Published online Sep 7, 2018. doi: 10.3748/wjg.v24.i33.3681
Peer-review started: May 5, 2018
First decision: May 17, 2018
Revised: June 5, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: September 7, 2018
Processing time: 124 Days and 9.9 Hours
Fecal calprotectin (FC) has emerged as one of the most useful tools for clinical management of inflammatory bowel diseases (IBD). Many different methods of assessment have been developed and different cut-offs have been suggested for different clinical settings. We carried out a comprehensive literature review of the most relevant FC-related topics: the role of FC in discriminating between IBD and irritable bowel syndrome (IBS) and its use in managing IBD patients In patients with intestinal symptoms, due to the high negative predictive value a normal FC level reliably rules out active IBD. In IBD patients a correlation with both mucosal healing and histology was found, and there is increasing evidence that FC assessment can be helpful in monitoring disease activity and response to therapy as well as in predicting relapse, post-operative recurrence or pouchitis. Recently, its use in the context of a treat-to-target approach led to a better outcome than clinically-based therapy adjustment in patients with early Crohn’s disease. In conclusion, FC measurement represents a cheap, safe and reliable test, easy to perform and with a good reproducibility. The main concerns are still related to the choice of the optimal cut-off, both for differentiating IBD from IBS, and for the management of IBD patients.
Core tip: This manuscript is a review of current literature on clinical use of fecal calprotectin in distinguishing irritable bowel syndrome from inflammatory bowel diseases and in the long-term management of inflammatory bowel disease patients, which includes monitoring of disease activity, response to therapy, disease relapse and post-operative recurrence. Concerns about the optimal cut-off in different settings have also been discussed.
- Citation: Mumolo MG, Bertani L, Ceccarelli L, Laino G, Di Fluri G, Albano E, Tapete G, Costa F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterol 2018; 24(33): 3681-3694
- URL: https://www.wjgnet.com/1007-9327/full/v24/i33/3681.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i33.3681
Calprotectin is a 36 kDa calcium and zinc binding protein, which represent about 60% of soluble proteins of the cytoplasm of granulocytes[1]. It is heat and proteolysis resistant heterocomplex of S100A8 and S100A9 consisting of 2 heavy (14 kDa) e 1 light (8 kDa) chains, each binding 2 Ca2+.
Functions of calprotectin include: competitive inhibition of zinc-dependent enzymes, potential biostatic activity against microbes through chelation of zinc ions, apoptosis induction in malignant cells, and regulation of the inflammatory process[2,3].
Fecal calprotectin (FC) is one of the most sensitive non-invasive marker in distinguishing inflammatory bowel diseases (IBD) from functional disorders. Several factors, however, may influence FC levels, such as colonic cleansing[4], age, diet, exercise[5], and the faecal amount of mucus or blood in stools[6].
A further limitation is a low specificity in discriminating ulcerative colitis (UC) from Crohn’s disease (CD), active IBD from non-IBD intestinal inflammation (infections, non-steroidal anti-inflammatory drugs-related damage, cancer, diverticulitis). FC is a more sensitive marker than C-reactive protein (CRP) for detection of mild mucosal inflammation, although in severely active cases CRP better reflects systemic inflammation[7,8].
Roseth, in 1992, demonstrated the stability of calprotectin in stools for up to 7 d at room temperature[9,10], which offers advantages for its use in clinical practice[11].
In a more recent study, however, calprotectin concentrations in stool samples were unchanged only for 3 d at room temperature, while after 7 d a significant decrease (P < 0.01) was found[12].
Day-to-day variation of FC was demonstrated by Husebay et al[13] in patients without colonic inflammation or neoplasm and confirmed by Moum et al[14], in patients with mild-to-moderate active CD, where significant differences in 63 pairs of stool samples collected in 2 consecutive days were found. A lower variability was observed in fecal samples collected for 3 d from 93 CD patients in clinical remission[15]. Dobrzanski et al[16] confirmed that variability seems to be relevant only in active IBD, particularly in UC where large amounts of mucus and blood are present in stools.
The most reliable results were provided by analyzing 3 in-wk samples from the first bowel movement in the morning[12]. Higher variability was found in patients with the highest levels of FC; further, the test results were influenced by the sample consistency and by the interval between the bowel movements, supposedly related to the accumulation of leukocyte-derived proteins in the gut lumen.
A good correlation was found between the FC concentrations assessed in two randomly different samples collected from the same bowel movement[12]. Calafat et al[6] did not find any influence of the timing of stools sampling, or the presence of blood on FC concentrations, in particular in patients with moderate-to-severe active UC, where the decision-making strategies based on single quantitative FC determinations are not advisable.
Different methods can be used for the quantitative assessment of FC, most of them based on the enzyme-linked immunosorbent assay (ELISA); chemiluminescence immunoassays (CLIA), fluoro enzyme immunoassays (FEIA) and particle enhanced turbidimetric immunoassays (PETIA) were also introduced.
Oyaert et al[17] compared six automated immunoassays: Thermo Fisher EliA Calprotectin assay on the Phadia 250 (Thermo Fisher Scientific, Uppsala, Sweden), Diasorin Calprotectin assay on the Liaison (Diasorin S.P.A., Saluggia, Italy), Inova QUANTA Flash Calprotectin (research use only) on the Inova BIO-FLASH instrument (Inova Diagnostics, San Diego, CA, United States), Bühlmann fCAL Turbo (Bühlmann Laboratories AG, Schönenbuch, Switzerland) on the Roche Cobas c501 (Roche Diagnostics, Mannheim, Germany), Euroimmun Calprotectin assay (Euroimmun; Lübeck, Germany), on an automated ELISA instrument (QUANTA-Lyser 2, Inova) and Orgentec Calprotectin assay on the Alegria (Orgentec Diagnostika, Mainz, Germany). The authors found that all assays had a sensitivity of 100% when the cut-off of the manufacturer was used (i.e., 50 μg/g), while the specificity at the same cut-off value ranged from 58.4% to 78.5%.
Furthermore, while qualitative correlation among the methods from the different manufacturers was found to be good, quantitative agreement was poor, which means that the result of one method cannot be replaced by the result of another. This data are in line with a study from the United Kingdom National External Quality Assessment Service, where up to 3.8-fold differences among methods from different manufacturers were observed[18]. This suggests that the antibodies used in the different assays were directed against different protein complexes. Alternatively, the difference could be explained by the use of different antibodies (monoclonal vs polyclonal) of different origins (recombinant vs native) with different immunoassay techniques (ELISA vs PETIA vs CLIA vs FEIA).
Further quantitative tests for calprotectin are available including the Quantum Blue® Calprotectin Rapid Test (Bühlmann Laboratories AG, Schönenbuch, Switzerland), which has been shown to be a suitable alternative to ELISA in a clinical setting[19], although an overestimation of FC levels in comparison with Calprest® ELISA test was found[20].
In pediatric IBD patients, an automated ELISA test (Bühlmann PhiCal Calprotectin-EIA) , an EliA (Phadia 250 EliA-Calprotectin), and Bühlmann immunochromatographic Point-of-Care Test (POCT ) displayed similar performance in predicting relapse[21].
Although many studies have suggested different cut-off values, which take into account the type of assay used and the population that the tests were applied to (Table 1), a cut/off value of 50 μg/g of FC has been the most commonly adopted both in literature and by commercially available ELISA kits, for adults and children over 4 years to differentiate IBD from other forms of inflammation[22]. Moreover, Lin et al[23] suggested 50 μg/g as a screening cut-off value for further endoscopy examination in clinical practice, with specificity of 60% and pooled sensitivity of 92%.
| Ref. | Patients and disease (n) | Cut-off | Sensitivity (%) | Specificity (%) |
| Lin et al[23] | 1471 IBD (active vs inactive) | 50 μg/g | 92 | 60 |
| 100 μg/g | 84 | 66 | ||
| 150 μg/g | 80 | 82 | ||
| Limburg et al[24] | 110 patients with chronic diarrhea (prediction of inflammation) | 100 μg/g | 83 | 83 |
| Von Roon et al[25] | IBD vs no IBD | |||
| 1267 | 50 μg/g | 89 | 81 | |
| 328 | 100 μg/g | 98 | 91 | |
| D’Haens et al[27] | 126 IBD (large ulcers) | 250 μg/g | 60.4 | 79.5 |
| 87 CD (Endoscopic remission) | 250 μg/g | 94 | 62.2 | |
| 39 UC (Active mucosal disease) | ||||
| 250 μg/g | 71 | 100 | ||
| Sipponen et al[29] | 77 CD (active vs inactive) | 50 μg/g | 91 | 44 |
| 100 μg/g | 81 | 69 | ||
| 200 μg/g | 70 | 92 | ||
| Kittanakom et al[21] | 40 inactive pediatric CD (prediction of relapse) | 400 μg/g | 100 | 75.9 |
| (PhiCal Calprotectin - EIA) | ||||
| 500 μg/g (Buhlmann POCT) | ||||
| 800 μg/g | 100 | 75.9 | ||
| (EliA-Calprotectin) | ||||
| 100 | 75.9 | |||
| Vazquez Moron et al[31] | 71 CD (active vs inactive) | 170 μg/g | 77.6 | 95.5 |
A single cut-off level of 100 μg/g was agreed by an expert panel as appropriate for this purpose based on results from previous studies, which reported increased diagnostic precision for discrimination of colorectal inflammation in patients with CD and UC at this cut-off[24,25]; a higher cut-off level would be desirable to maximize the negative predictive value (NPV) and reduce incorrect diagnoses of IBD.
A negative test result at the lowest cut-off level (30 to 50 μg/g) suggests a diagnosis of a non-inflammatory condition, such as irritable bowel syndrome (IBS). A positive result at the cut-off of 100 μg/g may indicate IBS, with the recommendation to repeat the test in 6 wk to confirm the initial result[26].
As the cut-off value increases, sensitivity becomes lower and specificity higher. A FC value of 250 μg/g was deemed appropriate for monitoring disease activity in IBD. D’Haens et al[27] examinated 126 IBD patients (87 CD and 39 UC) and proposed a FC cut-off of 250 μg/g for indicating IBD remission.
The same cut-off was recommended by a recent meta-analysis[28] to contemplate escalating therapy with pooled sensitivity of 80% and specificity of 82%; in UC the test performed better than in CD.
An earlier study by Sipponen et al[29] proposed a cut-off value of 200 μg/g for identification of endoscopically inactive CD. In another study of 115 CD patients, FC less than 300 μg/g was associated with a reduced risk of disease relapse[30].
Even a 400 μg/g cut-off was agreed by an expert panel with 1000 μg/g proposed for monitoring response to therapy in patients with severe active IBD[26].
In pediatric patients the optimal FC cut-offs to differentiate active from inactive IBD were 400, 500, and 800 μg/g measured by different methods (PhiCal Calprotectin-EIA, Bühlmann POCT, and EliA-Calprotectin)[21].
In CD, FC concentration of ≥ 170 μg/g predicted endoscopic activity with 77.6% sensitivity, 95.5% specificity and likelihood ratio +17.06, while values ≤ 71 μg/g were predictive of mucosal healing with sensitivity of 95.9%, specificity of 52.3% and likelihood ratio -0.08[31].
Larger prospective studies were suggested to be carried out to validate the cut-off values in clinical practice for UC, using endoscopy as a reference. Once established for UC, cut-off values for CD could then be developed and validated, although harder to establish due to the lack of consistent evidence. In addition, small bowel disease activity is reflected by FC correlates less reliably than in case of colonic involvement[32,33].
FC levels have a significant negative correlation with age[28]. Children in the first months of life have high FC concentrations, which could reflect an increased trans-epithelial migration of either granulocytes or newly recruited macrophages as well as inability to regulate the microbial gut flora related to immaturity of the mucosal barrier function[28]. The increase of intestinal permeability during the first weeks of life was suggested in healthy newborns with high FC levels[34]. The type of feeding also influences FC concentrations: breastfed infants have higher FC levels than non-breastfed ones in the first months of life, reflecting the influence of immunomodulatory factors in human milk on the gut mucosa[28].
A statistical difference was found between FC in healthy children aged 1-3 mo and those aged 3-6 mo (375.2 μg/g vs 217.9 μg/g, P < 0.001), as well as between 1-6 mo and 6-18 mo (median: 282.7 μg/g vs 114.9 μg/g; P < 0.001)[28]. The results clearly indicate that different cut-offs are necessary for children less than 4 years old. Oord et al[35] proposed 538 μg/g for 1-6 mo, 214 μg/g for 6 mo-3 years, and 75 μg/g for 3-4 years.
Finally, in newborns FC concentrations may increase up to 30% when the sample is collected from a diaper, which may be explained by the water absorption into the diaper[35].
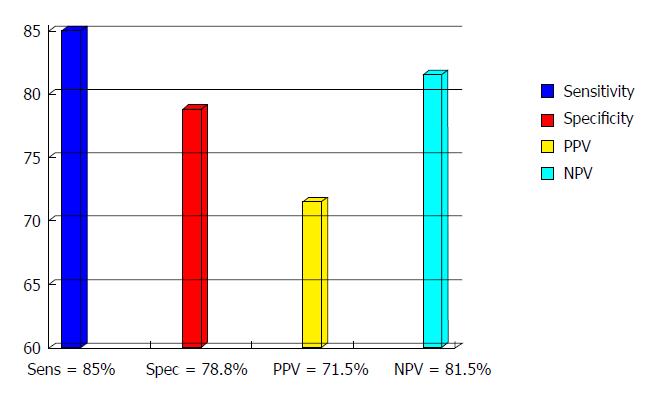
The role of FC as a screening test to differentiate patients with IBD from IBS was firstly proposed by Tibble and co-workers[36,37] who demonstrated the high sensitivity and high NPV of the test. In a population of 602 unselected patients with intestinal symptoms, FC levels < 30 μg/g and Rome I criteria positivity were highly predictive for not having IBD[38]. Since then, a considerable body of literature has been published corroborating these results (Figure 1), yet with wide variation in reported sensitivity and specificity, which may be related to the use of different ELISA kits, different patients populations and different cut off values. Summerton and colleagues[39] reported sensitivity and specificity in line with Tibble, while Carroccio et al[40] in a prospective study carried out in adults and children with chronic diarrhea of unknown origin found similar specificity (84%), but lower sensitivity (66%), attributed by the authors to the high number among their referrals of possible celiac patients, where FC levels are usually low. A major problem is represented by the assessment of the optimal threshold, as many authors used only the manifacturer’s recommended cut offs, mostly 50 μg/g. In the first study carried out on southern European patients by our group[41], the ROC curve showed that a value of 60 μg/g offered a diagnostic accuracy of 83% with sensitivity, specificity, positive predictive value (PPV) and NPV of 81%, 88%, 93% and 71% respectively. Li et al[42] found median FC concentrations of 466 μg/g in patients with chronic inflammation, 159 μg/g in colorectal cancer and 12.21 μg/g, not statistically different from healthy subjects and IBS patients. In a prospective study[8] where the accuracy of fecal markers, CRP, blood leucocytes, pASCA and pANCA for differentiating IBD from IBS was assessed, fecal tests performed best with overall accuracy of 90% and 89% for fecal lactoferrin (FL) and FC respectively. These results were confirmed by Otten et al[43], who found sensitivity and NPV of 100% for FC and 78% and 95% for FL, and by Langhorst et al[44] who reported in active UC significantly higher FL and FC levels (152 and 103.5 μg/g respectively) in comparison with IBS (8.3 and 18.6 μg/g)
In the first study which assessed the role of FC in routine general practice in 962 patients with persistent gastrointestinal symptoms[45], at the manufacturer’s cut-off of 50 μg/g the NPV was 98% while PPV dropped to a disappointing 28%, showing the impact of evaluating a population with a low prevalence of organic disease on the test performance. Increasing the cut off to 150 μg/g the PPV raised to 71%, saving an acceptable 69% sensitivity. In the systematic review by Waugh et al[46] evaluating FC testing for distinguishing between inflammatory and non-inflammatory bowel diseases, 28 studies in both adult and pediatric populations where included; at a cut off level of 50 μg/g, FC showed in adults a pooled sensitivity of 93% (83%-100%), while the specificity ranged between 60% and 100% with a pooled value of 94%. In pediatric patients, at the same cut off sensitivity and specificity ranged from 95% to 100% and from 44% to 93% respectively ; for overlap values between 50 μg/g and 150 μg/g repeated assessments were suggested; point-of-care and ELISA testing proved equally reliable and in a primary care setting FC turned out to reduce the number of referrals and endoscopies.
The potential of FC to discriminate between intestinal inflammation and functional disorders has been highlighted by a large number of further studies. Chang et al[47]confirmed significantly higher FC values in IBD than in IBS and healthy controls (P < 0.0001). Using the manufacturer cut off value Caviglia et al[48] found 100% sensitivity and NPV (with corresponding specificity and PPV 52.4% and 70.6%) for discriminating between patients with and without intestinal inflammation; similar results were reported by Banerjee et al[49]. In a systematic online database search, at ≤ 40 μg/g, less than 1% probability of having IBD was reported[50]. Lower values (76% sensitivity and 53% NPP) where reported by Fu et al[51] in a comparative study among fecal B cell-activating factor, FC and fecal occult blood test.
Adopting a cut off value > 164 μg/g Kalantari et al[52] found sensitivity and specificity of 57% and 75% respectively for discrimination between UC and IBS.
In conclusion, FC is currently the most widely used fecal marker for differentiating between IBD and IBS; due to the high NPV it is highly accurate in ruling out intestinal inflammation both in primary and secondary care. Among IBD patients apparently in remission with IBS-like symptoms, FC tends to be significantly higher than in IBS, suggesting the presence of an undercurrent low-grade inflammation[53,54].
As IBD are chronic relapsing diseases, regular monitoring is needed for prediction of imminent flares and for tailoring treatment[55]; it includes clinical, biochemical, endoscopic and histologic evaluations.
Many physicians treating IBD still adopt a clinically-based management[56], even if recent data suggest that many IBD patients in clinical remission still have subclinical mucosal inflammation[57]. FC is correlated with clinical activity evaluated either by Sutherland criteria[58] or Partial Mayo Score[59]. In a study by Xiang et al[58], FC concentrations were useful to discriminate patients with active UC, inactive UC and control, with a cut-off point of 50 μg/g showing 91.9% sensitivity and 79.4% specificity; in patients with UC, FC had a better correlation with clinical activity than CRP. Moreover, in a prospective study, FC assessment after 3 mo of the initial treatment could predict the clinical course of UC patients after 3 years of follow up[60].
Although colonoscopy is considered the gold standard to assess disease activity, current ECCO guidelines emphasize that routine endoscopy for IBD patients in clinical remission is unnecessary, unless it is likely to change patient management[61]. Therefore, a marker reflecting intestinal inflammation in patients in clinical remission is needed. Many studies showed that FC is the most promising noninvasive marker for assessing mucosal inflammation. In a study by D’Haens et al[27] FC had a significant correlation with endoscopic disease scores in both CD and in UC: a cut-off value of 250 μg/g suggested the presence of large ulcers with sensitivity of 60.4%, specificity of 79.5%, PPV 78.4% and NPV 62.0%) in CD, while in UC, a FC > 250 μg/g gave a sensitivity of 71.0% and a specificity of 100.0% (PPV 100.0%, NPV 47.1%) for mucosal disease activity (Mayo > 0). In UC, FC levels reflect the degree of inflammation rather than the disease extent[10].
Interestingly, FC were significantly related to symptom scores in UC (r = 0.561, P < 0.001), but not in CD. A study by Theede et al[62] found a strong correlation both with Mayo Endoscopic Score and Ulcerative Colitis Endoscopic Score. A correlation with Rachmilewitz and modified Baron Score was also demonstrated. Schoepfer and colleagues[63,64] found that FC was the only marker able to discriminate among mild, moderate and severe disease. A recent Korean study[59] highlighted how not only the ELISA, but also the Quantitative POCT predicted endoscopic inflammation (Mayo endoscopic score ≥ 1) in UC at a cut-off value of 201.3 μg/g and 150.5 μg/g respectively. In CD, a significant correlation with endoscopic activity was found both in colonic[33] and in small bowel CD[36], as well as with capsule endoscopy[65].
In a prospective study on 58 pediatric patients[66] FC showed a high correlation both with endoscopy (r = 0.655) and histology grading (r = 0.699); it proved the most accurate tool (sensitivity 94%, specificity 64%, PPV 81%, NPV 87%) to detect active mucosal inflammation when compared to clinical scores and serum markers. The highest accuracy was found in patients with apparent clinical and laboratory remission (sensitivity 100%, specificity 80%, PPV 67%, NPV 100%).
Guardiola et al[67] prospectively evaluated UC patients in clinical and endoscopic remission; those with histologic features of inflammation were reliably identified based on their FC levels at a cut off of 155 μg/g with a sensitivity of 78% and a specificity of 71%. More recently, Zittan et al[68] confirmed that FC could predict histological remission, with a cut-off of 100 μg/g. A recent study comparing the predictive value of FC measurement and histological scoring in IBD patients, found that FC performed better, especially in UC[69]. This finding was confirmed in a subsequent study by Theede et al[70], who showed that in UC baseline FC more than 321 μg/g predicted relapse both at 6-mo and 12-mo in contrast to histological activity, CRP, or length of remission. A study by Puolanne et al[71] confirmed the correlation between FC, clinical activity, and histopatologic findings in 72 patients with colonic IBD.
In an English study[72], calprotectin concentration in the colonic mucosa of UC patients correlated with histological remission; moreover, a median value > 5/HPF were independently associated with worse outcome (corticosteroid use, hospitalisation, or colectomy during a 6-year follow-up). Moreover, in a short report by Roseth et al[73], low FC levels were closely associated with mucosal healing.
A major challenge in managing IBD is a timely detection of patients at risk for impending clinical relapse. Tibble et al. firstly suggested that a high FC concentration could identify those IBD patients in remission who were at risk of early relapse without any difference between UC and CD[74]. Conversely, we showed a 14-fold increase in the relapse risk in patients with UC and a two-fold increase in CD patients in clinical remission with FC concentration higher than 150 μg/g concluding that FC was a stronger predictor of clinical relapse in UC than in CD[32]. In CD, D’Incà et al[33] found a significant correlation between a positive FC test and probability of relapse (P < 0.001) only for colonic localization at 130 μg/g cut-off level. On the other hand, a meta-analysis by Mao et al[3] was not able to demonstrate that the overall accuracy of FC for predicting relapse was different in UC and CD because of the heterogeneity across studies, due to different criteria to define remission and relapse; however, due to the limited data, the ileal involvement was not assessed.
In asymptomatic patients with IBD, Heida et al[75] found that increase of FC levels were correlated with increased (from 53% to 83%) probability of relapse within the next 2 mo to 3 mo, while consecutive normal FC values were associated with 67% to 94% probability of remission in the next 2 mo to 3 mo.
In patients under maintenance therapy with Infliximab (IFX), levels > 160 μg/g were related to probability of relapse higher than 60% over the following 8 wk[76].
In a subanalysis of the STORI trial, serial measurements of FC in CD patients in clinical remission after stopping IFX, showed that in those who relapsed, the FC levels had started to increase 4-6 mo earlier[77]. Despite the test reliability, the ideal FC threshold for monitoring disease relapse is still awaiting to be defined.
In clinical practice, “Treat-To-Target” is currently considered the most important strategy for therapy adjustment.
A study by Wagner et al[78] in patients with UC or CD treated with 5-aminosalicilic acid, prednisone or Azathioprine, showed that FC were correlated with clinical scores after 4 wk and 8 wk of treatment in UC and in CD, respectively, and in patients with complete response to therapy there was a significant decline in FC levels (P < 0.01) after 4 wk, which was not observed in partial or non-responders. In children with active disease treated with steroids, FC levels declined in line with clinical improvement but seldom fell within the normal range[79].
In the biologic era, many studies confirmed the role of FC in monitoring the effectiveness of therapy. Molander et al[80] demonstrated that a normal FC (< 100 μg/g) after induction therapy with anti-TNFα predicts sustained clinical remission in the majority of patients, both in CD and UC; a cut-off of 139 μg/g for FC had 72% sensitivity and 80% specificity to predict the risk of clinically active disease after 1 year. According to De Vos et al[81] two consecutive FC measurements over 300 μg/g are more specific than a single assessment for predicting relapse in UC patients under maintenance treatment with IFX.
Interestingly, even after discontinuation of anti-TNFα therapy, an increase of FC could predict clinical and endoscopic relapse[82]. This data is in accordance with the STORI study[30,77], where FC was comparable to endoscopic assessment in predicting the relapse risk after stopping TNFα-blocking therapy, starting to increase 4-6 mo before the clinical relapse. A prospective study[83] in IBD patients (20 UC and 52 CD) under treatment with anti-TNFα, showed that the diagnostic accuracy of rapid FC seems to be higher in predicting persistence of endoscopic lesions than clinical remission.
Both in monitoring of therapy and in prediction of relapses FC seems to be more effective in UC than in CD[32]. Nevertheless, in a prospective study of Laharie et al[84] in patients responding to IFX induction regimen, FC measurement at w14 could not predict CD clinical relapse at one year. In severe acute colitis, FC evaluation could be helpful in timely prediction of clinical course: Ho et colleagues[85] demonstrated that FC was higher in patients requiring colectomy with a trend toward significance when compared to responders, suggesting that FC in patients with severe acute colitis could be included among the prognostic criteria.
Shifting the therapeutic target from clinical remission to mucosal healing has been supported by population-based cohort studies, post hoc analysis of clinical trials, and meta-analysis, both for CD and UC[86-90]. The STRIDE recommendations[91] defined FC as an adjunctive target in IBD patients, while a Mayo Endoscopic Score ≤ 1 for UC and the resolution of ulcerations in CD are the best target to reach, besides patient reported outcome. The recently published CALM study[92], for the first time used FC as a target despite clinical activity in CD patients, in whom therapy was escalated if FC was ≥ 250 μg/g in a group of patients, while the control group was treated on the basis of clinical activity. The tight control algorithm led to rapid optimization of therapy and, therefore, to a higher proportion of patients achieving mucosal healing [CD Endoscopic Activity Index of Severity (CDEIS) < 4] and no deep ulcers on endoscopy, deep remission [CD Activity Index (CDAI) < 150 and CDEIS < 4 and no deep ulcers, no draining fistula, and no prednisone use for 8 wk or more], biological remission (FC < 250 μg/g, CRP < 5 mg/L, and CDEIS < 4), and steroid-free remission (CDAI < 150 with no prednisone for 8 wk). A limitation is represented by the discretional taper schedule of prednisone at study entry, that, affecting the treatment option at randomization (the use of prednisone defined treatment failure in the tight control group) could have led to an earlier introduction of adalimumab and positively affected the outcomes.
Despite the increasing use of immunosuppressants and biologics, IBD patients frequently need surgery. Approximately 80% of CD patients require intestinal surgery within 20 years after diagnosis and 10%-30% UC patients need colectomy, at 25 years following diagnosis[93]. Surgery is not curative, and is followed by post-operative recurrence (POR, in CD patients) and pouchitis (in UC patients) in a high percentage of cases. The post-operative monitoring, mainly based on endoscopy, is crucial to identify those patients who require early treatment. Non-invasive markers of intestinal inflammation, especially FC, represent an easy, quick and cheap tool for the early diagnosis of post-operative recurrence or pouchitis.
Post-operative recurrence: POR after ileo-colonic resection is a feature of CD. Early studies by the Leuven group reported an endoscopic and histological recurrence rate of 73% within one year from surgery although only 20% of the patients had symptoms[94]. A more recent review, focusing on historical population-based studies, showed that the cumulative risk of POR after 10 years is around 44%-55%[95]. As endoscopic recurrence occurs before the onset of symptoms[94], the early detection of asymptomatic endoscopic lesions may allow a timely treatment in post-operative CD patients. Conventional ileocolonscopy within 6-12 mo is currently recommended to evaluate CD recurrence, graded according to the Rutgeerts’ score. The Post-Operative Crohn’s Endoscopic Recurrence (POCER) study showed that postoperative endoscopic monitoring, together with treatment escalation for early recurrence, is superior to standard drug therapy alone in preventing disease recurrence, at least in the short term[96]. However, it is not established the timing of endoscopic re-evaluation. Ileocolonoscopy is expensive, time-consuming, often not well accepted by the patient and not devoid of risks. Moreover, endoscopic examination of the neo-terminal ileum is not always technically feasible[97].
Although the role of FC in early detection of POR is still to be established, several studies suggest that FC could avoid unnecessary endoscopies and facilitate earlier diagnosis. FC and FL assay have been suggested as non-invasive, inexpensive and reproducible biomarkers in post-operative CD patients[98].
Orlando et al[99] prospectively evaluated 50 CD patients who had undergone surgery; a FC value > 200 μg/g within 3 mo showed 63% sensitivity and 75% specificity in predicting endoscopic recurrence at one year, superior to ultrasound, whose sensitivity and specificity was 26% and 90% respectively.
In asymptomatic CD patients who had undergone ileo-colonic resection with a median follow-up of 40.5 mo, long term high levels of FL and FC were observed, interpreted as sign of ongoing intestinal inflammation, although partially influenced by the systemic post- operative inflammatory status[100].
In a small cohort of 13 post-operative CD patients followed for 1 year, FC and FL were more accurate in predicting clinical disease activity than CRP, platelet count or endoscopic appearance[101]. Accordingly, FC and FL levels positively correlated with both clinical recurrence and severity of endoscopic findings in the neo-terminal ileum who remained in remission during 6-12 mo after ileocolonic resection[102]. At 170 μg/g cut-off, sensitivity and specificity of FC were higher than FL (83% and 93% vs 67% and 71% respectively) in predicting risk of clinical relapse. More recently, the same authors showed that in asymptomatic patients after ileo-colonic resection for CD, sustained low FC levels predict low risk of endoscopic recurrence, avoiding unnecessary endoscopic examinations[103].
These data are in line with Boschetti et al[104], who found were significantly higher (473 ± 78 μg/g) FC levels in asymptomatic CD patients with endoscopic recurrence after ileo-colonic resection in the last 18 mo when compared with those in remission (115 ± 18 μg/g, P < 0.0001). Sensitivity analysis excluding patients with both ileal and colonic recurrence did not change the results (456 ± 68 μg/g vs 115 ± 18 μg/g; P < 0.0002). The best cutoff point for FC to distinguish between endoscopic remission and recurrence was 100 μg/g as determined by the ROC curve, and its sensitivity, specificity, PPV and NPV, as well as overall accuracy were 95%, 54%, 69%, 93%, and 77%, respectively. Taking into account the high NPV of FC, a threshold below 100 μg/g could avoid systematic ileocolonoscopies in 30% of patients.
In the retrospective study by Herranz Bachiller et al[105] 97 patients with CD and ileocolic resection who had undergone FC measurement and subsequent ileo-colonoscopy were included. FC was related to endoscopic recurrence more than any clinical or serological parameters. Unlike other studies, the optimal cut-off was 60 μg/g.
Lobatón et al[106], compared the accuracy of ELISA test with the new quantitative POCT for the prediction of endoscopic activity and POR in CD patients. FC levels correlated more closely with CDEIS than leucocytes, platelets or CRP. The prediction of endoscopic remission (CDEIS < 3), using the quantitative POCT (cut-off 272 μg/g) and the ELISA (cut-off 274 μg/g) presented an area under the curve of 0.933 and 0.935, respectively. Median POCT levels discriminated endoscopic (Rutgeerts) score i0-i1 from i2-i4 (98 μg/g vs 234.5 μg/g). These results suggest that FC determined by rapid quantitative test predicts endoscopic remission as well as endoscopic postoperative recurrence in CD patients.
Disappointing results came from a Swedish study[107], that found no significant difference in FC concentrations between patients in endoscopic remission and relapsing patients one year after ileoceacal resection. However, the significant variation over time of FC concentrations highly influenced these results, especially in patients with diarrhea, which implies that a single measurement of FC has limited clinical utility in predicting POR.
The sub-analysis of the POCER study by Wright et al[108], demonstrated that FC has good sensitivity and NPV to monitor CD recurrence after intestinal resection. Levels of FC were measured in 319 samples from 135 patients. FC concentration was markedly increased before surgery and decreased substantially after resection of all macroscopically involved segments at 6 mo. Combined 6- and 18-mo FC levels correlated significantly with endoscopic recurrence, whereas CRP and CDAI did not. A cutoff of FC > 100 μg/g detected patients with endoscopic recurrence with 89% sensitivity, 58% specificity and 91% NPV. In this cohort, colonoscopy could be avoided in 47% of cases with endoscopic remission, at the cost of missing 11% of patients with endoscopic recurrence. Also, FC decreased in patients who underwent therapy intensification supporting its role in treatment monitoring. A FC level < 51 μg/g in patients in remission at 6 mo after surgery predicted remission at 18 mo, with 79% NPV; sensitivity, specificity and PPV were less satisfying (50%, 68% and 36%, respectively), suggesting a limited value of FC measurement in long-term prediction of endoscopic recurrence.
Large scale studies should be carried out to clarify controversial points. The optimal cut-off value of FC as a surrogate marker of POR needs to be established and the measurement procedures to be standardized. Nevertheless, our overview suggests the use of FC as promising alternative to ileo-colonoscopy in POR , especially in asymptomatic CD patients after initial negative post-operative endoscopy, and in monitoring response to treatment.
Pouchitis: Ileal pouch anal anastomosis (IPAA) after restorative proctocolectomy is currently the preferred surgical treatment for refractory or complicated UC. De novo inflammation of the ileal reservoir, the so-called pouchitis, is reported in about half of the patients. Even though the etiology of pouchitis remains unknown, several influencing factors have been suggested, such as fecal stasis, bacterial overgrowth, dysbiosis, genetic susceptibility and immune alteration. More recently, a CD-like complication of the pouch, has been described which can involve up to 13% of the patients following proctocolectomy with IPAA for UC. This entity is characterized by inflammation in the afferent limb (prepouch ileitis), presence of proximal small bowel strictures, or perianal or internal fistulae unrelated to surgery[109].
In 1994, the pouchitis disease activity index (PDAI), a composite score evaluating symptoms, endoscopic and histologic alteration has been developed to standardize the definition of pouchitis and to assess its severity. Patients with a total PDAI score of ≥ 7 points are classified as having pouchitis. The diagnosis of pouchitis therefore requires endoscopic confirmation with mucosal biopsies. Few studies have evaluated the value of FC measurement in these patients. However, available data show possible benefit with accurate diagnosis and management of pouch disorders as well as cost reduction.
In the small study by Thomas et al[110], significantly increased FC levels were found in all 9 patients with endoscopic and histologic evidence of pouch inflammation compared with those without it. The first-morning FC levels correlated well (r = 0.91, P ≤ 0.0001) with 24-h stool collection, with endoscopic and histologic scores, and with the percentage of CD15+ mature neutrophils and CD14+ macrophages within the lamina propria.
These findings were confirmed in a larger study carried out in 46 patients with UC and in 8 with familial adenomatous polyposis, who had undergone restorative proctocolectomy[111]. Using a threshold of 92.5 μg/g, FC levels correlated closely with the PDAI with a sensitivity of 90% and a specificity of 76.5%.
In pediatric UC, FC levels after restorative proctocolectomy positively correlated with subsequent pouchitis (r = 0.468, P < 0.01), with mean FC values of 71.50 μg/g among patients with no history of pouchitis, 290 ± 131 μg/g among those with a single episode of pouchitis, and highest level 832 ± 422 μg/g among patients with recurrent pouchitis (P = 0.019 between recurrent pouchitis and no pouchitis). A history of recurrent pouchitis was a significant predictor of FC higher than 300 μg/g (OR = 51; 95%CI: 1.2-2200; P = 0.040). Sensitivity, specificity, PPV, and NPV for FC concentration over 300 μg/g in detecting recurrent pouchitis were 57%, 92%, 67%, and 89%, respectively[112].
Yamamoto et al[113] prospectively evaluated the serial monitoring of FC and FL for the early detection of pouchitis after restorative proctocolectomy. Stool samples were collected every 2 mo up to 12 mo from 60 patients who had undergone ileostomy closure following total proctocolectomy and IPAA for UC. Endoscopy was performed in all asymptomatic patients at 12 mo and as soon as symptoms suggestive of pouchitis occurred. In the 10 patients (17%) who developed pouchitis FC and FL levels were already increased 2 mo before the diagnosis of pouchitis, while in the others both markers remained constantly at low levels. At cut-off values of 56 μg/g for FC and 50 μg/g for FL, sensitivity and specificity were 100% and 84%, and 90% and 86% respectively. At the time of endoscopy, the median FC and FL levels were significantly higher in patients with pouchitis than those without. Nevertheless, several questions can be raised on how to implement these findings into clinical practice. Current guidelines do not recommend routine pouchoscopy in patients in clinical remission as symptoms seem to reflect underlying inflammation in the pouch[114]. The results by Yamamoto et al[113] are in line with these recommendation. None of the 47 asymptomatic patients developed pouchitis during the 12-mo follow-up period, whereas in 10/13 symptomatic patients the inflammation of the pouch was confirmed. Thus, the NPV of 100% of the PDAI score < 7 could be considered as referral criteria for pouchoscopy in symptomatic patients[115].
In conclusion, even in patients with IPAA FC could allow the early detection of subclinical inflammation. Prospective studies need to establish whether this strategy could reduce the rate of chronic pouchitis and subsequent pouch failure.
We reviewed the role of FC in various settings of IBD clinical management. About 20 years after the study by Roseth[10], FC has been confirmed as one of the most reliable, non-invasive diagnostic tools for management of IBD in clinical practice both in adults and children.
A considerable body of evidence confirms the high sensitivity and NPV of FC in distinguishing IBD from IBS in patients with clinical suspicion of intestinal inflammation. In those with established diagnosis of IBD, a growing number of studies suggest an increasingly recognized role of the test in monitoring disease activity and response to therapy, as well as in predicting disease relapse and POR, including pouchitis. The main concerns are still related to the choice of the optimal cut-off, both for ruling out intestinal inflammation and for the management of IBD patients.
Recently the CALM study[92] included FC measurement among the treatment failure criteria for escalating therapy in patients with early CD, and showed that adjustment of therapy based on the combination of clinical symptoms and biomarkers leads to better outcomes than symptoms-driven decision. These results support the use of FC in the context of the “Treat-To-Target” strategy and may open the way for a higher standard of care in IBD patients, if confirmed by further studies with a longer follow up. Finally, similarly designed studies are awaited in UC, where FC appears to perform best.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Can G, Esmat SM, Ribaldone DG S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Rodrigo L. [Fecal calprotectin]. Rev Esp Enferm Dig. 2007;99:683-688. [PubMed] |
| 2. | Dale I, Fagerhol MK, Naesgaard I. Purification and partial characterization of a highly immunogenic human leukocyte protein, the L1 antigen. Eur J Biochem. 1983;134:1-6. [PubMed] |
| 3. | Mao R, Xiao YL, Gao X, Chen BL, He Y, Yang L, Hu PJ, Chen MH. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. 2012;18:1894-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Kolho KL, Alfthan H, Hämäläinen E. Effect of bowel cleansing for colonoscopy on fecal calprotectin levels in pediatric patients. J Pediatr Gastroenterol Nutr. 2012;55:751-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:279-284. [PubMed] |
| 6. | Calafat M, Cabré E, Mañosa M, Lobatón T, Marín L, Domènech E. High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis. 2015;21:1072-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Ricanek P, Brackmann S, Perminow G, Lyckander LG, Sponheim J, Holme O, Høie O, Rydning A, Vatn MH; IBSEN II Study Group. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793-798. [PubMed] |
| 10. | Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176-180. [PubMed] |
| 11. | Joishy M, Davies I, Ahmed M, Wassel J, Davies K, Sayers A, Jenkins H. Fecal calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Lasson A, Stotzer PO, Öhman L, Isaksson S, Sapnara M, Strid H. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis. 2015;9:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Husebye E, Tøn H, Johne B. Biological variability of fecal calprotectin in patients referred for colonoscopy without colonic inflammation or neoplasm. Am J Gastroenterol. 2001;96:2683-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Moum B, Jahnsen J, Bernklev T. Fecal calprotectin variability in Crohn’s disease. Inflamm Bowel Dis. 2010;16:1091-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Naismith GD, Smith LA, Barry SJ, Munro JI, Laird S, Rankin K, Morris AJ, Winter JW, Gaya DR. A prospective single-centre evaluation of the intra-individual variability of faecal calprotectin in quiescent Crohn’s disease. Aliment Pharmacol Ther. 2013;37:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Dobrzanski C, Pedersen N, Fuglsang H, Munkholm P. Is there a diurnal variation in faecal calprotectin in inflammatory bowel disease patients? United European Gastroenterol J. 2013;1:A135-A587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Oyaert M, Boel A, Jacobs J, Van den Bremt S, De Sloovere M, Vanpoucke H, Van Hoovels L. Analytical performance and diagnostic accuracy of six different faecal calprotectin assays in inflammatory bowel disease. Clin Chem Lab Med. 2017;55:1564-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Whitehead SJ, French J, Brookes MJ, Ford C, Gama R. Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann Clin Biochem. 2013;50:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Coorevits L, Baert FJ, Vanpoucke HJ. Faecal calprotectin: comparative study of the Quantum Blue rapid test and an established ELISA method. Clin Chem Lab Med. 2013;51:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Caviglia GP, Ribaldone DG, Rosso C, Saracco GM, Astegiano M, Pellicano R. Fecal calprotectin: beyond intestinal organic diseases. Panminerva Med. 2018;60:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Kittanakom S, Shajib MS, Garvie K, Turner J, Brooks D, Odeh S, Issenman R, Chetty VT, Macri J, Khan WI. Comparison of Fecal Calprotectin Methods for Predicting Relapse of Pediatric Inflammatory Bowel Disease. Can J Gastroenterol Hepatol. 2017;2017:1450970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009;41:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Lin JF, Chen JM, Zuo JH, Yu A, Xiao ZJ, Deng FH, Nie B, Jiang B. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 24. | Limburg PJ, Ahlquist DA, Sandborn WJ, Mahoney DW, Devens ME, Harrington JJ, Zinsmeister AR. Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000;95:2831-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, Paraskeva P, Tekkis PP. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (2)] |
| 26. | Rogler G, Aldeguer X, Kruis W, Lasson A, Mittmann U, Nally K, Peyrin-Biroulet L, Schoepfer A, Vatn M, Vavricka S. Concept for a rapid point-of-care calprotectin diagnostic test for diagnosis and disease activity monitoring in patients with inflammatory bowel disease: expert clinical opinion. J Crohns Colitis. 2013;7:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 623] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 28. | Li F, Ma J, Geng S, Wang J, Liu J, Zhang J, Sheng X. Fecal calprotectin concentrations in healthy children aged 1-18 months. PLoS One. 2015;10:e0119574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 30. | Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63-70.e5; quiz e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 459] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 31. | Vázquez Morón JM, Pallarés Manrique H, Machancoses FH, Ramos Lora M, Ruiz Frutos C. Accurate cut-offs for predicting endoscopic activity and mucosal healing in Crohn’s disease with fecal calprotectin. Rev Esp Enferm Dig. 2017;109:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 33. | D'Incà R, Dal Pont E, Di Leo V, Benazzato L, Martinato M, Lamboglia F, Oliva L, Sturniolo GC. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007-2014. [PubMed] |
| 34. | Campeotto F, Butel MJ, Kalach N, Derrieux S, Aubert-Jacquin C, Barbot L, Francoual C, Dupont C, Kapel N. High faecal calprotectin concentrations in newborn infants. Arch Dis Child Fetal Neonatal Ed. 2004;89:F353-F355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Oord T, Hornung N. Fecal calprotectin in healthy children. Scand J Clin Lab Invest. 2014;74:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506-513. [PubMed] |
| 37. | Tibble JA, Bjarnason I. Non-invasive investigation of inflammatory bowel disease. World J Gastroenterol. 2001;7:460-465. [PubMed] |
| 38. | Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450-460. [PubMed] |
| 39. | Summerton CB, Longlands MG, Wiener K, Shreeve DR. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:841-845. [PubMed] |
| 40. | Carroccio A, Iacono G, Cottone M, Di Prima L, Cartabellotta F, Cavataio F, Scalici C, Montalto G, Di Fede G, Rini G. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem. 2003;49:861-867. [PubMed] |
| 41. | Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, Sterpi C, Marchi S, Maltinti G. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35:642-647. [PubMed] |
| 42. | Li XG, Lu YM, Gu F, Yang XL. [Fecal calprotectin in differential diagnosis of irritable bowel syndrome]. Beijing Da Xue Xue Bao Yi Xue Ban. 2006;38:310-313. [PubMed] |
| 43. | Otten CM, Kok L, Witteman BJ, Baumgarten R, Kampman E, Moons KG, de Wit NJ. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Langhorst J, Junge A, Rueffer A, Wehkamp J, Foell D, Michalsen A, Musial F, Dobos GJ. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Pavlidis P, Chedgy FJ, Tibble JA. Diagnostic accuracy and clinical application of faecal calprotectin in adult patients presenting with gastrointestinal symptoms in primary care. Scand J Gastroenterol. 2013;48:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Waugh N, Cummins E, Royle P, Kandala NB, Shyangdan D, Arasaradnam R, Clar C, Johnston R. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess. 2013;17:xv-xix, 1-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 47. | Chang MH, Chou JW, Chen SM, Tsai MC, Sun YS, Lin CC, Lin CP. Faecal calprotectin as a novel biomarker for differentiating between inflammatory bowel disease and irritable bowel syndrome. Mol Med Rep. 2014;10:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 48. | Caviglia GP, Pantaleoni S, Touscoz GA, Adriani A, Rosso C, Smedile A, Pellicano R, Astegiano M, Bresso F. Fecal calprotectin is an effective diagnostic tool that differentiates inflammatory from functional intestinal disorders. Scand J Gastroenterol. 2014;49:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Banerjee A, Srinivas M, Eyre R, Ellis R, Waugh N, Bardhan KD, Basumani P. Faecal calprotectin for differentiating between irritable bowel syndrome and inflammatory bowel disease: a useful screen in daily gastroenterology practice. Frontline Gastroenterol. 2015;6:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Menees SB, Powell C, Kurlander J, Goel A, Chey WD. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 51. | Fu Y, Wang L, Xie C, Zou K, Tu L, Yan W, Hou X. Comparison of non-invasive biomarkers faecal BAFF, calprotectin and FOBT in discriminating IBS from IBD and evaluation of intestinal inflammation. Sci Rep. 2017;7:2669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Kalantari H, Taheri A, Yaran M. Fecal calprotectin is a useful marker to diagnose ulcerative colitis from irritable bowel syndrome. Adv Biomed Res. 2015;4:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Keohane J, O’Mahony C, O’Mahony L, O’Mahony S, Quigley EM, Shanahan F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105:1788, 1789-1794; quiz 1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 54. | Quigley EM. Overlapping irritable bowel syndrome and inflammatory bowel disease: less to this than meets the eye? Therap Adv Gastroenterol. 2016;9:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 55. | Chen JM, Liu T, Gao S, Tong XD, Deng FH, Nie B. Efficacy of noninvasive evaluations in monitoring inflammatory bowel disease activity: A prospective study in China. World J Gastroenterol. 2017;23:8235-8247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (3)] |
| 56. | Schoepfer AM, Vavricka S, Zahnd-Straumann N, Straumann A, Beglinger C. Monitoring inflammatory bowel disease activity: clinical activity is judged to be more relevant than endoscopic severity or biomarkers. J Crohns Colitis. 2012;6:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Baars JE, Nuij VJ, Oldenburg B, Kuipers EJ, van der Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis. 2012;18:1634-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 58. | Xiang JY, Ouyang Q, Li GD, Xiao NP. Clinical value of fecal calprotectin in determining disease activity of ulcerative colitis. World J Gastroenterol. 2008;14:53-57. [PubMed] |
| 59. | Lee YW, Lee KM, Lee JM, Chung YY, Kim DB, Kim YJ, Chung WC, Paik CN. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J Intern Med. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | Lasson A, Simrén M, Stotzer PO, Isaksson S, Ohman L, Strid H. Fecal calprotectin levels predict the clinical course in patients with new onset of ulcerative colitis. Inflamm Bowel Dis. 2013;19:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 61. | Elkjaer M. E-health: Web-guided therapy and disease self-management in ulcerative colitis. Impact on disease outcome, quality of life and compliance. Dan Med J. 2012;59:B4478. [PubMed] |
| 62. | Theede K, Holck S, Ibsen P, Ladelund S, Nordgaard-Lassen I, Nielsen AM. Level of Fecal Calprotectin Correlates With Endoscopic and Histologic Inflammation and Identifies Patients With Mucosal Healing in Ulcerative Colitis. Clin Gastroenterol Hepatol. 2015;13:1929-1936.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 63. | Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, Armstrong D, Schmidt C, Trummler M, Pittet V, Vavricka SR. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013;19:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 64. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 65. | Kopylov U, Yung DE, Engel T, Avni T, Battat R, Ben-Horin S, Plevris JN, Eliakim R, Koulaouzidis A. Fecal calprotectin for the prediction of small-bowel Crohn’s disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Canani RB, Terrin G, Rapacciuolo L, Miele E, Siani MC, Puzone C, Cosenza L, Staiano A, Troncone R. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008;40:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 67. | Guardiola J, Lobatón T, Rodríguez-Alonso L, Ruiz-Cerulla A, Arajol C, Loayza C, Sanjuan X, Sánchez E, Rodríguez-Moranta F. Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol. 2014;12:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 68. | Zittan E, Kelly OB, Kirsch R, Milgrom R, Burns J, Nguyen GC, Croitoru K, Van Assche G, Silverberg MS, Steinhart AH. Low Fecal Calprotectin Correlates with Histological Remission and Mucosal Healing in Ulcerative Colitis and Colonic Crohn’s Disease. Inflamm Bowel Dis. 2016;22:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 69. | Mooiweer E, Severs M, Schipper ME, Fidder HH, Siersema PD, Laheij RJ, Oldenburg B. Low fecal calprotectin predicts sustained clinical remission in inflammatory bowel disease patients: a plea for deep remission. J Crohns Colitis. 2015;9:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 70. | Theede K, Holck S, Ibsen P, Kallemose T, Nordgaard-Lassen I, Nielsen AM. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 71. | Puolanne AM, Kolho KL, Alfthan H, Ristimäki A, Mustonen H, Färkkilä M. Rapid Fecal Calprotectin Test and Symptom Index in Monitoring the Disease Activity in Colonic Inflammatory Bowel Disease. Dig Dis Sci. 2017;62:3123-3130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Guirgis M, Wendt E, Wang LM, Walsh A, Burger D, Bryant RV, Kent A, Adamson R, Brain O, Travis SPL. Beyond Histological Remission: Intramucosal Calprotectin as a Potential Predictor of Outcomes in Ulcerative Colitis. J Crohns Colitis. 2017;11:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Røseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004;39:1017-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 74. | Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15-22. [PubMed] |
| 75. | Heida A, Park KT, van Rheenen PF. Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide. Inflamm Bowel Dis. 2017;23:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 76. | Ferreiro-Iglesias R, Barreiro-de Acosta M, Otero Santiago M, Lorenzo Gonzalez A, Alonso de la Peña C, Benitez Estevez AJ, Dominguez-Muñoz JE. Fecal Calprotectin as Predictor of Relapse in Patients With Inflammatory Bowel Disease Under Maintenance Infliximab Therapy. J Clin Gastroenterol. 2016;50:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 77. | de Suray N, Salleron J, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M. P274 Close monitoring of CRP and fecal calprotectin levels to predict relapse in Crohn's disease patients. A sub-analysis of the STORI study. ACAAI. 2012;6:S118-S119. [DOI] [Full Text] |
| 78. | Wagner M, Peterson CG, Ridefelt P, Sangfelt P, Carlson M. Fecal markers of inflammation used as surrogate markers for treatment outcome in relapsing inflammatory bowel disease. World J Gastroenterol. 2008;14:5584-5589; discussion 5588. [PubMed] |
| 79. | Kolho KL, Raivio T, Lindahl H, Savilahti E. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;41:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Molander P, af Björkesten CG, Mustonen H, Haapamäki J, Vauhkonen M, Kolho KL, Färkkilä M, Sipponen T. Fecal calprotectin concentration predicts outcome in inflammatory bowel disease after induction therapy with TNFα blocking agents. Inflamm Bowel Dis. 2012;18:2011-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 81. | De Vos M, Louis EJ, Jahnsen J, Vandervoort JG, Noman M, Dewit O, D’haens GR, Franchimont D, Baert FJ, Torp RA. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013;19:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 82. | Molander P, Färkkilä M, Ristimäki A, Salminen K, Kemppainen H, Blomster T, Koskela R, Jussila A, Rautiainen H, Nissinen M. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis. 2015;9:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Tursi A, Elisei W, Picchio M, Giorgetti G, Brandimarte G. Accuracy of Rapid Fecal Calprotectin Test in Monitoring Inflammatory Bowel Diseases Under Treatment with TNFα Antagonists. Dig Dis Sci. 2015;60:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Laharie D, Mesli S, El Hajbi F, Chabrun E, Chanteloup E, Capdepont M, Razaire S, de Lédinghen V, Zerbib F. Prediction of Crohn’s disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther. 2011;34:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 85. | Ho GT, Lee HM, Brydon G, Ting T, Hare N, Drummond H, Shand AG, Bartolo DC, Wilson RG, Dunlop MG. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol. 2009;104:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 86. | Colombel JF, Rutgeerts PJ, Sandborn WJ, Yang M, Camez A, Pollack PF, Thakkar RB, Robinson AM, Chen N, Mulani PM. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:414-422.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 87. | Colombel JF, Narula N, Peyrin-Biroulet L. Management Strategies to Improve Outcomes of Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:351-361.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 88. | Shah SC, Colombel JF, Sands BE, Narula N. Mucosal Healing Is Associated With Improved Long-term Outcomes of Patients With Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1245-1255.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 89. | Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43:317-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 277] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 90. | Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 873] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 91. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D’Haens G, Dotan I, Dubinsky M, Feagan B. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1408] [Article Influence: 140.8] [Reference Citation Analysis (115)] |
| 92. | Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, Danalioglu A, Novacek G, Armuzzi A, Hébuterne X. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 671] [Article Influence: 83.9] [Reference Citation Analysis (1)] |
| 93. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1561] [Article Influence: 111.5] [Reference Citation Analysis (1)] |
| 94. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956-963. [PubMed] |
| 95. | Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 756] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 96. | De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, Liew D, Prideaux L, Lawrance IC, Andrews JM. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 443] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 97. | Garcia-Planella E, Mañosa M, Cabré E, Marín L, Gordillo J, Zabana Y, Boix J, Sáinz S, Domènech E. Fecal Calprotectin Levels Are Closely Correlated with the Absence of Relevant Mucosal Lesions in Postoperative Crohn’s Disease. Inflamm Bowel Dis. 2016;22:2879-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 98. | Yamamoto T. The clinical value of faecal calprotectin and lactoferrin measurement in postoperative Crohn’s disease. United European Gastroenterol J. 2015;3:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Orlando A, Modesto I, Castiglione F, Scala L, Scimeca D, Rispo A, Teresi S, Mocciaro F, Criscuoli V, Marrone C. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn’s disease: a comparison with ultrasound. Eur Rev Med Pharmacol Sci. 2006;10:17-22. [PubMed] |
| 100. | Scarpa M, D’Incà R, Basso D, Ruffolo C, Polese L, Bertin E, Luise A, Frego M, Plebani M, Sturniolo GC. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn’s disease. Dis Colon Rectum. 2007;50:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 101. | Lamb CA, Mohiuddin MK, Gicquel J, Neely D, Bergin FG, Hanson JM, Mansfield JC. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg. 2009;96:663-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 102. | Yamamoto T, Shiraki M, Bamba T, Umegae S, Matsumoto K. Faecal calprotectin and lactoferrin as markers for monitoring disease activity and predicting clinical recurrence in patients with Crohn’s disease after ileocolonic resection: A prospective pilot study. United European Gastroenterol J. 2013;1:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 103. | Yamamoto T, Shimoyama T, Umegae S, Matsumoto K. Serial monitoring of faecal calprotectin for the assessment of endoscopic recurrence in asymptomatic patients after ileocolonic resection for Crohn’s disease: a long-term prospective study. Therap Adv Gastroenterol. 2016;9:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 104. | Boschetti G, Laidet M, Moussata D, Stefanescu C, Roblin X, Phelip G, Cotte E, Passot G, Francois Y, Drai J. Levels of Fecal Calprotectin Are Associated With the Severity of Postoperative Endoscopic Recurrence in Asymptomatic Patients With Crohn’s Disease. Am J Gastroenterol. 2015;110:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 105. | Herranz Bachiller MT, Barrio Andres J, Fernandez Salazar L, Ruiz-Zorrilla R, Sancho Del Val L, Atienza Sanchez R. The utility of faecal calprotectin to predict post-operative recurrence in Crohńs disease. Scand J Gastroenterol. 2016;51:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis. 2013;7:e641-e651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 107. | Lasson A, Strid H, Ohman L, Isaksson S, Olsson M, Rydström B, Ung KA, Stotzer PO. Fecal calprotectin one year after ileocaecal resection for Crohn’s disease--a comparison with findings at ileocolonoscopy. J Crohns Colitis. 2014;8:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 108. | Wright EK, Kamm MA, De Cruz P, Hamilton AL, Ritchie KJ, Krejany EO, Leach S, Gorelik A, Liew D, Prideaux L. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology. 2015;148:938-947.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 109. | Huguet M, Pereira B, Goutte M, Goutorbe F, Dubois A, Bommelaer G, Buisson A. Systematic Review With Meta-Analysis: Anti-TNF Therapy in Refractory Pouchitis and Crohn’s Disease-Like Complications of the Pouch After Ileal Pouch-Anal Anastomosis Following Colectomy for Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 110. | Thomas P, Rihani H, Røseth A, Sigthorsson G, Price A, Nicholls RJ, Bjarnason I. Assessment of ileal pouch inflammation by single-stool calprotectin assay. Dis Colon Rectum. 2000;43:214-220. [PubMed] |
| 111. | Johnson MW, Maestranzi S, Duffy AM, Dewar DH, Forbes A, Bjarnason I, Sherwood RA, Ciclitira P, Nicholls JR. Faecal calprotectin: a noninvasive diagnostic tool and marker of severity in pouchitis. Eur J Gastroenterol Hepatol. 2008;20:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 112. | Pakarinen MP, Koivusalo A, Natunen J, Ashorn M, Karikoski R, Aitola P, Rintala RJ, Kolho KL. Fecal calprotectin mirrors inflammation of the distal ileum and bowel function after restorative proctocolectomy for pediatric-onset ulcerative colitis. Inflamm Bowel Dis. 2010;16:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Yamamoto T, Shimoyama T. Can Fecal Biomarkers Detect Ileal Inflammation in Inflammatory Bowel Disease? Am J Gastroenterol. 2015;110:1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 114. | Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, Beaugerie L, Gomollón F, Häuser W, Herrlinger K. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 340] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 115. | Schoepfer A, Reinisch W. Serial Fecal Calprotectin and Lactoferrin Measurements for Early Diagnosis of Pouchitis After Proctocolectomy for Ulcerative Colitis: Is Pouchoscopy No Longer Needed? Am J Gastroenterol. 2015;110:888-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |