Published online Aug 14, 2018. doi: 10.3748/wjg.v24.i30.3440
Peer-review started: May 31, 2018
First decision: June 15, 2018
Revised: June 18, 2018
Accepted: June 30, 2018
Article in press: June 30, 2018
Published online: August 14, 2018
Processing time: 74 Days and 1.3 Hours
To introduce a novel, modified primary closure technique of laparoscopic extralevator abdominal perineal excision (LELAPE) for low rectal cancer.
We retrospectively analyzed data from 76 patients with rectal cancer who underwent LELAPE from March 2013 to May 2016. Patients were classified into the modified primary closure group (32 patients) and the biological mesh closure group (44 patients). The total operating time, reconstruction time, postoperative stay duration, total cost, postoperative complications and tumor recurrence were compared.
All surgery was successfully performed. The pelvic reconstruction time was 14.6 ± 3.7 min for the modified primary closure group, which was significantly longer than that of the biological mesh closure group (7.2 ± 1.9 min, P < 0.001). The total operating time was not different between the two groups (236 ± 20 min vs 248 ± 43 min, P = 0.143). The postoperative hospital stay duration was 8.1 ± 1.9 d, and the total cost was 9297 ± 1260 USD for the modified primary closure group. Notably, both of these categories were significantly lower in this group than those of the biological mesh closure group (P = 0.001 and P = 0.003, respectively). There were no differences observed between groups when comparing other perioperative data, long-term complications or oncological outcomes.
The modified primary closure method for reconstruction of the pelvic floor in LELAPE for low rectal cancer is technically feasible, safe and cost-effective.
Core tip: The modified primary closure approach requires laparoscopic closure of the pelvic peritoneum and layered closure of the perineal defect. By using this modified approach, the length of hospital stay and the total cost were decreased significantly, while other clinical outcomes did not differ, except for a relatively longer time for pelvic reconstruction (14.6 ± 3.7 min vs 7.2 ± 1.9 min). We conclude that the modified primary closure method for reconstruction of the pelvic floor in laparoscopic extralevator abdominal perineal excision for low rectal cancer is technically feasible, safe and cost-effective.
- Citation: Wang YL, Zhang X, Mao JJ, Zhang WQ, Dong H, Zhang FP, Dong SH, Zhang WJ, Dai Y. Application of modified primary closure of the pelvic floor in laparoscopic extralevator abdominal perineal excision for low rectal cancer. World J Gastroenterol 2018; 24(30): 3440-3447
- URL: https://www.wjgnet.com/1007-9327/full/v24/i30/3440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i30.3440
To improve the oncological outcome of patients with low rectal cancer, extralevator abdominal perineal excision (ELAPE) has been introduced to reduce the rate of positive circumferential margin and intraoperative perforation[1-4]. Assisted by laparoscopy, ELAPE can minimize physical invasion while ensuring oncological benefits[5,6]. However, the extended resection of ELAPE may increase the risk of severe perineal wound complications, including perineal hernia, with a reported incidence of 20%-26%[7]. Thus, how to reconstruct the pelvic floor and close the perineum after massive resection has become a major concern and challenge in laparoscopic ELAPE (LELAPE). The established reconstruction methods include: primary perineal closure, omentoplasty, biological or synthetic mesh placement, myocutaneous flaps, and negative wound pressure therapy[8-12]. These methods all have their own advantages as well as restrictions, and no consensus has been reached so far. In traditional abdominoperineal resection (APR), the pelvic peritoneum is usually closed prior to reconstruction of the pelvic floor, in order to separate the small intestine from the presacral operating field, which is technically challenging in LELAPE. We have recently modified the primary closure technique by adding the laparoscopic pelvic peritoneum suture procedure, and applied it to LELAPE. In the present study, we compare this method with biological mesh closure in the reconstruction of the pelvic floor after LELAPE, and evaluate its feasibility, safety and cost-effectiveness.
We retrospectively analyzed the data from 76 patients with rectal cancer undergoing LELAPE from March 2013 to May 2016. Patients were classified into the modified primary closure group (32 patients) and the biological mesh closure group (44 patients). Total operating time, reconstruction time, postoperative stay duration, total cost, postoperative complications and tumor recurrence were compared. The protocol was approved by the Ethics Committee of Qilu Hospital, Shandong University, Jinan, China.
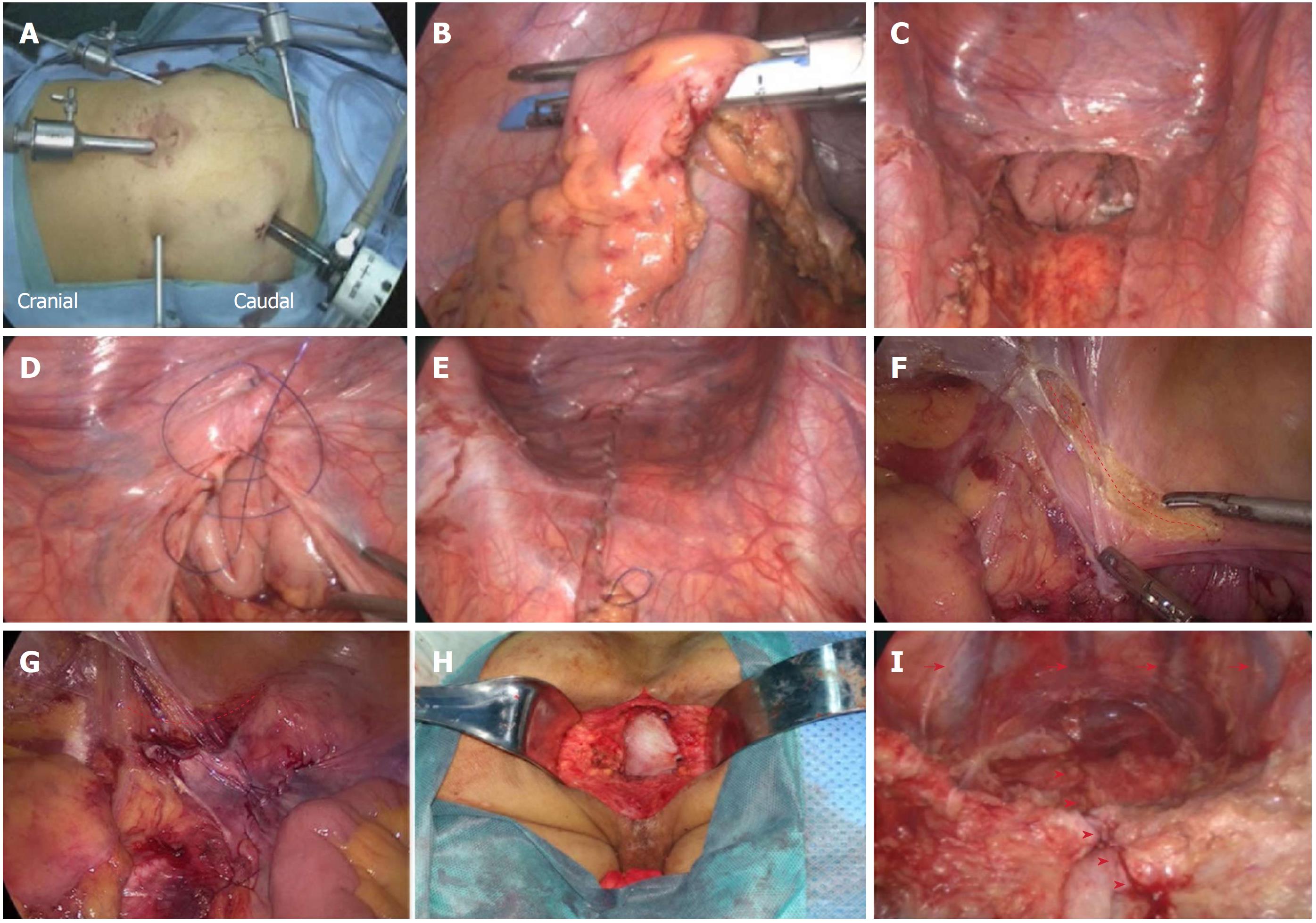
We have described the LELAPE procedure in a previous report[13]. The abdominal procedure was performed with the patient being placed in the Trendelenburg position, and the port placement was set up as shown in Figure 1A. After laparoscopic exploration, dissection and division of the pedicle of the inferior mesenteric vessels were performed. The sigmoid colon was mobilized from medial to lateral, and the rectum was mobilized following the total mesorectal excision principle. The sigmoid mesentery was trimmed at the rectosigmoid junction, where the rectum was transected with an endoscopic linear stapler (Figure 1B). The distal rectum and mesorectum were pushed down to the pelvic cavity (Figure 1C).
For modified primary closure, the pelvic peritoneum was closed with continuous suturing using a barbed suture (Covidien, Shanghai, China) (Figure 1D and E) before creation of a colostomy. For tension-free suturing, the adjacent pelvic peritoneum was dissected to reduce tension if necessary (Figure 1F and G). The patient was turned over to the prone jackknife position. The levator ani was transected at its origin, and a cylindrical specimen was removed. The procedure was completed with both the placement of one negative-pressure drainage tube in the presacral space, and the layered closure of the ischiorectal fat and skin. The drainage tube was removed when the drainage fluid was clear and < 10 mL in volume. The coccyx was not routinely removed.
For biological mesh closure, the patient was changed into a prone position for perineal dissection after creation of a colostomy. The levator ani was transected at its origin, and a cylindrical specimen was removed. A human acellular dermal matrix mesh (Ruinuo, Qingyuanweiye BioTissue Engineering Ltd., Beijing, China) was implanted and fixed to the tendinous arch by continuous prolene sutures (Covidien) for reconstruction of the pelvic floor (Figure 1H). The procedure was completed with both the placement of one negative-pressure drainage tube below the mesh, and the layered closure of the ischiorectal fat and skin. All the operations were finished by the same surgical group.
Numerical data were expressed as mean ± SD and analyzed with Student’s t tests. Categorical data were analyzed with the χ test or Fisher’s exact test. Repeated measures analysis of variance was performed for postoperative drainage and temperature change. All analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered to be statistically significant.
The baseline characteristics, including male/female ratio, age, body mass index, neoadjuvant therapy, distance to anal verge, and postoperative TNM staging, were comparable between the two groups (P > 0.05 each, Table 1). All patients were successfully followed up postoperatively for one year.
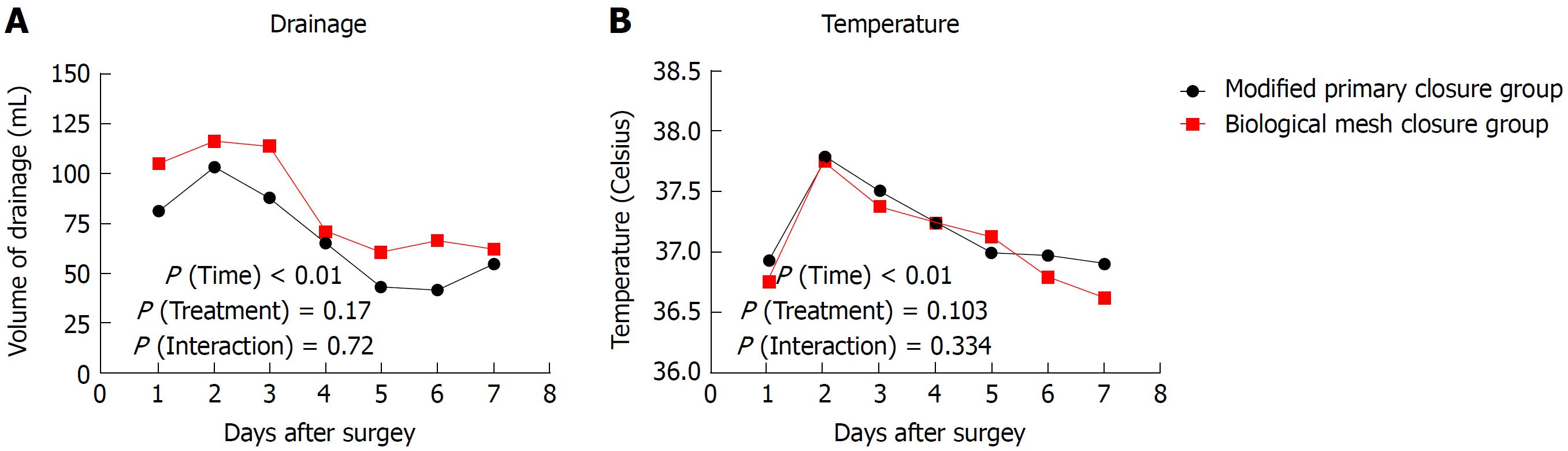
| Modified primary closure(n = 32) | Biological mesh closure(n = 44) | P value | |
| Male/female | 24/8 | 31/13 | 0.662 |
| Age (yr) | 52.8 ± 12.2 | 58.2 ± 12.5 | 0.137 |
| BMI | 26.8 ± 3.2 | 25.7 ± 2.7 | 0.097 |
| Neoadjuvant therapy | 8 | 7 | 0.326 |
| Tumor location, Distance to anal verge (cm) | 2.6 ± 0.8 | 2.8 ± 0.9 | 0.278 |
| Postoperative TNM staging | |||
| II | 23 | 29 | 0.581 |
| III | 9 | 15 | |
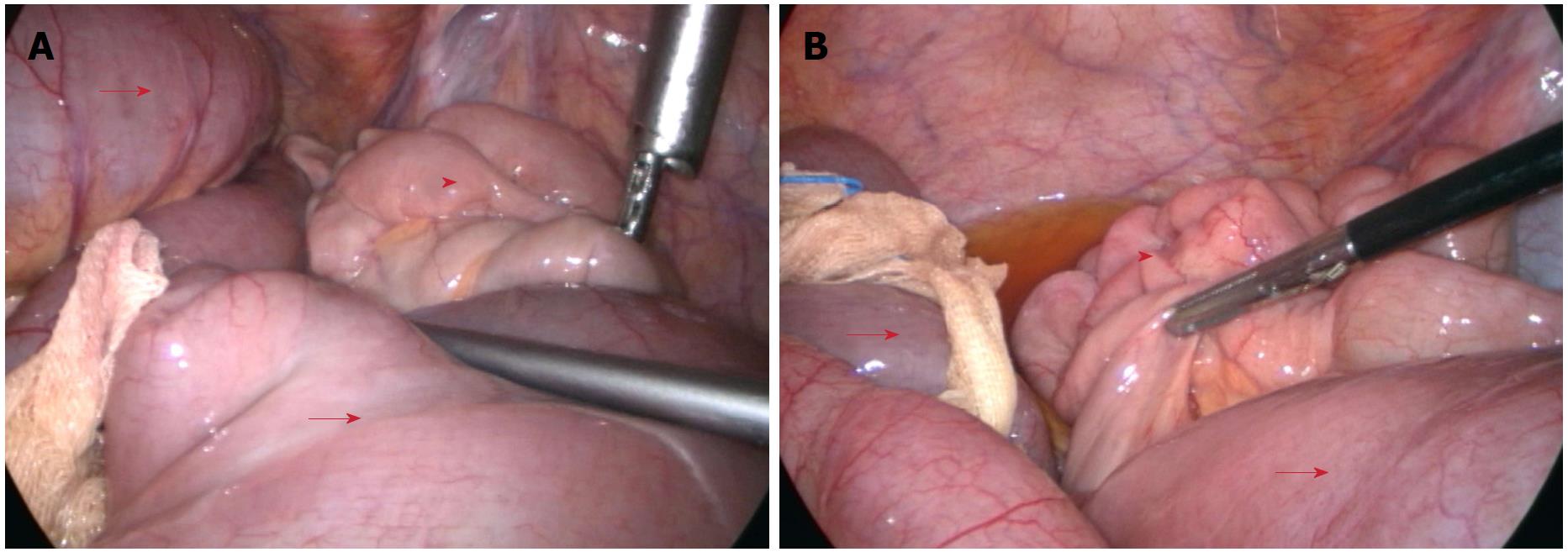
All operations were successfully performed without serious intraoperative complications. The pelvic reconstruction time was 14.6 ± 3.7 min for the modified primary closure group, which was significantly longer than that of the biological mesh closure group (7.2 ± 1.9 min, P < 0.001). The total operating time did not differ between the two groups (236 ± 20 min vs 248 ± 43 min, P = 0.143). One patient in the biological mesh closure group developed bowel perforation due to a large tumor within the anterior wall of the rectum. No positive circumferential margin was observed in either group. Intraoperative blood loss and recovery of bowel function were comparable between the two groups (both P > 0.05) (Table 2). The drainage tube was removed postoperatively at 6.6 ± 1.1 d in the modified primary closure group, which was earlier than in the biological mesh closure group (7.3 ± 2.0 d, P = 0.094). The volume of drainage fluid peaked at 2 d postoperatively and then decreased gradually, without any difference between the two groups (P treatment > 0.05, P interaction > 0.05) (Figure 2A). The temperature changes after operation showed a similar pattern to drainage volume, with no difference between the groups (P treatment > 0.05, P interaction > 0.05) (Figure 2B). Postoperative hospital stay was 8.1 ± 1.9 d, and the total cost was 9297 ± 1260 USD for the modified primary closure group. Both of these categories in this group were significantly less than those of the biological mesh closure group (P = 0.001 and P = 0.003, respectively) (Table 2). One patient in the biological mesh closure group developed postoperative intestinal obstruction at 40 d. Conservative therapy did not work, and a laparoscopic exploration was performed at 42 d. The middle part of the ileum, approximately 100 cm to the ileocecal junction, adhered to the pelvic floor, leading to dilation of the proximal small intestine (Figure 3). The patient was healed by decompression of the small intestine and intestinal rearrangement under laparotomy.
| Modified primary closure(n = 32) | Biological mesh closure(n = 44) | P value | |
| Reconstruction time (min) | 14.6 ± 3.7 | 7.2 ± 1.9 | < 0.001 |
| Total operative time (min) | 236 ± 20 | 248 ± 43 | 0.143 |
| Intraoperative blood loss (mL) | 165 ± 57 | 149 ± 52 | 0.242 |
| Positive CRM | 0 | 0 | N/A |
| Bowel perforation | 0 | 1 | 1.000 |
| Recovery of bowel function (h) | 22.8 ± 4.7 | 23.6 ± 5.0 | 0.475 |
| Intestinal obstruction | 0 | 1 | 1.000 |
| Drainage removal (days after surgery) | 6.6± 1.1 | 7.3 ± 2.0 | 0.094 |
| Postoperative hospital stay (d) | 8.1 ± 1.9 | 10.1 ± 2.8 | 0.001 |
| Cost (USD) | 9297 ± 1260 | 10719 ± 2360 | 0.003 |
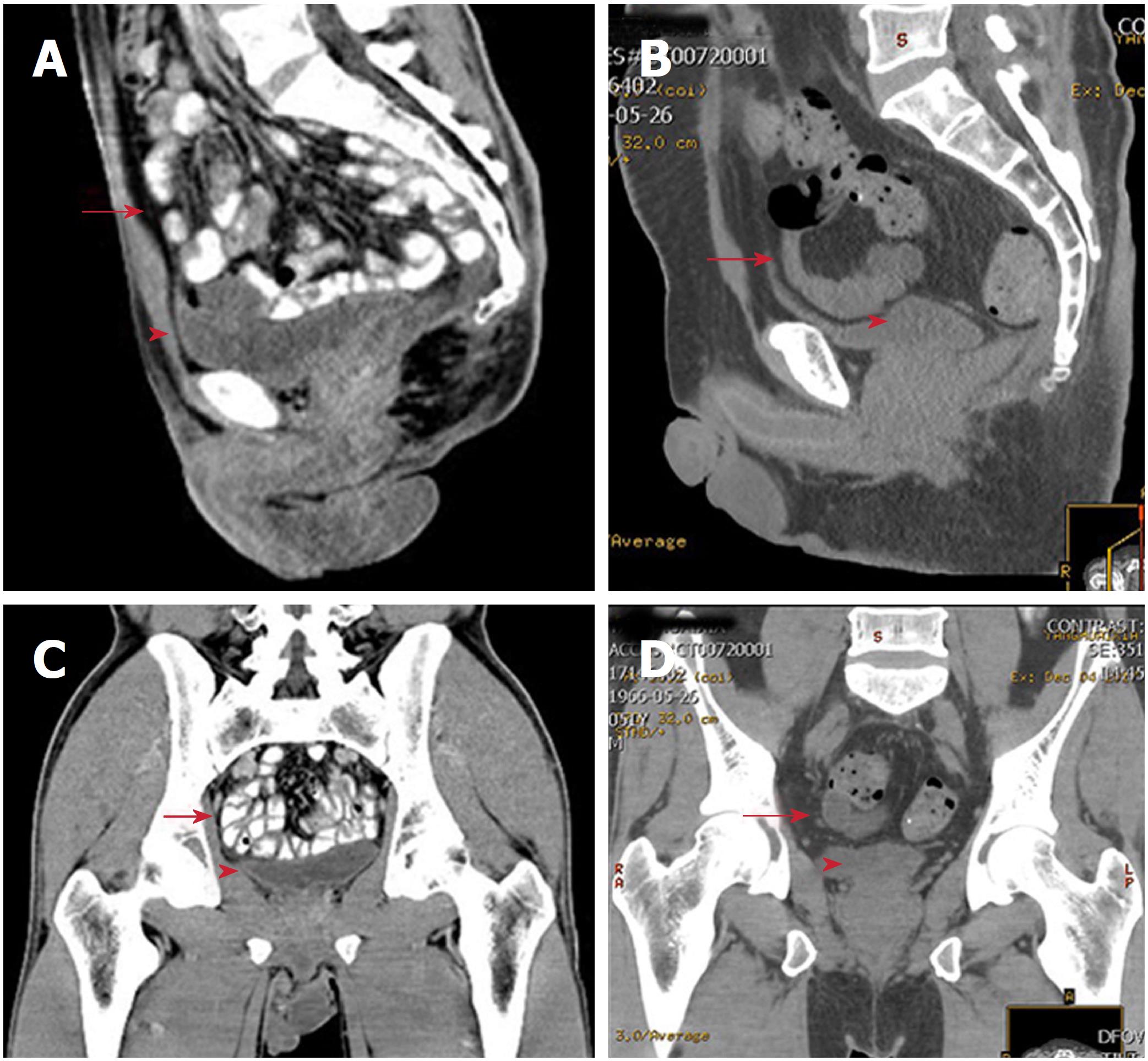
In the modified primary closure group, five patients had perineal wound infection. Within the first 10 d postoperatively, three patients had haemoserous discharge from the perineal wound, and were healed following potassium permanganate hip bath after 1 mo. At 12 d, one patient showed purulent discharge, which was solved after daily dressing change and thermal therapy. At 15 d, the perineal wound broke down in one female patient with type 2 diabetes. Debridement and secondary suturing were performed at 33 d after daily dressing change. Likewise, in the biological mesh closure group, five patients had perineal wound infection within the first 10 d (Table 3), and recovered within 60 d after appropriate treatment. No difference in infection rate or grade was found between the two groups (P > 0.05 each). Perineal hernia is theoretically expected to be more frequent without the placement of meshes. However, at 12 mo postoperatively, no perineal hernia occurred in either of the groups. Notably, four patients in the modified primary closure group and two in the biological mesh group experienced the feeling of bulging. Computed tomography at 12 mo showed that in the modified primary closure group, the small intestine was kept in the pelvic cavity with a clear descent of the pelvic peritoneum. In the biological mesh group, without suturing the pelvic peritoneum, the small intestine was also kept in the presacral space. No obvious postoperative differences were detected in the computed tomography scans between the two groups at 12 mo (Figure 4).
| Modified primary closure(n = 32) | Biological mesh closure(n = 44) | P value | |
| Normal perineal wound healing1 | |||
| 10 d postoperatively | 29 | 39 | 0.546 |
| 30 d postoperatively | 27 | 41 | 0.270 |
| 60 d postoperatively | 32 | 44 | 1.000 |
| Perineal wound infection | 5 | 5 | 0.734 |
| Clear or haemoserous discharge | 3 | 1 | 0.304 |
| Pus/purulent discharge | 1 | 2 | 1.000 |
| Deep infection with or without tissue breakdown | 1 | 2 | 1.000 |
| Postoperative perineal hernia (12 mo) | 0 | 0 | N/A |
| Postoperative feeling of bulge (12 mo) | 4 | 2 | 0.233 |
| Postoperative chemotherapy | 23 | 28 | 0.330 |
| Postoperative radiotherapy | 7 | 14 | 0.339 |
| Postoperative local recurrence (12 mo) | 0 | 1 | 1 |
| Postoperative liver/lung metastasis (12 mo) | 2 | 3 | 1 |
| Postoperative death (12 mo) | 0 | 0 | N/A |
Postoperative chemotherapy (the XELOX or FOLFOX regimen) and radiotherapy were given to 23 and seven patients in the modified primary closure group, as well as 28 and 14 patients in the biological mesh closure group, respectively (P = 0.330 and P = 0.339). In the biological mesh closure group, local recurrence occurred in one patient, who received only postoperative chemotherapy with the XELOX regimen, and the patient was subsequently treated with radiotherapy. Three patients had minor liver metastases and were cured with local ablative treatment. In the modified primary closure group, minor lung metastasis and minor liver metastases were found in two patients, respectively. Both of these patients received local ablative treatment. No patients died in either of the two groups.
The necessity of reconstruction of the pelvic floor after ELAPE has been widely accepted in order to avoid postoperative perineal complications[14,15]. However, the feasibility and superiority of various methods proposed for this reconstruction remain to be investigated. In the present study, we compared two methods, modified primary closure and biological mesh closure, in 76 patients with lower rectal cancer undergoing LELAPE. The major findings were that modified primary closure required longer reconstruction time, shorter postoperative hospital stay and was more cost-effective when compared to biological mesh closure. No difference in other perioperative data, long-term complications or oncological outcomes was observed.
Various methods have been developed for perineal wound healing after ELAPE. Of these, perineal closure with myocutaneous flaps, biological or synthetic mesh placement, and omentoplasty with perineal closure are currently the most widely performed[8-12]. Myocutaneous flaps can be obtained by various approaches, including gluteal rotation/advancement flaps[16], inferior gluteal artery myocutaneous island transposition flaps (IGAM)[17], transverse rectus/vertical rectus abdominis (TRAM/VRAM)[18,19], and gracilis[20]. Myocutaneous flaps have the benefit of delivering good perfusion and oxygenation, thus facilitating the healing process of large perineal defects. However, this approach requires plastic surgeons and may cause additional complications (e.g., a donor site hernia)[14]. Mesh repair has the advantage of reducing operative duration, and is therefore more cost-effective compared to myocutaneous flaps[21]. However, it should be noted that the inertness of biomesh might be a reason for small bowel obstruction[22], and synthetic mesh carries the potential for fistula formation[23]. By contrast, omentoplasty with perineal closure represents a safer approach for the reconstruction of the pelvic floor. Owing to its rich lymphovascular supply, the mobilized omentum in the pelvic cavity inhibits regional fluid collection, and hence prevents small intestine adhesion to the pelvic floor, thus dramatically reducing related complications[24]. For some patients, this technique may not apply when it is not technically feasible to mobilize the omentum to reach the pelvic cavity, or when the omentum has been resected previously.
The major strength of the modified primary closure method is the reconstruction of the pelvic peritoneum, which keeps the small intestine in the abdominal and pelvic cavities, thus avoiding adhesion to extraperitoneal tissues. In the present study, one case of intestinal obstruction in the biological mesh closure group appeared, which was caused by adhesion of the small intestine to the pelvic floor. However, the rate of postoperative intestinal obstruction did not show any difference between the two groups, which is likely due to insufficient study power. Compared with biological mesh closure, modified primary closure reduced postoperative hospital stay duration and total cost.
The pelvic floor is usually left open after APR due to the concern that incomplete closure of the pelvic floor may cause pelvic floor hernias and intestinal obstruction. However, APR is associated with clinically significant perineal hernias, albeit < 1% of the incidence[23]. ELAPE requires extensive resection of the pelvic floor, and thus contributes to the development of perineal hernias, with an incidence of 2.8% vs 0.8% compared to traditional APR[1]. As to LELAPE, perineal hernias could occur in nearly half of the patients without reconstruction of the pelvic floor[25]. In LELAPE, closure of the pelvic peritoneum is more challenging because the distal rectum has not been removed at that time. When mobilizing the sigmoid and rectum, the peritoneum on both sides should be intentionally preserved for re-approximation of the pelvic peritoneum. One possible concern is that the intentionally preserved peritoneum may lead to compromised oncological outcomes. However, in the present study, the oncological outcomes did not show any difference between the two groups. The rectum should be transected at the sigmoidorectal junction area, or even lower if possible. A continuous suture with barbed thread is recommended to facilitate the procedure. In obese patients, the peripheral peritoneum should be dissected to reduce tension. The perineal wound was directly sutured in layers. No pelvic floor hernias and perineal hernias occurred in all of our patients, with a mean followup of 12 mo. With regard to patients with rigid peritoneums after neoadjuvant radiotherapy or large pelvic peritoneum defects, this procedure may not be eligible and other reconstructive methods should be applied.
In conclusion, based on our preliminary experience, the modified primary closure method for reconstruction of the pelvic floor is technically feasible, safe and cost-effective. However, as the present study was retrospective, the safety and feasibility of this method still warrants high evidence-level research.
Laparoscopic extralevator abdominal perineal excision (LELAPE) was introduced to reduce the rate of positive circumferential margins and intraoperative perforation, however its extensive dissection requires reconstruction of the pelvic floor.
To introduce a novel modified primary closure technique of LELAPE for low rectal cancer.
To assess the feasibility, safety and cost-effectiveness of the newly introduced technique by comparing it with the traditional method.
Data from 76 patients with rectal cancer undergoing LELAPE from March 2013 to May 2016 were retrospectively analyzed. Patients were classified into the modified primary closure group (32 patients) and the biological mesh closure group (44 patients). Total operating time, reconstruction time, postoperative stay duration, total cost, postoperative complications and tumor recurrence were compared.
The modified primary closure of the pelvic floor requires longer reconstruction time, but total operating time was not different compared with the biological mesh closure group. The postoperative length of hospital stay and the total cost were both less in the modified primary closure group. No differences in other perioperative data, long-term complications or oncological outcomes were observed.
The modified primary closure method for reconstruction of the pelvic floor in LELAPE for low rectal cancer is technically feasible, safe and cost-effective.
Future multicentered randomized controlled trials should be performed to confirm the conclusions made in the present study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Manuscript source: Unsolicited manuscript
P- Reviewer: Higgins PD, Knittel T, Michael HJ, Tomiyasu A S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | West NP, Anderin C, Smith KJ, Holm T, Quirke P; European Extralevator Abdominoperineal Excision Study Group. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer. Br J Surg. 2010;97:588-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 283] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 2. | Yu HC, Peng H, He XS, Zhao RS. Comparison of short- and long-term outcomes after extralevator abdominoperineal excision and standard abdominoperineal excision for rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2014;29:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Yang Y, Xu H, Shang Z, Chen S, Chen F, Deng Q, Luo L, Zhu L, Shi B. Outcome of extralevator abdominoperineal excision over conventional abdominoperineal excision for low rectal tumor: a meta-analysis. Int J Clin Exp Med. 2015;8:14855-14862. [PubMed] |
| 4. | Stelzner S, Hellmich G, Sims A, Kittner T, Puffer E, Zimmer J, Bleyl D, Witzigmann H. Long-term outcome of extralevator abdominoperineal excision (ELAPE) for low rectal cancer. Int J Colorectal Dis. 2016;31:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Chi P, Chen ZF, Lin HM, Lu XR, Huang Y. Laparoscopic extralevator abdominoperineal resection for rectal carcinoma with transabdominal levator transection. Ann Surg Oncol. 2013;20:1560-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Kipling SL, Young K, Foster JD, Smart NJ, Hunter AE, Cooper E, Francis NK. Laparoscopic extralevator abdominoperineal excision of the rectum: short-term outcomes of a prospective case series. Tech Coloproctol. 2014;18:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Christensen HK, Nerstrøm P, Tei T, Laurberg S. Perineal repair after extralevator abdominoperineal excision for low rectal cancer. Dis Colon Rectum. 2011;54:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Musters GD, Buskens CJ, Bemelman WA, Tanis PJ. Perineal wound healing after abdominoperineal resection for rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. 2014;57:1129-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Peirce C, Martin S. Management of the Perineal Defect after Abdominoperineal Excision. Clin Colon Rectal Surg. 2016;29:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Sumrien H, Newman P, Burt C, McCarthy K, Dixon A, Pullyblank A, Lyons A. The use of a negative pressure wound management system in perineal wound closure after extralevator abdominoperineal excision (ELAPE) for low rectal cancer. Tech Coloproctol. 2016;20:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Alam NN, Narang SK, Köckerling F, Daniels IR, Smart NJ. Biologic Mesh Reconstruction of the Pelvic Floor after Extralevator Abdominoperineal Excision: A Systematic Review. Front Surg. 2016;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Ge W, Jiang SS, Qi W, Chen H, Zheng LM, Chen G. Extralevator abdominoperineal excision for rectal cancer with biological mesh for pelvic floor reconstruction. Oncotarget. 2017;8:8818-8824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Wang YL, Dai Y, Jiang JB, Yuan HY, Hu SY. Application of laparoscopic extralevator abdominoperineal excision in locally advanced low rectal cancer. Chin Med J (Engl). 2015;128:1340-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Butt HZ, Salem MK, Vijaynagar B, Chaudhri S, Singh B. Perineal reconstruction after extra-levator abdominoperineal excision (eLAPE): a systematic review. Int J Colorectal Dis. 2013;28:1459-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Jensen KK, Rashid L, Pilsgaard B, Møller P, Wille-Jørgensen P. Pelvic floor reconstruction with a biological mesh after extralevator abdominoperineal excision leads to few perineal hernias and acceptable wound complication rates with minor movement limitations: single-centre experience including clinical examination and interview. Colorectal Dis. 2014;16:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Anderin C, Martling A, Lagergren J, Ljung A, Holm T. Short-term outcome after gluteus maximus myocutaneous flap reconstruction of the pelvic floor following extra-levator abdominoperineal excision of the rectum. Colorectal Dis. 2012;14:1060-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Boccola MA, Rozen WM, Ek EW, Grinsell D, Croxford MA. Reconstruction of the irradiated extended abdominoperineal excision (APE) defect for locally advanced colorectal cancer. J Gastrointest Cancer. 2011;42:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Petrie N, Branagan G, McGuiness C, McGee S, Fuller C, Chave H. Reconstruction of the perineum following anorectal cancer excision. Int J Colorectal Dis. 2009;24:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | McMenamin DM, Clements D, Edwards TJ, Fitton AR, Douie WJ. Rectus abdominis myocutaneous flaps for perineal reconstruction: modifications to the technique based on a large single-centre experience. Ann R Coll Surg Engl. 2011;93:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Shibata D, Hyland W, Busse P, Kim HK, Sentovich SM, Steele G Jr, Bleday R. Immediate reconstruction of the perineal wound with gracilis muscle flaps following abdominoperineal resection and intraoperative radiation therapy for recurrent carcinoma of the rectum. Ann Surg Oncol. 1999;6:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Peacock O, Pandya H, Sharp T, Hurst NG, Speake WJ, Tierney GM, Lund JN. Biological mesh reconstruction of perineal wounds following enhanced abdominoperineal excision of rectum (APER). Int J Colorectal Dis. 2012;27:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Jess P, Bulut O. Small bowel obstruction after reconstruction of the pelvic floor with porcine dermal collagen (Permacol) after extended abdominoperineal extirpation for rectal cancer: report of two cases. Colorectal Dis. 2010;12:e178-e179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Mjoli M, Sloothaak DA, Buskens CJ, Bemelman WA, Tanis PJ. Perineal hernia repair after abdominoperineal resection: a pooled analysis. Colorectal Dis. 2012;14:e400-e406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Hultman CS, Sherrill MA, Halvorson EG, Lee CN, Boggess JF, Meyers MO, Calvo BA, Kim HJ. Utility of the omentum in pelvic floor reconstruction following resection of anorectal malignancy: patient selection, technical caveats, and clinical outcomes. Ann Plast Surg. 2010;64:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Sayers AE, Patel RK, Hunter IA. Perineal hernia formation following extralevator abdominoperineal excision. Colorectal Dis. 2015;17:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |