Published online Aug 21, 2018. doi: 10.3748/wjg.v24.i31.3538
Peer-review started: May 11, 2018
First decision: May 16, 2018
Revised: May 25, 2018
Accepted: June 25, 2018
Article in press: June 25, 2018
Published online: August 21, 2018
Processing time: 98 Days and 8 Hours
To investigate the expression and clinical significance of B7 homolog 3 (B7-H3) and β-1,3-galactosyltransferase-4 (B3GALT4) in colorectal cancer (CRC) patients.
Using tissue microarray, we identified the expression of B7-H3 and B3GALT4 in 223 CRC patient samples by immunohistochemistry and evaluated the possible correlation between B7-H3 and B3GALT4 and clinical outcomes. Further, the mRNA and protein expression were identified to establish the regulatory relationship of B7-H3 with B3GALT4 in vitro.
A significant positive correlation between B7-H3 and B3GALT4 was observed in CRC specimens (r = 0.219, P = 0.001). High expression of B7-H3 was identified as a significant independent predictor of poor overall survival (OS) [hazard ratio (HR) = 1.781; 95%CI: 1.027-3.089; P = 0.040]. Moreover, high expression of B3GALT4 was also recognized as an independent predictor of inferior OS (HR = 1.597; 95%CI: 1.007-2.533; P = 0.047). Additionally, CRC patients expressing both high B7-H3 and high B3GALT4 contributed to a significant decrease in OS (HR = 2.283; 95%CI: 1.289-4.042; P = 0.005). In CRC cell lines with stable expression of high B7-H3, the mRNA and protein expressions of B3GALT4 were significantly upregulated. Similarly, the expression of B3GALT4 was significantly reduced when expression of B7-H3 was knocked down.
The expression of B3GALT4 in CRC is positively correlated with B7-H3 expression in vitro. B7-H3/B3GLAT4 may be used as dual prognostic biomarkers for CRC.
Core tip: The present study for the first time revealed the expression of β-1,3-galactosyltransferase-4 (B3GALT4) in colorectal cancer (CRC) and its correlation with B7 homolog 3 (B7-H3) in vitro. Overall, the findings of the present study suggest that B7-H3 and B3GALT4 are novel prognostic biomarkers for CRC and highlight the significance of both B7-H3 and B3GALT4 as promising therapeutic targets for CRC. Thus, here we present our preliminary work on the relationship of the immune function and glycosylation of tumor-associated protein in CRC.
- Citation: Zhang T, Wang F, Wu JY, Qiu ZC, Wang Y, Liu F, Ge XS, Qi XW, Mao Y, Hua D. Clinical correlation of B7-H3 and B3GALT4 with the prognosis of colorectal cancer. World J Gastroenterol 2018; 24(31): 3538-3546
- URL: https://www.wjgnet.com/1007-9327/full/v24/i31/3538.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i31.3538
Colorectal cancer (CRC) is the most prevalent gastrointestinal tract malignancy worldwide among both men and women. Overall CRC incidence and mortality rates have been declining over the recent decades, yet CRC ranks third overall among other malignancies in men and women[1]. In China, there were approximately 376300 new cases and 191000 potential deaths for CRC in 2015[2]. Despite several therapeutic advancements including surgical resection, neoadjuvant chemoradiotherapy, and targeted therapy, the prognosis of CRC patients remains relatively poor. With unprecedented survival benefits in selected patients, immunotherapy has become a promising treatment strategy and continuous progress has been made with immunomodulatory agents that target immune system checkpoints such as anti-programmed death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4)[3].
B7 homolog 3 (B7-H3), also known as CD276, is a type I transmembrane glycoprotein that belongs to the B7/CD28 immunoglobulin superfamily. B7-H3 mRNA is widely expressed on many tissues and cell types. However, B7-H3 protein is not constitutively expressed on T-cells, natural killer cells and antigen-presenting cells, and its expression can be induced on immune cells. While limited expression has been noticed on normal tissues, overexpression is observed in a majority of human malignancies including CRC[4] and has been correlated with the poor prognosis of patients[5]. Moreover, B7-H3 has been suggested to promote tumor growth, migration, invasion, and metastasis. Thus, it may be a potential therapeutic target for active immunotherapy.
The β-1,3-galactosyltransferase-4 (B3GALT4) gene belongs to the β-1,3-galactosyltransferase (β3GalT) gene family, which encodes type II membrane-bound glycoproteins and is located in the centromeric segment of the human MHC class II region[6]. This gene is abundantly expressed in human organs and tissues, predominantly in the brain and involved in GM1/GD1 ganglioside synthesis[7]. The β3GalT family plays an essential role in the O-glycosylation process. The surface of cancer cells express glycoproteins, which are rich in O-glycosylation domains[8]. Thus, the family may be closely related to the tumor. Besides, Seko et al[9] had confirmed that B3GALT4 could be used as a novel biomarker for the diagnosis of gynecological cancers. However, there are insufficient reports about the correlation between B3GALT4 and CRC.
Therefore, the present study aimed to investigate the clinical correlation of B7-H3 and B3GALT4 with CRC. Further, correlation between the expression of B7-H3 and B3GALT4 was evaluated to determine their prognostic significance in CRC.
The medical records of patients who received a histopathological diagnosis and underwent surgery for CRC at Affiliated Hospital of Jiangnan University between June 2008 and December 2011 were retrieved. A total of 223 formalin-fixed paraffin-embedded CRC tissue samples were included in the study. These patients did not receive radiotherapy or chemotherapy before the surgery. Only histologically confirmed cases were included in the study. However, patients who received chemo- or radiotherapy before surgery and cases with incomplete clinical data were excluded from the study. The study was approved by the Medical Ethics Committee of Affiliated Hospital of Jiangnan University, and written informed consent was obtained from all patients. All the patients were followed up by telephone up to October 31, 2017, to obtain the survival data. The median follow-up was 79 mo (range, 6-114 mo).
Two experienced pathologists examined the section stained with hematoxylin and eosin and marked the carcinoma sites in the corresponding paraffin block. Using a manual tissue microarrayer (Quick-Ray, UNITMA, Seoul, Korea), tissue cylinders with a 1.0 mm diameter were punched from representative tissue areas of each donor tissue block and implanted into the hole of the premade recipient paraffin block (UNITMA, Seoul, Korea). The tissue microarray paraffin block was then constructed and cut into 4 μm thick continuous sections, which were attached to anti-dewaxing slides and stored at room temperature.
The tissue paraffin blocks were serially sectioned into a 4 μm thickness, dewaxed in xylene, and hydrated in an ethanol gradient. Antigen retrieval was performed by heating the tissue sections at 100 °C for 30 min in citrate buffer. Moreover, endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide for 10 min. Subsequently, the sections were incubated in 5% bovine serum albumin at room temperature for 30 min. Primary antibodies, mouse anti-human B7-H3 monoclonal antibody (1:200, Santa Cruz, Dallas, TX, United States) and rabbit anti-human B3GALT4 monoclonal antibody (1:50, Abcam, Cambridge, MA, United States) respectively were added drop-wise followed by overnight incubation at 4 °C. The slides were washed and incubated with a horseradish peroxidase-conjugated secondary antibody for 30 min. The immunostaining was carried out by staining with 3,3’-diaminobenzidine tetrahydrochloride solution (GTVisionII Immunohistochemistry Detection Kit for Rabbit/Mouse, Gene Tech, Shanghai, China), counter-stained with hematoxylin, dehydrated, and mounted. Sections were examined under a microscope.
Two independent pathologists performed a blinded manner review of the sections. Both the intensity and extent of immunological staining were analyzed semi-quantitatively. According to the percentage of positively stained cells, the sections were graded by five levels: 0 (≤ 5%), 1 (6%-25%), 2 (26%-50%), 3 (51%-75%) and 4 (> 76%). The staining intensity was scored similarly, with 0 used for negative staining, 1 for weakly positive, 2 for moderately positive and 3 for strongly positive. The scores for the percentage of positive cells and the staining intensity were multiplied to generate an immune-reactive score for each specimen (0-12). Based on the total score, scores of 0-3 were considered as the low expression group, and scores of 4-12 were defined as the high expression group.
The two human CRC cell lines, SW480 and Caco-2, which exhibited different expression levels of B7-H3 were maintained in our lab. We constructed SW480 cells that expressed a high level of B7-H3 (SW480-B7-H3) by transfection with an overexpression plasmid, and Caco-2 cells were stably transfected with a B7-H3 shRNA (Caco-2-shB7-H3). Cells transfected with a mock vector were used as negative controls (SW480-NC and Caco-2-shNC). SW480-NC and SW480-B7-H3 were cultured in L-15 medium (HyClone GE Healthcare Life Sciences, South Logan, UT, United States). Caco-2-NC and Caco-2-shB7-H3 were maintained in RPMI-1640 medium (the same as above). All the media were supplemented with 10% fetal bovine serum (Clark Bioscience, Houston, TX, United States). The cells were incubated at 37 °C in a humidified atmosphere with 5% CO2.
Total RNA was extracted from 1 × 106 cells using TRIzol (Invitrogen, Carlsbad, CA, United States), according to the manufacturer’s protocol. A NanoDrop 2000 Spectrophotometer (Thermo Scientific, Waltham, MA, United States) was used to estimate the purity and concentration of total RNA by measuring the optical density. The first strand cDNA was generated from 1 μg RNA using the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) following the manufacturer’s instructions.
The expression levels of B7-H3 and B3GALT4 were detected using the QuantiNovaTM SYBR Green PCR Kit (QIAGEN, Hilden, Germany) on ViiATM 7 Real-Time PCR System (Applied Biosystems, Foster City, CA, United States). β-actin was used as an internal control for normalization of the expression data. The first strand cDNA was amplified in a total 20 μL PCR reaction mixture: 10 μL 2 x QuantiNova SYBR Green PCR Master Mix, 0.1 μL QN ROX Reference Dye, 0.4 μL of each specific primer set, 2 μL cDNA and ddH2O added to 20 μL. The sequences of primers were as follows: β-actin 5’-CATGTACGTTGCTATCCAGGC-3’ (sense), 5’-CTCCTTAATGTCACGCACGAT-3’ (antisense); B7-H3 5’-AGCACTGTGGTTCTGCCTCACA-3’ (sense), 5’-CACCAGCTGTTTGGTATCTGTCAG-3’ (antisense); B3GALT4 5’-ACTCCTACCGCAACCTCA-3’ (sense), 5’- CACATCATCGTCCGTCTT-3’ (antisense). Each sample and internal control gene were run in triplicate, using an amplification condition of denaturation at 95 °C for 2 min, followed by 40 cycles of 95 °C for 5 s and 60 °C for 10 s. The 2-ΔΔCt method was used to calculate the relative expression level of the target genes.
Total proteins were extracted from 1 × 106 cells by using ice-cold RIPA lysis buffer containing a cocktail of protease inhibitors (KeyGEN BioTECH, China) for 30 min. The concentrations of whole cell proteins were determined using a BCA Protein Assay Kit (CWBio, Beijing, China) and adjusted to the same concentration. For western blotting assay, the proteins of equal quantity were separated by 10% SDS-PAGE and then transferred onto a PVDF membrane (Merck Millipore, Germany). After blocking with 5% nonfat dry milk for 1 h at room temperature, the membranes were incubated with the primary antibodies at a concentration of 1:1000 at 4 °C overnight. The antibodies included rabbit anti-human B7-H3 monoclonal antibody (Abcam, Cambridge, MA, United States), rabbit anti-human B3GALT4 monoclonal antibody (Abcam, Cambridge, MA, United States) and mouse anti-human β-actin monoclonal antibody (Beyotime, Nantong, China). After washing with TBST (TBS with 0.1% Tween), the membranes were incubated with a corresponding goat-anti-mouse or goat-anti-rabbit IgG-horseradish peroxidase secondary antibody (1:1000, Beyotime, Nantong, China) for 1 h at room temperature. The membranes were finally treated with enhanced chemiluminescence (ECL) assay reagents (absin, Shanghai, China) and the immunoreactive bands were visualized using the ChemiDocTM XRS+ system with Image LabTM Software (Bio-Rad, Hercules, CA, United States). Quantitative analysis was carried out with Quantity One (Bio-Rad, Hercules, CA, United States).
Statistical analysis of clinical data was performed using the software package SPSS 19.0 software (IBM, Chicago, IL, United States). Chi-square test analyzed the association between B7-H3 or B3GALT4 and the clinicopathological data. The non-parametric Spearman test evaluated the correlation of B7-H3 and B3GALT4 expression. Overall survival (OS) was plotted using the Kaplan-Meier method and was compared using the log-rank test. Multivariate survival analysis was performed using the Cox proportional hazard model. All the in vitro experiments were performed in triplicate. A non-paired t-test analyzed differences in mean values between groups. All data were analyzed using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, United States). A P-value less than 0.05 indicated a statistically significant difference.
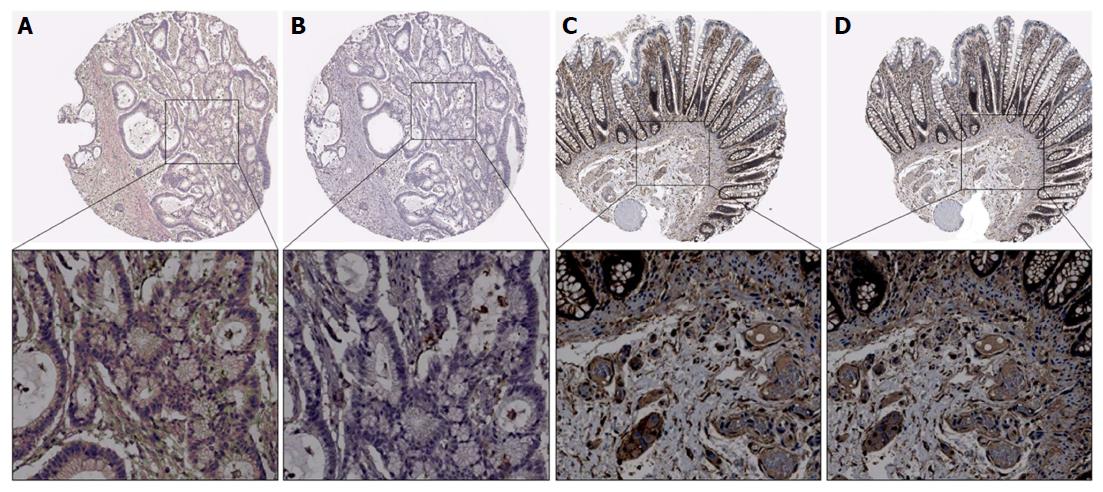
The expression of B7-H3 and B3GALT4 was analyzed using immunochemistry in CRC tissue. Positive staining of B7-H3 was predominantly detected in the membrane and cytoplasm (Figure 1C), and the location of B3GALT4 expression was identified in the cytoplasm (Figure 1D). The positive rates of the B7-H3 and B3GALT4 expressions were 70.4% (157/223) and 54.7% (122/223), respectively. Further, a highly significant correlation between the positive expression of B7-H3 with the depth of tumor invasion (P = 0.049), distant metastasis (P = 0.034) and differentiation (P = 0.017) was observed. However, the expression of B3GALT4 had no significant correlation with clinicopathological parameters (Table 1).
| Clinicopathological parameter | Case (n) | B7-H3 expression | P value | B3GALT4 expression | P value | ||
| Low | High | Low | High | ||||
| Gender | 0.616 | 0.213 | |||||
| Male | 124 | 35 | 89 | 63 | 61 | ||
| Female | 99 | 31 | 68 | 42 | 57 | ||
| Age (yr) | 0.259 | 0.791 | |||||
| < 60 | 87 | 22 | 65 | 40 | 47 | ||
| ≥ 60 | 136 | 44 | 92 | 65 | 71 | ||
| Tumor location | 0.361 | 0.905 | |||||
| Colon | 88 | 23 | 65 | 41 | 47 | ||
| Rectum | 135 | 43 | 92 | 64 | 71 | ||
| Colon cancer site | 0.119 | 0.352 | |||||
| Right-sided | 37 | 7 | 30 | 20 | 17 | ||
| Left-sided | 186 | 59 | 127 | 85 | 101 | ||
| Depth of tumor invasion | 0.049 | 0.527 | |||||
| T1/2 | 64 | 25 | 39 | 28 | 36 | ||
| T3/4 | 159 | 41 | 118 | 77 | 82 | ||
| Lymph node metastasis | 0.433 | 0.145 | |||||
| N0 | 116 | 37 | 79 | 60 | 56 | ||
| N1/2 | 107 | 29 | 78 | 45 | 62 | ||
| Distant metastasis | 0.034 | 0.425 | |||||
| Yes | 16 | 1 | 15 | 6 | 10 | ||
| No | 207 | 65 | 142 | 99 | 108 | ||
| TNM stage | 0.297 | 0.068 | |||||
| I/II | 113 | 37 | 76 | 60 | 53 | ||
| III/IV | 110 | 29 | 81 | 45 | 65 | ||
| Neural invasion | 0.146 | 0.683 | |||||
| Yes | 32 | 6 | 26 | 14 | 18 | ||
| No | 191 | 60 | 131 | 91 | 100 | ||
| Vascular invasion | 0.079 | 0.585 | |||||
| Yes | 35 | 6 | 29 | 15 | 20 | ||
| No | 188 | 60 | 128 | 90 | 98 | ||
| Mucinous adenocarcinoma | 0.579 | 0.506 | |||||
| Yes | 20 | 7 | 13 | 8 | 12 | ||
| No | 203 | 59 | 144 | 97 | 106 | ||
| Differentiation | 0.017 | 0.186 | |||||
| Poor | 73 | 14 | 59 | 39 | 34 | ||
| Moderate/well | 150 | 52 | 98 | 66 | 84 | ||
Of the 223 CRC cases, 97 (43.5%) patients exhibited higher expression of both B7-H3 and B3GALT4, and 41 (18.4%) showed lower expression of both. Also, there were 85 (38.1%) cases with either B7-H3 low or B3GALT4 low. Spearmen’s Correlation was used to examine the association of B7-H3 expression with B3GALT4 expression in CRC. The result showed that there was a highly significant positive correlation between B7-H3 and B3GALT4 expression (r = 0.219, P = 0.001, Table 2).
| B3GALT4 expression | B7-H3 expression | r | P value | |
| Low | High | |||
| Low | 41 | 60 | 0.219 | 0.001 |
| High | 25 | 97 | ||
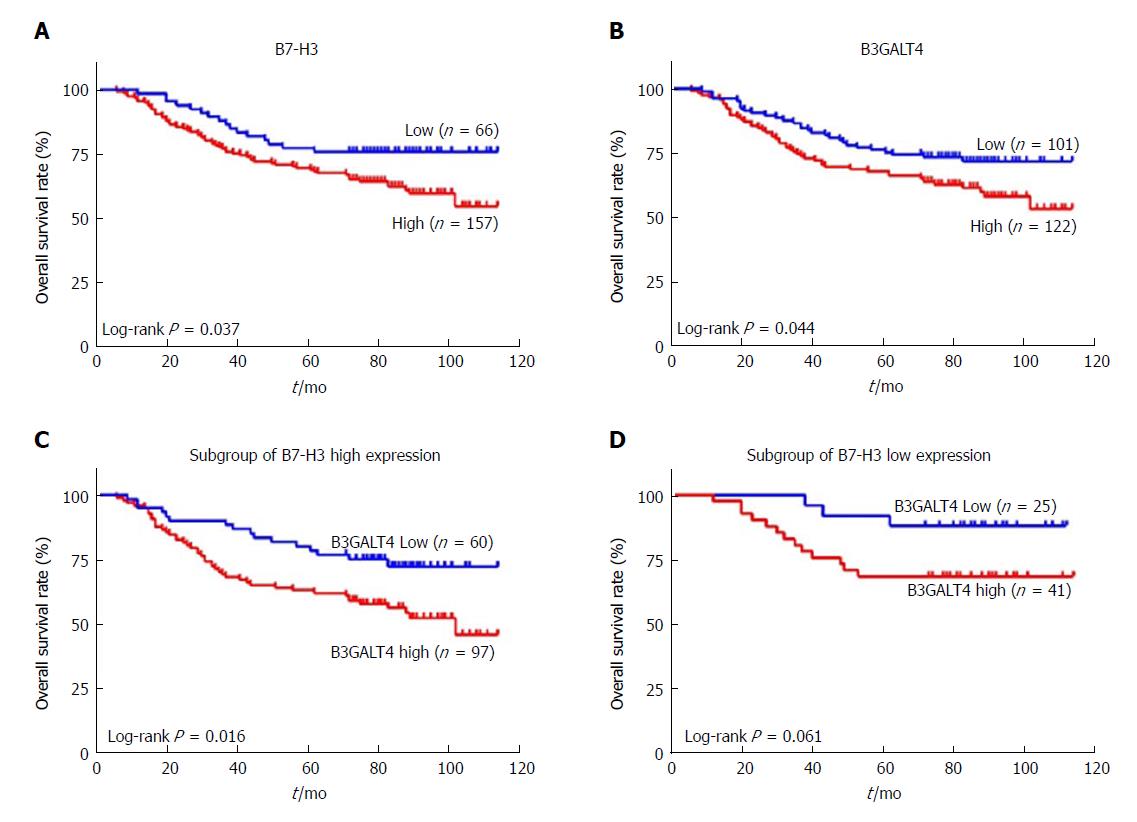
Notably, patients with high expression of B7-H3 had a significantly worse OS as compared to patients with low expression (P = 0.037) (Figure 2A). Similarly, the OS in the high B3GALT4 expression group was significantly inferior to that in the low expression group (P = 0.044) (Figure 2B). Moreover, a subgroup of patients with high expression of B7-H3 and high B3GALT4 exhibited a significantly worse prognosis as compared to patients with a low expression of B3GALT4 (P = 0.016) (Figure 2C). However, in a subgroup of patients with low expression of both B7-H3 and B3GALT4 prognosis of patients with CRC was unaffected (P = 0.061) (Figure 2D).
Furthermore, the univariate Cox proportional hazard model analysis was performed to evaluate the risk factors related to the prognosis of patients with CRC. As shown in Table 3, high expression of B7-H3, high expression of B3GALT4, high expression of both B7-H3 and B3GALT4, depth of tumor invasion (T3/4), lymph node metastasis (N1/2), distant metastasis, TNM stage (III/IV), neural invasion, and vascular invasion were correlated with the OS of patients with CRC. However, there was no correlation of OS with gender, age, tumor location, colon cancer site, mucinous adenocarcinoma, differentiation, and B7-H3 low expression group (Table 3). Besides, the multivariate analysis revealed that distant metastasis, TNM stage (III/IV), and vascular invasion were significant independent prognostic factors for OS of patients with CRC (Table 3).
| Clinical parameter | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender (male vs female) | 0.999 (0.637-1.566) | 0.995 | ||
| Age (≥ 60 vs < 60) (yr) | 0.951 (0.603-1.500) | 0.829 | ||
| Tumor location (rectum vs colon) | 1.351 (0.863-2.117) | 0.188 | ||
| Colon cancer site (left-sided vs right-sided) | 0.970 (0.534-1.760) | 0.919 | ||
| Depth of tumor invasion (T3/4 vs T1/2) | 2.522 (1.362-4.670) | 0.003 | 1.807 (0.949-3.443) | 0.072 |
| Lymph node metastasis (N1/2 vs N0) | 2.922 (1.801-4.739) | 0.000 | 0.322 (0.077-1.341) | 0.119 |
| Distant metastasis (yes vs no) | 9.353 (5.206-16.804) | 0.000 | 4.635 (2.224-9.660) | 0.000 |
| TNM stage (III/IV vs I/II) | 3.497 (2.114-5.783) | 0.000 | 7.490 (1.636-34.280) | 0.009 |
| Neural invasion (yes vs no) | 2.240 (1.320-3.802) | 0.003 | 0.800 (0.410-1.561) | 0.514 |
| Vascular invasion (yes vs no) | 2.531 (1.529-4.188) | 0.000 | 1.962 (1.082-3.558) | 0.026 |
| Mucinous adenocarcinoma (yes vs no) | 1.063 (0.488-2.314) | 0.877 | ||
| Differentiation (poor vs moderate/well) | 0.799 (0.504-1.267) | 0.340 | ||
| B7-H3 (high vs low) | 1.781 (1.027-3.089) | 0.040 | 1.289 (0.721-2.307) | 0.392 |
| B3GALT4 (high vs low) | 1.597 (1.007-2.533) | 0.047 | 1.209 (0.721-2.307) | 0.392 |
| B7-H3 and B3GALT4 (high vs low) | 0.737 (0.552-0.983) | 0.038 | 1.153 (0.845-1.572) | 0.369 |
| B7-H3 high (B3GALT4 high vs low) | 2.283 (1.289-4.042) | 0.005 | ||
| B7-H3 low (B3GALT4 high vs low) | 0.321 (0.091-1.127) | 0.076 | ||
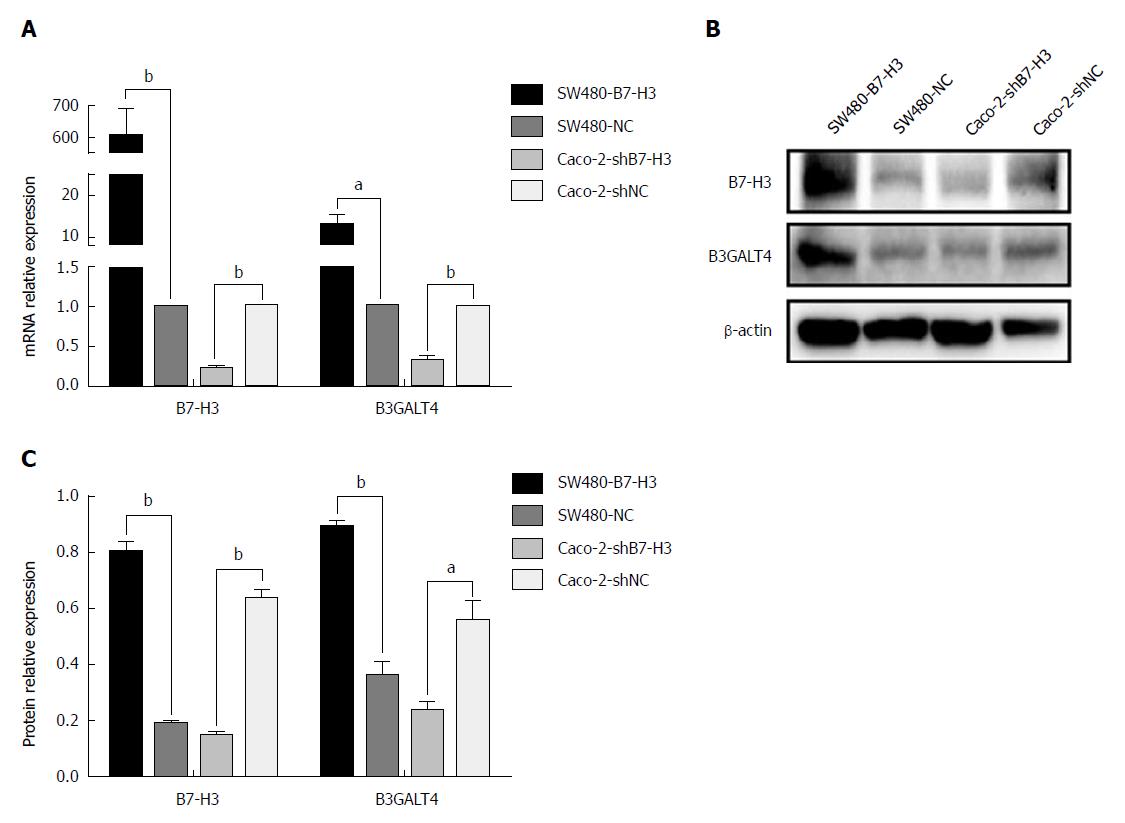
We further investigated the relationship between expression of B7-H3 and B3GALT4 in vitro. The mRNA and protein expression was detected in the CRC cell lines with different B7-H3 expression levels, which were maintained in our lab. Both the mRNA and protein expression of B7-H3 and B3GALT4 were significantly upregulated in SW480-B7-H3 cell line expressing a high level of B7-H3 as compared to SW480-NC cell line. Similarly, as compared to Caco-2-shNC cell line, Caco-2-shB7-H3 cell line with low expression of B7-H3 significantly downregulated the expression of B3GALT4. Therefore, B7-H3 and B3GALT4 had a positive correlation both in mRNA and protein expression in vitro (Figure 3).
CRC continues to be a major cause of cancer-related morbidity and mortality with over 1200000 cases diagnosed every year, and more than 600000 patients succumb to the disease worldwide. Moreover, five-year survival rates for stage I CRC was more than 90% where as for stage IV was 10%[10]. Given the poor prognosis of CRC, the search for novel diagnostic and prognostic biomarkers is highly desirable to prevent CRC-related deaths.
Immune checkpoint signaling pathways are most frequently modulated in cancer to inhibit the nascent anti-tumor immune response. Checkpoint antibody inhibitors, such as CTLA-4 and PD-1/PD-L1 are the most extensively studied novel class of inhibitors. Currently, drugs inhibiting these pathways are employed for a wide variety of malignancies and have demonstrated durable anti-tumor activities in a subset of cancer patients[11,12]. Furthermore, investigations on new inhibitory pathways are also undergoing, and drugs are being investigated including those targeting B7-H3[13]. As an immune checkpoint protein, B7-H3 has been recognized as an immunoregulatory molecule for T cell activation, proliferation, and cytokine production[14]. Thus far, overexpression of B7-H3 has been reported in several different cancer types and the expression level of B7-H3 correlated with tumor growth, invasion, metastasis, malignant stage, and recurrence rate[15]. Furthermore, several groups have generated anti-B7-H3 antibodies and reported overall suppression of tumor growth in vitro and in vivo[16]. Therefore, it is expected that B7-H3 plays a crucial role in cancer diagnosis and treatment. The present study revealed that B7-H3 was highly expressed in CRC tissue (70.4%) and significantly associated with the depth of tumor invasion (P = 0.049), distant metastasis (P = 0.034) and differentiation (P = 0.017). These results were consistent with previous findings[17,18].
Furthermore, B7-H3 is a type I transmembrane glycoprotein and plays a role in immunosuppression. Chen et al[19] demonstrated that glycoprotein B7-H3 was involved in the pathological process and tumor growth of oral squamous cell carcinoma, and the glycans of B7-H3 from oral cancer cells comprised of terminal α-galactoses and more diverse N-glycan structures with higher fucosylation than that of healthy cells. In addition, the presence of a carbohydrate-lectin receptor interaction between the tumor-associated B7-H3 from oral cancer cells and immune cells was detected[19]. B3GALT4, encoding a UDP-galactose: β-N-acetylgalactosamine β-1,3-galactosyltransferase activity, belongs to the human β-3-galactosyltransferase gene family[7]. Galactosyltransferase (GT) of the glycosyltransferase family, is the most common Golgi “marker” enzyme, catalyzes the transfer of galactose to glycoprotein-bound acetylglucosamine. The enzyme is provided with one N-linked oligosaccharide and palmitate residues and plays a vital role in the process of O-glycosylation. Moreover, in patients suffering from ovarian and breast cancer, increased expression of GT enzyme activity has been reported[20]. Enzymatic activity of B3GALT4 and T5 in ovarian cancer tissues, and significant overexpression of B3GALT4/T5 mainly in stage I uterine corpus cancers, indicated that B3GALT4/T5 is a novel tumor marker for uterine corpus cancer and other gynecological cancers[9]. Consequently, B7-H3 and B3GALT4 were both correlated with glycosylation and tumor progession. However, there was a paucity of literature on the expression and association of B7-H3 and B3GALT4 in CRC. Therefore, the association of B7-H3 and B3GALT4 expression in CRC tissues and cell lines was investigated.
The present study revealed that high expression of B7-H3 or B3GALT4 reduced the OS rate of CRC patients independently (P = 0.037, P = 0.044, respectively). Furthermore, overexpression of both B7-H3 and B3GALT4 in CRC resulted in poorer prognosis (P = 0.016). Besides, there was a significant positive correlation between B7-H3 and B3GALT4 (r = 0.219, P = 0.001). Taken together, B7-H3 and B3GALT4 could serve as a novel, reliable prognostic marker for CRC. Furthermore, we detected that the expression of B3GALT4 was positively altered following B7-H3 expression in vitro. Combined with the results of clinical samples, this study indicated that B7-H3 could positively regulate B3GALT4 at the mRNA as well as the protein level. However, the underlying mechanism of regulation of B7-H3 and B3GALT4 in CRC warrants further investigation.
In summary, the present study for the first time revealed the expression of B3GALT4 in CRC and its correlation with B7-H3 in vitro. Overall, the findings of the present study suggested that B7-H3 and B3GALT4 are novel prognostic biomarkers for CRC and highlight the significance of both B7-H3 and B3GALT4 as promising therapeutic targets for CRC. Thus, here we present our preliminary work on the relationship of the immune function and glycosylation of tumor-associated protein in CRC.
Colorectal cancer (CRC) is the most prevalent gastrointestinal tract malignancy worldwide. The prognosis of CRC patients remains relatively poor. B7 homolog 3 (B7-H3) mRNA is widely expressed on many tissues and cell types. However, B7-H3 protein is not constitutively expressed on T-cells, natural killer cells and antigen-presenting cells. The β-1,3-galactosyltransferase-4 (B3GALT4), which belongs to β-1,3-galactosyltransferase (β3GalT) gene family is abundantly expressed in human organs and tissues, predominantly in brain and involved in GM1/GD1 ganglioside synthesis. The β3GalT family may be closely related to the tumor.
There are insufficient reports about the correlation between B3GALT4 and CRC.
The aim of the present study is to investigate the clinical correlation of B7-H3 and B3GALT4 with CRC, and the correlation between the expression of B7-H3 and B3GALT4 was evaluated to determine their prognostic significance in CRC.
The authors identified the expression of B7-H3 and B3GALT4 in 223 CRC patient samples by immunohistochemistry and evaluated the possible correlation between B7-H3 and B3GALT4 and clinical outcomes. The mRNA and protein expression were also identified to establish the regulatory relationship of B7-H3 with B3GALT4 in vitro.
A significant positive correlation between B7-H3 and B3GALT4 was observed in CRC specimens. High expression of B7-H3 was identified as a significant independent predictor of poor overall survival (OS). High expression of B3GALT4 was also recognized as an independent predictor of inferior OS. In CRC cell lines with the stable expression of high B7-H3, the mRNA and protein expressions of B3GALT4 were significantly upregulated. The expression of B3GALT4 was significantly reduced when expression of B7-H3 was knocked down.
The expression of B3GALT4 in CRC and its positive correlation with B7-H3 in vitro was revealed, as well as B7-H3/B3GLAT4 as dual prognostic biomarkers for CRC.
The present study suggested that B7-H3 and B3GALT4 are novel prognostic biomarkers for CRC, and the significance of both B7-H3 and B3GALT4 as promising therapeutic targets for CRC are highlighted.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bordonaro M, Sipos F, Sulkowski S S- Editor: Wang JL L- Editor: Filipodia E- Editor: Huang Y
| 1. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2911] [Article Influence: 363.9] [Reference Citation Analysis (3)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13205] [Article Influence: 1467.2] [Reference Citation Analysis (3)] |
| 3. | Zhang Y, Chen Z, Li J. The current status of treatment for colorectal cancer in China: A systematic review. Medicine (Baltimore). 2017;96:e8242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Castellanos JR, Purvis IJ, Labak CM, Guda MR, Tsung AJ, Velpula KK, Asuthkar S. B7-H3 role in the immune landscape of cancer. Am J Clin Exp Immunol. 2017;6:66-75. [PubMed] |
| 5. | Ye Z, Zheng Z, Li X, Zhu Y, Zhong Z, Peng L, Wu Y. B7-H3 Overexpression Predicts Poor Survival of Cancer Patients: A Meta-Analysis. Cell Physiol Biochem. 2016;39:1568-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Shiina T, Kikkawa E, Iwasaki H, Kaneko M, Narimatsu H, Sasaki K, Bahram S, Inoko H. The beta-1,3-galactosyltransferase-4 (B3GALT4) gene is located in the centromeric segment of the human MHC class II region. Immunogenetics. 2000;51:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Amado M, Almeida R, Carneiro F, Levery SB, Holmes EH, Nomoto M, Hollingsworth MA, Hassan H, Schwientek T, Nielsen PA. A family of human beta3-galactosyltransferases. Characterization of four members of a UDP-galactose:beta-N-acetyl-glucosamine/beta-nacetyl-galactosamine beta-1,3-galactosyltransferase family. J Biol Chem. 1998;273:12770-12778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennett EP, Mandel U, Ragupathi G, Livingston PO, Hollingsworth MA, Taylor-Papadimitriou J. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010;70:1306-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 386] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 9. | Seko A, Kataoka F, Aoki D, Sakamoto M, Nakamura T, Hatae M, Yonezawa S, Yamashita K. Beta1,3-galactosyltransferases-4/5 are novel tumor markers for gynecological cancers. Tumour Biol. 2009;30:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2289] [Article Influence: 208.1] [Reference Citation Analysis (1)] |
| 11. | Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol. 2018;8:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 972] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 12. | Abdo J, Cornell DL, Mittal SK, Agrawal DK. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front Oncol. 2018;8:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 584] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 14. | Picarda E, Ohaegbulam KC, Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res. 2016;22:3425-3431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 390] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 15. | Li G, Quan Y, Che F, Wang L. B7-H3 in tumors: friend or foe for tumor immunity? Cancer Chemother Pharmacol. 2018;81:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Shi J, Zhang DL, Cui ZC, Wang HM. Preparation and application of a novel monoclonal antibody specific for human B7-H3. Mol Med Rep. 2016;14:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, Wang HT, Lu BF, Zhang XG. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Mao Y, Sun J, Wang WP, Zhang XG, Hua D. Clinical significance of costimulatory molecule B7-H3 expression on CD3(+) T cells in colorectal carcinoma. Chin Med J (Engl). 2013;126:3035-3038. [PubMed] |
| 19. | Chen JT, Chen CH, Ku KL, Hsiao M, Chiang CP, Hsu TL, Chen MH, Wong CH. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc Natl Acad Sci USA. 2015;112:13057-13062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Strous GJ. Golgi and secreted galactosyltransferase. CRC Crit Rev Biochem. 1986;21:119-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 1.9] [Reference Citation Analysis (0)] |