cancer treatment guidelines, surgical resection should be performed after non-curative resection of early gastric cancer[5]. However, for patients with differentiated carcinoma in which the positive lateral margin is the only non-curative factor, secondary ESD can be performed given the low risk of lymph node metastasis in such cases.
Primary ESD is a relatively established technique that dissects along the loose submucosal layer. However, secondary ESD is difficult because the submucosal layer is eliminated by the previous endoscopic resection and is replaced with fibrosis. In patients who have positive lateral margin after primary ESD, early secondary ESD is performed within a few days. This can be technically difficult because the orientation of the residual lesion might be confusing and development of submucosal fibrosis may have begun[6]. Late secondary ESD is performed a few months after the primary ESD and is also technically demanding because of severe submucosal fibrosis. Until recently, there have only been a few reports of secondary ESD for residual or locally recurrent tumors[6-8]. Therefore, this study aimed to investigate the feasibility and safety of secondary ESD procedures for residual or locally recurrent gastric neoplasms.
Between January 2010 and February 2017, 1623 consecutive patients underwent gastric ESD for gastric neoplasms at Gangnam Severance Hospital in Seoul, Korea. Among these, 28 patients received secondary ESD for residual or locally recurrent tumors. We compared clinicopathologic factors between secondary ESD (28 patients) and primary ESD (1595 patients). The prospectively collected database of ESD and medical records were then reviewed and analyzed retrospectively to determine the feasibility and safety of ESD for residual or locally recurrent tumors. The Institutional Review Board (IRB) of Gangnam Severance Hospitals approved this study.
Procedures were primarily performed using one or two ESD knives, including a DualKnife (Olympus Co., Tokyo, Japan), FlexKnife (Olympus Co., Tokyo, Japan), HookKnife (Olympus Co., Tokyo, Japan), and/or an insulated-tip knife (Olympus Co., Tokyo, Japan). Following circumferential marking (via argon plasma coagulation), a mixture of indigo carmine, epinephrine, and 10% glycerol (Cerol; JW Pharmaceutical Co, Seoul, South Korea) was used to inject the submucosa. The mucosa surrounding each lesion was then incised and dissection of the submucosal layer ensued. Hemostasis during ESD and ablation of visible vessels at the post-ESD ulcer site were achieved using hemostatic forceps (Coagrasper; Olympus Co, Tokyo, Japan). For the electrosurgical unit, the VIO300D (ERBE, Tuebingen, Germany) was used. Mucosal incision was performed using the Endocut I current (effect 2, cut duration 4, interval 3), and submucosal dissection was carried out with the swift coagulation current (effect 3, 40 W). The visible vessels were ablated using the soft coagulation current (effect 3, 60 W).
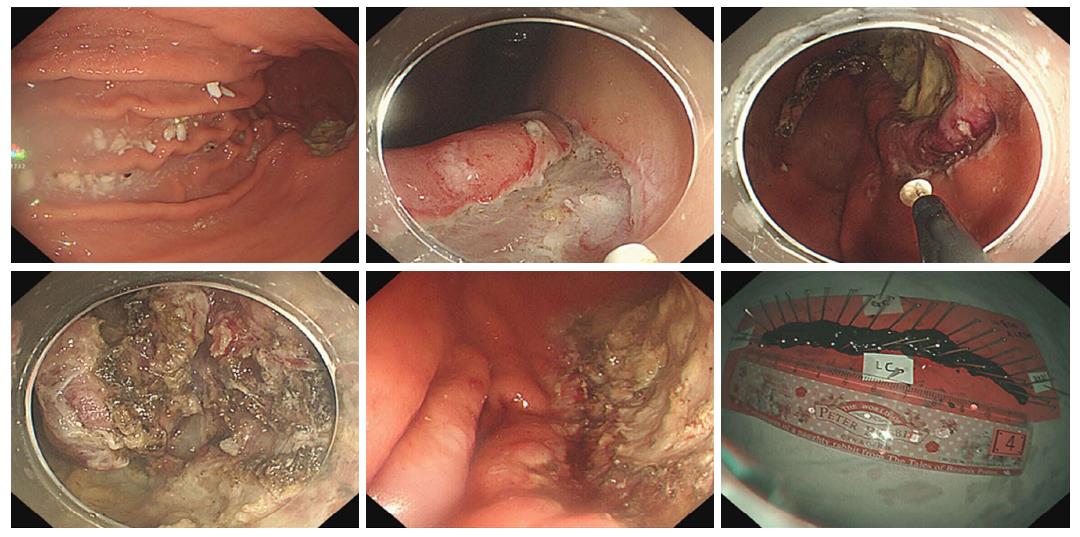
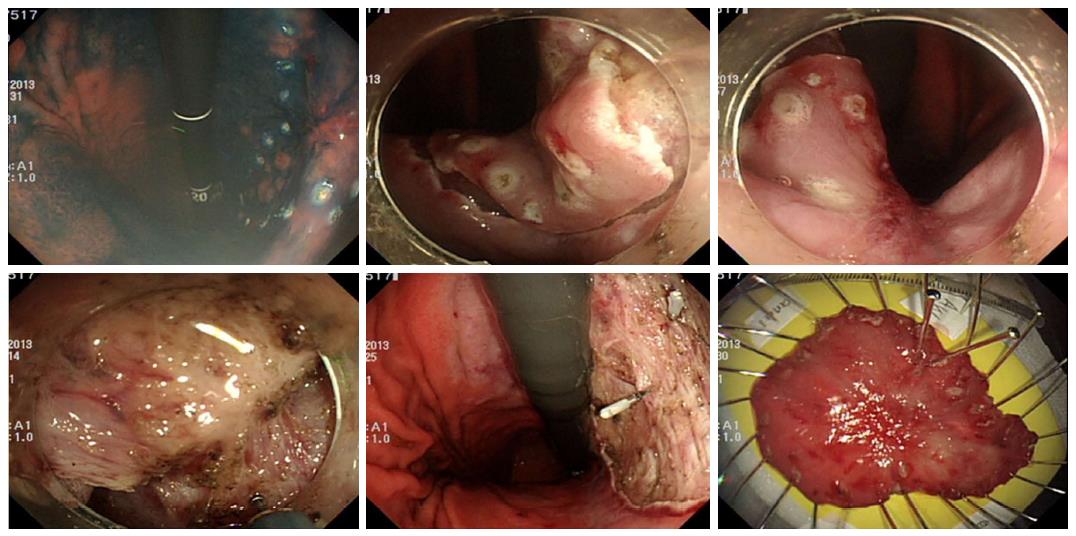
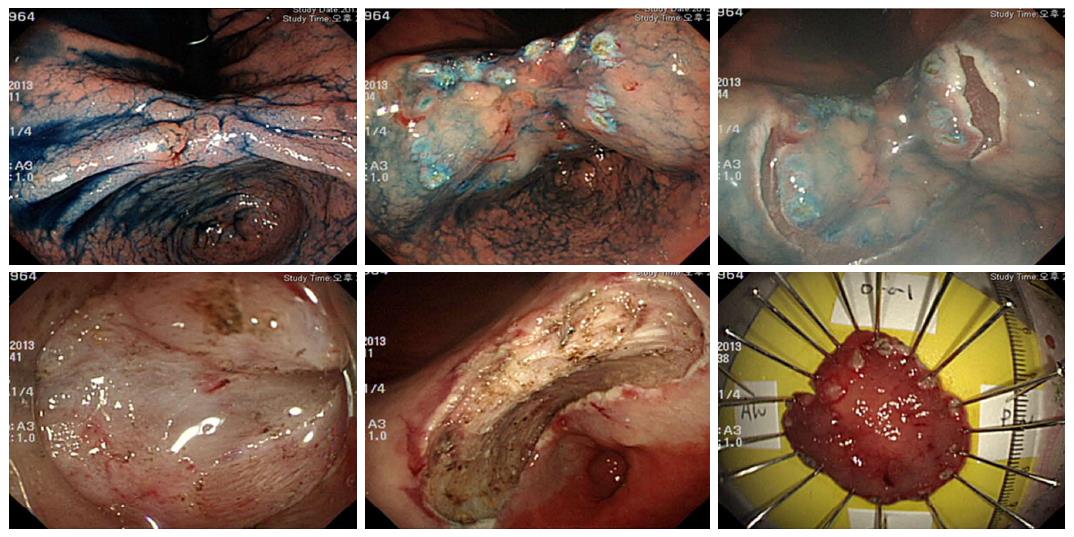
In this study, secondary ESD refers to an additional ESD procedure for residual or locally recurrent tumors. Early salvage ESD was performed immediately after histological confirmation of positive lateral margins of the initial ESD specimen (Figure 1). Late salvage ESD was prescribed when the positive lateral margin was histologically confirmed after the initial ESD, and was performed after complete healing of the artificial ulcer caused by the initial ESD (Figure 2). Late secondary ESD was performed when local recurrence was proven histologically during the follow-up period after initially curative primary ESD (Figure 3). The general sequence of early or late salvage and late secondary ESD were similar to that of the primary ESD. However, sodium hyaluronate (LG Life Science Co., Seoul, South Korea) was frequently used as a submucosal injection material to overcome non-lifting signs induced by ulceration and fibrosis of the submucosal layer caused by the primary ESD[9]. In general, a non-insulated knife was used for dissecting fibrotic areas that were not lifted due to fibrosis. Additionally, to achieve an appropriate angle for a dissecting view of the submucosa in fibrotic area, the submucosal dissection was initiated in a non-fibrotic area of sufficient distance from the fibrotic area.
En bloc resection was defined as a tumor that was removed whole as a single piece. Curative resection was defined as expanded indications according to the Japanese gastric cancer treatment guidelines[5]. According to the Japanese gastric cancer treatment guidelines, the expanded indications for curative endoscopic resection (ER) were en bloc resection, negative lateral and vertical margins, no lymphovascular invasion (LVI) and one of the following: (1) Tumor size > 2 cm, differentiated type, mucosa, and ulcer (-); (2) tumor size ≤ 3 cm, differentiated type, mucosa, and ulcer (+); (3) tumor size ≤ 2 cm, undifferentiated type, mucosa, and ulcer (-); or (4) tumor size ≤ 3 cm, differentiated type, and submucosa1 (SM1, < 500 μm from the muscularis mucosa).
Comparison of clinicopathologic factors between primary ESD and secondary ESD was performed using the chi-square test and Fisher’s exact test. The Student’s t-test was used for intergroup comparison of non-categorical variables. The accepted significance level was a P value < 0.05. All statistical analyses were performed using the software SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, United States).
Among 1623 patients, 1595 patients received primary ESD and 28 patients underwent secondary ESD. Six of these patients (21.4%) and eight of these patients (28.6%) underwent early and late salvage ESD for residual lesions, respectively, while fourteen of these patients (50.0%) underwent late secondary ESD for locally recurrent lesions. Baseline characteristics of the gastric lesions treated by ESD are shown in Table 1. There were significantly more depressed macroscopic lesions in the secondary ESD group and there were no significant differences in lesion location or size between the two groups. We investigated the initial lesion corresponding to secondary ESD. The initial lesions corresponding to secondary ESD were dysplasia in 13 patients, and carcinoma in 15 patients. A total of 15 patients with carcinoma had mucosal cancer without lymphovascular invasion.
| Characteristics | ESD | Secondary ESD | P value |
| (n = 1595, %) | (n = 28, %) | ||
| Gender | 0. 836 | ||
| Male | 1117 (70.0) | 19 (67.9) | |
| Female | 478 (30.0) | 9 (32.1) | |
| Age [yr, mean (SD)] | 64.8 (10.4) | 63.5 (9.2) | 0.528 |
| Lesion location | 0.217 | ||
| Upper | 127 (8.0) | 3 (11.1) | |
| Middle | 633 (39.7) | 15 (53.6) | |
| Lower | 835 (52.4) | 10 (35.7) | |
| Multiplicity | 250 (15.7) | 2 (7.1) | 0.296 |
| Lesion size [mm, mean (SD)] | 17.4 (12.8) | 16.4 (13.2) | 0.664 |
| Macroscopic type | < 0.001 | ||
| Elevated | 674 (42.3) | 7 (25.0) | |
| Flat | 685 (42.9) | 9 (32.1) | |
| Depressed | 236 (14.8) | 12 (42.9) | |
| WHO classification | < 0.001 | ||
| Low grade dysplasia | 610 (38.2) | 8 (28.6) | |
| High grade dysplasia | 221 (13.9) | 4 (14.3) | |
| Well differentiated | 356 (22.3) | 4 (14.3) | |
| Moderately differentiated | 268 (16.8) | 7 (25.0) | |
| Poorly differentiated | 76 (4.8) | 3 (10.7) | |
| Signet ring cell carcinoma | 64 (4.0) | 0 (0.0) | |
| No residual lesion | 0 (0.0) | 2 (7.1) | |
| Depth of invasion | < 0.001 | ||
| Dysplasia | 831 (52.1) | 12 (42.9) | |
| Mucosal cancer | 609 (38.2) | 14 (50.0) | |
| Submucosal cancer | 155 (9.7) | 0 (0.0) | |
| No residual lesion | 0 (0.0) | 2 (7.1) | |
| LVI | 1.000 | ||
| No | 1546 (96.9) | 28 (100.0) | |
| Yes | 49 (3.1) | 0 (0.0) | |
| Lateral margin | 0.381 | ||
| Negative | 1521 (95.4) | 26 (92.9) | |
| Positive | 74 (4.6) | 2 (7.1) | |
| Vertical margin | 0.615 | ||
| Negative | 1542 (96.7) | 27 (96.4) | |
| Positive | 53 (3.3) | 1 (3.6) |
When we compared the procedure outcomes of the 1595 patients in the primary ESD group and the 28 patients in the secondary ESD group, the resected specimen size in the secondary ESD group was significantly larger than that of the primary ESD group, though the lesion size of two groups was not different. Next, significant differences in procedure outcomes were identified by comparing the primary ESD group and the secondary ESD group (Table 2). Patients in the secondary ESD group had a longer mean ESD procedure time than patients in the primary ESD group (78.2 min vs 55.1 min, P = 0.004). Additionally, the dissection times for the secondary ESD group were significantly longer than that of the primary ESD group (63.4 min vs 36.7 min, P < 0.001). Furthermore, the dissection speed was significantly lower in the secondary ESD group than in the primary ESD group (22.1 mm2/min vs 38.7 mm2/min, P < 0.001). The adverse events rate in the secondary ESD group was not significantly different but trended slightly higher than that in the primary ESD group (10.7% vs 3.8%, P = 0.095). There were no perforations in the secondary ESD group.
| ESD | Secondary ESD | P value | |
| (n = 1595, %) | (n = 28, %) | ||
| Lesion size [mm, mean (SD)] | 17.4 (12.8) | 16.4 (13.2) | 0.664 |
| Specimen size [mm, mean (SD)] | 38.1 (14.6) | 47.8 (19.6) | 0.001 |
| Whole procedure time [min, mean (SD)] | 55.1 (41.5) | 78.2 (38.0) | 0.004 |
| Dissection time [min, mean (SD)] | 36.7 (34.7) | 63.4 (38.0) | < 0.001 |
| Dissection speed [mm2/min, mean (SD)] | 38.7 (32.8) | 22.1 (12.7) | < 0.001 |
| Complication | 61 (3.8) | 3 (10.7) | 0.095 |
| Perforation | 37 (2.3) | 0 (0.0) | |
| Bleeding | 15 (0.9) | 1 (3.6) | |
| Aspiration pneumonia | 13 (0.8) | 2 (7.1) | |
| Hospital stay [d, mean (SD)] | 3.6 (3.2) | 3.9 (2.5) | 0.639 |
| En bloc resection | 1574 (98.7) | 26 (92.9) | 0.058 |
| Curative resection | 1383 (86.7) | 25 (89.3) | 1.000 |
| Additive salvage treatment | 104 (6.5) | 3 (10.7) | 0.425 |
| Additive surgery | 85 (5.3) | 2 (7.1) | |
| Redo ESD | 16 (1.0) | 1 (3.6) | |
| Argon plasma coagulation | 3 (0.2) | 0 (0.0) |
The en bloc resection and curative rates of resection of secondary ESD were 92.9% and 89.3%, respectively. These rates were comparable with those of primary ESD, which had an en bloc resection rate of 98.7% and a curative resection rate of 86.7% (Table 2). Three patients in the secondary ESD group had additive salvage treatments due to a positive lateral margin (n = 2) and other synchronous lesions that had vertical margin involvement after secondary ESD (n = 1). One patient in the secondary ESD group showed a positive vertical margin with a very focal extension of dysplasia. This patient was 75 years old and decided to be observed and was followed up closely. During follow-up, one patient who received secondary ESD presented with metachronous recurrence 25 months after secondary ESD. We evaluated the outcomes according to an institutional learning curve based upon the initial phase and the late phase of the study period. The en bloc resection rate was not significantly different (initial phase vs late phase, 98.4% vs 98.8%, P = 0.533). Curative resection rate was significantly lower in initial phase vs late phase (84.6% vs 88.8%, P = 0.013). When we evaluated the outcomes in the secondary ESD group, the en bloc and curative resection rates were not statistically different (initial phase vs late phase, 100% vs 88.2%, P = 0.505 and 90.9 % vs 88.2%, P = 1.000, respectively).
We categorized secondary ESD into three groups according to the surgical strategy (early salvage, late salvage, or late secondary ESD) (Table 3). The median interval times from the primary ESD to the secondary ESD were 6.5, 129.0, and 712.8 d in patients who received early salvage, late salvage, or late secondary ESD, respectively. Hospital stay was significantly longer in early salvage ESD group compared with late salvage and late secondary ESD groups (7.2 d vs 3.0 d and 3.0 d, P ≤ 0.001). There was no other significant difference in procedure outcomes among the three groups (Table 4). The en bloc and curative resection rates of early and late salvage groups were both 100%. However, the en bloc and curative resection rates of the late secondary ESD group tended to be lower than that of the other groups (Table 4).
| Characteristics | Early salvage ESD (n = 6, %) | Late salvage ESD (n = 8, %) | Late secondary ESD (n = 14, %) | P value |
| Gender | 0. 909 | |||
| Male | 4 (66.7) | 5 (62.5) | 10 (71.4) | |
| Female | 2 (33.3) | 3 (37.5) | 4 (28.6) | |
| Age [yr, mean (SD)] | 65.4 (10.8) | 61.6 (7.3) | 63.8 (9.9) | 0.749 |
| Lesion location | 0.255 | |||
| Upper | 2 (33.3) | 0 (0.0) | 1 (7.1) | |
| Middle | 2 (33.3) | 4 (50.0) | 9 (64.3) | |
| Lower | 2 (33.3) | 4 (50.0) | 4 (28.6) | |
| Multiplicity | 0 (0.0) | 1 (12.5) | 1 (7.1) | 0.668 |
| Lesion size [mm, mean (SD)] | 19.8 (22.4) | 13.6 (10.9) | 16.4 (9.8) | 0.701 |
| Initial method | 0.341 | |||
| EMR | 0 (0.0) | 0 (0.0) | 2 (14.3) | |
| ESD | 6 (100.0) | 8 (100.0) | 12 (85.7) | |
| Macroscopic type | 0.014 | |||
| Elevated | 0 (0.0) | 0 (0.0) | 7 (50.0) | |
| Flat | 4 (66.7) | 2 (25.0) | 3 (21.4) | |
| Depressed | 2 (33.3) | 6 (75.0) | 4 (28.6) | |
| WHO classification | 0.081 | |||
| Low grade dysplasia | 2 (33.3) | 3 (37.5) | 3 (21.4) | |
| High grade dysplasia | 0 (0.0) | 1 (12.5) | 3 (21.4) | |
| Well differentiated | 1 (16.7) | 0 (0.0) | 3 (21.4) | |
| Moderately differentiated | 1 (16.7) | 4 (50.0) | 2 (14.3) | |
| Poorly differentiated | 0 (0.0) | 0 (0.0) | 3 (21.4) | |
| Signet ring cell carcinoma | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| No residual lesion | 2 (33.3) | 0 (0.0) | 0 (0.0) | |
| Depth of invasion | 0.090 | |||
| Dysplasia | 2 (33.3) | 4 (50.0) | 6 (42.9) | |
| Mucosal cancer | 2 (33.3) | 4 (50.0) | 8 (57.1) | |
| Submucosal cancer | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| No residual lesion | 2 (33.3) | 0 (0.0) | 0 (0.0) | |
| LVI | 1.000 | |||
| No | 6 (100.0) | 8 (100.0) | 14 (100.0) | |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Lateral margin | 0.341 | |||
| Negative | 6 (100.0) | 8 (100.0) | 12 (85.7) | |
| Positive | 0 (0.0) | 0 (0.0) | 2 (14.3) | |
| Vertical margin | 0.617 | |||
| Negative | 6 (100.0) | 8 (100.0) | 13 (92.9) | |
| Positive | 0 (0.0) | 0 (0.0) | 1 (7.1) |
| Early salvage ESD (n = 6, %) | Late salvage ESD (n = 8, %) | Late secondary ESD (n = 14, %) | P value | |
| Lesion size [mm, mean (SD)] | 19.8 (22.4) | 13.6 (10.9) | 16.4 (9.8) | 0.701 |
| Specimen size [mm, mean (SD)] | 63.0 (33.0) | 46.4 (11.6) | 42.1 (12.8) | 0.084 |
| Whole procedure time [min, mean (SD)] | 81.7 (48.5) | 85.9 (24.3) | 72.3 (41.1) | 0.714 |
| Dissection time [min, mean (SD)] | 73.2 (46.8) | 73.0 (23.4) | 53.8 (40.7) | 0.421 |
| Dissection speed [mm2/min, mean (SD)] | 19.4 (6.8) | 19.0 (7.5) | 24.9 (12.7) | 0.512 |
| Method | 0.341 | |||
| ESD only | 6 (100.0) | 8 (100.0) | 12 (85.7) | |
| ESD plus snaring | 0 (0.0) | 0 (0.0) | 2 (14.3) | |
| Complication | 0 (0.0) | 1 (12.5) | 2 (14.3) | 0.627 |
| Perforation | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Bleeding | 0 (0.0) | 0 (0.0) | 1 (7.1) | |
| Aspiration pneumonia | 0 (0.0) | 1 (12.5) | 1 (7.1) | |
| Hospital stay [d, mean (SD)] | 7.2 (2.9) | 3.0 (1.8) | 3.0 (1.2) | < 0.001 |
| Median interval [d, mean (SD)] | 6.5 (3.1) | 129.0 (133.6) | 712.8 (546.5) | 0.001 |
| En bloc resection | 6 (100.0) | 8 (100.0) | 12 (85.7) | 0.341 |
| Curative resection | 6 (100.0) | 8 (100.0) | 11 (78.6) | 0.186 |
| Additive treatment | 0 (0.0) | 0 (0.0) | 3 (21.4) | 0.186 |
| Additive surgery | 0 (0.0) | 0 (0.0) | 2 (14.3) | |
| Redo ESD | 0 (0.0) | 0 (0.0) | 1 (7.1) |
ESD is now widely performed for gastric neoplasms and has favorable outcomes. However, the incomplete resection rate of ESD is 2.2% to 26.3%[10-21] and the local recurrence rate after ESD is 0% to 3%[15,16,22-24]. Therefore, the management of residual or locally recurrent gastric neoplasms after ESD continues to be problematic. Secondary ESD for a locally recurrent tumor is difficult to perform due to severe fibrosis and ulcer scar formation, and there are few published reports about secondary ESD for residual or locally recurrent gastric neoplasms. Bae et al[6] reported a total of 16 early secondary ESD cases in which the curative resection rate was 93.8% (15/16). Therefore, they asserted that early secondary ESD was a feasible and useful treatment in patients with a positive lateral margin. Additionally, secondary ESD was also determined to be an effective treatment for locally recurrent lesions after endoscopic mucosal resection (EMR)[7]. Oka et al[7] reported that the complete resection rate of ESD for a residual or locally recurrent lesion is 93.3% (14/15). Therefore, they suggested that secondary ESD was an effective and minimally invasive procedure for patients with residual or local recurrent tumors after EMR.
Secondary ESD is technically difficult due to the severe fibrosis and scar formation that results from primary ESD[25]. In this study, the mean procedure time and dissection time of secondary ESD was longer than that for primary ESD. In addition, the dissection speed was lower in the secondary ESD group than in the primary ESD group, while the adverse events rate of secondary ESD was slightly higher than that of primary ESD. That is, secondary ESD is associated with a greater technical difficulty, longer procedure time, and increased incidence of adverse events. However, in this study, bleeding after secondary ESD was managed endoscopically, without the need for further emergent operation; aspiration pneumonia was treated with antibiotics and conservative care. The aspiration pneumonia occurred in 15 (0.9%) patients. The procedure time was longer in patients with aspiration pneumonia than others (83.3 min vs 55.1 min, P = 0.125). However, the difference was not statistically significant. Although many endoscopists worry that submucosal fibrosis might cause perforation, in this study, there were no perforations reported in the secondary ESD group. We suspected that deep excavation by benign peptic ulcers would not preserve the muscle layer. However, the artificial ulcer caused by ESD was contracted, preserving the muscle layer, which was thicker than before ESD. Therefore, this observation may explain the rarity of perforation. Primary ESD procedures in this study were performed by one of five ESD endoscopists. Two of them were fully skilled experts (YYH, far more than a thousand cases of ESD experience; KJH, more than five hundreds cases of ESD experience) and the other three were less-skilled ESD endoscopists whose ESD experience were less than a hundred cases. However, secondary ESD in this study was exclusively performed only by the two fully skilled ESD experts (YYH and KJH). That might be a reason why the outcomes of the secondary ESD group were relatively good despite the technical difficulty.
ESD has multiple advantages compared to other treatment options for residual or locally recurrent tumors, such as gastrectomy and additional endoscopic treatment. Compared to gastrectomy, ESD offers improved quality of life, decreased morbidity, longer length of recovery, and lower healthcare costs. It also has advantages over other endoscopic treatments, such as argon plasma coagulation, including a high chance of en bloc resection, which allows for complete histologic assessment. Therefore, the application of secondary ESD for residual or locally recurrent tumors is an important treatment consideration.
When we categorized secondary ESD according to the time interval from primary ESD, the main difference between early secondary ESD and late secondary ESD was scar formation. Early secondary ESD is performed while the ESD ulcer is still open, therefore, there is minimal fibrosis. However, by the time late secondary ESD is performed, the ulcer has healed with severe fibrosis[26]. In this study, we compared three groups: early salvage ESD, late salvage ESD, and late secondary ESD, and hospital stay of the early salvage ESD group was the longest. This difference was due to many patients in the early salvage ESD group that underwent primary and secondary ESD successively during a single hospitalization. There was no other significant difference in procedure outcomes regarding the whole procedure time, dissection time, and dissection speed among the three groups. Although the late salvage ESD and late secondary ESD groups had severe fibrosis and scar formations, no perforations occurred.
According to this study, secondary ESD for residual or locally recurrent tumors is a safe, minimally invasive, and effective treatment. However, secondary ESD should be performed by an experienced endoscopist with precautions, including the appropriate electrosurgical knife, the appropriate dissection depth, and the careful gradual dissection of the severe fibrotic tissue along the plane of the deep submucosa or superficial proper muscle. Furthermore, the surrounding non-fibrotic tissue should first be dissected sufficiently to make a flap of the specimen and directly visualize the plane of the submucosa from the non-lifting fibrotic area to the fibrotic area[27,28]. Therefore, in this study, we found that the specimen size of the secondary ESD group was significantly larger than the primary ESD group. In addition, one recommendation for dissecting the fibrotic area is to dissect deeply along the surface of the muscle fiber because the ESD ulcer scar has an intact muscle layer.
Correct diagnosis of the depth of invasion of residual or locally recurrent tumors is often difficult due to scar formation after ESD. Therefore, residual or locally recurrent tumors with submucosal invasion are sometimes misdiagnosed as mucosal cancer[29]. In this study, the depth of invasion of all secondary ESD was within the mucosa.
In previous reports, the curative resection rate for residual gastric neoplasms was 92% to 100%[6,26,30] and curative resection rate for locally recurrent gastric neoplasms was 76% (35/46) to 87% (13/15)[26,28]. In this study, the en bloc and curative resection rates of secondary ESD were 92.9% and 89.3%, respectively, which were comparable with those reported previously for secondary ESD. Two patients in the early salvage ESD group had no residual tumor. Although, they showed histological confirmation of positive lateral margins of the initial ESD specimen, no residual lesion was detected due to burning effects on the tissue by electrosurgical unit.
Early and late salvage ESD, which were both performed after histological confirmation of positive lateral margins of the primary ESD specimen, did not involve en bloc resection of the primary lesion. Therefore, the risks of local recurrence and lymph node metastasis resulting from piecemeal resection were always taken into consideration. Hence, careful surveillance after early and late salvage ESD is also very important. In this study, there was no local recurrence during the mean follow-up period in any patient who received secondary ESD.
There is no consensus on the timing of salvage ESD. Until now, there were few reports about the feasibility and effectiveness of early salvage ESD[6,26,31]. In this study, we compared the clinicopathologic factors between early salvage ESD and late salvage ESD and found no significant differences in procedure or oncologic outcomes. However, further study about the timing of salvage ESD will be needed.
Although this study was retrospective with a small number of patients, this is the first study to report the feasibility and safety of secondary ESD according to the timing of ESD.
In conclusion, we found that secondary ESD appears to be a feasible and curative treatment. When we consider the morbidity of gastrectomy, secondary ESD of residual or locally recurrent gastric neoplasms is a good therapeutic option worth pursuing, although the procedure time is longer and associated with higher technical difficulty.
Endoscopic submucosal dissection (ESD) is an accepted curative treatment option for gastric tumors with very low local recurrence. However, residual or locally recurrent tumors occur rarely after ESD. Although secondary ESD is technically demanding, it can be applied to residual or recurrent tumors with scar and dense fibrotic submucosa. We investigated the feasibility and safety of secondary ESD for gastric tumors. We also categorized secondary ESD into three groups according to the surgical strategy (early salvage, late salvage, or late secondary ESD) and analyzed them.
There is no consensus on the timing of salvage ESD. Until now, there were few reports about the feasibility and effectiveness of early salvage ESD.
To investigate the feasibility and safety of secondary ESD for residual or locally recurrent gastric tumors.
Between 2010 and 2017, 1623 consecutive patients underwent ESD for gastric neoplasms at a single tertiary referral center. Among these, 28 patients underwent secondary ESD for a residual or locally recurrent tumor. Our analysis compared clinicopathologic factors between primary ESD and secondary ESD groups.
The en bloc resection and curative rate of resection of secondary ESD were 92.9% and 89.3%. The average procedure time of secondary ESD was significantly longer than primary ESD, and the adverse events rate was not statistically different but trended slightly higher in the secondary ESD group compared to the primary ESD group. Patients who received secondary ESD had favorable outcomes without severe adverse events. During a mean follow-up period, no local recurrence occurred in patients who received secondary ESD.
Although it requires greater technical efficiency and longer procedure time, secondary ESD of residual or locally recurrent gastric tumors appears to be feasible and curative treatment.
Secondary ESD of residual or locally recurrent gastric tumors is a feasible and curative treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akiho H, Nishida T S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Benefits of endoscopic submucosal dissection according to size and location of gastric neoplasm, compared with conventional mucosal resection. J Gastroenterol Hepatol. 2009;24:1102-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77 Suppl 1:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Otsuka Y, Niwa Y, Ohmiya N, Ando N, Ohashi A, Hirooka Y, Goto H. Usefulness of magnifying endoscopy in the diagnosis of early gastric cancer. Endoscopy. 2004;36:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Zhao Z, Yin Z, Wang S, Wang J, Bai B, Qiu Z, Zhao Q. Meta-analysis: The diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. J Gastroenterol Hepatol. 2016;31:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1914] [Article Influence: 239.3] [Reference Citation Analysis (1)] |
| 6. | Bae SY, Jang TH, Min BH, Lee JH, Rhee PL, Rhee JC, Kim JJ. Early additional endoscopic submucosal dissection in patients with positive lateral resection margins after initial endoscopic submucosal dissection for early gastric cancer. Gastrointest Endosc. 2012;75:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kanao H, Kawamura T, Yoshida S, Yoshihara M, Chayama K. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38:996-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Sekiguchi M, Suzuki H, Oda I, Abe S, Nonaka S, Yoshinaga S, Taniguchi H, Sekine S, Kushima R, Saito Y. Favorable long-term outcomes of endoscopic submucosal dissection for locally recurrent early gastric cancer after endoscopic resection. Endoscopy. 2013;45:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 476] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 11. | Akasaka T, Nishida T, Tsutsui S, Michida T, Yamada T, Ogiyama H, Kitamura S, Ichiba M, Komori M, Nishiyama O. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc. 2011;23:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Song HJ, Lee GH. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 13. | Shin KY, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, Chung YJ, Kwon JG, Jung JT, Kim EY. Clinical outcomes of the endoscopic submucosal dissection of early gastric cancer are comparable between absolute and new expanded criteria. Gut Liver. 2015;9:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Nakamura K, Honda K, Akahoshi K, Ihara E, Matsuzaka H, Sumida Y, Yoshimura D, Akiho H, Motomura Y, Iwasa T. Suitability of the expanded indication criteria for the treatment of early gastric cancer by endoscopic submucosal dissection: Japanese multicenter large-scale retrospective analysis of short- and long-term outcomes. Scand J Gastroenterol. 2015;50:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, Tanioka D, Tsuzuki T, Yagi S, Kato J. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 527] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 17. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 18. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 520] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 19. | Goto O, Fujishiro M, Kodashima S, Ono S, Omata M. Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy. 2009;41:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc. 2010;24:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, Arai M, Suzuki T, Matsumura T, Bekku D. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009;41:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Park JC, Lee SK, Seo JH, Kim YJ, Chung H, Shin SK, Lee YC. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc. 2010;24:2842-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Kakushima N, Fujishiro M, Kodashima S, Kobayashi K, Tateishi A, Iguchi M, Imagawa A, Motoi T, Yahagi N, Omata M. Histopathologic characteristics of gastric ulcers created by endoscopic submucosal dissection. Endoscopy. 2006;38:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Hoteya S, Iizuka T, Kikuchi D, Mitani T, Matsui A, Ogawa O, Furuhata T, Yamashta S, Yamada A, Kaise M. Secondary endoscopic submucosal dissection for residual or recurrent tumors after gastric endoscopic submucosal dissection. Gastric Cancer. 2014;17:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Shimamura Y, Ishii N, Nakano K, Ikeya T, Nakamura K, Takagi K, Fukuda K, Suzuki K, Fujita Y. Repeat endoscopic submucosal dissection for recurrent gastric cancers after endoscopic submucosal dissection. World J Gastrointest Endosc. 2013;5:600-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Yokoi C, Gotoda T, Hamanaka H, Oda I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc. 2006;64:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Okada K, Fujisaki J, Kasuga A, Omae M, Yoshimoto K, Hirasawa T, Ishiyama A, Yamamoto Y, Tsuchida T, Hoshino E. Endoscopic ultrasonography is valuable for identifying early gastric cancers meeting expanded-indication criteria for endoscopic submucosal dissection. Surg Endosc. 2011;25:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Higashimaya M, Oka S, Tanaka S, Numata N, Sanomura Y, Yoshida S, Arihiro K, Chayama K. Endoscopic submucosal dissection for residual early gastric cancer after endoscopic submucosal dissection. Gastrointest Endosc. 2013;77:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Kikuchi D, Iizuka T, Hoteya S, Yamada A, Furuhata T, Yamashita S, Domon K, Nakamura M, Matsui A, Mitani T. Safety and efficacy of secondary endoscopic submucosal dissection for residual gastric carcinoma after primary endoscopic submucosal dissection. Digestion. 2012;86:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |