Published online Aug 14, 2018. doi: 10.3748/wjg.v24.i30.3398
Peer-review started: May 11, 2018
First decision: June 11, 2018
Revised: June 17, 2018
Accepted: June 28, 2018
Article in press: June 28, 2018
Published online: August 14, 2018
Processing time: 94 Days and 20.1 Hours
To develop a novel hepatocyte serum-free medium based on sericin, and to explore the effect of sericin on the hepatocyte transcriptome.
A controlled trial comparing novel serum-free medium and other media: C3A cells were cultured in our novel serum-free medium, HepatoZYME, complete medium (DMEM/F12 with 100 mL/L FBS), and DMEM/F12, and then cell attachment, proliferation, and function as well as the biocompatibility of the media were assessed. A comparative study of serum-free media with or without 2 mg/mL sericin: the effect of sericin on C3A growth was assessed by cell viability and proliferation, the effect of sericin on C3A cell cycle distribution was determined by flow cytometry, and the effect of sericin on the C3A transcriptome was assessed by gene-chip array and RT-qPCR.
More C3A cells attached to the plate containing our serum-free medium than to those containing HepatoZYME and DMEM/F12 at 24 h post-seeding. Both the viability and proliferation rate of C3A cells in sericin-based serum-free medium were superior to those of cells in HepatoZYME and DMEM/F12 (P < 0.001). The content of albumin and urea in our serum-free medium was significantly higher than that in HepatoZYME and DMEM/F12 throughout the whole culture period (P < 0.001) and was similar to that in complete medium at day 3, 4, and 5. In part 2, cell viability and proliferation were greater in the presence of 2 mg/mL sericin (P < 0.001), as was the proportion of cells in S phase (16.21% ± 0.98% vs 12.61% ± 0.90%, P < 0.01). Gene-chip array analysis indicated that the expression of CCR6, EGFR, and FOS were up-regulated by 2 mg/mL sericin, and RT-qPCR revealed that the expression of CCR6, EGFR, FOS, AKT1, JNK1, NFkB1, MMP-9, MEK2, ERK1/2 and MYC was up-regulated by 2 mg/mL sericin (P < 0.05).
We developed a novel hepatocyte serum-free medium. Sericin probably enhances cell attachment through the CCR6-Akt-JNK-NF-κB pathway and promotes cell proliferation through CCR6-mediated activation of the ERK1/2-MAPK pathway.
Core tip: In recent decades, few studies have focused on the development of hepatocyte serum-free medium. In this study, we developed a novel hepatocyte serum-free medium suitable for in vitro culture of C3A cells and applied an advanced method, gene-chip array, to explore the effect of sericin on the hepatocyte transcriptome. We found that sericin probably enhanced cell attachment through the CCR6-Akt-JNK-NF-κB pathway and promoted cell proliferation through CCR6-mediated activation of the ERK1/2-MAPK pathway. These findings inspired the following study on the mechanism by which sericin promotes cell attachment and proliferation.
- Citation: Huang Y, Peng Q, Li HY, Jia ZD, Li Y, Gao Y. Novel sericin-based hepatocyte serum-free medium and sericin’s effect on hepatocyte transcriptome. World J Gastroenterol 2018; 24(30): 3398-3413
- URL: https://www.wjgnet.com/1007-9327/full/v24/i30/3398.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i30.3398
The bioartificial liver support system (BALSS) is a novel and ideal therapy for hepatic insufficiency, which can provide additional liver function for patients with acute liver injury and end-stage liver failure[1]. During the BALSS operation, hepatocytes in the bioreactor perform various functions such as albumin synthesis, ammonia elimination, and bilirubin metabolism, which can decrease the symptoms of liver failure[2].
The BALSS is mainly composed of a hepatocyte culture module and an extracorporeal circulation device[3]. At the present time, the cells used in BALSS are mainly primary porcine hepatocytes[4] and immortalized cells, such as HepG2 and C3A[5]. C3A is a human hepatocellular carcinoma cell line, with high albumin production and excellent ability of ammonia elimination. Therefore, C3A is selected as the hepatocyte in the extracorporeal liver assist device (ELAD), which has proven to be effective in liver support and biocompatible in patients in clinical trials[6].
Normally, in vitro culture of hepatocytes requires serum of animal-origin. However, the serum possesses several shortcomings, including immunogenicity, allergenicity and exposure to microorganisms[7]. During the operation of the BALSS, the hepatocyte culture medium is in contact with the patient’s plasma in the bioreactor, resulting in the potential for a variety of adverse reactions such as anaphylaxis and bacteremia. Therefore, serum-free medium suitable for hepatocyte culture in the BALSS has been needed within recent decades. However, few studies have focused on this topic.
HepatoZYME-SFM, the most popular of all hepatocyte serum-free media, is a serum-free medium for the long-term maintenance of hepatocyte phenotypic expression including the active and inducible forms of cytochrome P450 and active phase II enzymes[8]. However, it is mainly used for serum-free primary hepatocyte culture, and serum is required for the adherence of hepatocytes at the early stage of serum-free culture with HepatoZYME.
Generally, serum-free medium comprises nutrients, growth factors, adherence-promoting factors, hormones, and trace elements. Advanced DMEM/F-12 (Dulbecco’s Modified Eagle Medium/Ham’s F-12) is a widely used basal medium that allows the culture of mammalian cells with reduced (10-50 mL/L) fetal bovine serum (FBS) supplementation, so it is often selected as the basal medium of the serum-free medium. Growth factor is the key component of serum-free culture medium, as it promotes cell growth. Hepatocyte growth factor (HGF) is a key ligand that elicits G1/S progression of epithelial cells, including hepatocytes, by up-regulating cyclin-E1 via the proline-mTOR pathway[9]. Epidermal growth factor (EGF) is not only a promoter of the growth of epithelial cells but also an important regulator that promotes CYP3A4 expression in hepatocytes[10]. Dexamethasone affects the growth of hepatocytes in a dose-dependent manner. HGF-induced DNA synthesis and proliferation in primary cultures of adult rat hepatocytes are promoted by dexamethasone at the concentration of 10-10 mol/L, but are inhibited at the concentration of 10-8 mol/L[11]. Furthermore, dexamethasone effectively induces gluconeogenesis in malignant hepatocytes both in vitro and in vivo by up-regulating PEPCK and G6Pase expression[12].
Sericin, a silk-derived protein that constitutes 20%-30% of silk, is soluble in the water and envelops the fibroin fibers on the surface of silk. Sericin is widely used in the garment industry, cosmetics, pharmaceuticals, and biomedical engineering because of its moisture-regulating ability, ultraviolet (UV) resistance, and antibacterial, anticancer, and anticoagulant properties[13-15]. It is well known that sericin has the ability to promote the attachment and proliferation of several mammalian cells[16], and as a consequence, sericin has been widely used in cell cultures. In DMEM containing 100 mL/L FBS, cell viability and proliferation of normal animal cells, tumor cells, hybridoma cells and normal mouse fibroblast L929 cells were improved by adding sericin at a certain concentration[17].
Along with the development of culture technology, sericin has also been used for 3D cell culture. Mandal et al[18] reported that a novel biopolymeric matrix fabricated by chemically cross-linking polyvinyl alcohol (PVA) with silk sericin was superior to PVA with respect to swellability, mechanical strength and flexibility, and cell attachment and viability. In another study, a silk sericin/gelatin 3-D scaffold showed enhanced mechanical strength, and higher compressibility, swellability, and porosity than a 2-D film. Moreover, improved cell attachment and viability, and low immunogenicity have suggested that a sericin/gelatin 3-D scaffold may be an ideal biomedical material[19]. Sericin has also been used for hepatocyte culture and cryopreservation, the glucose consumption, urea secretion rate, and intracellular albumin content of HepG2 cells were increased in sericin-alginate-chitosan microcapsules[20]. Miyamoto et al[21] demonstrated that a serum-free solution containing sericin and maltose improved the attachment capability of cryopreserved primary hepatocytes.
Although sericin has been shown to promote cell attachment, viability, and proliferation, the mechanism has been clarified. It is probably that the promotion of viability and proliferation is based on enhanced cell attachment. Aramwit et al[22] showed that sericin had the capability to improve the production of type-I collagen in the mouse fibroblast cell line L929 and that sericin increased the production of collagen in a dose-dependent manner within the range of 0.2-1.0 mg/mL.
Despite the advantages of sericin, it has not been used in the development of serum-free medium. The purpose of this study was to develop a novel serum-free medium suitable for in vitro hepatocyte culture based on sericin, growth factors, and other additives. In addition, the effects of sericin on the gene expression of hepatocytes were also investigated using a gene-chip array in order to explore the mechanism by which sericin promotes cell viability and proliferation.
There were two parts to this study. Part 1 was a controlled trial comparing the novel serum-free medium to other media. Part 2 was a comparative study between serum-free medium with or without sericin.
Sericin, insulin-transferrin-sodium selenite liquid media supplement (ITS), and 3-(4,5-dimethylthiazol-2)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich. Advanced DMEM/F12, HepatoZYME, L-glutamine, FBS, and phosphate-buffered saline (PBS) were purchased from Thermo Fisher Scientific. Penicillin-streptomycin and 2.5 g/L trypsin-EDTA solution were purchased from Leagene. HGF, EGF, and dexamethasone were purchased from R&D Systems. Dimethyl sulfoxide (DMSO) was purchased from MP Biomedicals. A Live/Dead kit (calcein AM/PI) was purchased from Dojindo. SYBR Green qPCR SuperMix (a qPCR kit) was purchased from Invitrogen. TRIzol reagent and the RNeasy Plus Mini kit (reagents for extracting RNA from cells) were purchased from Ambion and Qiagen, respectively. An Olympus phase-contrast microscope and fluorescence microscope were used for cell observation. A Bio-Tek ELx800 absorbance reader was used for the MTT assay. An Aeroset automated biochemistry analyzer was used for the measurement of albumin and urea. A BioPhotometer plus (Eppendorf) and an ABI PRISM®7500 Sequence Detection System (Thermo Fisher Scientific) were used for RT-qPCR. A BD FACSVerse was used for flow cytometry.
Sericin, HGF, and EGF were dissolved into PBS, and dexamethasone (Dex) was dissolved into DMSO at a certain concentration to create stock solutions, respectively. DMEM/F12 and HepatoZYME were employed as the basal medium for their corresponding group. The medium for each group in part 1 of the study was prepared by adding the supplements into the basal medium as shown in Table 1. In part 2, the medium used for group A was the same medium used for group A in part 1, while the medium used for group B was sericin knock-out (Table 2).
| Group A | Group B | Group C | Group D |
| DMEM/F12 | HepatoZYME | DMEM/F12 | DMEM/F12 |
| ITS 10 mL/L | L-Glutamine 2 μmol/mL | FBS 100 mL/L | PEN-SM 100 U/mL |
| HGF 20 ng/mL | PEN-SM 100 U/mL | PEN-SM 100 U/mL | |
| EGF 10 ng/mL | |||
| Dex 1 nmol/mL | |||
| Sericin 2 mg/mL | |||
| PEN-SM 100 U/mL |
| Group A (sericin) | Group B (control) |
| DMEM/F12 | DMEM/F12 |
| ITS 10 mL/L | ITS 10 mL/L |
| HGF 20 ng/mL | HGF 20 ng/mL |
| EGF 10 ng/mL | EGF 10 ng/mL |
| Dex 1 nmol/mL | Dex 1 nmol/mL |
| Sericin 2 mg/mL | PEN-SM 100 U/mL |
| PEN-SM 100 U/mL |
The human hepatocellular carcinoma cell line C3A was obtained from ATCC (ATCC® CRL-10741). C3A cells were inoculated into 25 cm2 flasks after thawing and cultured in DMEM containing FBS (100 mL/L) and penicillin-streptomycin (100 U/mL) under 50 mL/L CO2 at 37 °C. The medium was changed every two days. Once the cells reached confluence, they were harvested using 2.5 g/L trypsin-EDTA, followed by the addition of fresh culture medium to create a new single-cell suspension for further incubation. The concentration of FBS was gradually reduced from 100 mL/L to 50 mL/L, then to 20 mL/L, and finally to 10 mL/L. After stable growth was achieved in DMEM containing 10 mL/L FBS, the cells were cultured in the corresponding medium for each group.
Cell attachment after inoculation was evaluated by fluorescence microscopy using a Live/Dead kit. This method allows the simultaneous detection of both live and dead cells with calcein acetoxymethyl (calcein AM) and propidium iodide (PI) dyes. Calcein AM is a nonfluorescent and permeable reagent, which is converted by intracellular esterases to the intensely green fluorescent calcein. Propidium iodide enters dead cells through damaged membranes and produces a bright red fluorescence when bound to nucleic acids.
The cells were inoculated into 24-well plates at a density of 1 × 105 cells/well, and then cultured in the respective medium. Twenty-four hours later, the medium was discarded, and the cells were washed three times with PBS and incubated with calcein AM and PI under 50 mL/L CO2 at 37 °C for 15 min. The morphology and quantity of the cells were observed using a fluorescence microscope at the wavelengths of 490 nm and 545 nm, respectively. The number of live cells in each photo was counted using the ImageJ software.
Cell growth was investigated every 24 h for seven days. Briefly, the cells were inoculated into 12-well plates at a density of 1 × 105 cells/well, and cultured in the respective medium under 50 mL/L CO2 at 37 °C. Every 24 h, the medium was changed, and the cells in each group were counted using a phase-contrast microscope in triplicate.
The viability and the proliferation capacity of the cells were quantitatively assessed by MTT assay. This assay is based on the reduction of MTT (a tetrazolium salt solution) to purple formazan by metabolically active cells. For this analysis, 5 × 103 cells were inoculated into each well of 96-well plates, and subsequently cultured in the respective medium under 50 mL/L CO2 at 37 °C for seven days. Every 24 h, the cells were incubated with 1 mg/mL MTT for 4 h. After solubilization in DMSO for 10 min, the concentration of the formazan produced by the metabolically active cells was quantified at 490 nm with a Bio-Tek ELx800 absorbance reader in quintuplicate.
Cell function was assessed by the amount of albumin and urea produced by metabolically active cells. The cells were inoculated into 24-well plates at a density of 1 × 105 cells/well and cultured in the respective medium under 50 mL/L CO2 at 37 °C for seven days. The supernatant was collected every 24 h for quantitative testing of albumin and urea using an Aeroset automated biochemistry analyzer in triplicate.
The biocompatibility of the media was evaluated by aspartate transaminase (AST) and lactate dehydrogenase (LDH), which were released into the culture medium by the cells through damaged membranes after suffering an acute injury. Their concentrations in the culture media are correlated with membrane damage and the biocompatibility of the medium.
Every 24 h post-seeding, the supernatant was harvested for quantitative testing of AST and LDH using an Aeroset automated biochemistry analyzer in triplicate for seven days.
Cells were inoculated into 6-well plates at a density of 5 × 105 cells/well, and cultured in the respective medium under 50 mL/L CO2 at 37 °C. Seventy-two hours later, the cells were detached by trypsinization, centrifuged, and the pellet of cells was immediately fixed with ice-cold 700 mL/L ethanol. Afterwards, cells were washed three times with cold PBS to remove the ethanol, and finally stained with PI using a standard method[23]. Cells were analyzed by flow cytometry using BD FACSVerse.
The differences in the transcriptomes of the cells cultured in the presence of sericin were quantitatively assessed using the GeneChip® PrimeView™ Human Gene Expression Array. The cells were inoculated into 6-well plates at a density of 1.5 × 106 cells/well, and subsequently cultured in the respective medium under 50 mL/L CO2 at 37 °C. Seventy-two hours post seeding, the cells were harvested and total RNA was extracted using a Qiagen RNeasy Plus Mini kit. After the quality was verified using a Thermo Nanodrop 2000 and Agilent 2100 Bioanalyzer, the RNA was analyzed using GeneChip® PrimeView™ to reveal the differentially expressed genes related to proliferation. The expression differences of up-regulated genes were presented as fold-change (group A/group B ratio), and differences between samples were considered statistically significant at a value of fold-change > 1.5.
RT-qPCR was used to distinguish the differences of genes related to hepatocyte function in each group of part 1, and to verify the differentially expressed genes in the gene chip array of part 2. The cells were inoculated into 6-well plates at a density of 1.5 × 106 cells/well, and subsequently cultured in the respective medium under 50 mL/L CO2 at 37 °C. At each set time point, the cells were harvested and total RNA was extracted using a Qiagen RNeasy Plus Mini kit. After RNA quality verification, RT-qPCR was performed using an ABI PRISM®7500 Sequence Detection System and SYBR Green qPCR SuperMix. The genes related to hepatocyte function were assessed two, four, and six days after seeding, and included uridinediphosphate-glucuronosyl transferase (UGT: The enzyme catalyzing the conversion of unconjugated bilirubin into conjugated bilirubin), glutathione S-transferase (GST: The enzyme catalyzing the conjugation of the reduced form of glutathione to xenobiotic substrates for the purpose of detoxification), glutamate-ammonia ligase (GLUL: The key enzyme in glutamine synthesis), glucose-6-phosphatase (G6P: The key enzyme in gluconeogenesis and glycogenolysis), albumin, carbamoyl phosphate synthetase I (CPS1: The key enzyme in the production of urea), and cytochrome P-450 (CYP3A4, CYP2D6: the oxidizing enzymes in drug metabolism). The genes screened by the gene chip array were assessed one, two, three, and four days after seeding. The relative expression of each gene was presented as the value of 2-ΔΔCt.
The statistical analyses of the data in parts 1 and 2 were performed using one-way ANOVA and a t-test, respectively, with SPSS version 20.0 software. The results were expressed as the mean ± standard deviation (SD) using GraphPad Prism Software. Differences between samples were considered statistically significant at a value of P < 0.05.
In part 1, group A is our novel serum-free medium, group B is HepatoZYME, group C is the complete medium (DMEM/F12 with 100 mL/L FBS), and group D is DMEM/F12 (Table 1).
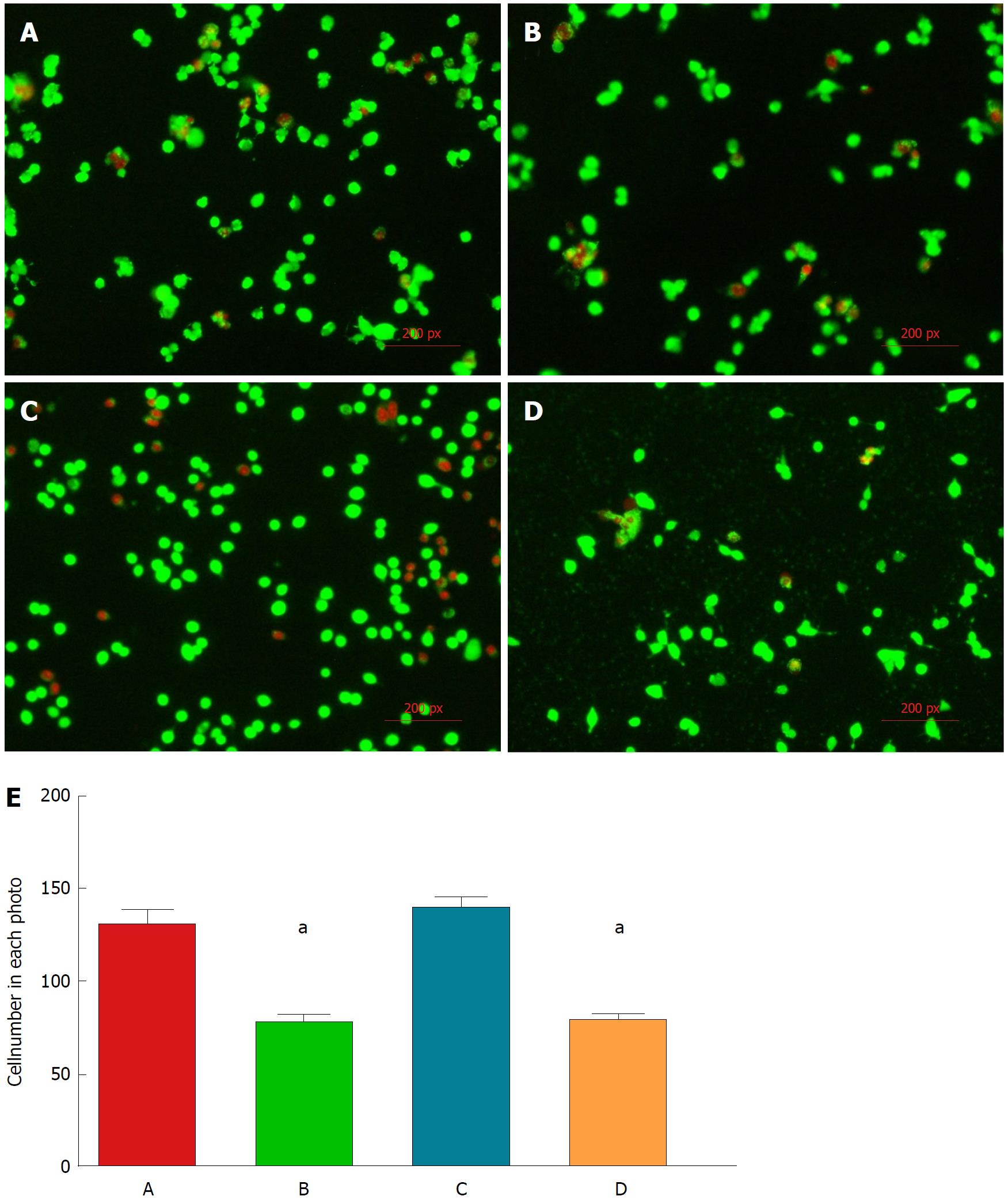
Live/dead fluorescence microscopy assay: Cell behavior in terms of attachment and viability was qualitatively investigated after 24 h of culture under standard conditions by fluorescence microscopy, based on the simultaneous staining of live (green-labeled) and dead (red-labeled) cells (Figure 1).
The density of attached living cells in group A was obviously higher than those in group B and group D, and almost approached that of group C. C3A cells in group A possessed a greater attachment capability than those in group B and group D (P < 0.01).
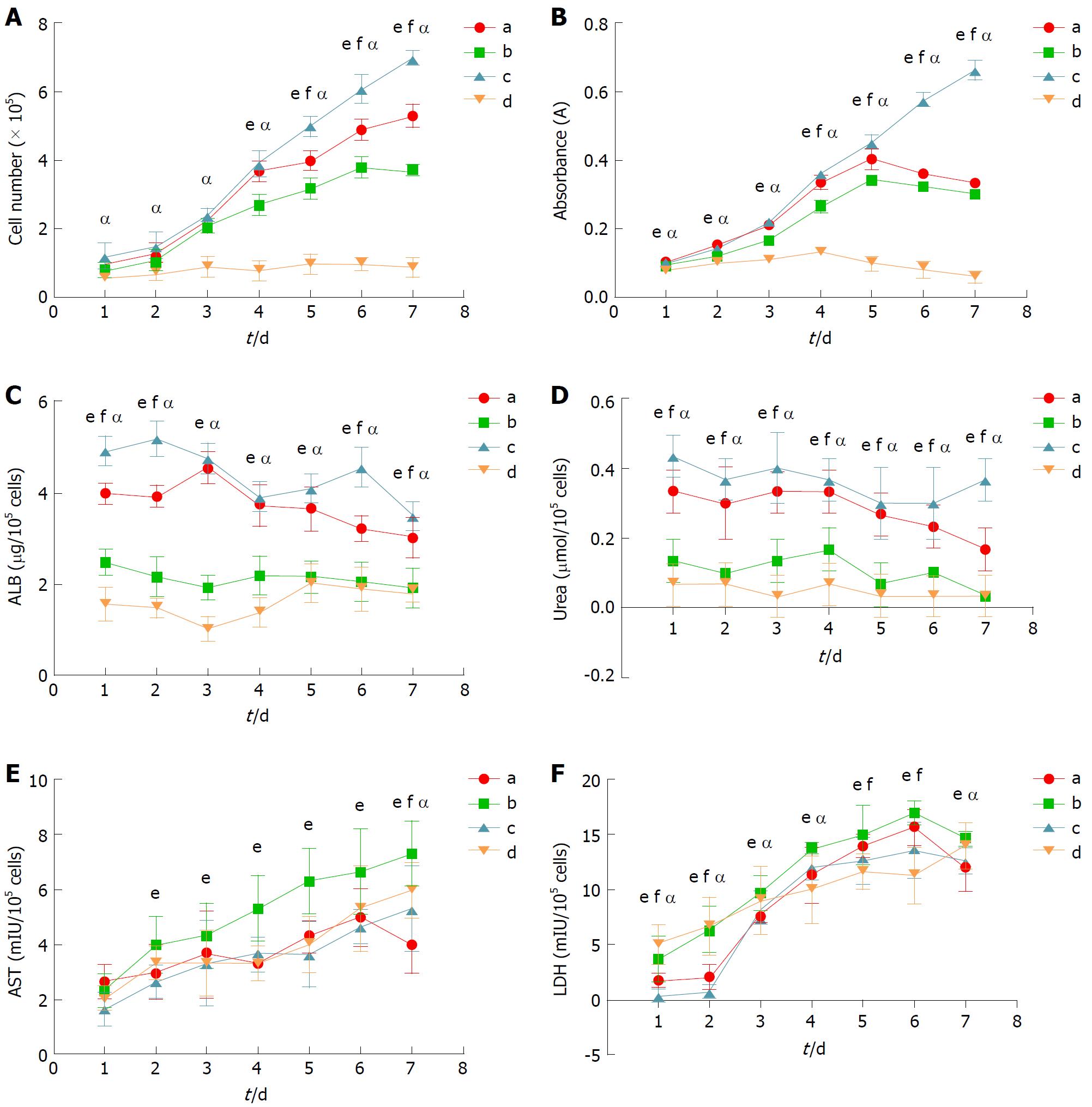
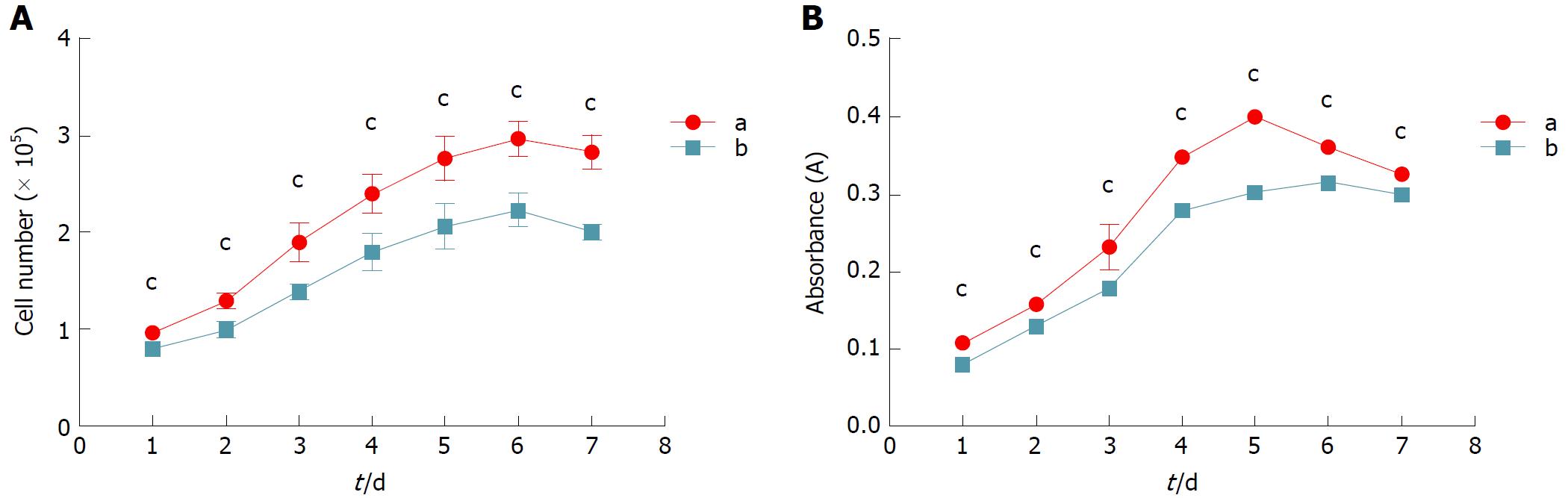
Cell growth curves: Cell growth was directly measured by counting the cell number in each group with standard hemocytometry every 24 h. During the seven days post-seeding, the cell number in each group increased gradually at different rates (Figure 2A). Within three days post-seeding, the difference in the cell numbers among groups A, B and C were not significant. Beginning on the fourth day post-seeding, the cell number in group A was significantly higher than those in group B and group D at each time point (P < 0.001). Although the daily cell number in group A was lower than that in group C starting on the fifth day post-seeding (P < 0.001), they were close in number during the first four days of culture.
Cell viability and proliferation assessment: To examine cell viability and the proliferation rate, the MTT assay was employed in which the number of metabolically active cells is linearly associated with the absorbance at 490 nm within a certain range. During the whole culture period, the curve of each group rose gradually at different rates (Figure 2B). Within three days post-seeding, the cell viability and proliferation of group A were similar to group C. Afterwards, the number of metabolically active cells in group A was less than that in group C (P < 0.001), and reached the maximum at day 5. The viabilities of groups A, B and D declined after day 5. Nevertheless, the daily number of metabolically active cells in group A was significantly greater than those in group B and group D during the whole culture period (P < 0.001).
Cell function assessment: Cell function was assessed by the quantity of albumin and urea produced by C3A cells. With respect to albumin (Figure 2C), the curves of group A and group C showed a slightly declining trend throughout the whole culture period. On days 1, 2, 6, and 7, the albumin in the supernatant of group A was lower than that in group C (P < 0.001), but there was no significant difference on days 3, 4, and 5. Throughout the culture process, the quantity of albumin secreted by C3A cells in group A was greater than that in group B and group D at each time point (P < 0.001). With respect to urea (Figure 2D), the content of urea in the supernatant of each group decreased gradually during the culture period. At each time point, the urea synthesized by the C3A cells in group A was lower than that in group C (P < 0.001), but it was significantly greater than that in group B and group D (P < 0.001).
Biocompatibility assessment for media: As they reflect hepatocyte injury, AST and LDH were selected to assess the biocompatibility of each medium with C3A cells. With respect to AST leakage (Figure 2E), there were no differences among the groups during the first 24 h. Beginning on day 2, AST leakage in groups A, C, and D was similar until day 6, and significantly lower than that in group B at each time point (P < 0.001). AST leakage in group A was lower than that in all the other groups on day 7 (P < 0.001). With respect to LDH leakage (Figure 2F), on days 1, 2, 5, and 6, LDH leakage in group A was greater than that in group C, but they shared a similar level at the other time points. During the whole culture period, LDH leakage in group B was significantly greater than that in group A and group C (P < 0.001). In the early stage of culture, the LDH content in the supernatant of group D was greater than that in group A and group C, and unexpectedly, LDH levels in group D was the lowest from day 4 to day 6 (P < 0.001).
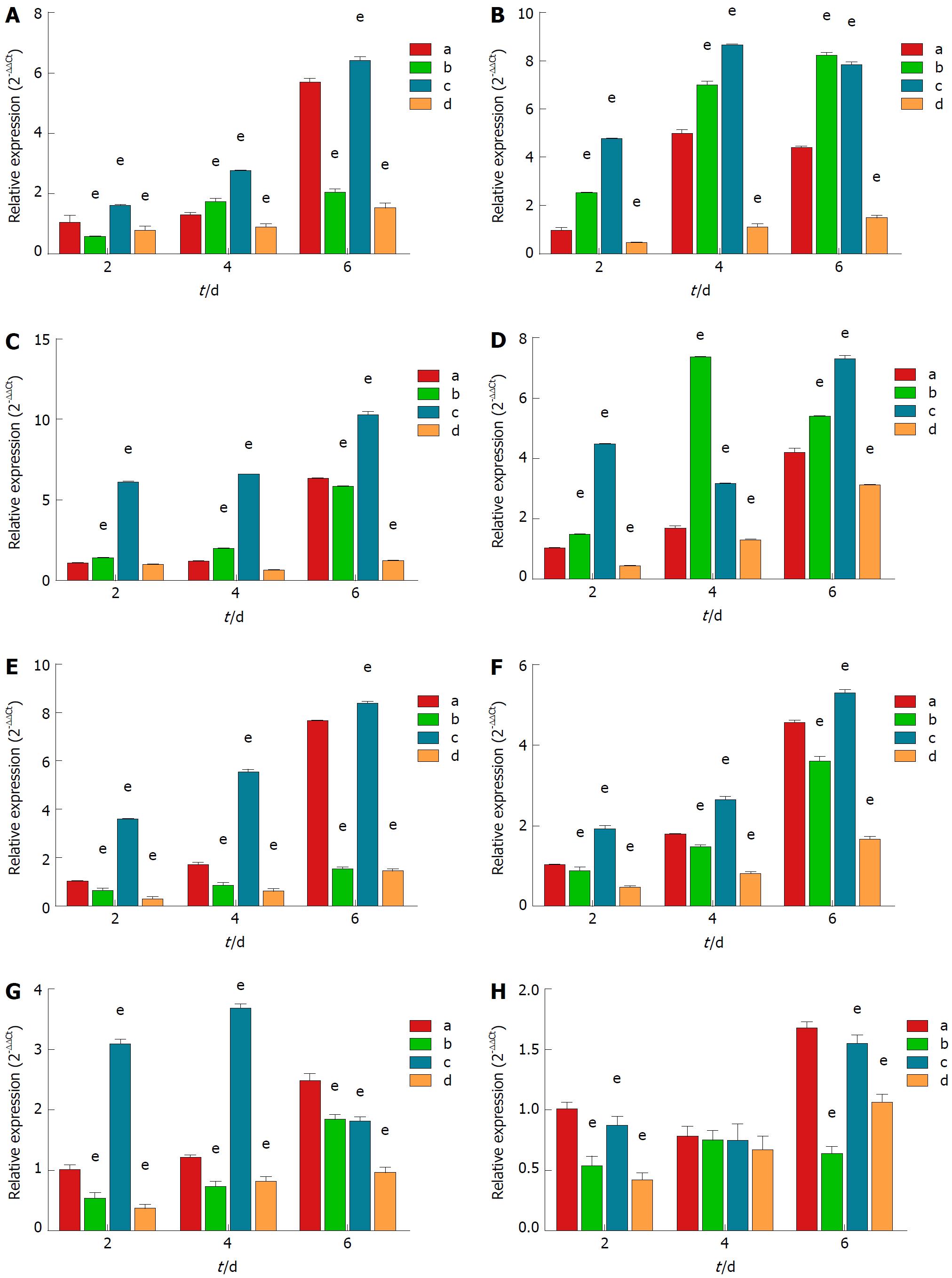
Expression of genes related to hepatocyte functions: The differences in gene expression related to hepatocyte functions were estimated by using RT-qPCR, a highly sensitive and effective method to detect the expression of specific genes. Uridinediphosphate-glucuronosyl transferase (UGT) (Figure 3A): On day 2 and day 6, the relative expression in group A was lower than that in group C, but higher than that in group B and group D (P < 0.001). On day 4, the relative expression in group A was slightly lower than that in group B and group C, but still higher than that of group D (P < 0.001). Glutathione S-transferase (GST) (Figure 3B): At each time point, the relative expression of group A was lower than that of group B and group C, but greater than that of group D (P < 0.001). Glutamate-ammonia ligase (GLUL) (Figure 3C): The relative expression in group C was higher than that in the other groups at all time points. The relative expression in group A was lower than that in group B at day 2 and 4, but significantly higher at day 6 (P < 0.002). Glucose-6-Phosphatase (G6P) (Figure 3D): At each time point, the expression in group A was lower than that in group B and group C, but higher than that in group D (P < 0.001). Albumin (Figure 3E): The relative expression in group A was lower than that in group C, but significantly higher than that in the other groups (P < 0.001). Carbamoyl phosphate synthetase 1 (CPS1) (Figure 3F): The expression in group A was greater than that in group B and group D, although lower than that in group C (P < 0.001). Cytochrome P 3A4 (CYP3A4) (Figure 3G): At each time point, the expression in group A was greater than that in group B and group D (P < 0.001). The expression in group A was lower than that in group C on days 2 and 4, but higher than that in the other groups on day 6 (P < 0.001). Cytochrome P 2D6 (CYP2D6) (Figure 3H): On day 2 and 6, the expression in group A was significantly higher than that in the other groups (P < 0.001); however, there were no significant differences on day 4.
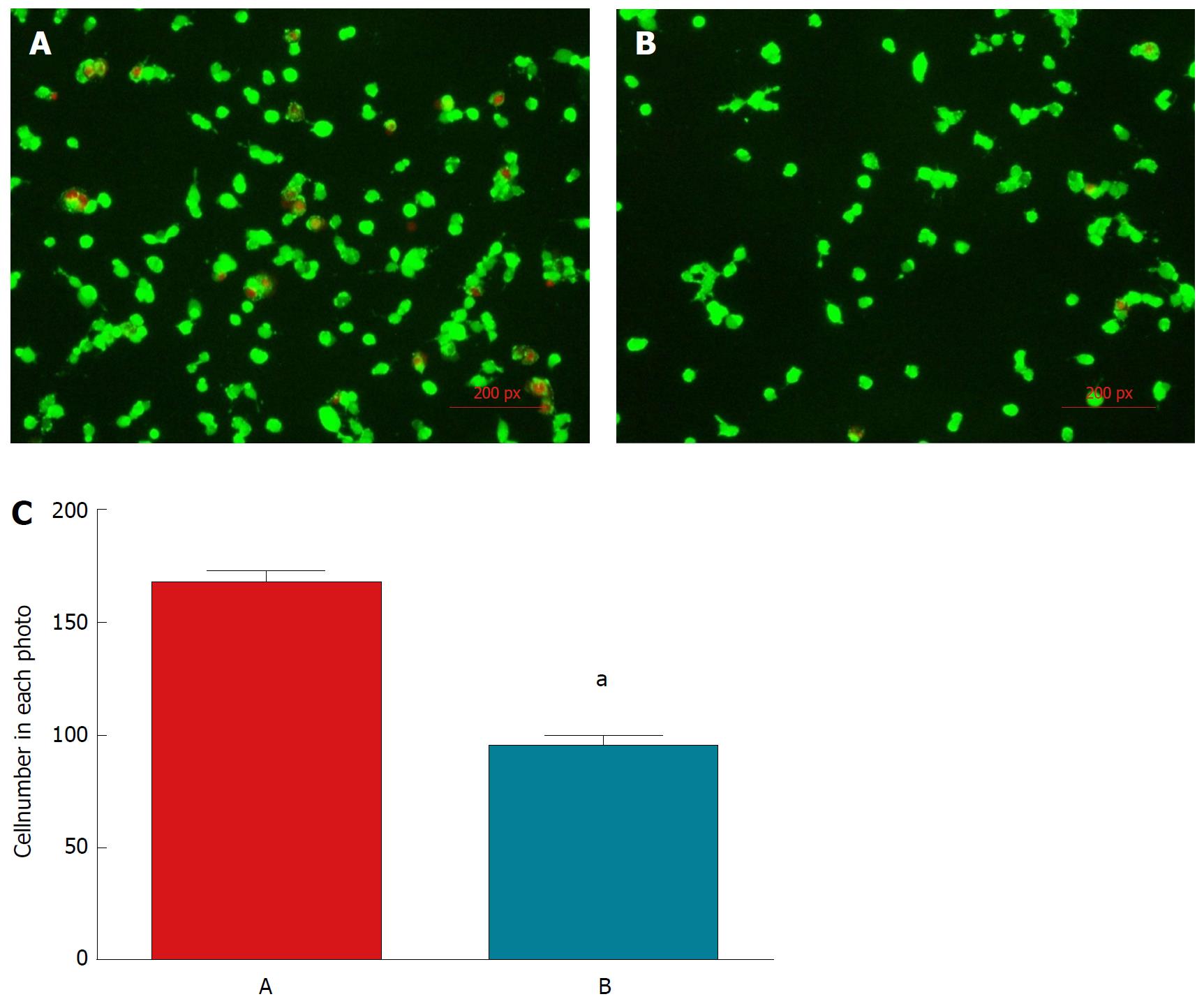
Live/dead fluorescence microscopy assay: The attachment capability of C3A cells was evaluated by the amount of live cells adhering to the plate (green-labeled) 24 h post-seeding. In the presence of sericin, a larger number of cells adhered to the surface (P < 0.01), and afterwards proceeded with proliferation as well as normal physiological functions (Figure 4).
Cell growth curves: Daily cell counting revealed that the cell growth curves of both groups rose gradually in the early stage of culture, then reached a plateau at day 5 (Figure 5A). At every time point, the cell growth in group A was superior to that in group B (P < 0.001).
Cell viability and proliferation assessment: The absorbance curves in both groups rose gradually within four days post-seeding (Figure 5B). On day 5, the viability of group A reached a peak value and then declined slightly, while that of group B reached a plateau. Over the whole culture period, there were more metabolically active cells in group A than in group B (P < 0.001).
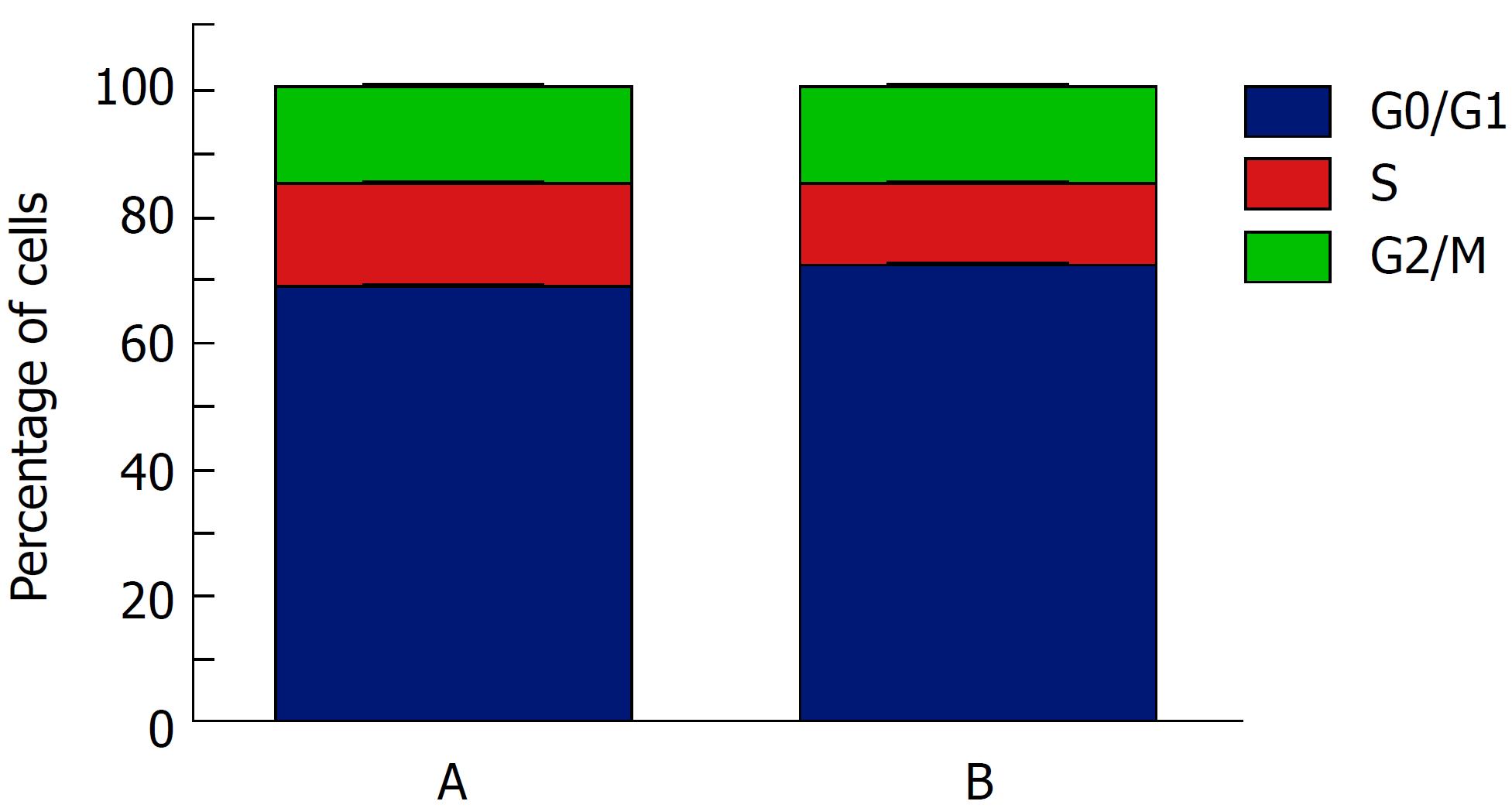
Cell cycle analysis: The cell cycle was divided as G0/G1, S, and G2/M phase, and S phase was the DNA synthesis period. Therefore, the percentage of cells in S phase of the whole cell population was used to evaluate cell proliferation. The cell cycle analysis indicated that the proportion of cells in S phase in group A was 16.21% ± 0.98%, while that in group B was 12.61% ± 0.90% (P = 0.009) (Figure 6). These results indicated that the cells in group A possessed a stronger proliferative capability than those in group B.
Gene chip array: Since the previous experiments verified that the attachment and proliferative capabilities of C3A cells were significantly enhanced by 2 mg/mL sericin, we decided to proceed with exploring the impact of sericin on the C3A transcriptome. The differential gene expression profile between the two groups was analyzed by gene chip array, and the expression differences in up-regulated genes were presented as fold-change (group A/group B ratio). A cut-off of > 1.5-fold increase was applied, and the results revealed that a total of 250 genes were significantly up-regulated by sericin.
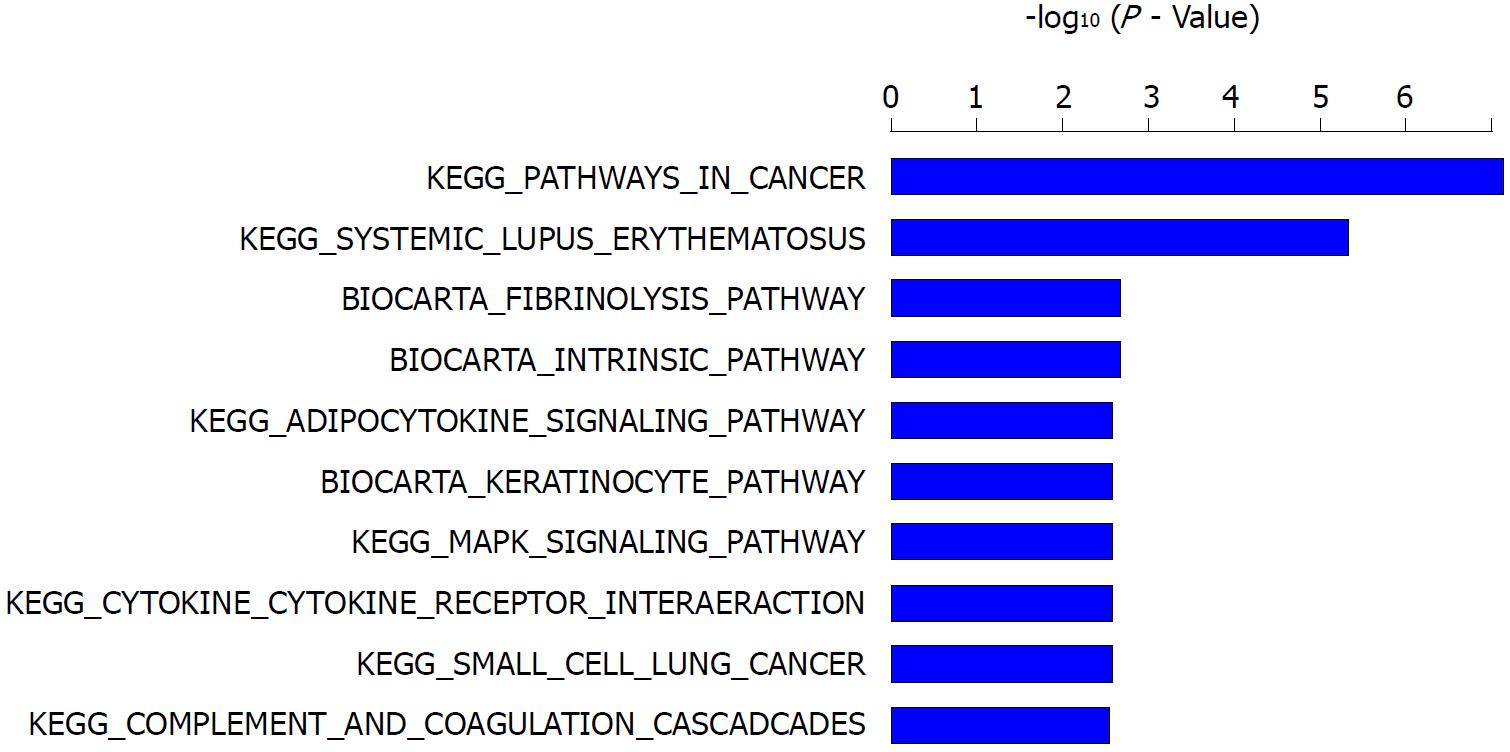
To characterize the signaling pathways modulated by sericin in C3A cells, a pathway enrichment analysis was conducted using Ingenuity Pathway Analysis (IPA) according to the KEGG and BIOCARTA databases. The top significantly enriched pathways modulated by sericin were determined (Figure 7), among which KEGG-pathways in cancer and the MAPK signaling pathway were associated with cell proliferation. The genes related to cell proliferation were COL4A6, MAX, EGFR, FOS, CDC42, FGF12, and SLC2A1 within the pathways in cancer, and EGFR, FOS, and MAPK13 within the MAPK signaling pathway. The classic MAPK signaling pathway is included in the pathways in cancer, so the genes included in both pathways, EGFR and FOS (Table 3), were considered to be the genes affecting the proliferation of C3A cells with the most potential.
| Up-regulated genes | Pathways involved | Fold change |
| CCR6 | Cytokine-cytokine receptor interaction | 1.99 |
| EGFR | Pathways in cancer, MAPK signaling pathway | 1.55 |
| FOS | Pathways in cancer, MAPK signaling pathway | 1.59 |
Subsequently, the other significantly up-regulated genes in group A in comparison with group B were screened by literature review to determine the genes related to proliferation. It has been demonstrated that CCR6 and the MAPK pathway are associated with the migration and proliferation of breast epithelial cells. Thus, RT-qPCR was used to verify the gene expressions of CCR6, EGFR, and c-FOS, as well as the molecules in the MAPK pathway, including Src, PI3K, AKT1, JNK1, NFkB1, MMP-9, GRB2, SHC2, K-RAS, RAF1, MEK2, ERK1/2, c-myc and cyclinE1.
mRNA expression of CCR6 and the MAPK pathway: The early stage is the key period for attachment and proliferation, so RT-qPCR was performed on the samples at days 1, 2, 3, and 4 post-seeding. The expression difference at each time point was presented as fold-change [fold-change = 2-(ΔCt groupA - ΔCt groupB)] in Table 4. CCR6: The expression of CCR6 in group A was greater than that in group B from inoculation until day 3 (P < 0.02), and CCR6 showed a 2.36-fold increase in group A compared to group B on day 3. EGFR: The expression in group A was greater at all time points (P < 0.01), and the increases of EGFR in group A were 7.71- and 2.34-fold compared to group B on days 2 and 4, respectively. FOS: On day 1 and day 3, the expression in group A was higher than that in group B (P < 0.001). Src: Beginning on day 2, the mRNA expression in group A was significantly higher than that in group B (P < 0.001), and the increases of Src in group A were 2.44- and 3.67-fold compared to group B on days 2 and 4, respectively. PI3K: The significant increases in expression in group A ranged from 3.00- to 3.10-fold compared to group B on days 1, 2 and 4 (P < 0.001). AKT1: At each time point, the expression in group A was greater than that in group B (P < 0.01), and the increase of AKT1 in group A was 2.04-fold compared to group B on day 3. JNK1: In the early stage of culture, the expression of the two groups was similar, but the expression in group A significantly increased 2.69- and 2.09-fold compared to group B on days 3 and 4 (P < 0.001). NFkB1: The expression in group A was greater than that in group B on days 2 and 3 (P < 0.001). MMP-9: The expression was greater in group A at all time points except on day 2 (P < 0.006). GRB2: On days 2 and day 3 the expression was significantly greater in the presence of sericin (P < 0.001). SHC2: Beginning on day 3, the expression was greater in group A (P < 0.001). K-RAS: The expression in group A was higher than that in group B at each time point except on day 1 (P < 0.01). RAF1: Beginning on day 2, the expression in group A was greater than that in group B (P < 0.001). MEK2: The significant increase in expression in group A ranged from 2.38- to 8.45-fold compared to group B (P < 0.001). ERK1: The expression of ERK1 in group A was greater than that in group B on the first two days (P < 0.001). ERK2: The expression was greater in group A at every time point (P < 0.001), and the fold change ranged from 1.63 to 5.15. c-myc: In the first three days, the mRNA expression of c-myc was higher in the presence of sericin (P < 0.001), and the fold change on day 1 was 2.32. cyclinE1: The expression in group A was greater except on day 1 (P < 0.001), and the fold change on day 3 was 2.21.
| Genes | FC in gene chip | FC1 | FC2 | FC3 | FC4 |
| CCR6 | 1.99 | 1.36 ± 0.091 | 1.40 ± 0.01 | 2.36 ± 0.041 | 0.75 ± 0.01 |
| EGFR | 1.55 | 1.76 ± 0.041 | 7.71 ± 0.491 | 1.46 ± 0.131 | 2.34 ± 0.241 |
| FOS | 1.59 | 1.22 ± 0.091 | 0.96 ± 0.07 | 1.77 ± 0.051 | 0.96 ± 0.01 |
| Src | 1.15 | 0.84 ± 0.161 | 2.44 ± 0.081 | 1.93 ± 0.701 | 3.67 ± 0.401 |
| PI3K | 1.24 | 3.00 ± 0.271 | 3.01 ± 0.391 | 1.43 ± 0.15 | 3.10 ± 0.141 |
| AKT1 | 1.13 | 1.22 ± 0.041 | 1.21 ± 0.051 | 2.04 ± 0.101 | 1.59 ± 0.061 |
| JNK1 | 1.09 | 0.98 ± 0.07 | 1.07 ± 0.11 | 2.69 ± 0.091 | 2.09 ± 0.041 |
| NFκB1 | 1.03 | 0.97 ± 0.07 | 1.51 ± 0.021 | 1.77 ± 0.041 | 0.84 ± 0.02 |
| MMP-9 | 1.22 | 1.38 ± 0.151 | 1.10 ± 0.06 | 1.51 ± 0.041 | 1.56 ± 0.041 |
| GRB2 | 1.05 | 1.04 ± 0.10 | 1.48 ± 0.031 | 1.34 ± 0.011 | 1.06 ± 0.05 |
| SHC2 | 1.30 | 0.98 ± 0.03 | 1.13 ± 0.06 | 1.39 ± 0.211 | 1.47 ± 0.171 |
| K-RAS | 1.09 | 1.16 ± 0.05 | 1.21 ± 0.081 | 1.42 ± 0.041 | 1.53 ± 0.011 |
| RAF1 | 1.01 | 1.09 ± 0.10 | 1.24 ± 0.021 | 1.48 ± 0.021 | 1.22 ± 0.031 |
| MEK2 | 1.02 | 5.86 ± 1.221 | 4.61 ± 1.111 | 2.38 ± 1.051 | 8.45 ± 1.001 |
| ERK1 | 1.07 | 1.80 ± 0.621 | 1.29 ± 0.031 | 1.02 ± 0.26 | 1.19 ± 0.02 |
| ERK2 | 1.18 | 2.58 ± 0.211 | 2.52 ± 0.621 | 5.15 ± 0.261 | 1.63 ± 0.061 |
| c-myc | 1.17 | 2.32 ± 0.211 | 1.67 ± 0.271 | 1.41 ± 0.301 | 0.06 ± 0.011 |
| cyclinE1 | 1.04 | 1.04 ± 0.13 | 1.52 ± 0.031 | 2.21 ± 0.021 | 1.48 ± 0.011 |
Serum is a necessary supplement for the in vitro culture of mammalian cells, because of its capability to simulate a suitable microenvironment similar to that of in vivo culture. However, serum is always removed from the culture for certain purposes, such as avoiding the impacts of serum on the experiment results and immunological rejection in heterogeneous animal in vivo experiments. To maintain normal attachment, proliferation, synthesis, and other cellular physiological processes, serum-free medium needs to contain a number of essential factors including basic nutrients, attachment promoters, growth factors, trace elements, and hormones[24,25].
In previous experiments, we selected several biomaterials including sericin, HGF, EGF, and dexamethasone as supplements in serum-free medium, followed by exploring their optimal concentrations in the serum-free medium. Finally, the most suitable serum-free medium was successfully prepared for subsequent controlled trials, and included HepatoZYME, DMEM/F12 and DMEM/F12 supplemented with 100 mL/L FBS (complete medium).
Sericin, the “glue” on the surface of the silk fibers, has been proven to be one of the perfect substitutes for serum[26]. Sericin has the ability to promote the attachment and proliferation of mammalian cells[27]. Our experiment exploring the best concentration of sericin in serum-free culture medium indicated that C3A cells proliferated at the highest rate in the medium containing 2 mg/mL sericin. In contrast, the C3A cells in the medium containing 5 mg/mL sericin exhibited growth arrest. This result corresponded to that reported by Terada et al[16], which demonstrated that an overload of sericin was harmful to the cells.
In this study, cell attachment, cell viability and proliferation, hepatocyte function, biocompatibility of the medium with the cells, and the expression of genes related to hepatocyte functions were examined in the different media. For hepatocytes, attachment to a carrier is necessary for biological processes. During the first 24 h after inoculation, a larger number of C3A cells adhered to the surface of the plate in our serum-free medium compared with HepatoZYME and DMEM/F12. In addition, the adherence approached that observed for a complete medium. The excellent adherence of C3A cells in our serum-free medium can be mostly attributed to the attachment-promoting capability of sericin, which has been shown in a number of other studies. Akturk et al[28] demonstrated that a sericin/collagen membrane had the ability to enhance the initial attachment of keratinocytes 24 h post-seeding, and adhesion proteins such as collagen, laminin, and fibronectin were also present in their sericin/collagen membrane, which even contained the arginine-glycine-aspartic (RGD) acid sequence recognized by the cell surface receptors integrins.
During the culture period, the daily cell number in our serum-free medium was similar to that in the complete medium in the early period, and greater than that in HepatoZYME and DMEM/F12 during the whole culture period. Furthermore, this result was consistent with the cell viability and proliferation assessment. As previously mentioned, sericin promotes cell proliferation, making our serum-free medium superior to other media with respect to cell growth. Although HGF and EGF are also mitogens for hepatocytes, the results of the experiments in part 2 indicated that sericin was the dominant growth promoter in our serum-free medium. However, in the later stage of culture, the viability of hepatocytes in the serum-free medium declined slightly, indicating that the promotion of proliferation by the serum-free medium on hepatocytes was a short-term (within the first four-five days of culture) effect.
Biocompatibility with the cells, tissues, and organs is an important evaluation standard for biomaterials. Sericin has been proven to be a biocompatible material for a variety of cells, as it does not cause cell cycle arrest and it releases few inflammatory mediators[19,29]. AST and LDH concentrations in the supernatant are commonly employed as indicators of acute hepatocyte damage because of their leakage from hepatocytes after injury. In this study, the AST concentration in our serum-free medium and the complete medium were similar during almost the whole culture period, and significantly lower than that in HepatoZYME at each time point. Similarly, LDH leakage in the serum-free medium was less than that in HepatoZYME. These results indicated that there was excellent biocompatibility of our serum-free medium with hepatocytes. However, low AST leakage should be attributed to not only the excellent biocompatibility of sericin but also the protection of HGF. Glanemann et al[30] demonstrated that pretreatment with HGF significantly reduced AST leakage in rat hepatocytes and reactive oxygen intermediate formation by increasing glutathione synthesis during inflammation.
As the core of the BALSS, the hepatocyte functions attract the most attention. In this study, biochemical assays and RT-qPCR were employed to evaluate hepatocyte functions. The albumin synthesis in the serum-free medium was similar to that in the complete medium in the middle of the culture period, and greater than that in HepatoZYME and DMEM/F12 at every time point. This result was consistent with the mRNA expression of albumin assessed by RT-qPCR. However, during long-term culture, the albumin synthesized by C3A cells was on a downward trend. In other words, the hepatocytes used in BALSS should be replaced every four-five days in order to ensure persistent albumin synthesis. The eximious ALB synthesis was associated with HGF in our serum-free medium, as shown by Hou et al[31]. Hou et al[31] manufactured a HGF/heparin-immobilized collagen system as a synthetic extracellular matrix for hepatocyte culture, in which albumin synthesis was greater than that in heparin-immobilized collagen, revealing that HGF promotes albumin synthesis in hepatocytes.
Though lower than that in complete medium, urea production in our serum-free medium was significantly greater than that in HepatoZYME and DMEM/F12, as indicated by both the quantitative detection of urea and the mRNA expression of CPS1.
UGT is the primary phase II enzyme catalyzing the conjugation of glucuronic acid to the xenobiotics, with polar groups facilitating their clearance[32]. The UGT expression in our serum-free medium was greater than that in HepatoZYME and DMEM/F12, and approached that in complete medium. CYP450 are phase I enzymes responsible for the metabolism of at least 90% of drugs[33], among which CYP3A4 and CYP2D6 are the most important. Our study showed excellent CYP450 expression in the serum-free medium, close to and even sometimes superior to that in the complete medium. Dexamethasone is considered an inducer of CYP3A4[34]. In sheep small intestine, dexamethasone caused a significant enhancement of CYP3A apoprotein level in the duodenal mucosa[35]. Thus, we suggest that dexamethasone played an important role in the excellent CYP450 expression in the serum-free medium. Although it was shown that dexamethasone could effectively up-regulate G6Pase expression[12], the result in our study was the opposite, probably because of the different concentration of dexamethasone used in the studies.
Compared with HepatoZYME and DMEM/F12, our serum-free medium exhibited advantages in C3A cell attachment, proliferation, functions, and biocompatibility, making it a perfect medium for serum-free hepatocyte culture.
Since the C3A cells exhibited excellent proliferation in our serum-free medium, the key factor, sericin, became the focus of the study. In part 2, we designed a controlled trial with two groups: the serum-free medium with or without sericin. Twenty-four hours after inoculation, more C3A cells were attached to the surface of the plate in the presence of 2 mg/mL sericin, followed by better proliferation and viability during the whole culture period, which corroborated the conclusions of other studies[19,20,36]. Based on its positive effects on cell proliferation, sericin has been added to antimicrobial creams and wound dressings to ameliorate wound healing[28,37].
Considering that sericin promotes cell proliferation, the experiments in part 2 were designed to explore the underlying mechanisms. As a key cyclin for liver regeneration[38], cyclinE1 is an important regulator of G1/S progression in hepatocytes. Up-regulated cyclinE1 could prompt cells to proceed from G1 phase to S phase, when DNA is synthesized in a large amount for the subsequent mitosis[9]. Our study clarified that the percentage of S phase cells was elevated in the presence of sericin, while that of cells in G0/G1 phase was reduced accordingly. In addition, the expression of cyclinE1 was significantly higher in the presence of sericin, indicating that the promotion of sericin for cell proliferation might result from active G1/S progression mediated by cyclinE1.
To explore the mechanism by which sericin promotes cell proliferation, we performed gene chip array analysis to distinguish the differences in the transcriptome between the two groups. Among the most significantly up-regulated genes, CCR6, EGFR, and FOS were implicated in cell proliferation.
Chemokine receptor 6 (CCR6) is the unique receptor of chemokine (C-C motif) ligand 20 (CCL20), which has been proven to promote the proliferation of malignant cells[39]. The up-regulation of CCR6 promotes spontaneous intestinal tumorigenesis[40]. Brand et al[41] demonstrated that CCR6 mediated the activation of Akt, ERK-1/2, and SAPK/JNK MAP kinases, resulting in increased intestinal epithelial cell migration and proliferation. In breast epithelial cells, CCL20/CCR6 binding promotes cell migration and proliferation by activating the MAPK pathway[42]. Fujii et al[43] also reported that CCL20 enhanced the growth of HuH7 cells via phosphorylation of p44/42 MAPK in vitro. As signaling molecules in the MAPK pathway, up-regulated EGFR and c-FOS have been demonstrated to be promotors of proliferation. EGFR is the receptor for EGF, the phosphorylation and activation of which induces proliferation of several cells[44,45]. Mitogenic effects of many substances are mediated by c-FOS[46], and its overexpression could increase the proliferation of human hepatocytes by stabilizing nuclear Cyclin D1[47], while its blockage inhibits the proliferation and invasion of cancer cells[48].
Based on the gene chip array results and conclusion of the other studies, we assessed CCR6 and molecules in the MAPK pathway by RT-qPCR to identify differential expression between the two groups. As a transmembrane receptor, the expression of CCR6 was up-regulated in the early post-seeding period, resulting in increased expression of molecules in the MAPK pathway at different time points. At the transcription level, it was speculated that sericin promoted attachment and proliferation by two pathways: CCR6 on the C3A cell membrane was activated in the presence of sericin; subsequently the signal was transduced through the activation of Src, PI3K, AKT1, JNK1 and NFkB1, and finally MMP-9 was up-regulated. MMP-9 is associated with cell migration because of its capability to remodel the extracellular matrix[49]. MMP-9 up-regulation through a Src-dependent pathway is consistent with previous studies showing that oleic acid and denatured type-IV collagen induced MMP-9 secretion and invasion in breast cancer cells[50,51]. The enhanced attachment of C3A cells by sericin was supposed to result from the increased migration. On the other hand, the CCL20/CCR6 binding transactivated the EGFR on the membrane, leading to a chain up-regulation of GRB2, SHC2, K-RAS, RAF1, MEK2 and ERK1/2. Furthermore, ERK1/2 translocates into the nucleus and enhances transcription of early-response genes including c-myc and c-FOS[52]. Subsequently, the up-regulation of c-myc and c-FOS increased the expression of cyclinE1, promoting the G1/S progression and proliferation of C3A cells. However, this inference has not been proven by protein expression, so more effort should be made to clarify the mitogenic mechanism of sericin.
There are some limitations in this study. Although it is proven that our serum-free medium is suitable for in vitro culture of C3A cells, and sericin promotes the attachment and proliferation of C3A cells, it is still not clear whether the results obtained are restricted to C3A cells. In the further study, we are going to verify whether this serum-free medium is suitable for other hepatocytes, and determine the effect of sericin on other hepatocytes, such as HepG2, HuH7 and primary porcine hepatocytes. Hyperammonemia and hyperbilirubinemia are the clinical features of patients with liver failure. However, the urea production under an overload of NH4+ and the ability to convert non-conjugated bilirubin into conjugated bilirubin of hepatocytes were not assessed in this study. These functions of hepatocytes will be assessed in the further study.
In summary, a novel serum-free medium for hepatocytes was developed in this study, and it exhibits excellent biocompatibility, and an enhanced capability of promoting cell attachment and proliferation, and provides a suitable microenvironment for hepatocyte functioning. It raises the possibility of large-scale serum-free culture of hepatocytes in the BALSS. In addition, the mechanism by which sericin promotes cell proliferation was explored, and it is speculated that sericin enhances cell attachment through the CCR6-Akt-JNK-NF-κB pathway, and promotes cell proliferation through CCR6-mediated activation of the ERK1/2-MAPK pathway.
A serum-free medium suitable for hepatocyte culture in the bioartificial liver support system (BALSS) has been needed within recent decades, but few studies have focused on the development of hepatocyte serum-free medium. Sericin was proven to promote cell attachment and proliferation, but the mechanism is not clarified.
Poor adherence and proliferation were often observed in the serum-free culture. Sericin has the ability to promote the attachment and proliferation of several mammalian cells, so it was selected as a key supplement in our serum-free medium. The mechanism how sericin promotes the attachment and proliferation of hepatocytes was not clarified. So, the effect of sericin on the hepatocyte transcriptome was explored in this study.
To develop a novel serum-free hepatocyte medium and to clarify the effect of sericin on the hepatocyte transcriptome.
Part 1 is a controlled trial comparing the novel serum-free medium and other media: C3A cells were cultured in our novel serum-free medium, HepatoZYME, complete medium (DMEM/F12 with 100 mL/L FBS), and DMEM/F12, then cell attachment, proliferation, and function as well as the biocompatibility of the media were assessed. Part 2 is a comparative study of serum-free media with or without 2 mg/mL sericin: The effect of sericin on C3A growth was assessed by cell viability and proliferation, the effect of sericin on C3A cell cycle distribution was determined by flow cytometry, and the effect of sericin on the C3A transcriptome was assessed by gene-chip array and RT-qPCR.
More C3A cells attached to the plate containing our serum-free medium than to those containing HepatoZYME and DMEM/F12 at 24 h post-seeding. Both the viability and proliferation rate of C3A cells in sericin-based serum-free medium were superior to those of cells in HepatoZYME and DMEM/F12. The content of albumin and urea in our serum-free medium was significantly higher than that in HepatoZYME and DMEM/F12 throughout the whole culture period, and was similar to that in complete medium at day 3, 4, and 5. In part 2, cell viability and proliferation were greater in the presence of 2 mg/mL sericin, as was the proportion of cells in S phase. Gene-chip array analysis indicated that the expression of CCR6, EGFR, and FOS were up-regulated by 2 mg/mL sericin, and RT-qPCR revealed that the expression of CCR6, EGFR, FOS, AKT1, JNK1, NFkB1, MMP-9, MEK2, ERK1/2 and C-MYC was up-regulated by 2 mg/mL sericin.
We developed a novel serum-free hepatocyte medium in this research and demonstrated that sericin probably enhances cell attachment through the CCR6-Akt-JNK-NF-kB pathway and promotes cell proliferation through CCR6-mediated activation of the ERK1/2-MAPK pathway.
In future studies, we will use the novel serum-free hepatocyte medium in large scale hepatocyte culture in the BALSS and assess the biocompatibility, immunogenicity and allergenicity in animal and clinical experiments. To clarify the mechanism of promotion of sericin on cell attachment and proliferation, we are going to study the protein level expression of CCR6-Akt-JNK-NF-kB pathway and ERK1/2-MAPK pathway components.
Our thanks are due to the staff of the Department of Hepatobiliary Surgery II and Institute of Regenerative Medicine in Zhujiang Hospital, Southern Medical University.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chmiela M, Grassi G, Jung Y S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | You S, Zhu B, Liu H, Rong Y, Liu W, Zang H, Zhang A, Wan Z, Xin S. Safety of Human Hepatoma Cell-Line Constructing Bioartificial Liver Supporting System Treating Patients with Liver Failure. Hepatogastroenterology. 2014;61:933-936. [PubMed] |
| 2. | Zhang Z, Zhao YC, Cheng Y, Jian GD, Pan MX, Gao Y. Hybrid bioartificial liver support in cynomolgus monkeys with D-galactosamine-induced acute liver failure. World J Gastroenterol. 2014;20:17399-17406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 3. | Lee JH, Lee DH, Park JK, Kim SK, Kwon CH, Lee SK. Potentiality of immobilized pig hepatocyte spheroids in bioartificial liver system. Transplant Proc. 2012;44:1012-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Sheil AG, Sun J, Mears DC, Waring M, Woodman K, Johnston B, Horvat M, Watson J, Koutalistras N, Wang L. Positive biochemical effects of a bioartificial liver support system (BALSS) in a porcine fulminant hepatic failure (FHF) model. Int J Artif Organs. 1998;21:43-48. [PubMed] |
| 5. | Tsiaoussis J, Newsome PN, Nelson LJ, Hayes PC, Plevris JN. Which hepatocyte will it be? Hepatocyte choice for bioartificial liver support systems. Liver Transpl. 2001;7:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Thompson J, Jones N, Al-Khafaji A, Malik S, Reich D, Munoz S, MacNicholas R, Hassanein T, Teperman L, Stein L. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: A multinational, prospective, controlled, randomized trial. Liver Transpl. 2018;24:380-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 7. | van der Valk J, Brunner D, De Smet K, Fex Svenningsen A, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML. Optimization of chemically defined cell culture media--replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. 2010;24:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 398] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 8. | Catania JR, McGarrigle BP, Rittenhouse-Olson K, Olson JR. Induction of CYP2B and CYP2E1 in precision-cut rat liver slices cultured in defined medium. Toxicol In Vitro. 2007;21:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Oka K, Ohya-Shimada W, Mizuno S, Nakamura T. Up-regulation of cyclin-E(1) via proline-mTOR pathway is responsible for HGF-mediated G(1)/S progression in the primary culture of rat hepatocytes. Biochem Biophys Res Commun. 2013;435:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Yin M, Yang H, Su X, Li Z, Yue Z, Zhang X, Sun D, Shi Y, Li D. Identification of EGF as an important regulator for promoting CYP3A4 expression in human embryonic stem cell-derived hepatocytes using TALEN-based gene targeting. J Genet Genomics. 2014;41:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Kimura M, Moteki H, Ogihara M. Inhibitory effects of dexamethasone on hepatocyte growth factor-induced DNA synthesis and proliferation in primary cultures of adult rat hepatocytes. J Pharmacol Sci. 2011;115:390-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Ma R, Zhang W, Tang K, Zhang H, Zhang Y, Li D, Li Y, Xu P, Luo S, Cai W. Switch of glycolysis to gluconeogenesis by dexamethasone for treatment of hepatocarcinoma. Nat Commun. 2013;4:2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Zhaorigetu S, Yanaka N, Sasaki M, Watanabe H, Kato N. Inhibitory effects of silk protein, sericin on UVB-induced acute damage and tumor promotion by reducing oxidative stress in the skin of hairless mouse. J Photochem Photobiol B. 2003;71:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Kurioka A, Kurioka F, Yamazaki M. Characterization of sericin powder prepared from citric acid-degraded sericin polypeptides of the silkworm, Bombyx Mori. Biosci Biotechnol Biochem. 2004;68:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Sano M, Tamada Y, Niwa K, Morita T, Yoshino G. Sulfated sericin is a novel anticoagulant influencing the blood coagulation cascade. J Biomater Sci Polym Ed. 2009;20:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Terada S, Nishimura T, Sasaki M, Yamada H, Miki M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology. 2002;40:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Cao TT, Zhang YQ. Viability and proliferation of L929, tumour and hybridoma cells in the culture media containing sericin protein as a supplement or serum substitute. Appl Microbiol Biotechnol. 2015;99:7219-7228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Mandal BB, Ghosh B, Kundu SC. Non-mulberry silk sericin/poly (vinyl alcohol) hydrogel matrices for potential biotechnological applications. Int J Biol Macromol. 2011;49:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Mandal BB, Priya AS, Kundu SC. Novel silk sericin/gelatin 3-D scaffolds and 2-D films: fabrication and characterization for potential tissue engineering applications. Acta Biomater. 2009;5:3007-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Nayak S, Dey S, Kundu SC. Silk sericin-alginate-chitosan microcapsules: hepatocytes encapsulation for enhanced cellular functions. Int J Biol Macromol. 2014;65:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Miyamoto Y, Teramoto N, Hayashi S, Enosawa S. An improvement in the attaching capability of cryopreserved human hepatocytes by a proteinaceous high molecule, sericin, in the serum-free solution. Cell Transplant. 2010;19:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Aramwit P, Kanokpanont S, De-Eknamkul W, Kamei K, Srichana T. The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J Biomater Sci Polym Ed. 2009;20:1295-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Darzynkiewicz Z, Halicka HD, Zhao H. Analysis of cellular DNA content by flow and laser scanning cytometry. Adv Exp Med Biol. 2010;676:137-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Bowen WC, Michalopoulos AW, Orr A, Ding MQ, Stolz DB, Michalopoulos GK. Development of a chemically defined medium and discovery of new mitogenic growth factors for mouse hepatocytes: mitogenic effects of FGF1/2 and PDGF. PLoS One. 2014;9:e95487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Fernández-Fígares I, Shannon AE, Wray-Cahen D, Caperna TJ. The role of insulin, glucagon, dexamethasone, and leptin in the regulation of ketogenesis and glycogen storage in primary cultures of porcine hepatocytes prepared from 60 kg pigs. Domest Anim Endocrinol. 2004;27:125-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Sahu N, Pal S, Sapru S, Kundu J, Talukdar S, Singh NI, Yao J, Kundu SC. Non-Mulberry and Mulberry Silk Protein Sericins as Potential Media Supplement for Animal Cell Culture. Biomed Res Int. 2016;2016:7461041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Dinescu S, Gălăţeanu B, Albu M, Lungu A, Radu E, Hermenean A, Costache M. Biocompatibility assessment of novel collagen-sericin scaffolds improved with hyaluronic Acid and chondroitin sulfate for cartilage regeneration. Biomed Res Int. 2013;2013:598056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Akturk O, Tezcaner A, Bilgili H, Deveci MS, Gecit MR, Keskin D. Evaluation of sericin/collagen membranes as prospective wound dressing biomaterial. J Biosci Bioeng. 2011;112:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Aramwit P, Kanokpanont S, De-Eknamkul W, Srichana T. Monitoring of inflammatory mediators induced by silk sericin. J Biosci Bioeng. 2009;107:556-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 30. | Glanemann M, Knobeloch D, Ehnert S, Culmes M, Seeliger C, Seehofer D, Nussler AK. Hepatotropic growth factors protect hepatocytes during inflammation by upregulation of antioxidative systems. World J Gastroenterol. 2011;17:2199-2205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Hou YT, Ijima H, Matsumoto S, Kubo T, Takei T, Sakai S, Kawakami K. Effect of a hepatocyte growth factor/heparin-immobilized collagen system on albumin synthesis and spheroid formation by hepatocytes. J Biosci Bioeng. 2010;110:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Yang N, Sun R, Liao X, Aa J, Wang G. UDP-glucuronosyltransferases (UGTs) and their related metabolic cross-talk with internal homeostasis: A systematic review of UGT isoforms for precision medicine. Pharmacol Res. 2017;121:169-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Shimada T. Inhibition of Carcinogen-Activating Cytochrome P450 Enzymes by Xenobiotic Chemicals in Relation to Antimutagenicity and Anticarcinogenicity. Toxicol Res. 2017;33:79-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Teo YL, Saetaew M, Chanthawong S, Yap YS, Chan EC, Ho HK, Chan A. Effect of CYP3A4 inducer dexamethasone on hepatotoxicity of lapatinib: clinical and in vitro evidence. Breast Cancer Res Treat. 2012;133:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Maté ML, Lifschitz A, Sallovitz J, Ballent M, Muscher AS, Wilkens MR, Schröder B, Lanusse C, Virkel G. Cytochrome P450 3A expression and function in liver and intestinal mucosa from dexamethasone-treated sheep. J Vet Pharmacol Ther. 2012;35:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Terada S, Sasaki M, Yanagihara K, Yamada H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J Biosci Bioeng. 2005;100:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Aramwit P, Palapinyo S, Srichana T, Chottanapund S, Muangman P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch Dermatol Res. 2013;305:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Nevzorova YA, Tschaharganeh D, Gassler N, Geng Y, Weiskirchen R, Sicinski P, Trautwein C, Liedtke C. Aberrant cell cycle progression and endoreplication in regenerating livers of mice that lack a single E-type cyclin. Gastroenterology. 2009;137:691-703, 703.e1-703.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Wallace AE, Catalano RD, Anderson RA, Jabbour HN. Chemokine (C-C) motif ligand 20 is regulated by PGF(2α)-F-prostanoid receptor signalling in endometrial adenocarcinoma and promotes cell proliferation. Mol Cell Endocrinol. 2011;331:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Nandi B, Pai C, Huang Q, Prabhala RH, Munshi NC, Gold JS. CCR6, the sole receptor for the chemokine CCL20, promotes spontaneous intestinal tumorigenesis. PLoS One. 2014;9:e97566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Brand S, Olszak T, Beigel F, Diebold J, Otte JM, Eichhorst ST, Göke B, Dambacher J. Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J Cell Biochem. 2006;97:709-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Marsigliante S, Vetrugno C, Muscella A. CCL20 induces migration and proliferation on breast epithelial cells. J Cell Physiol. 2013;228:1873-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Fujii H, Itoh Y, Yamaguchi K, Yamauchi N, Harano Y, Nakajima T, Minami M, Okanoue T. Chemokine CCL20 enhances the growth of HuH7 cells via phosphorylation of p44/42 MAPK in vitro. Biochem Biophys Res Commun. 2004;322:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Gao M, Zhan YQ, Yu M, Ge CH, Li CY, Zhang JH, Wang XH, Ge ZQ, Yang XM. Hepassocin activates the EGFR/ERK cascade and induces proliferation of L02 cells through the Src-dependent pathway. Cell Signal. 2014;26:2161-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Lanaya H, Natarajan A, Komposch K, Li L, Amberg N, Chen L, Wculek SK, Hammer M, Zenz R, Peck-Radosavljevic M. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat Cell Biol. 2014;16:972-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 46. | Biasin V, Chwalek K, Wilhelm J, Best J, Marsh LM, Ghanim B, Klepetko W, Fink L, Schermuly RT, Weissmann N. Endothelin-1 driven proliferation of pulmonary arterial smooth muscle cells is c-fos dependent. Int J Biochem Cell Biol. 2014;54:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Güller M, Toualbi-Abed K, Legrand A, Michel L, Mauviel A, Bernuau D, Daniel F. c-Fos overexpression increases the proliferation of human hepatocytes by stabilizing nuclear Cyclin D1. World J Gastroenterol. 2008;14:6339-6346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Long Y, Wu Z, Yang X, Chen L, Han Z, Zhang Y, Liu J, Liu W, Liu X. MicroRNA-101 inhibits the proliferation and invasion of bladder cancer cells via targeting c-FOS. Mol Med Rep. 2016;14:2651-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Cheng X, Yang Y, Fan Z, Yu L, Bai H, Zhou B, Wu X, Xu H, Fang M, Shen A. MKL1 potentiates lung cancer cell migration and invasion by epigenetically activating MMP9 transcription. Oncogene. 2015;34:5570-5581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Cortes-Reynosa P, Robledo T, Macias-Silva M, Wu SV, Salazar EP. Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol. 2008;27:220-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Soto-Guzman A, Navarro-Tito N, Castro-Sanchez L, Martinez-Orozco R, Salazar EP. Oleic acid promotes MMP-9 secretion and invasion in breast cancer cells. Clin Exp Metastasis. 2010;27:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |