Published online Aug 21, 2018. doi: 10.3748/wjg.v24.i31.3547
Peer-review started: April 19, 2018
First decision: June 6, 2018
Revised: July 11, 2018
Accepted: July 16, 2018
Article in press: July 16, 2018
Published online: August 21, 2018
Processing time: 120 Days and 7.2 Hours
To elucidate the prevalence and risk of mortality of nonalcoholic liver cirrhosis (LC) patients with coronary artery disease (CAD).
The study cohort included newly diagnosed nonalcoholic LC patients age ≥ 40 years old without a diagnosis of CAD from 2006 until 2011 from a longitudinal health insurance database. The mean follow-up period for the study cohort was 1152 ± 633 d. The control cohort was matched by sex, age, residence, and index date. Hazard ratios (HRs) were calculated using the Cox proportional hazard model and the Kaplan-Meier method.
After exclusion, a total of 3409 newly diagnosed nonalcoholic cirrhotic patients were identified from one million samples from the health insurance database. We found that CAD (5.1% vs 17.4%) and hyperlipidemia (20.6% vs 24.1%) were less prevalent in nonalcoholic LC patients than in normal subjects (all P < 0.001), whereas other comorbidities exhibited an increased prevalence. Among the comorbidities, chronic kidney disease exhibited the highest risk for mortality (adjusted HR (AHR) = 1.76; 95%CI: 1.55-2.00, P < 0.001). Ascites or peritonitis exhibited the highest risk of mortality among nonalcoholic cirrhotic patients (AHR = 2.34; 95%CI: 2.06-2.65, P < 0.001). Finally, a total of 170 patients developed CAD after a diagnosis of nonalcoholic LC. The AHR of CAD in nonalcoholic LC patients was 0.56 (95%CI: 0.43-0.74, P < 0.001). The six-year survival rates for nonalcoholic LC patients with and without CAD were 52% and 50%, respectively (P = 0.012).
We conclude that CAD was less prevalent and associated with a reduced risk of mortality in nonalcoholic cirrhotic patients.
Core tip: Coronary artery disease (CAD) is less prevalent and associated with a reduced risk of mortality in nonalcoholic liver cirrhosis (LC) patients. Nonalcoholic LC patients with CAD exhibit an increased six-year survival rate compared to cirrhotic patients without CAD. The LC complication rates did not differ between nonalcoholic LC patients with and without CAD. Of note, nonalcoholic LC patients with ascites or peritonitis exhibited the highest risk of mortality among LC complications.
- Citation: Tsai MC, Yang TW, Wang CC, Wang YT, Sung WW, Tseng MH, Lin CC. Favorable clinical outcome of nonalcoholic liver cirrhosis patients with coronary artery disease: A population-based study. World J Gastroenterol 2018; 24(31): 3547-3555
- URL: https://www.wjgnet.com/1007-9327/full/v24/i31/3547.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i31.3547
Liver cirrhosis (LC) is the 14th leading cause of death in adults and accounted for one million deaths in 2010 worldwide[1]. In Taiwan, LC and chronic liver disease constituted the 8th most common cause of death in 2011[2]. These conditions contribute to numerous lethal complications and comorbidities that result in poor clinical outcomes. Variceal bleeding, systemic infection, hepatic encephalopathy, and hepatocellular carcinoma (HCC) are the primary complications of LC and causes of mortality[3,4]. In addition, LC is associated with systemic comorbidities, such as hemorrhagic stroke and digestive tract malignancies[5-7].
Controversy has emerged regarding the association between LC and coronary artery disease (CAD) in recent decades[7-17]. Several comorbidities of cirrhotic patients have been regarded as cardioprotective factors (e.g., low blood pressure, coagulopathy, thrombocytopenia, malnutrition, and lower serum cholesterol levels)[8,18-20]. However, case-controlled studies have revealed conflicting results and an increased prevalence of CAD in LC patients compared to the normal population[7,9,17,21,22]. A different etiology with an increased proportion of alcoholic and nonalcoholic steatohepatitis (NASH)-related LC in Western populations may have contributed to this result. Furthermore, the application of interferon and nucleot(s)ide analogues for viral hepatitis aided in prolonging survival in recent decades[23,24]. Age-related risks associated with CAD also may have led to an increased prevalence of CAD.
There is a paucity of available data regarding the association between nonalcoholic LC and CAD. Taiwan is an endemic area of viral hepatitis where the majority of LC in patients is caused by hepatitis B (HBV) and hepatitis C (HCV) viral infections[25]. Hence, the nonalcoholic LC population in Taiwan is an ideal cohort in which the confounding effect of alcohol consumption is minimized.
The aim of our study was to elucidate the association between nonalcoholic LC and CAD using a national population-based database in Taiwan. Additionally, we analyzed the prevalence, hazard ratio (HR), and survival for concomitant comorbidities and complications.
This study was a nationwide retrospective longitudinal population-based cohort study based on the Taiwanese National Health Insurance research database (NHIRD). The National Health Insurance program has provided compulsory medical insurance for greater than 99% of the Taiwanese population since March 1, 1995. We identified all LC patients with a first-time diagnosis of LC using International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes (ICD-9-CM code: 571.5) for the period from 2004 to 2011. The following patients were excluded from the study: patients who had LC or CAD before the end of 2005, had LC after diagnosis of CAD from 2006 to 2011, were younger than 40 years old, had a diagnosis of alcoholic LC (ICD-9-CM code: 571.2) or biliary LC (ICD-9-CM code: 571.6), and were outpatients with less than two follow-up visits after the first visit. The control subjects were matched with the patients in the study cohort at a ratio of five to one (5 control subjects per case patient) in terms of sex, age (40-49, 50-59, 60-69, and > 69 years), residence, and entry year.
Other comorbidity data and the ICD-9-CM codes are summarized in Table Appendix 1. The diagnosis of comorbidities was defined as having three outpatient visits or one admission. Hyperlipidemia was defined as having a lipid profile with any one of the following factors: total cholesterol (TC) ≥ 2000 mg/L, low-density lipoprotein cholesterol (LDL-C) ≥ 1600 mg/L, or a triglyceride (TG) level ≥ 1500 mg/L. Complications of LC that occurred after enrollment included esophageal varices (EV) (ICD-9-CM codes: 456-456.21), EV with bleeding (ICD-9-CM codes: 456.0, 456.20) concomitant with ICD-9 procedure codes for ligation of EV or sclerotherapy, ascites (ICD-9-CM codes: 789.5 and 568.82) or peritonitis (ICD-9-CM codes: 567.xx), and hepatic encephalopathy (ICD-9-CM code: 572.2).
We followed up all the subjects enrolled from the index date to the end of 2011. The mean follow-up period for the newly diagnosed nonalcoholic LC cohort (3409 patients) was 1152 ± 633 d with a median of 1169 d, maximum of 2920 d, and minimum of 7 d. Patients with newly diagnosed CAD (one inpatient or three outpatient codes) were categorized based on the first incidence of CAD. The first-time diagnosis of CAD was identified as the primary end point, and the death of the subject served as the secondary end point. All-cause mortality was analyzed in both the study and control cohorts.
Microsoft SQL Server 2008 R2 (Microsoft Corporation, Redmond, WA, United States) was used to perform data processing. Statistical analyses were conducted using SPSS software 19.0 (SPSS, Inc., Chicago, IL, United States). Chi-square tests and analysis of variance (ANOVA) were used to analyze the demographic data, concomitant comorbidities, and complications of cirrhosis. A Cox proportional hazards model was developed to calculate the overall mortality HRs of all comorbidities and complications in cirrhotic patients. The results are expressed in unadjusted and adjusted HRs (AHRs) with 95% confidence intervals (CI). The six-year cumulative survival and survival curve were calculated using the Cox regression method and Kaplan-Meier method. A two-tailed P value less than or equal to 0.05 was considered statistically significant.
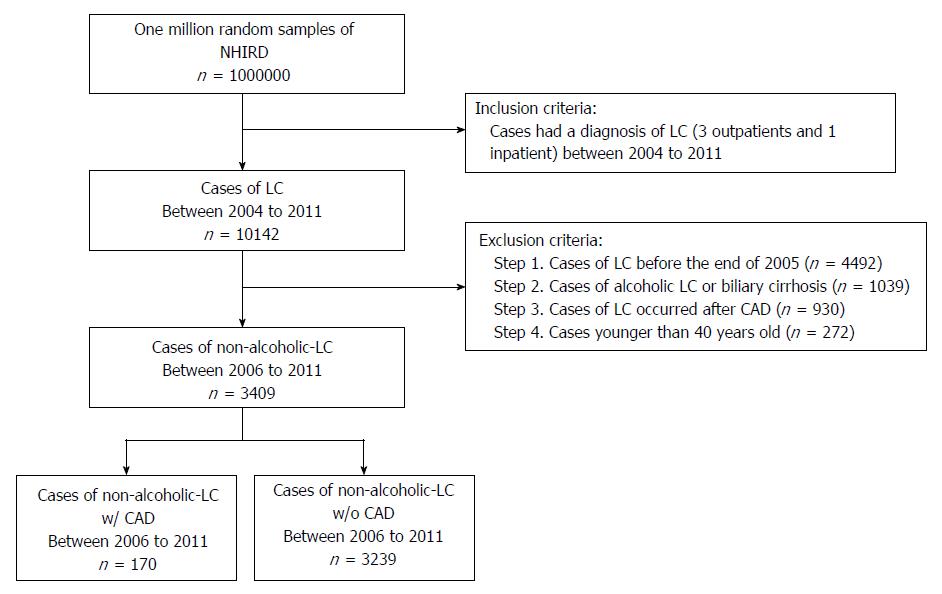
A total of 10142 cases of LC were retrieved from one million random samples in the NHIRD from 2004 to 2011. To ensure that only newly diagnosed LC cases were included, we excluded patients who met the following criteria: LC diagnosed before the end of 2005, CAD occurred before LC, alcoholic or biliary LC, and age younger than 40 years old. Finally, a total of 3409 newly diagnosed nonalcoholic LC patients between 2006 and 2011 were identified and included in the study (Figure 1). To select age-, sex-, residence-, and entry year-matched controls, we further excluded 173 cirrhotic patients to achieve 100% randomization. A total of 3236 patients with nonalcoholic LC and 16180 matched controls were analyzed for all demographic characteristics (Table 1). Patients with nonalcoholic LC were more likely to have comorbidities including hemorrhagic stroke, hypertension, heart failure (HF), diabetes mellitus (DM), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD) (all P < 0.001), and ischemic stroke (P = 0.005). In contrast, hyperlipidemia was less prevalent in nonalcoholic LC patients (P < 0.001). During six years of follow-up, 165 (5.1%) patients in the nonalcoholic LC cohort and 2814 (17.4%) subjects in the control cohort developed CAD (P < 0.001).
| Variable | Non-alcoholic LC patients, n = 3236 | Control, n = 16180 | P value |
| Sex | 1.000 | ||
| Male | 2083 (64.4) | 10415 (64.4) | |
| Female | 1153 (35.6) | 5765 (35.6) | |
| Age (yr) | 1.000 | ||
| 40-49 | 565 (17.5) | 2825 (17.5) | |
| 50-59 | 885 (27.3) | 4425 (27.3) | |
| 60-69 | 766 (23.7) | 3830 (23.7) | |
| > 69 | 1020 (31.5) | 5100 (31.5) | |
| Residence | 1.000 | ||
| Metropolis | 1834 (56.7) | 9170 (56.7) | |
| General area | 1362 (42.1) | 6810 (42.1) | |
| Remote areas | 40 (1.2) | 200 (1.2) | |
| Coronary artery disease | 165 (5.1) | 2814 (17.4) | < 0.001 |
| Cerebrovascular disease | 413 (12.8) | 1580 (9.8) | < 0.001 |
| Hemorrhage | 110 (3.4) | 283 (1.7) | < 0.001 |
| Ischemia | 333 (10.3) | 1413 (8.7) | 0.005 |
| Hypertension | 1620 (50.1) | 6672 (41.2) | < 0.001 |
| Heart failure | 252 (7.8) | 972 (6.0) | < 0.001 |
| Diabetes mellitus | 1075 (33.2) | 3141 (19.4) | < 0.001 |
| Chronic kidney disease | 616 (19.0) | 1216 (7.5) | < 0.001 |
| Hyperlipidemia | 668 (20.6) | 3903 (24.1) | < 0.001 |
| Chronic obstructive pulmonary disease | 534 (16.5) | 1976 (12.2) | < 0.001 |
As shown in Table 2, we stratified nonalcoholic LC patients (n = 3409) into two groups according to the presence of CAD. Nonalcoholic LC patients with CAD were older (≥ 60 years old), more likely female, and exhibited increased concomitant comorbidities of ischemic stroke, hypertension, HF, DM, CKD, hyperlipidemia, and COPD (all P < 0.001). When we compared the complications of liver cirrhosis between the two groups, nonalcoholic LC patients with CAD had fewer LC-related complications, including EV with bleeding, ascites or peritonitis, and hepatic encephalopathy (Supplementary Table 1). However, no significant differences were noted between the two groups.
| Variable | Non-alcoholic LC with CAD, n = 170 | Non-alcoholic LC without CAD, n = 3239 | P value |
| Sex | 0.094 | ||
| Male | 99 (58.2) | 2091 (64.6) | |
| Female | 71 (41.8) | 1148 (35.4) | |
| Age (yr) | < 0.001 | ||
| 40-49 | 18 (10.6) | 554 (17.1) | |
| 50-59 | 25 (14.7) | 877 (27.1) | |
| 60-69 | 46 (27.1) | 751 (23.2) | |
| > 69 | 81 (47.6) | 1057 (32.6) | |
| Residence | 0.484 | ||
| Metropolis | 89 (52.4) | 1830 (56.5) | |
| General area | 79 (46.5) | 1358 (41.9) | |
| Remote areas | 2 (1.2) | 51 (1.6) | |
| Cerebrovascular disease | 46 (27.1) | 400 (12.3) | < 0.001 |
| Hemorrhage | 9 (5.2) | 103 (3.2) | 0.132 |
| Ischemia | 37 (21.8) | 327 (10.1) | < 0.001 |
| Hypertension | 139 (81.8) | 1594 (49.2) | < 0.001 |
| Heart failure | 54 (31.8) | 215 (6.6) | < 0.001 |
| Diabetes mellitus | 84 (49.4) | 1051 (32.4) | < 0.001 |
| Chronic renal failure | 68 (40.0) | 587 (18.1) | < 0.001 |
| Hyperlipidemia | 54 (31.8) | 656 (20.3) | < 0.001 |
| Chronic obstructive pulmonary disease | 49 (28.8) | 521 (16.1) | < 0.001 |
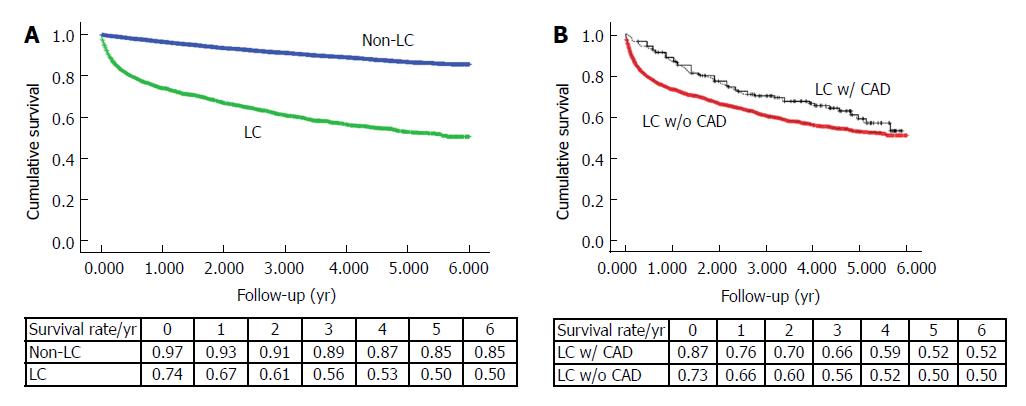
We analyzed 3409 nonalcoholic LC patients and 16180 control subjects with a mean follow-up period of six years, and six-year survival rates were 50% and 85%, respectively (P < 0.001) (Figure 2A). When we compared nonalcoholic LC patients with and without CAD, the six-year survival rates were 52% and 50%, respectively (P = 0.012) (Figure 2B). The Cox regression proportional hazard model corresponding to each group also demonstrated a better survival rate for the nonalcoholic LC with CAD group than for those without CAD (AHR: 0.56; 95%CI: 0.43-0.74; P < 0.001) (Table 3).
| Variable | Unadjusted hazard ratio | Adjusted hazard ratio1 | ||||
| Risk ratio | 95%CI | P value | Risk ratio | 95%CI | P value | |
| Sex | ||||||
| Male | 1.20 | 1.07-1.35 | 0.002 | 1.34 | 1.19-1.51 | < 0.001 |
| Female (reference) | ||||||
| Age (yr) | ||||||
| 40-49 (reference) | ||||||
| 50-59 | 1.04 | 0.85-1.27 | 0.688 | 1.08 | 0.89-1.32 | 0.446 |
| 60-69 | 1.29 | 1.06-1.57 | 0.013 | 1.40 | 1.14-1.72 | < 0.001 |
| > 69 | 2.51 | 2.10-3.00 | < 0.001 | 2.73 | 2.25-3.30 | < 0.001 |
| Coronary artery disease | ||||||
| No (reference) | ||||||
| Yes | 0.71 | 0.55-0.93 | 0.012 | 0.56 | 0.43-0.74 | < 0.001 |
| Cerebrovascular disease | ||||||
| No (reference) | ||||||
| Yes | 1.25 | 1.08-1.45 | 0.004 | 1.08 | 0.93-1.27 | 0.323 |
| Hypertension | ||||||
| No (reference) | ||||||
| Yes | 0.98 | 0.88-1.09 | 0.709 | 0.75 | 0.66-0.85 | < 0.001 |
| Heart failure | ||||||
| No (reference) | ||||||
| Yes | 1.50 | 1.26-1.78 | < 0.001 | 1.23 | 1.02-1.48 | 0.027 |
| Diabetes mellitus | ||||||
| No (reference) | ||||||
| Yes | 1.03 | 0.92-1.16 | 0.609 | 1.12 | 0.99-1.26 | 0.082 |
| Chronic kidney disease | ||||||
| No (reference) | ||||||
| Yes | 1.89 | 1.67-2.13 | < 0.001 | 1.76 | 1.55-2.00 | < 0.001 |
| Hyperlipidemia | ||||||
| No (reference) | ||||||
| Yes | 0.51 | 0.44-0.60 | < 0.001 | 0.52 | 0.44-0.62 | < 0.001 |
| Chronic obstructive pulmonary disease | ||||||
| No (reference) | ||||||
| Yes | 1.30 | 1.13-1.48 | < 0.001 | 0.99 | 0.858-1.141 | 0.888 |
Table 3 presents the HRs of mortality in LC patients with different comorbidities. Increased HRs were observed for males, older patients, cerebrovascular disease (CVD), HF, DM, CKD and COPD. Among these factors, CKD exhibited the highest AHR of mortality (AHR: 1.76; 95%CI: 1.55-2.00). The AHRs of CAD, hypertension, and hyperlipidemia were reduced. Hyperlipidemia exhibited the lowest AHR of 0.52 (95%CI: 0.44-0.62).
When we analyzed the HRs of LC complications that occurred after enrollment, all complications exhibited increased values for both unadjusted HRs and AHRs. Ascites or peritonitis exhibited the highest AHR at 2.34 (95%CI: 2.06-2.65) (Supplementary Table 2).
Finally, we compared the effect of the number of comorbidities and complications on the survival rate in LC patients. Cirrhotic patients with one, two, or three comorbidities had HRs of 1.35, 1.72, and 1.95 (95%CI: 1.19-1.55, 1.47-2.00, and 1.57-2.43), respectively (Supplementary Table 3). However, the HRs for one, two, or t three LC complications were 3.56, 4.63, and 5.15 (95%CI: 3.14-4.03, 3.94-5.44, and 3.82-6.94), respectively (Supplementary Table 4).
In the present study, we observed a reduced prevalence of CAD and an increased survival rate for concomitant CAD in nonalcoholic LC patients. A lower prevalence of atherosclerosis and CAD was first described in an autopsy-based study in 1960[10]. A reduced prevalence of ischemic events was also reported for ischemic stroke[8,26]. However, conflicting data have been reported in some retrospective cohort and case-controlled studies[8,9,13,16,17,21,22]. First, the increased prevalence of CAD found in LC patients might be due to the different etiologies of LC in these Western population-based cohorts. An increased proportion of NASH and alcohol-related LC was noted compared to cohorts in viral hepatitis endemic areas[13,16,21,22]. NASH and alcohol consumption are generally associated with increased CAD risk factors. Nonetheless, the prevalence of CAD was reduced in end-stage liver disease patients assessed for liver transplantation who had alcoholic LC when compared to those with nonalcoholic LC[27]. Although moderate consumption of alcohol (< 30 g/d) has been reported as a protective factor against CAD[12], controversy still exists regarding heavy drinkers. In comparison, most subjects in our study had viral hepatitis-related LC. Few metabolic disorders were associated with steatohepatitis (such as hyperlipidemia), and no alcohol consumption was reported in our study cohort.
Second, lower blood pressure, better serum lipid profiles (including lower LDL-C, TC, and TG), and coagulopathy in LC patients were regarded as protective factors against CAD[8,18-20,22]. However, viral hepatitis per se can influence vessel function and cause endothelial dysfunction, which is correlated with atherosclerotic disease progression[28]. A retrospective cohort study indicated that hepatitis C virus-infected subjects exhibited an increased risk for CAD, although better blood pressure and lipid profiles were observed in the population[29,30]. Consistent with previous reports, we observed a reduced prevalence of hyperlipidemia in cirrhotic patients. The proportion of comorbidities, including CVD, hypertension, HF, DM, CKD, hyperlipidemia, and COPD, was increased in the nonalcoholic LC with CAD patients[7]. The increase in these age-related comorbidities may be attributed to the older age in this study cohort. Third, CAD is an age-related disease. We hypothesize that better liver function may contribute to increased survival in the cirrhotic patients with CAD cohort. When survival is prolonged, age-related CAD risks also increase[31].
Ischemic stroke is a systemic thromboembolic event. Compared to normal subjects, the prevalence of ischemic stroke was reduced in cirrhotic patients[32]. In contrast, hemorrhagic events increased[7,33]. However, in our study, both ischemic and hemorrhagic stroke were significantly increased in nonalcoholic LC subjects. Of note, the prevalence of hemorrhagic stroke in nonalcoholic LC patients was two-fold higher than that in normal subjects (3.4% vs 1.7%, P < 0.001), which was considerably increased compared to ischemic stroke (10.3% vs 8.7%, P = 0.005). Older age and higher proportions of concomitant hypertension, DM, and COPD (as a result of smoking) in our nonalcoholic LC cohort might explain this result. These comorbidities have been reported as significant risk factors for all types of stroke[34].
We analyzed the association among comorbidities, complications, and HRs in LC patients. Consistent with previous studies, cirrhosis concomitant with CVD, HF, CKD, or COPD was regarded as a risk factor for mortality in both the Charlson comorbidity index and the Cirrhosis-specific Comorbidity Scoring System (CirCom)[35-37]. In addition, CKD had the highest HR in our study and exhibited increased risk scores in the two prognostic scoring systems. All the complications in LC patients were risk factors in our study. However, CAD, hypertension, and hyperlipidemia were associated with a lower HR and improved survival in this study. Although acute myocardial infarction was found to be a risk factor of mortality in nonalcoholic LC patients[36], the relationship among chronic stable CAD, hypertension, hyperlipidemia, and LC mortality remains unclear. Among these factors, cirrhotic patients with greater than three comorbidities exhibited the worst prognosis with at least a 1.954-fold increased risk of mortality.
There were some limitations in our study. First, this is a retrospective cohort study. Although nonalcoholic LC and CAD subjects were retrieved from the Taiwanese NHIRD database according to ICD-9 codes, data were lacking regarding the severity of cirrhosis (e.g., Child-Turcotte-Pugh scores). In this study, we excluded patients who had less than three outpatient visits with the same diagnosis. Furthermore, we analyzed the complications of cirrhosis to evaluate the severity. Second, the severity of concomitant comorbidities, such as hypertension, DM, and hyperlipidemia, cannot be evaluated. In addition, other known risk factors, such as smoking status and family history of CAD, were not recorded in the database. Third, the severity of CAD was not recorded in the database. A prospective case-controlled study revealed an increased prevalence of nonobstructive CAD (i.e., narrowing < 50%) in cirrhotic patients compared to normal controls using computerized coronary angiography. This feature may contribute to the increased survival rates for LC patients with CAD. The severity of CAD should be further evaluated in future studies[17]. Finally, the different follow-up periods for each patient may impact the HRs of mortality in nonalcoholic LC patients.
In conclusion, CAD was less prevalent in nonalcoholic LC patients. Among cirrhotic patients, an increased number of concomitant comorbidities was found in cirrhotic patients with CAD. However, improved survival was found in nonalcoholic LC patients with CAD when compared to those without CAD.
The risk of mortality in nonalcoholic liver cirrhosis (LC) patients with coronary artery disease (CAD) is unclear. Previous case-control studies demonstrated conflicting results potentially due to the different etiologies of LC. LC patients with alcoholic and nonalcoholic fatty liver disease-related metabolic disorders exhibit an increased risk of CAD. In contrast, hemostatic defects that occur in LC, such as thrombocytopenia, coagulopathy, and low blood pressure, are not considered as potential protective factors of cirrhosis against atherosclerotic events.
The results of CAD risk in LC patients are controversial. In contrast to the present study, previous works using alcoholic LC-based cohorts may have been confounded by the increased risk of metabolic syndrome in heavy drinkers. To the best of our knowledge, the risk of CAD in nonalcoholic cirrhotic patients is not well established.
The aim of the study was to elucidate the prevalence and risk of mortality in nonalcoholic cirrhotic patients. The comorbidities and LC-related complications were also important prognostic factors among cirrhotic patients. The result of this study can provide a better understanding of CAD risk in LC patients.
We collected 10142 LC patients diagnosed from 2004 to 2011 using the Taiwanese National Health Insurance research database. After exclusion of subjects who were treated before the end of 2005, had alcoholic or biliary cirrhosis, had LC occurring after CAD, and were younger than 40 years old, a total of 3409 LC patients were enrolled in the study. The comorbidities and complications of LC were collected. The first-time diagnosis of CAD was identified as the primary end point, and the death of the subject served as the secondary end point. All-cause mortality was analyzed in both the study and control cohorts. A Cox proportional hazards model was developed to calculate the overall mortality hazard ratios of all comorbidities and complications in cirrhotic patients. The six-year cumulative survival and survival curve were calculated using the Cox regression method and the Kaplan-Meier method.
CAD was less prevalent in nonalcoholic LC patients than in controls. Nonalcoholic LC patients with CAD were associated with a reduced risk of mortality. The six-year survival rates were increased in patients with CAD compared to patients without CAD in the nonalcoholic LC cohort. As this is a retrospective cohort study, further prospective studies are needed to confirm this finding.
In this study, we demonstrate that CAD is less prevalent and associated with a reduced risk for mortality in nonalcoholic LC patients. This result confirms previous studies regarding the lower risk for atherosclerotic events (i.e. ischemic stroke and coronary artery disease) in alcohol-related and nonalcohol-related LC cohorts. Although viral hepatitis can cause endothelial dysfunction, which correlates with atherosclerotic disease progression, we propose that LC has a more powerful protective effect against atherosclerotic events based on the favorable cardiovascular risk profiles, such as thrombocytopenia, coagulopathy, and low blood pressure. The strengths of the study include the large sample size cohort and risk adjustments for comorbidities and complications. Finally, we conclude that nonalcoholic LC patients with CAD exhibit a favorable outcome.
Future prospective research should focus on the advantages and disadvantages of antiplatelet therapy in the prevention of CAD in nonalcoholic LC patients. Additionally, it is advised that future studies should compare alcoholic and nonalcoholic LC cohorts and evaluate the effect of viral treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chang ST, Ciccone MM, Posadas-Sánchez R S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 1. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9574] [Article Influence: 736.5] [Reference Citation Analysis (0)] |
| 2. | Department of Health EY, ROC . Statistics of Causes of Death. 2011;. |
| 3. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1311] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 4. | Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ. Causes of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet. 2006;368:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Sun LM, Lin MC, Lin CL, Liang JA, Jeng LB, Kao CH, Lu CY. Nonalcoholic Cirrhosis Increased Risk of Digestive Tract Malignancies: A Population-Based Cohort Study. Medicine (Baltimore). 2015;94:e2080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Jepsen P, Lash TL, Vilstrup H. The clinical course of alcoholic cirrhosis: development of comorbid diseases. A Danish nationwide cohort study. Liver Int. 2016;36:1696-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Parikh NS, Navi BB, Kumar S, Kamel H. Association between Liver Disease and Intracranial Hemorrhage. J Stroke Cerebrovasc Dis. 2016;25:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Berzigotti A, Bonfiglioli A, Muscari A, Bianchi G, Libassi S, Bernardi M, Zoli M. Reduced prevalence of ischemic events and abnormal supraortic flow patterns in patients with liver cirrhosis. Liver Int. 2005;25:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Tiukinhoy-Laing SD, Rossi JS, Bayram M, De Luca L, Gafoor S, Blei A, Flamm S, Davidson CJ, Gheorghiade M. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am J Cardiol. 2006;98:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | HOWELL WL, MANION WC. The low incidence of myocardial infarction in patients with portal cirrhosis of the liver: A review of 639 cases of cirrhosis of the liver from 17,731 autopsies. Am Heart J. 1960;60:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Carey WD, Dumot JA, Pimentel RR, Barnes DS, Hobbs RE, Henderson JM, Vogt DP, Mayes JT, Westveer MK, Easley KA. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation. 1995;59:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 183] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Mukamal KJ, Rimm EB. Alcohol’s effects on the risk for coronary heart disease. Alcohol Res Health. 2001;25:255-261. [PubMed] |
| 13. | Kalaitzakis E, Björnsson E. Coronary artery disease in liver cirrhosis: does the aetiology of liver disease matter? J Hepatol. 2009;51:962-963; author reply 963-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Marui A, Kimura T, Tanaka S, Miwa S, Yamazaki K, Minakata K, Nakata T, Ikeda T, Furukawa Y, Kita T. Coronary revascularization in patients with liver cirrhosis. Ann Thorac Surg. 2011;91:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, Fix OK, Kay N, Abecassis MI, Gheorghiade M. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011;58:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Gologorsky E, Pretto EA Jr, Fukazawa K. Coronary artery disease and its risk factors in patients presenting for liver transplantation. J Clin Anesth. 2013;25:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | An J, Shim JH, Kim SO, Lee D, Kim KM, Lim YS, Lee HC, Chung YH, Lee YS. Prevalence and prediction of coronary artery disease in patients with liver cirrhosis: a registry-based matched case-control study. Circulation. 2014;130:1353-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 951] [Article Influence: 67.9] [Reference Citation Analysis (1)] |
| 19. | D’Arienzo A, Manguso F, Scaglione G, Vicinanza G, Bennato R, Mazzacca G. Prognostic value of progressive decrease in serum cholesterol in predicting survival in Child-Pugh C viral cirrhosis. Scand J Gastroenterol. 1998;33:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Lisman T, Caldwell SH, Burroughs AK, Northup PG, Senzolo M, Stravitz RT, Tripodi A, Trotter JF, Valla DC, Porte RJ; Coagulation in Liver Disease Study Group. Hemostasis and thrombosis in patients with liver disease: the ups and downs. J Hepatol. 2010;53:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 21. | Kadayifci A, Tan V, Ursell PC, Merriman RB, Bass NM. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: a comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J Hepatol. 2008;49:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Kalaitzakis E, Rosengren A, Skommevik T, Björnsson E. Coronary artery disease in patients with liver cirrhosis. Dig Dis Sci. 2010;55:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 23. | Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, Lee WM, Di Bisceglie AM, Bonkovsky HL, Dienstag JL. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 24. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2400] [Article Influence: 184.6] [Reference Citation Analysis (0)] |
| 25. | Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 720] [Cited by in RCA: 748] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 26. | Kane WC, Aronson SM. Cerebrovascular disease in an autopsy population. 4. Reduced frequency of stroke in patients with liver cirrhosis. Trans Am Neurol Assoc. 1971;96:259-260. [PubMed] |
| 27. | Patel S, Kiefer TL, Ahmed A, Ali ZA, Tremmel JA, Lee DP, Yeung AC, Fearon WF. Comparison of the frequency of coronary artery disease in alcohol-related versus non-alcohol-related endstage liver disease. Am J Cardiol. 2011;108:1552-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Ciccone MM, Principi M, Ierardi E, Di Leo A, Ricci G, Carbonara S, Gesualdo M, Devito F, Zito A, Cortese F. Inflammatory bowel disease, liver diseases and endothelial function: is there a linkage? J Cardiovasc Med (Hagerstown). 2015;16:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 30. | Roed T, Lebech AM, Kjaer A, Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging. 2012;32:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, Berger JS. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol. 2013;61:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 32. | Chen YH, Chen KY, Lin HC. Non-alcoholic cirrhosis and the risk of stroke: a 5-year follow-up study. Liver Int. 2011;31:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Lai CH, Cheng PY, Chen YY. Liver cirrhosis and risk of intracerebral hemorrhage: a 9-year follow-up study. Stroke. 2011;42:2615-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 2175] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 35. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38228] [Article Influence: 1006.0] [Reference Citation Analysis (0)] |
| 36. | Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sørensen HT. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology. 2008;48:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Jepsen P, Vilstrup H, Lash TL. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology. 2014;146:147-156; quiz e15-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |